Abstract
Slow lorises (Nycticebus spp.) are one of six venomous mammals, and the only known venomous primate. In the wild envenomation occurs mainly during conspecific competition for mates and territory, but may also be used as an application against parasites or for predator defense. Envenomation in humans is documented, with the most extreme accounts detailing near-fatal anaphylactic shock. From September 2016 – August 2017, we received questionnaire responses from 80 wild animal practitioners working with Nycticebus spp. in zoos, rescue centres and in the wild. We identified 54 practitioners who had experience of being bitten or were otherwise affected by slow loris venom, and an additional 26 incomplete entries. No fatalities were reported. Fifteen respondents noted that medical intervention was required, 12 respondents indicated no reaction to being bitten (9 of these indicated they were wearing gloves). Symptoms for those affected included: anaphylactic shock, paraesthesia, haematuria, dyspnoea, extreme pain, infection and general malaise. Impact of slow loris bites ranged from instantaneous to long-persisting complications, and healing time ranged from 1 day to >8 months. Extremities, including hands and arms, were mostly affected from the bites. Six of nine species of slow loris were reported to bite, with N. pygmaeus being the most common in our sample. We make suggestions regarding the use of these highly threatened yet dangerous primates as unsuitable tourist photo props and zoo animal ambassadors. We discuss the medical complications experienced in relation to protein sensitisation, and bacterial pathogenesis. We recommend future work to ascertain the protein content of slow loris venom to aid in enabling mitigation of risks posed.
Keywords: Venomous mammal, primate, slow loris, anaphylactic shock
Introduction
Questionnaire surveys of patients are demonstrably beneficial when assessing medical symptoms and concepts, such as pain (Breivik et al, 2006; Freynhagen et al, 2006). Amongst medical complications, surveys assessing trauma are well-represented (Pédrono et al, 2016; Fekete et al, 2017). Surveys have been utilised to obtain information effectively from victims of many animal-induced injuries, including animal bites (Rajkumar et al, 2016). Qualitative information from respondents who have received trauma from animal bites has helped to identify and prevent risks to human health (Pédrono et al, 2016; Shaikh et al, 2016). Regarding venomous animals, surveys are frequently employed, and are particularly beneficial in identifying the nature of venom, the medical complications experienced and potential impacts on public health (Lam et al, 2016; Williams et al, 2011).
Recipient surveys have been demonstrated as particularly useful for obtaining retrospective information from recipients of toxin-induced medical complications (Chan et al, 2010). Long-term physical and psychological ailments have been identified that were not available for immediate prognosis, and which often remain under-represented in medical reports despite animal bites having a long-term post-traumatic effect on psychological wellbeing (Williams et al 2011). Retrospective surveys allow additional insight into an event when the recipient is not affected by the immediate or recent effects of envenomation – allowing increased clarity of respondent’s medical afflictions.
Slow lorises (Nycticebus spp.) are one of six venomous mammal taxa, and the only known venomous primate (Nekaris et al, 2013). There are nine species of slow loris that occur in South and Southeast Asia, where they are threatened predominantly by deforestation, the illegal pet and live trade for tourist photo souvenirs (Nekaris and Starr, 2015). Although anti-predator and anti-parasitic functions for the venom have been proposed, in the wild envenomation occurs in conspecifics during antagonistic intraspecific competition, usually for territory or mates. Intraspecific envenomation is often fatal, causing extreme necrosis, festering and secondary infections (Fuller et al, 2017). Amongst professionally-housed captive animals, similar accounts are documented with intraspecific aggression being a significant cause of premature mortality (Sutherland-Smith and Stalis, 2001; Fuller et al, 2014). Slow lorises are regularly held in zoological collections and, more recently rescue centres. Their increased popularity, including on social media, results in them being exposed to an increasingly large audience, and, despite their venomous nature, direct interactions between slow lorises and the public are common.
Slow loris venom is a dual composite consisting of saliva and brachial gland exudate. Brachial gland exudate is most observed when animals are stressed, and is often produced when they are handled by humans (Nekaris et al, 2013). A threatened slow loris will raise its arms above its head in a defensive pose allowing the secretion of the brachial gland to be accessed easily by mouth. The fluid is then sequestered in the oral cavity and is amalgamated with salivary fluid to form a potent venom (Alterman, 1995). The venom is administered into the target by specially modified dentition in the form of an adapted toothcomb that provides effective venom administration through capillary action (Alterman, 1995).
