Abstract
Despite evidence that tripeptide glycyl-ʟ-histidyl-ʟ-lysine (GHK) is an endogenous antioxidant, its mechanism and importance are not fully understood. In the present study, the ability of GHK to reduce levels of reactive oxygen species (ROS) in Caco-2 cells was evaluated by flow cytometry with the oxidation-sensitive fluorescent dye 2’,7’-dichlorodihydrofluorescein diacetate. Further, types of ROS diminished by GHK were assessed by utilizing an electron spin resonance (ESR) spin-trapping technique. GHK reduced the tert-butyl hydroperoxide-induced increase in ROS levels in Caco-2 cells at concentrations of 10 µM or less. Experiments utilizing an ESR spin-trapping technique revealed that, among hydroxyl (·OH), superoxide (O2 -·), and peroxyl (ROO·) radicals generated by respective chemical reaction systems, GHK diminished signals of both ·OH and ROO·, but not O2 -·. Additionally, the GHK effect on the signal of ·OH was much stronger than those of other well-known antioxidative, endogenous peptides, carnosine and reduced glutathione. These results suggest that GHK can function as an endogenous antioxidant in living organisms, possibly by diminishing ·OH and ROO·.
Keywords: Glycyl-ʟ-histidyl-ʟ-lysine, hydroxyl radical, peroxyl radical, oxidative stress, endogenous antioxidant
Introduction
The natural tripeptide glycyl-ʟ-histidyl-ʟ-lysine (GHK) was first isolated by Pickart and Thaler [1]. GHK is liberated from extracellular matrix proteins, especially the α-II chain of collagen in response to soft tissue damage [2-4]. Numerous studies have demonstrated that this simple molecule improves wound healing and tissue regeneration (e.g., skin, hair follicles, bones, stomach, intestinal linings, and liver tissues), increases collagen and glycosaminoglycans, and increases angiogenesis and nerve outgrowth [5-8]. Interestingly, Bobyntsev et al. [9] also reported that the administration of GHK has marked anxiolytic effects on behavioral responses of rats. Thus, GHK has many beneficial effects on the body, but the mechanisms underlying these effects are not fully understood.
Substantial evidence has surfaced regarding the harmful effects of reactive oxygen species (ROS) generated endogenously and exogenously. Certain antioxidants may have physiological and pharmacological functions, without any side effects, in preventing oxidative stress-induced damage. GHK has been reported to quench the toxic products of lipid peroxidation, α,β-4-hydroxy-trans-2-nonenal and acrolein, which play important roles in the pathogenesis of several age-related conditions [10,11]. However, to the best of our knowledge, the effect of GHK on ROS levels in living cells and on various types of ROS are unknown.
In this study, we examined the concentration-dependent effects of GHK on ROS levels in Caco-2 cells by using flow cytometry with the oxidation-sensitive fluorescent dye 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA). We also utilized an electron-spin resonance spectrometry (ESR) spin-trapping technique [12] to evaluate the specific effects of GHK on hydroxyl (·OH), superoxide (O2 -·), and peroxyl (ROO·) radicals generated by respective chemical reaction systems and compared these effects with those of other well-known antioxidative, endogenous peptides, carnosine and reduced glutathione (GSH) [13,14].
Materials and methods
Materials
GHK, H2O2, mannitol, SOD, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), xanthine, xanthine oxidase (XO), tert-butyl hydroperoxide (t-BOOH, 70%, w/w, aqueous solution), GSH, and diethylenetriaminepentaacetic acid (DTPA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). DCFH-DA was obtained from Life Technologies Corporation (Carlsbad, CA, USA). 5,5-Dimethyl-1-pyrroline-N-oxide (DMPO) and α-(4-pyridyl 1-oxide)-N-tert-butylnitrone (POBN) were purchased from Enzo Life Technologies (Farmingdale, NY, USA). N-Acetyl-ʟ-cysteine (NAC), Ce(SO4)2·4H2O, and FeSO4·7H2O were obtained from Wako Pure Chemical Industries, Limited (Osaka, Japan). All other reagents were of analytical grade. The ultrapure water was prepared using a compact ultrapure water system (Milli-Q®; Merck Millipore, Burlington, MA, USA).
Measurement of ROS in Caco-2 cells
Measurements of ROS in Caco-2 cells were performed following previously reported methods [15]. Caco-2 cells were purchased from the European Collection of Cell Cultures (Salisbury, Wilts, UK) and cultured in Minimum Essential Medium (Life Technologies Corporation) supplemented with 10% fetal bovine serum (Nichirei Biosciences Inc., Tokyo, Japan) and 1% non-essential amino acids (Life Technologies Corporation). The cells were maintained in a humidified atmosphere of 5% carbon dioxide at 37°C.
