Abstract
Objectives: The management of pediatric mid-dermal burns is challenging. Anecdotal evidence suggests Biobrane™ (UDL Laboratories, Inc., Sugar Land, TX) may expedite epithelization, reducing the requirement for skin grafting. Our standard management for burns of this depth is Acticoat™ (Smith and Nephew, St. Petersburg, Fl, USA). No publications are known to compare Biobrane™ to Acticoat™ for treatment of mid-dermal burns. Methods: A prospective, randomised controlled pilot study was conducted, comparing Biobrane™ to Acticoat™ for mid-dermal burns affecting ≥ 1% Total Body Surface Area (TBSA) in children. Mid-dermal burns were confirmed using Laser Doppler Imaging within 48 hours of injury. Participants were randomized to Biobrane™ with an Acticoat™ overlay or Acticoat™ alone. Results: 10 participants were in each group. Median age and TBSA were similar; 2.0 (Biobrane™) and 1.5 years (Acticoat™), 8% (Biobrane™) and 8.5% TBSA (Acticoat™). Use of Biobrane™ had higher infection rates (6 children versus 1) (P = 0.057) and more positive wound swabs, although not significant (7 children versus 4) (P = 0.37). Healing time was shorter in the Biobrane™ group, this was not significant (19 days versus 26.5 days, P = 0.18). Median dressing changes were similar (5 versus 5.5) (P = 0.56). Skin grafting requirement was greater in the Acticoat™ group (7 versus 4 children, P = 0.37) and similar in % TBSA (1.75% TBSA). Conclusion: This pilot study suggests that the use of Biobrane™ for mid-dermal burns in children may be associated with increased risk of infection but appears to decrease the time to healing and therefore the need for skin grafting compared to Acticoat™ alone.
Keywords: Burns, pediatric, biobrane™, acticoat™
Introduction
Management of mid-dermal burns in children can be extremely challenging. Even with Laser Doppler Imaging (LDI, Moor Instruments, Axminster, UK), which is a highly sensitive tool that can be used to predict time to healing [1,2], it is often difficult to determine whether these burns will heal within a 14 day period. Current management in our Burn Unit (BU) is to graft burns that do not heal by day 14 post-injury. This is based on studies that show that there is a correlation with hypertrophic scarring in those children with scald burns who take more than 14 days to heal [3,4]. The best treatment for mid-dermal depth burns in children is not known [5,6]. If there were a dressing able to expedite healing, this would be highly beneficial.
There are many studies that compare a range of dressings for partial thickness burns [5-10], however none that specifically look at the management of mid-dermal burns in children, and none that confirm and standardised the depth of the burn using LDI.
The standard treatment for mid-dermal depth burns in our BU is with Acticoat™ or Acticoat 7™ (Smith and Nephew, St. Petersburg, Fl, USA). There is some evidence that Biobrane™ (UDL Laboratories, Inc., Sugar Land, TX) may be superior for the treatment of mid-dermal burns [11]. There are many studies that compare Biobrane™ to other products, including silver dressing products [7,9,12-16]. There are none that compare Biobrane™ to our standard of care, Acticoat™.
Biobrane™ is a semi-permeable silicone membrane. It consists of a nylon mesh embedded with porcine collagen components [11,17]. It is applied to cleansed burn wound surface in an aseptic environment [11,17]. It is gently applied in a stretched fashion and secured to the wound with sterile tapes or glue [18]. In our BU, Acticoat 7™ is then applied directly over the top of Biobrane™, theoretically reducing wound colonisation and subsequent risk of infection.
One potential significant complication that may be linked with Biobrane™, as it does not have any specific antimicrobial properties, is that of wound infection [19-21]. Acticoat™ has well recognised antimicrobial properties, reducing wound colonisation and therefore theoretically expediting healing. One potential complication of silver based dressings is that the mechanism of action which produces its antimicrobial activity may also have a deleterious effect on wound epithelisation, and in fact delay epithelisation due to cytotoxic effects [22,23], leading to an increase in the days to healing and the requirement for skin grafting.