Laboratory tests detail fatality in mice within three minutes of intravenous venom administration (Alterman, 1995). The venom is lethal to arthropods in laboratory tests indicating an ecto-parasite reduction function. To slow loris conspecifics, envenomation causes sustained, often fatal, wounds in both the wild and captivity (Fuller et al, 2014; Grow and Nekaris, 2015; Fuller et al, 2017). The brachial gland component of the venom contains numerous volatile components, including a variation of the major cat allergen Fel-D1 that may act in olfactory communication (Hagey et al, 2007). Human envenomation usually occurs in the upper extremities (hands) as a result of handling and feeding slow lorises. Accounts of human envenomation are documented but sparse, with three slow loris species currently known to have caused negative reactions: N. bengalensis, N. pygmaeus, and N. kayan. Symptoms occurring in humans include oedema, haematuria, pain and near-fatal anaphylactic shock (Wilde, 1972; Kallimulah et al, 2008; Madani and Nekaris, 2014; Fung and Wong, 2016). Traditional folklore regarding death and amputations resulting from bites of all known taxa are documented in slow loris range countries where slow lorises are often feared or revered (Nekaris et al, 2013). The development of anaphylaxis and severe infections require immediate emergency medical treatment. In the present study, we aim to assess qualitative accounts of the nature of the physiological manifestations of bites experienced by slow loris husbandry professionals, as well as include one medically documented case study.
Materials and Methods
We created a survey using Survey Monkey, from which we collected 80 responses from March 2016 to August 2017. We asked 23 questions within four topics, including demographic information about the bite recipient, characteristics of the bite (if any), description of the slow loris, and the opportunity to add other details. Questions ranged from numeric scale, dichotomous, Likert scale, and open (Newing, 2010) (Supplementary Table 1). In addition, we provide the detailed medical records including photographs of one of our respondents. The Oxford Brookes University Research Ethics Committee approved our methods, which followed the guidelines of the 1999 Commonwealth ‘Ethical Guidelines for Good Research Practice’. Because data were not normally distributed, we analysed results using descriptive statistics with SPSS V.24, setting the p-value at ± 0.05. We performed a multinomial logistic regression to determine whether the slow loris bite pain level reported by respondents could be predicted by individual or situational factors. Multinomial logistic regression has no assumption about the distribution of predictor variables and the predictors do not need to be normally distributed (Tabachnick and Fidell, 1996).
Results
Eighty respondents (females, 69%, n=55, males, 31%, n=25) ranging in age from 18 to 65+, completed the questionnaire. Respondents reported working with slow lorises from three weeks to more than 40 years. All respondents were involved with slow loris husbandry in a professional capacity. From the 80 responses, 54 respondents reported receiving a bite, and 26 respondents failed to complete the questionnaire in its entirety. Two respondents reported working at facilities with slow lorises for over 11 and 21 years and never came across a co-worker with an adverse reaction. Another respondent who had worked with lorises for 15 years had an anaphylactic reaction and extreme infection from a bite, despite no previous symptoms.
Amongst bite recipients (n=54), 78% (n=42) reported symptoms resulting from the bite (Table 1). Symptoms included: nausea, facial and air-way swelling, infection and festering as repeated symptoms and a suite of differing individual physiological effects including: haematuria, lethargy, inflammation, paraesthesia, anaphylaxis, impaired blood coagulation, cephalgia (headache), general malaise, involuntary tremors, nausea and increased sensitivity to other allergens (i.e., bamboo fibre). Medical intervention was recorded by 15 respondents; antibiotics were prescribed in five instances and antihistamines in three instances. One person who had been bitten and experienced severe reactions continued to work with slow lorises and felt fine if they took anti-histamines 10minutes before handling an animal. Additionally, six respondents detailed medical complications from proximity/contact with slow lorises without receiving a bite. Non-bitten medical complications documented include: numbness of extremities following physical contact with a slow loris (including petting an animal), lethargy and nausea. When comparing the pain of the bite to similarly-sized animals, 47 respondents answered; 41% (n=19) perceived the bite as being more painful, 30% (n=14) perceived the pain as being similar and 29% (n=13) perceived the bite as less painful. Five respondents described the bite as less painful than a variety of other animals including: aye ayes, dwarf lemurs, sugar gliders, spotted genets, meerkats or dogs. Two respondents said the bite was similar to the sting of a wasp. Five respondents described it as more painful than coatis, squirrel monkeys, marmosets, giant fruit bats, dogs or cats, with four saying it was much more painful than the bite from animals of similar size. Of eleven respondents wearing gloves, five reported that the bite penetrated the glove. Two of these respondents perceived less pain whilst wearing gloves. These two respondents also reported that the bite went through a glove and a finger nail. Another respondent barely bitten through the seam of a glove reported that their finger turned purple.
Table 1.