ROS levels were measured by flow cytometry with the oxidation-sensitive fluorescent dye, DCFH-DA. The cells (1.0 × 106 cells/28 cm2 dish) were incubated with the test reagents for 6 h, and then DCFH-DA was added at a final concentration of 10 μM. After incubation for 30 min, the cells were collected by centrifugation (4°C and 200 × g for 5 min) and washed twice. The samples were filtered through a nylon mesh (37 μm) and subjected to flow cytometry (FACSAria™ III Flow Cytometer; Becton Dickinson, Basel, Switzerland).
ESR measurement
The ESR spectrometer TE-2100 (JEOL, Tokyo, Japan) and a JEOL flat quartz cell were used. The conditions were as follows: field, 336 ± 5 mT width; power, 2 mW (DMPO-OH· signal) and 4 mW (DMPO-OOH·, and POBN adduct signals); field modulation, 0.200 mT; time constant, 0.1; and amplitude, 300. A manganese signal was used as the external standard.
Observation of DMPO-OH· reflecting ·OH
The Fenton reaction was initiated by adding H2O2 (final concentration, 0.5 mM) to a mixture of DMPO (final concentration, 10 mM) and FeSO4·7H2O (final concentration, 0.25 mM) in 0.1 M sodium phosphate buffer (pH 7.4) in a total volume of 0.5 mL. The spin-trapped DMPO-OH· signal reflecting ·OH was measured 1 min after the addition of H2O2.
Observation of DMPO-OOH· reflecting O2 -·
The xanthine/XO reaction was started by adding xanthine (final concentration, 200 mM) to a mixture of DMPO (final concentration, 220 mM), XO (final concentration, 0.1 U/mL), and DTPA (final concentration, 0.1 mM) in 0.1 M sodium phosphate buffer (pH 7.4) in a total volume of 0.5 mL. The spin-trapped DMPO-OOH· signal reflecting O2 -· was measured 1 min after the addition of xanthine.
Observation of POBN-adduct signal reflecting t-BOO·
The Ce4+/t-BOOH reaction was started by adding t-BOOH (final concentration, 400 mM) to a mixture of POBN (final concentration, 10 mM) and Ce(SO4)2·4H2O (final concentration, 0.2 mM) in 0.1 M sodium phosphate buffer (pH 7.4) in a total volume of 0.5 mL. The POBN adduct signal reflecting t-BOO· were measured 1 min after the addition of t-BOOH.
Statistical analysis
The results are expressed as means ± standard errors of the mean. Significant differences between two groups were assessed using t-tests, and differences between multiple groups were assessed by one-way analysis of variance, followed by Scheffé’s multiple comparison tests. P-values less than 0.05 were considered statistically significant.
Results and discussion
GHK potently diminishes ROS levels in viable cells
Figure 1A shows the effects of t-BOOH with or without GHK and NAC on intracellular ROS generation in Caco-2 cells, as determined by flow cytometry with the redox-sensitive fluorescent dye DCFH-DA. NAC is often used as an antioxidant in cell experiments [16]. The addition of t-BOOH (50 µM) to Caco-2 cells shifted the mean fluorescence intensity (MFI, dashed lines in Figure 1A) to the right, indicating an increase in ROS levels based on DCF fluorescence. The increment in MFI induced by t-BOOH was reduced by the addition of 10 μM GHK. Figure 1B summarizes the MFI results obtained by the method described in Figure 1A. The addition of 10 μM NAC significantly diminished the t-BOOH-induced increase in MFI (28% inhibition). At the same concentration, GHK significantly reduced the t-BOOH-induced increase in MFI (10 μM GHK, 48% inhibition). The inhibitory effect of 10 μM GHK was stronger than that of NAC, with a statistically significance. This means that GHK reduces the t-BOOH-induced increase in ROS levels in living cells, even at concentrations of 10 µM or less.
Figure 1.