This is the first study to compare Biobrane™ to Acticoat™, which is the current standard of care for many BUs.
Methods
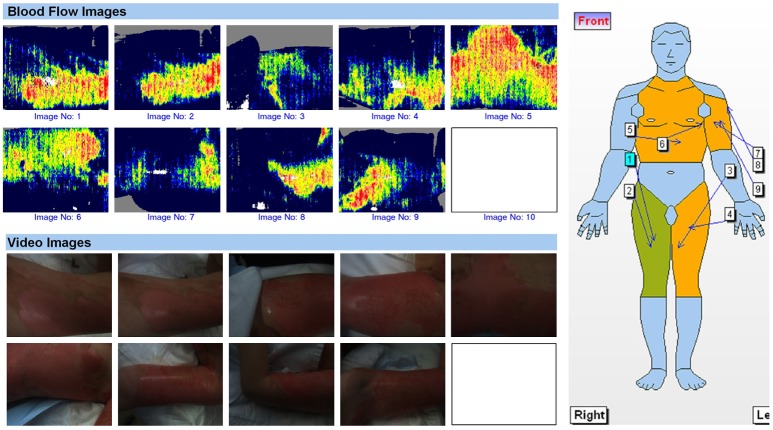
A prospective randomized controlled pilot study was undertaken from July 2012-February 2015, involving 20 children (≤ 16 years) who presented to the BU at the Children’s Hospital at Westmead (CHW) with an LDI confirmed mid-dermal burn involving more than 1% total body surface area (TBSA). Informed consent was obtained and computer generated randomization determined the treatment group to which each child was assigned on day 1 or 2 post injury. Children were then randomized into either a 7 day Acticoat™ dressing alone or a Biobrane™ dressing with a 7 day Acticoat™ overlay. LDI and application of the dressings were undertaken either in the outpatient’s clinic under sedation or in the operating theatre under a general anaesthetic (Figure 1).
Figure 1.
LDI images of a child with a mixed depth scald burn, showing a > 1% TBSA mid-dermal component.
Data collected included patient demographics (gender, age, mechanism of injury, first aid adequacy and %TBSA affected), the presence of infection requiring oral or intravenous antibiotic treatment, a positive swab result, the number of days to complete wound healing, the number of dressing changes, those children who required skin grafting and the %TBSA grafted.
Infection was defined as a moderate or greater growth on a wound swab of a pathological organism (i.e. not suggestive of non-pathological multiple skin commensals) and a confirmatory clinical assessment of infection by a burns clinician. A positive wound swab was defined as a light growth or greater of a pathological organism. The days to complete healing was determined by clinical assessment of the burn by an experienced burns clinician. Adequate first aid was defined as > 20 minutes of cool running water.
A statistical analysis of the pilot data was undertaken using the Fisher exact test and the Student’s t-test. A p-value of < 0.05 was considered statistically significant. This study was approved by the Sydney Children’s Hospitals Network Human Ethics Research Committee.
Results
10 children were randomized into each group. Both groups were evenly distributed with no significant differences. The median age in the Biobrane™ group was 2.0 years and 1.5 years in the Acticoat™ group. The median %TBSA affected was similar in both groups (8% Biobrane™ and 8.5% in the Acticoat™ group). There were 7 boys enrolled in the Biobrane™ group and 8 in the Acticoat™ group. Most in the Biobrane™ group sustained their injuries secondary to scalds and in addition one flame and one contact burn. All in the Acticoat™ group sustained their injuries due to scalds. Most children received adequate first aid (9 in each group). One child in the Biobrane™ group received < 5 minutes of cool running water, and one had toothpaste applied prior to 20 minutes of cool running water. One child in the Acticoat™ group received only 15 minutes of cool running water.
Six out of ten children in the Biobrane™ group had a clinically significant infection requiring oral or intravenous antibiotics, compared with only one of the ten children in the Acticoat group™ (P = 0.057). The number of children with a positive swab result in the Biobrane™ group was seven out of the ten versus, four out of ten in the Acticoat™ group (P = 0.37).