Detailing the mean pain level, instances where the recipient deemed the bite to be more painful than a similarly sized animal, other slow lorises present and mean time working with slow lorises, by species. Five recipients were bitten by multiple species, and 7 did not answer.
| Species | Total no. | Mean pain level | Bite comparison (worse) | Other loris present | Mean time working with lorises (months) |
|---|---|---|---|---|---|
| N. pygmaeus | 26 | 3.9 | 8 | 19 | 83 |
| N. bengalensis | 5 | 7.5 | 3 | 3 | 116 |
| N. coucang | 2 | 4.0 | 0 | 2 | 90 |
| N. javanicus | 2 | 6.0 | 1 | 0 | 72 |
| N. menagensis | 3 | 4.3 | 2 | 0 | 60 |
| N. hilleri | 1 | 4.0 | 0 | 0 | 228 |
| Unknown/multi spp. | 8 | 4.8 | 3 | 6 | 53 |
Bitten respondents who could identify the species that bit them (n=46) reported: N. javanicus (n=3), N. pygmaeus (n=26), N. hilleri (n=1), N. coucang (n=2), N. borneanus (n=1), N. menagensis (n=3) and N. bengalensis (n=5). Five respondents identified multiple species, three respondents did not recall the species and seven did not answer (Table 1). The bite types were classified as nips (n=19), locked-jaw (n=11), both nip and locked-jaw (n=6), puncture (n=1), multiple (n=5) and ‘other’ (n=7). One respondent noted that they had only had or heard of symptoms generated from bites of N. bengalensis, and never from those of N. pygmaeus. Bites where the slow loris combined brachial gland exudate with saliva were identified by two respondents. Bites occurred from animals in isolation (n=16), those in proximity to other slow lorises (n=21), those alone during the bite, but housed with others (n=10) (Table 1.). Respondents indicated slow lorises ‘warned’ prior to the bite in 56% of bite instances (n= 29). Three respondents mentioned that a bite occurred during feeding time, and suggested that the loris accidentally bit them.
Of 46 individuals identified to species, animals were house in complete isolation (34.9%), with conspecifics (45.7%) or individually (19.6%) but in proximity to one or more other slow lorises. Slow lorises that bit respondents were male (55%, n=27), female (31%, n=15) and of unidentified sex (14%, n=7). Primarily adult slow lorises bit respondents, accounting for 73% of recorded bites (n=36), with infants, juveniles and sub-adults identified in 6% of instances (n=3).
The mean pain level described by bitten respondents (n=54) was 5.3 (range 0-10). Respondents described pain as being in the highest quartile (>8 in 0-10 Likert scale) in 48% (n=27) of bites recorded, and within this 3.5% of respondents (n=2) described the pain to be of the highest possible option (10 in a 0 -10 Likert scale). Both male and female respondents experienced similar levels of pain (female, mean pain level 4.5, range 0-10, males, mean 4.5, range 0-9). Some respondents provided additional qualitative information such as “much more painful than a cat or dog or monkey bite – extreme throbbing and pulsing”. Of bitten respondents, 75% (n=42) experienced bleeding following the bite. Although low sample size of larger versus smaller species meant that we could not statistically compare our results, we found a trend for the largest species (N. bengalensis and N. javanicus) also to be associated with more painful bites.
We ran a multinomial logistic regression, where the pain levels (low, medium, and high) acted as the response variable. Proximity to other lorises, and participant allergies acted as the independent variables. The model fit was significant χ2(6)=13.57, p=0.035, indicating that the predictors, as a pair, reliably distinguish the three slow loris bite pain levels. The overall pain level prediction rate is 50%. The model correctly classified the pain level low 65% of the time, medium 50% of the time and high 29% of the time. Proximity to other slow lorises as a predictor was statistically different (p=0.047) in those respondents who reported a medium level of pain relative to those who reported a high level of pain (Table 2.).
Table 2.
Parameter estimates for a multinomial logistic regression, with pain level categories as the response variable (χ2 (6) = 13.57, p = .035).
| Low Pain Level | Medium Pain Level | |||||
|---|---|---|---|---|---|---|
| Variables | B | OR (95%CI) | SE | B | OR (95%CI) | SE |
| Proximity to other lorises | ||||||
| Absent | −0.64 | 0.53 (0.04/7.13) | 1.33 | −0.17 | 0.84 (0.06/11.60) | 1.34 |
| Present | −1.41 | 0.24 (0.02/2.59) | 1.21 | −2.73 | 0.06 (0.01/0.97)* | 1.38 |
| Allergies | ||||||
| Present | −1.80 | 0.164 (0.01/1.75) | 1.21 | −1.30 | 0.27 (0.02/3.88) | 1.36 |
Reference group: High Pain Level. OR = Odds Ratio. SE = Standard Error. 95% CI = Confidence Interval. * p ± .05
Case study
We detail medical data from a 37-year-old female patient. The patient weighed 55kg and had no existing health problems or known allergies. She was previously bitten by a pygmy slow loris (N. pygmaeus) over two years prior to the presented incident, from which she experienced only mild localised oedema and immediate short-term bleeding.
The patient was bitten by a male Bengal slow loris (N. bengalensis) at a rescue centre in Sathorn District, Bangkok, Thailand, on 29 November 2016. The male Bengal slow loris was housed with a female of the same species, present at the time of the bite. The bite occurred during routine husbandry practices. The animal bit the patient’s digit with locked jaws, for approximately 30seconds to one minute. Bleeding was immediate, with intense pain and a burning sensation localised proximally to the inflicted area. The wound was immediately rinsed with water, washed with soap and water, after which an antiseptic ointment was applied. Tetanus and rabies injections were administered at a local hospital within one hour, after a thorough cleaning of the wound. Daily cleaning was performed at the hospital and antibiotics were prescribed.