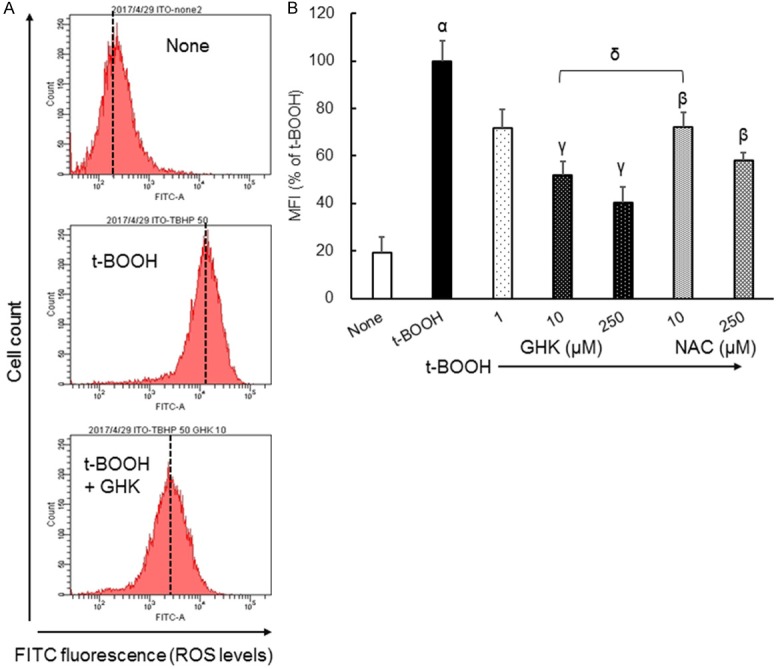
Alterations in ROS levels in Caco-2 cells treated with t-BOOH in combination with GHK. (A) Representative univariate histograms of flow cytometry data. (B) Quantitative assessment of the MFI flow cytometry data. Results are presented as means ± standard errors of the mean (n = 3-12). α P < 0.01 vs. None. β P < 0.01, γ P < 0.01 vs. t-BOOH. δ P < 0.01 vs. NAC. MFI, mean fluorescence intensity (shown as dashed lines in part A); GHK, glycyl-ʟ-histidyl-ʟ-lysine; t-BOOH, tert-butylhydroperoxide; NAC, N-acetyl-ʟ-cysteine.
GHK diminishes the amount of spin signal adduct of ·OH more potently than do carnosine and GSH
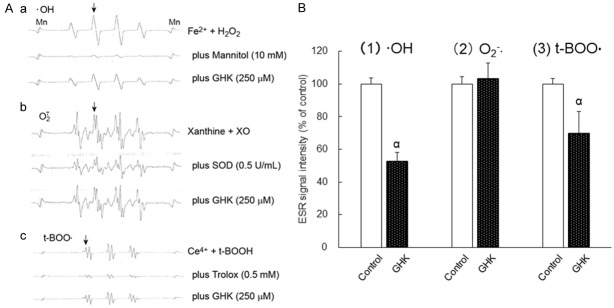
A direct method for measuring free radicals in aqueous conditions is ESR spectroscopy [14]. Figure 2A shows the ESR spectra of the spin signal adducts from ·OH, O2 -·, and t-BOO· using their respective spin trapping reagents.
Figure 2.
Effect of GHK on the amounts of spin signal adducts of ·OH, O2 -·, and t-BOO· generated by their respective chemical reaction systems. A. Representative ESR signal spectra of (a) DMPO-OH reflecting ·OH, (b) DMPO-OOH reflecting O2 -·, and (c) POBN adduct signal reflecting t-BOO·. (a) Fe2+, 0.25 mM; H2O2, 0.5 mM. (b) xanthine, 200 mM; XO, 0.1 U/mL. (c) Ce4+, 0.2 mM; t-BOOH, 400 mM. B. The effect of GHK on the amounts of spin signal adducts of ·OH, O2 -·, and t-BOO·. The radical intensity was defined as the ratio of the peak height of respective signal [indicated as arrows in part Aa-Ac] to that of Manganese (Mn). Results are presented as means ± standard errors of the mean (n = 3-8). α P < 0.01 vs. Control. GHK, 250 μM. ·OH, hydroxyl radicals; O2 -·, superoxide radicals; t-BOO·, tert-butyl peroxyl radicals; GHK, glycyl-ʟ-histidyl-ʟ-lysine; XO, xanthine oxidase; SOD, superoxide dismutase; ESR, electron spin resonance; t-BOOH, tert-butyl hydroperoxide.