The median number of dressing changes required was similar in both groups (Biobrane™ 5, versus Acticoat™ 5.5; P = 0.56, 95% Cl -3.17-1.77). The days to healing was a week less in the Biobrane™ group (19 days) compared with the Acticoat™ group (26.5 days), although this was not statistically significant (P = 0.18, 95% Cl -20.93-4.33). Less children (4/10) in the Biobrane™ group required skin grafting than the Acticoat™ group (7/10), although this was not significant. The %TBSA grafted was exactly the same in both groups (1.75% TBSA).
Discussion
There appears to be limited data about the use of Biobrane™ compared to Acticoat™, despite both products commonly being used in many BUs. Acticoat™ remains the standard product used in our BU for the treatment of mid-dermal burns. There is some limited evidence that Biobrane™ many expedite healing and therefore reduce the need for skin grafting in those children with mid-dermal burns [13,20].
There were two interesting trends noted in our pilot study. The time to complete healing in the Biobrane™ group was almost a week less than the Acticoat™ group, with a corresponding reduction in the need for subsequent skin grafting [3,4].
The second trend of note is that there were more clinically significant infections and positive wound swabs in the Biobrane™ group. Perhaps surprisingly, this did not result in an increased healing time. In our BU, we use the unusual technique of overlay Biobrane™ with Acticoat™, in an attempt to reduce colonization. From these data, it is unclear whether this makes any impact.
Not only are mid-dermal burns difficult to manage, they are also difficult to diagnose, and hence we felt it important to standardize these participants by undertaking an LDI scan to ensure that they sustained at least a mid-dermal burn of ≥ 1% TBSA. This, we believe, would be a burn significant enough to be studied. This is one of the first studies to attempt to standardize mid-dermal burns using LDI.
Conclusions
Biobrane™ may expedite healing of mid-dermal burns in children, however, those children who are treated with Biobrane™ may also develop more clinically significant infections than those treated with Acticoat™ alone.
Acknowledgements
The authors would like to acknowledge the children, parents and staff of the Burn Unit at The Children’s Hospital at Westmead.
Disclosure of conflict of interest
None.
References
- 1.La Hei ER, Holland AJ, Martin HC. Laser Doppler imaging of paediatric burns: burn wound outcome can be predicted independent of clinical examination. Burns. 2006;32:550–553. doi: 10.1016/j.burns.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen K, Ward D, Lam L, Holland AJ. Laser Doppler imaging prediction of burn wound outcome in children: is it possible before 48 h? Burns. 2010;36:793–798. doi: 10.1016/j.burns.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns. 2006;32:992–999. doi: 10.1016/j.burns.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Kishikova L, Smith MD, Cubison TC. Evidence based management for paediatric burn: new approaches and improved scar outcomes. Burns. 2014;40:1530–1537. doi: 10.1016/j.burns.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Vloemans AF, Hermans MH, van der Wal MB, Liebregts J, Middelkoop E. Optimal treatment of partial thickness burns in children: a systematic review. Burns. 2014;40:177–190. doi: 10.1016/j.burns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Wasiak J, Cleland H, Campbell F, Spinks A. Dressings for superficial and partial thickness burns. Cochrane Database Syst Rev. 2013:CD002106. doi: 10.1002/14651858.CD002106.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cassidy C, St Peter SD, Lacey S, Beery M, Ward-Smith P, Sharp RJ, Ostile DJ. Biobrane versus duoderm for the treatment of intermediate thickness burns in children: a prospective, randomized trial. Burns. 