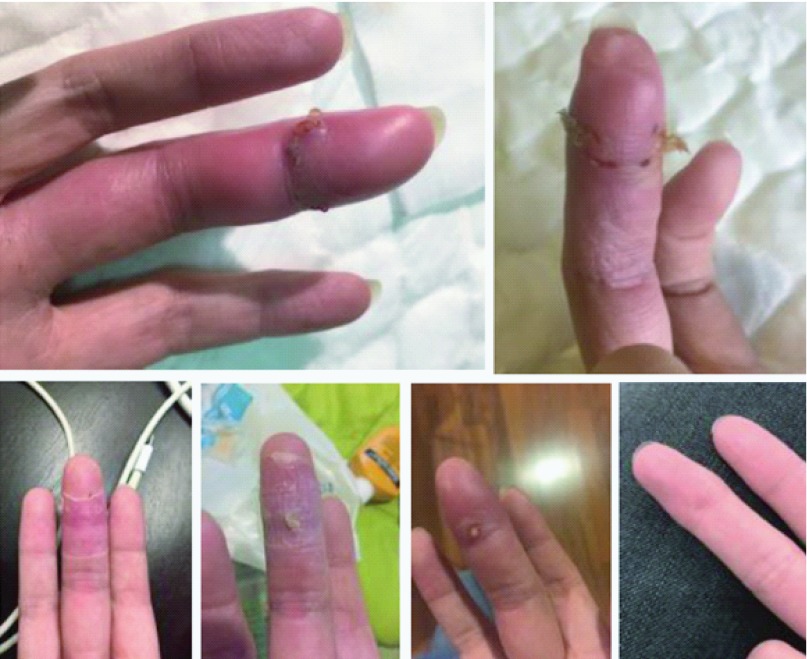
By 1 December, i.e. two days later, pain and oedema had significantly increased (Figure 1). Minor surgery was performed under anaesthetic to drain the wound, as the dactyl had become infected, discharging a yellow-milky coloured viscous fluid (puss). The strength of antibiotics prescribed was increased. Post-surgery daily wound cleaning at the hospital was performed.
Figure 1.
Detailing the wound from a male Nycticebus bengalensis bite on 29 November 2016 from one respondent. From right to left: top 1 December, 6 December, bottom: 15 December, 20 December, 30 December and 31 July.
On 3 December, during a wound clean visit, the patient was admitted to hospital and an antibiotic intravenous drip was administered as the oral antibiotics were not influencing the wound’s healing. Regular draining, flushing and cleaning of the wound were performed. Lidocaine was prescribed for the pain. Physical therapy was being practiced as the finger was not able to bend, and involuntarily tremored when moved. The mental stress of invasive procedures caused anxiety and fear in the patient. On 6 December, the patient was discharged from hospital under instruction to wash and soak the wound, perform physical therapy, and regularly remove dead skin to promote healing. The patient was instructed to visit the hospital every 2-3 days for the wound to be thoroughly cleaned.
By 14 December the finger had re-swollen and was red and by 17 December, a pustule had formed that was drained and cleaned. Antibiotic ointment was prescribed on 20 December and regular soaking in hydrogen peroxide and dead-skin removal was performed. Puss oozing, and occasional bleeding continued throughout December. By 31 December (32 days after the bite) the finger still caused intense pain if ‘knocked’, despite physical therapy the digit was still unable to grip or perform usual functions. By 14 February the wound had fully closed. The digit remained weak and unable to grip, despite continued physical therapy. Occasional pain was still experienced, and oedema was still present around the proximal interphalangeal, which possessed protrusions from internal scar tissue.
By 31 July (244 days after the bite) the digit was still unable to exercise a precision grip fully, mild pain was still experienced when the digit exerted pressure. The proximal interphalangeal joint remained protrusive from internal scarring. The patient has developed a previously non-existent aversion to injections and the finger remains permanently scarred. Permanent damage to the digit has been diagnosed resulting from the slow loris bite wound.
Discussion
Through the utilisation of retrospective surveys and one case study, we have demonstrated slow loris (Nycticebus spp.) bites have varied physiological effects on humans ranging from mild to severe, and potentially life-threatening. Symptoms of the bites parallel an autoimmune response, and bacterial pathogenesis. The physiological symptoms of slow loris bites highlight the fact these animals present a real risk to human health when exposed to the public as animal ambassadors, or as illegal pets. Although the causative mechanism of envenomation cannot be distinguished, slow lorises are demonstrably a risk to humans in contact with them. The case study highlights the potential for permanent disfigurement, and medical complications.