Figure 2Aa shows the ESR spectra obtained by the Fenton reaction and DMPO with and without mannitol, a specific ·OH scavenger [17,18]. The 1:2:2:1 quartet pattern shows a hyperfine coupling at 1.50 mT, the same value as that of DMPO-OH· reported previously [19,20]. The signal of DMPO-OH· was quenched almost completely with 10 mM mannitol. Figure 2Ab shows the spin signal adducts of DMPO-OOH· reflecting O2 -· in the absence or presence of 0.5 U/mL SOD. The hyperfine fit parameters for the DMPO adducts were as follows: a(N) = 1.42 mT, a(H)β = 1.14 mT, and a(H)γ = 0.12 mT, consistent with previous values reported for DMPO-OOH· [19,20]. The involvement of O2 -· was confirmed by the reduction in the DMPO-OOH· signal intensity in the presence of 0.5 U/mL SOD. Figure 2Ac shows the spin signal adduct of t-BOO· [a(N) = 1.51 mT, a(H) = 0.23 mT] by the reaction of the Ce4+/t-BOOH system with POBN. The hyperfine fit parameters are identical to those previously reported [21,22]. An obvious quenching by 0.5 mM Trolox, which is a potent peroxyl radial scavenger [23,24], supported the identity of the product. Positive correlations between the disappearance of the signals of DMPO-OH·, DMPO-OOH·, and POBN adduct signal of t-BOO·, and their respective scavenger concentrations were observed (Supplementary Data). Figure 2A and Supplementary Data collectively demonstrate that these three experimental conditions using the ESR apparatus can be used to assess the quenching efficacies of specific substances against ·OH, O2 -·, and t-BOO·. Figure 2A also showed representative ESR spectra in the presence of GHK. GHK at 250 μM reduced both spin signal adducts of ·OH (1) and t-BOO· (3), but not of O2 -· (2).
Figure 2B shows the effect of GHK on the amounts of spin signal adducts of ·OH (1), O2 -· (2), and t-BOO· (3) detected by the methods described in Figure 2Aa-Ac. GHK at 250 µM quenched the spin signal adducts of both ·OH (47% inhibition) and t-BOO· (30% inhibition), but did not influence the spin signal adduct of O2 -·.
The effect of GHK on the amount of spin signal adduct of ·OH was investigated further. The diminishing effect of GHK on the amount of spin signal adduct of ·OH was compared to that of an equimolar mixture (G+H+K, 1:1:1) of the GHK constitutive amino acids (Table 1). A mixture of G, H, and K (250 µM each) did not have any effect on the spin signal adduct of ·OH, whereas GHK at the same concentration did. These results indicate that GHK results in a cooperative or synergistic reduction in the spin signal adduct of ·OH when the amino acids were linked in the peptide structure.
Table 1.
Effects of G plus H plus K as well as GHK on the generation of hydroxyl radicals
The radical intensity was defined as the ratio of the peak height of the signal [indicated as arrows in Figure 2Aa-Ac] to that of Manganese (Mn). Results are presented as means ± standard errors of the mean (n = 4-10).
P < 0.01, significantly different from Control;
P < 0.01, significantly different from G+H+K.
GHK, 250 μM; G, H or K, 250 μM. G, glycine; H, histidine; K, lysine.
Many studies have examined the antioxidant and metal ion chelation effects of carnosine (β-alanyl-ʟ-histidine), a dipeptide, and GSH (γ-glutamyl-cysteinyl-glycine), a tri-peptide [13,14]. Figure 3 illustrates the ·OH diminishing efficacy of GHK in comparison with those of carnosine and GSH. All three small peptides dose-dependently diminished ·OH, but the effect of GHK was much stronger than those of the other two peptides (IC50 value: GHK, approximately 250 µM; carnosine, approximately 500 µM; GSH, greater than 1000 µM). This finding shows that GHK diminishes the spin signal adduct of ·OH more strongly than do carnosine and GSH.
Figure 3.
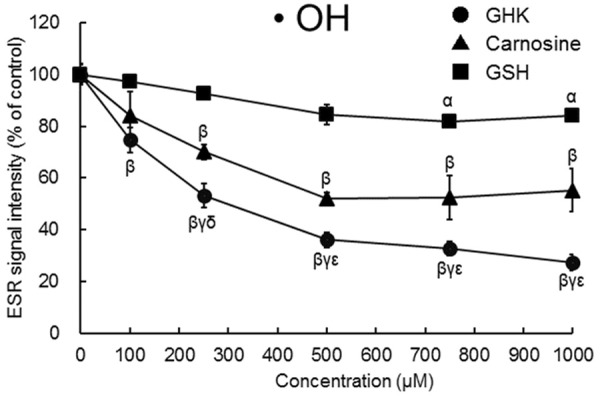
Effects of carnosine and GSH as well as GHK on the amounts of spin signal adduct of ·OH generated by the Fenton-type reaction. The radical intensity was defined as the ratio of the peak height of the signal [indicated as arrows in Figure 2Aa] to that of Manganese (Mn). Results are presented as means ± standard errors of the mean (n = 3-8). α P < 0.05, β P < 0.01 vs. None., γ P < 0.01 vs. GSH. δ P < 0.05, εP < 0.01 vs. carnosine. ·OH, hydroxyl radicals; GHK, glycyl-ʟ-histidyl-ʟ-lysine; GSH, reduced glutathione.