2005;31:890–893. doi: 10.1016/j.burns.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Lesher AP, Curry RH, Evans J, Smith VA, Fitzgerald MT, Cina RA, Streck CJ, Hebra AV. Effectiveness of biobrane for treatment of partial-thickness burns in children. J Pediatr Surg. 2011;46:1759–1763. doi: 10.1016/j.jpedsurg.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barret JP, Dziewulski P, Ramzy PI, Wolf SE, Desai MH, Herndon DN. Biobrane versus 1% silver sulfadiazine in second-degree pediatric burns. Plast Reconstr Surg. 2000;105:62–65. doi: 10.1097/00006534-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Mandal A. Paediatric partial-thickness scald burns--is biobrane the best treatment available? Int Wound J. 2007;4:15–19. doi: 10.1111/j.1742-481X.2006.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwood JE, Clausen J, Kavanagh S. Experience with biobrane: uses and caveats for success. Eplasty. 2009;9:e25. [PMC free article] [PubMed] [Google Scholar]
- 12.El-Khatib HA, Hammouda A, Al-Ghol A, Habib B, Al-Basti Aldehyde-treated porcine skin versus biobrane as biosynthetic skin substitutes for excised burn wounds: case series and review of the literature. Ann Burns Fire Disasters. 2007;20:78–82. [PMC free article] [PubMed] [Google Scholar]
- 13.Greenwood JE. A randomized, prospective study of the treatment of superficial partialthickness burns: AWBAT-S versus biobrane. Eplasty. 2011;11:e10. [PMC free article] [PubMed] [Google Scholar]
- 14.Krezdorn N, Konneker S, Paprottka FJ, Tapking C, Mett TR, Broisch GH, Boyce M, Ipaktchi R, Vogt PM. Biobrane versus topical agents in the treatment of adult scald burns. Burns. 2017;43:195–199. doi: 10.1016/j.burns.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 15.Rahmanian-Schwarz A, Beiderwieden A, Willkomm LM, Amr A, Schaller HE, Lotter O. A clinical evaluation of Biobrane ((R)) and Suprathel ((R)) in acute burns and reconstructive surgery. Burns. 2011;37:1343–1348. doi: 10.1016/j.burns.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Gerding RL, Emerman CL, Effron D, Lukens T, Imbembo AL, Fratianne RB. Outpatient management of partial-thickness burns: biobrane versus 1% silver sulfadiazine. Ann Emerg Med. 1990;19:121–124. doi: 10.1016/s0196-0644(05)81793-7. [DOI] [PubMed] [Google Scholar]
- 17.Solanki NS, Nowak KM, Mackie IP, Greenwood JE. Using biobrane: techniques to make life easier. Eplasty. 2010;10:e70. [PMC free article] [PubMed] [Google Scholar]
- 18.Hyland EJ, Maze D, Lawrence T, Harvey JG, Holland AJ. Biobrane and skin staples: beware of necrotic ulceration. Int Wound J. 2016;13:878–879. doi: 10.1111/iwj.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubik DJ, Wasiak J, Paul E, Cleland H. Biobrane: a retrospective analysis of outcomes at a specialist adult burns centre. Burns. 2011;37:594–600. doi: 10.1016/j.burns.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Lal S, Barrow RE, Wolf SE, Chinkes DL, Hart DW, Heggers JP, Herndon DN. Biobrane improves wound healing in burned children without increased risk of infection. Shock. 2000;14:314–318. doi: 10.1097/00024382-200014030-00013. discussion 318-319. [DOI] [PubMed] [Google Scholar]
- 21.Ou LF, Lee SY, Chen YC, Yang RS, Tang YW. Use of Biobrane in pediatric scald burns--experience in 106 children. Burns. 1998;24:49–53. doi: 10.1016/s0305-4179(97)00049-1. [DOI] [PubMed] [Google Scholar]
- 22.Qian LW, Fourcaudot AB, Leung KP. Silver sulfadiazine retards wound healing and increases hypertrophic scarring in a rabbit ear excisional wound model. J Burn Care Res. 2017;38:e418–e422. doi: 10.1097/BCR.0000000000000406. [DOI] [PubMed] [Google Scholar]
- 23.Aziz Z, Abu SF, Chong NJ. A systematic review of silver-containing dressings and topical silver agents (used with dressings) for burn wounds. Burns. 2012;38:307–318. doi: 10.1016/j.burns.2011.09.020. [DOI] [PubMed] [Google Scholar]