As frequent ambassador animals in zoos and photo props on tourist beaches, slow lorises are exposed to, and even handled by members of the public as an educational or amusement attraction. Our results detail even professional handling gloves may not prevent a bite penetrating the skin, meaning personal protective equipment would not negate the risk posed to the public. Considering our results from experienced and trained slow loris husbandry personnel, this practice is putting members of the public at risk of ill-health and even death. Due to the ‘cute’ appearance of slow lorises, the public may be ignorant of their venomous bite, so will therefore not act cautiously around this animal. Additionally, our results demonstrate a bite is not required for medical complications to occur; just being near a slow loris exposes a potential handler to an unnecessary risk of medical complications. We would like to urge the cessation of slow lorises as interactive animals on these grounds.
We identified 42 respondents who had clear negative reactions to a slow loris bite. Some of these became extremely ill, with only 12 bitten people reporting no affect and an additional 26 whom did not receive a bite. An additional 6 respondents identified physiological reactions to close-proximity/contact with a slow loris without receiving a bite. We document here for the first time a suite of differing reactions to slow loris bites, and proximity ranging from mild pain and oedema to near-fatal anaphylactic shock and permanent disfigurement.
The literature on slow loris bites in humans has focused on anaphylaxis as an effect of the venom (e.g., Madani and Nekaris, 2014). Five of our participants experienced symptoms synonymous with anaphylactic shock adding to this literature. Our results indicate a suite of additional medical complications including, paresthesia, dyspnoea, lethargy, haematuria, general malaise and nausea, additionally pain experienced was perceived by 40% as being worse than a similarly-sized animal. Although associated with venomous bites of some species (e.g., Ihama et al, 2014), these symptoms are more commonly the result of an allergic reaction to the venom (e.g., Bilo and Bonifazi, 2008). The brachial gland component of slow loris venom contains a variant of the major cat Felis domesticus allergen 1 (Fel-D 1) (Hagey et al, 2007; Krane et al, 2007). The isolation of Fel-D 1 within slow loris venom poses a plausible explanation for experienced symptoms including anaphylactic shock. In the absence of identification of additional causative mechanisms through protein identification within the venom, protein sensitisation and the mal-effects of the venom cannot be distinguished. In fact, anaphylactic shock following a mammalian bite is well-documented in the absence of venom; anaphylaxis response to bites is documented from the bites of hamsters (Mesocricetus spp.) (Borges et al, 2014), rats (Rattus spp.) (Kampitak and Betschel, 2016), rabbits (Orytolagus spp), mice (Apodemus spp.) (Kampitak and Betschel, 2016) and gerbils (Meriones spp.) (Watson et al, 2018). Thus, such complications following a slow loris bite cannot be confidently attributed as an effect of the venom.
Protein sensitivity (allergic reaction) must be considered in light of documented medical complications. Although human exposure to slow lorises is not significant, cross reactional allergies could still occur. Cross-reactional allergies are well-documented, whereby similar proteins present in different organisms increase sensitisation, causing allergic reaction (Díaz-Perales et al, 2006; Mattison et al, 2016). Prevalence of allergic reactions to many mammalian species is exacerbated by sensitisation (Feary and Cullinan, 2016). Sensitisation increases the rate of auto-immune responses including anaphylaxis, i.e. the body has encountered proteins such as Fel-d1 so ‘is prepared’ to diffuse molecules from the point of entry around the body thus reducing immediate bodily risk. This response is Immunoglobulin E antibody-mediated relying on chemical ‘rules’ that can be counterproductive to the host organism (Profet, 1991).
The Fel D-1 protein, a variant of which is present in slow loris brachial gland exudate is commonly referred to as the ‘cat allergen’ and is known to induce hypersensitisation in humans (Krane et al, 2003). Due to the popularity of cats as companion animals, rates of Fel D-1 protein sensitisation have increased (Custovic et al, 2003). A variant of Fel D-1 is present in several other mammals and can induce anaphylaxis and other complications, as experienced by our survey respondents (Smith et al, 2004). An increase in allergic susceptibility to exotic animals has risen with increasing adoption of new and unusual species (Díaz-Perales et al, 2006), a group to which slow lorises belong (Fuller et al, 2017). This relationship is especially concerning in areas such as Japan where ownership of slow lorises is high (Musing et al, 2015), as is the occurrence of Fel D-1 protein sensitisation (increased exposure) (Ichikawa et al, 1999).
Amongst animal bites bacterial infection is cited as a primary cause of complications, including infections, oedema, delayed healing time and extreme pain (Damborg et al, 2016; Rasmussen et al, 2017). Mammalian bites often contain high level of bacterial anaerobes and aerobes (Rasmussen et al, 2017; Kennedy et al, 2015). Even domestic species harbour dangerous bacterial agents; 56% of domestic cat and dog bites contain both anaerobes and aerobes (Jha et al, 2014) with 38% of domestic cat bites resulting in infection, 48% of human-induced bites cause infection, and even fatality from introduced bacteria (Mahida et al, 2015). The bacterial presence in slow loris venom is unknown, although bacteria have been cited as a potential reason for the negative effects of slow loris bites. Even if the slow loris is found to harbour potentially dangerous bacterial agents in its saliva, this would not mean they are not venomous. Necrotic effects are recorded for other venomous taxa with bacterial agents in their saliva including: gila monsters (Heleoderma suspectum), gastropods (i.e., Conus spp.) (Peraud et al, 2009), arachnids (i.e., Oedothorax gibbosus) (Vanthournout and Hendrickx, 2015) and hymenopteran species (e.g., Solenopsis invicta) (Tufts and Bextine, 2009). The possibility of bacterial pathogenesis cannot be discounted as the protagonist of complications from slow loris bite wounds. This possibility does not undermine the potency and potential danger of a slow loris bite, which is potentially fatal.
We recorded a single instance of haematuria, persisting for 3 days, from a male respondent, aged 40-55, which is the only medical effect not commonly caused by protein sensitivity or bacterial pathogenesis. Captive slow lorises have been documented as possessing a high mortality rate from renal failure and associated complication (Fuller et al, 2014). It is tempting to speculate the possession of a nephrotoxin within slow loris venom; however further research is required to characterise the venom.
It has been demonstrated within the evolutionary context of intraspecific competition that Nycticebus venom has constructed an effective mechanism for inflicting lasting, and often fatal, wounds to conspecifics. Whether by administering venom-derived antagonistic proteins, bacterial pathogenesis or stimulating an allergic reaction, a bite may pose more danger to humans bitten when the biting slow loris is in proximity to other slow lorises. We found a statistically significant correlation with the proximity of other slow lorises and the pain level experienced. The potency of a bite being higher when potential conspecific competitors or mates are present supports the hypothesised ecological use of intraspecific competition. These findings suggest two hypotheses. Either slow lorises produce more venom when in proximity to another slow loris to enable intraspecific competitive success against a rival loris, or they meter their venom when a threat is posed to inclusive fitness through intraspecific proximity of mates and/or offspring. Both scenarios require further investigation, and may influence captive-housing arrangements.
Our respondents’ results detail a complex suite of differing reactions to slow loris bites, from very mild to severe. Individual variation in the recipients’ sensitisation levels, and the lorises’ venom potency should also be considered in the variability of reactions in humans. We have argued the symptoms experienced, although plausibly an effect of envenomation, requires further research to discount other causative mechanisms including human protein sensitisation, and bacterial pathogenesis. Despite the ambiguity of causative mechanisms, the varying suite of medical complications experienced by professional slow loris husbandry personnel highlight the unsuitability of the species being exposed to untrained personnel as pets, as interactive animals whereby untrained people may handle them, or by any persons without adequate personal protective apparel. Furthermore, stronger gloves may be needed when dealing with the larger stronger species, which can deliver more powerful and painful bites. The fact that slow lorises are kept (albeit illegally) as pets, used as photo props and used as ambassador animals in zoos presents a risk to the public (Nekaris and Starr, 2015).
Acknowledgements
We would like to thank The European Association of Zoos and Aquaria, The Little Fireface Project, The American Association of Zoo Keepers, Love Wildlife, the Bangladesh Slow Loris Research and Conservation Project, Project Anoulak and the Endangered Primate Rescue Centre Vietnam and the anonymous respondents and their respective organisations for partaking in and distributing our survey. Funding for aspects of this research was provided by Augsburg Zoo, Chicago Board of Trustees Conservation Fund, Cleveland Metroparks Zoo, Cleveland Zoo Society, Columbus Zoo and Aquarium, Disney Worldwide Conservation Fund, Mohamed bin al Zayed Species Conservation Fund (152511813), Moody Gardens Zoo, Naturzoo Rhein, Omaha’s Henry Doorly Zoo, People’s Trust for Endangered Species, Phoenix Zoo, Shaldon Wildlife Trust, Primate Action Fund, and Margot Marsh Biodiversity Fund.
List of Abbreviations
- Fel D-1:
Felis domesticus allergen 1
Competing Interests
None declared.
References
- Alterman L. Toxins and toothcombs: potential allospecific chemical defenses in Nycticebus and Perodicticus. In: Alterman L, Gerald A., Doyle GA, Izard MK, editors. Creatures of the Dark. Springer; New York, USA: 1995. pp. 413–424. [Google Scholar]
- Bilò B.M., Bonifazi F. Epidemiology of insect-venom anaphylaxis. Curr Opin Allergy Clin Immunol. 2008;8:330–337. doi: 10.1097/ACI.0b013e32830638c5. [DOI] [PubMed] [Google Scholar]
- Borges L, Silva DB, Gonçalves TRT, et al. Anaphylaxis after bitten by domestic hamster: a case report. J Allergy Clin Immunol. 2014;133:AB28. [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Chan HY, Chan YC, Tse M L, Lau FL. Venomous fish sting cases reported to Hong Kong Poison Information Centre: a three-year retrospective study on epidemiology and management. Hong Kong J Emerg Med. 2010;17:40–44. [Google Scholar]
- Custovic A, Simpson BM, Simpson A, et al. Current mite, cat, and dog allergen exposure, pet ownership, and sensitization to inhalant allergens in adults. J Allergy Clin Immunol. 2003;111:402–407. doi: 10.1067/mai.2003.55. [DOI] [PubMed] [Google Scholar]
- Damborg P, Broens EM, Chomel BB, et al. Bacterial zoonoses transmitted by household pets: state-of-the-art and future perspectives for targeted research and policy actions. J Comp Pathol. 2016;155:S27–S40. doi: 10.1016/j.jcpa.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Díaz‐Perales A, Lombardero M, Sánchez‐Monge R, et al. Lipid‐transfer proteins as potential plant panallergens: cross‐reactivity among proteins of Artemisia pollen, Castanea nut and Rosaceae fruits, with different IgE‐binding capacities. Clin Exp Allergy. 2000;30:1403–1410. doi: 10.1046/j.1365-2222.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- Feary J, Cullinan P. Laboratory animal allergy: a new world. Curr Opin Allergy Clin Immunol. 2016;16:107–112. doi: 10.1097/ACI.0000000000000256. [DOI] [PubMed] [Google Scholar]
- Fekete C, Post MW, Bickenbach J, et al. A structured approach to capture the lived experience of spinal cord injury: data model and questionnaire of the International Spinal Cord Injury community survey. Am J Phys Med Rehabil. 2017;96:S5–S16. doi: 10.1097/PHM.0000000000000622. [DOI] [PubMed] [Google Scholar]
- Freynhagen R, Baron R, Gockel U, Tölle TR. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- Fuller G, Eggen WF, Wirdateti W, Nekaris KAI. Welfare impacts of the illegal wildlife trade in a cohort of confiscated greater slow lorises, Nycticebus coucang. J Appl An Welfare Sci. 2017:1–15. doi: 10.1080/10888705.2017.1393338. [DOI] [PubMed] [Google Scholar]
- Fuller G, Lukas KE, Kuhar C, Dennis PM. A retrospective review of mortality in lorises and pottos in North American zoos, 1980-2010. Endanger Species Res. 2014;23:205–217. [Google Scholar]
- Fung HT, Wong OF. Clinical quiz: a potentially toxic primate bite. Hong Kong J Emergency Medicine. 2016;23:301–303. [Google Scholar]
- Grow NB and Nekaris KAI. Does toxic defence in Nycticebus spp. relate to ectoparasites? The lethal effects of slow loris venom on arthropods. Toxicon. 2015;95:1–5. doi: 10.1016/j.toxicon.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Hagey L, Fry B, Fitch-Snyder H. Talking defensively, a dual use for the brachial gland exudate of slow and pygmy lorises. In: Gursky-Doyen S, Nekaris KA I, editors. Primate anti-predator strategies. Springer Science & Business Media; New York, USA: 2007. pp. 253–272. [Google Scholar]
- Ichikawa K, Iwasaki E, Baba M, Chapman MD. High prevalence of sensitization to cat allergen among Japanese children with asthma, living without cats. Clin Exp Allergy. 1999;29:754–761. doi: 10.1046/j.1365-2222.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- Ihama Y., Fukasawa M., Ninomiya K., Kawakami Y., Nagai T., Fuke C., Miyazaki T. Anaphylactic shock caused by sting of crown-of-thorns starfish (Acanthaster planci) Forensic Sci. 2014;236:e5–e8. doi: 10.1016/j.forsciint.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Jha S, Khan WS, Siddiqui NA. Suppl 1: Mammalian bite injuries to the hand and their management. Open Orthop J. 2014;8:194–198. doi: 10.2174/1874325001408010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimullah EA, Schmidt SM, Schmidt MJ, Lu JJ. Beware the pygmy slow loris? Clinical Toxicology. 2008;46(602) [Google Scholar]
- Kampitak T, Betschel SD. Anaphylaxis in laboratory workers because of rodent handling: two case reports. J Occup Health. 2016;58:381–383. doi: 10.1539/joh.16-0053-CS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SA, Stoll LE, Lauder AS. Human and other mammalian bite injuries of the hand: evaluation and management. J Am Acad Orthop Surg. 2015;23:47–57. doi: 10.5435/JAAOS-23-01-47. [DOI] [PubMed] [Google Scholar]
- Krane S, Itagaki Y, Nakanishi K, Weldon PJ. " Venom" of the slow loris: sequence similarity of prosimian skin gland protein and Fel d 1 cat allergen. Naturwissenschaften. 2003;90:60–62. doi: 10.1007/s00114-002-0394-z. [DOI] [PubMed] [Google Scholar]
- Lam A, Camara B, Kane O, Diouf A, Chppaux JP. Epidemiology of snakebites in Kédougou region (eastern Senegal): comparison of various methods for assessment of incidence and mortality. J Venom Anim Toxins Incl Trop Dis. 2016;22(9) doi: 10.1186/s40409-016-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani G, Nekaris KAI. Anaphylactic shock following the bite of a wild Kayan slow loris (Nycticebus kayan): implications for slow loris conservation. J Venom Anim Toxins Incl Trop Dis. 2014;20(43) doi: 10.1186/1678-9199-20-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida N, Anthony L, Martin N, Gupta A, Andrewartha F. Human bite leading to fatal Neisseria meningitidis septicaemia and pericarditis. JMM Case Rep. 2015;2 [Google Scholar]
- Mattison CP, Khurana T, Tarver M, et al. Termite proteins cross-react with cockroach allergens. J Allergy Clin Immunol. 2016;137:AB266. [Google Scholar]
- Musing L, Suzuki K, Nekaris KAI. Crossing international borders: the trade of slow lorises, Nycticebus spp., as pets in Japan. Asian Primates. 2015;5:12–24. [Google Scholar]
- Nekaris KAI, Starr CR. Conservation and ecology of the neglected slow loris: priorities and prospects. Endanger Species Res. 2015;28:87–95. [Google Scholar]
- Nekaris KAI, Moore RS, Rode EJ, Fry BG. Mad, bad and dangerous to know: the biochemistry, ecology and evolution of slow loris venom. J Venom Anim Toxins Incl Trop Dis. 2013;19(21) doi: 10.1186/1678-9199-19-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newing H. Conducting research in conservation: Social science methods and practice. Routledge; Abbington, UK: 2010. [Google Scholar]
- Pédrono G, Ricard C, Bouilly M, Beata C, Sarcey G, Thélot B. 483 Dog bites: severity and sequelae, a multicenter survey, France, September 2010–December 2011. Inj Prev. 2016;22:A175–A175. [Google Scholar]
- Peraud O, Biggs JS, Hughen RW, et al. Microhabitats within venomous cone snails contain diverse actinobacteria. Appl Environ Microbiol. 2009;75:6820–6826. doi: 10.1128/AEM.01238-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- Rajkumar K, Bhattacharya A, David S, et al. Socio-demographic study on extent of knowledge, awareness, attitude, and risks of zoonotic diseases among livestock owners in Puducherry region. Vet World. 2016;9(1018) doi: 10.14202/vetworld.2016.1018-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen D, Landon A, Powell J, Brown GR. Evaluating and treating mammalian bites. JAAPA. 2017;30:32–36. doi: 10.1097/01.JAA.0000512233.61549.2b. [DOI] [PubMed] [Google Scholar]
- Shaikh A, Phadke CP, Ismail F, Boulias C. Relationship between botulinum toxin, spasticity, and pain: a survey of patient perception. Can J Neurol Sci. 2016;43:311–315. doi: 10.1017/cjn.2015.321. [DOI] [PubMed] [Google Scholar]
- Smith W, Butler AJL, Hazell LA, et al. Fel d 4, a cat lipocalin allergen. Clin Exp Allergy. 2004;34:1732–1738. doi: 10.1111/j.1365-2222.2004.02090.x. [DOI] [PubMed] [Google Scholar]
- Sutherland-Smith M, Stalis JL. I: Health. In: Fitch-Snyder H, Schulze H, editors. Husbandry Manual for Asian Lorisines (Nycticebus & Loris ssp) Zoological Society of San Diego; San Diego, USA: 2001. pp. 60–71. [Google Scholar]
- Tabachnick B. G., Fidell L. S. Using multivariate statistics. 3rd edition. Harper Collins; New York: 1996. [Google Scholar]
- Tufts DM., Bextine B. Identification of bacterial species in the hemolymph of queen Solenopsis invicta (Hymenoptera: Formicidae) Environ Entomol. 2009;38:1360–1364. doi: 10.1603/022.038.0502. [DOI] [PubMed] [Google Scholar]
- Vanthournout B, Hendrickx F. Endosymbiont dominated bacterial communities in a dwarf spider. PloS One. 2015;10:e0117297. doi: 10.1371/journal.pone.0117297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J, Schobitz E, Davis J. Gerbil bite anaphylaxis- a rare case report. Am J Emerg Med. 2018;36:171.e5–171. doi: 10.1016/j.ajem.2017.10.040. [DOI] [PubMed] [Google Scholar]
- Wilde H. Anaphylactic shock following bite by a ‘slow loris,’ Nycticebus coucang. The Am J Trop Med Hyg. 1972;21:592–594. doi: 10.4269/ajtmh.1972.21.592. [DOI] [PubMed] [Google Scholar]
- Williams SS, Wijesinghe CA, Jayamanne SF, et al. Delayed psychological morbidity associated with snakebite envenoming. PLoS Negl Trop Dis. 2011;5:e1255. doi: 10.1371/journal.pntd.0001255. [DOI] [PMC free article] [PubMed] [Google Scholar]