GHK has been co-isolated from human plasma in association with the albumin and α-globulin fractions at about 200 ng/mL (0.6 µM) [1]. However, GHK has been reported to be liberated from extracellular matrix proteins, especially the α-II chain of collagen in response to even soft tissue damage [2-4]; accordingly, GHK level is likely to be detect at a broad range of concentrations, from nanomolar up to micromolar under certain pathophysiological conditions. The present study showed that GHK, even at concentration of 10 µM or less, significantly reduces ROS levels initiated by t-BOOH. Thus, it is possible that GHK acts as an antioxidant under such pathophysiological conditions.
Acrolein, a well-known carbonyl toxin, is produced by lipid peroxidation of polyunsaturated fatty acids. GHK has been reported to effectively reduce the formation of both acrolein and another product of oxidation, 4-hydroxynonenal [10,11]. These previous studies make us expect a direct scavenging capacity of GHK against ·OH and ROO·. But, it has been shown that GHK interacts with an approximately equimolar amount of copper and an approximately 1/5 molar amount of iron [17,25]. Accordingly, it seems likely that GHK chelates Fe and Ce in the chemical reaction systems for the generation of ·OH and ROO·, and this effect is stronger than those of carnosine and GSH. Additional studies are needed to clarify the mechanism by which GHK diminishes the generation of ·OH and ROO·.
Conclusion
GHK significantly reduced t-BOOH-induced increases in ROS levels in Caco-2 cells, and this effect was stronger than that of NAC. GHK also diminished the generation of ·OH and ROO· by using an ESR method. We believe that our study makes a significant contribution to the literature because, to the best of our knowledge, this is the first time that GHK has the potential to reduce oxidative stress in living organisms, possibly by diminishing ·OH and ROO·.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Pickart L, Thaler MM. Tripeptide in human serum which prolongs survival of normal liver cells and stimulates growth in neoplastic liver. Nat New Biol. 1973;234:85–87. [PubMed] [Google Scholar]
- 2.Pickart L, Vasquez-Soltero JM, Margolina A. The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: implications for cognitive health. Oxid Med Cell Longev. 2012;2012:324832–324839. doi: 10.1155/2012/324832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kukowska M, Dzierzbicka K. Developments in the synthesis and biological activity of Glycyl-L-Histydyl-L-Lysine derivatives. Curr Med Chem. 2014;221:1505–1521. doi: 10.2174/0929867321666131218095056. [DOI] [PubMed] [Google Scholar]
- 4.Kukowska M, Kukowska-Kaszuba M, Dzierzbicka K. In vitro studies of antimicrobial activity of Gly-His-Lys conjugates as potential and promising candidates for therapeutics in skin and tissue infections. Bioorg Med Chem Lett. 2015;25:542–546. doi: 10.1016/j.bmcl.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Pickart L. The human tri-peptide GHK and tissue remodeling. J Biomater Sci Polym Ed. 2008;19:969–988. doi: 10.1163/156856208784909435. [DOI] [PubMed] [Google Scholar]
- 6.Pickart L. The human tripeptide GHK (Glycyl-L-Histidyl-L-Lysine). The copper switch, and the treatment of the degenerative conditions of aging. In: Klatz R, Goldman R, editors. American Academy of Anti-Aging Medicine. 2009. pp. 301–312. [Google Scholar]
- 7.Choi HR, Kang YA, Ryoo SJ, Shin JW, Na JI, Huh CH, Park KC. Stem cell recovering effect of copper-free GHK in skin. J Pept Sci. 2012;18:685–690. doi: 10.1002/psc.2455. [DOI] [PubMed] [Google Scholar]
- 8.Jose S, Hughbanks ML, Binder BY, Ingavle GC, Leach JK. Enhanced trophic factor secretion by mesenchymal stem/stromal cells with Glycine-Histidine-Lysine (GHK)-modified alginate hydrogels. Acta Biomater. 2014;10:1955–1964. doi: 10.1016/j.actbio.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bobyntsev II, Chernysheva OI, Dolgintsev ME, Smakhtin MY, Belykh AE. Anxiolytic effects of Gly-His-Lys peptide and its analogs. Bull Exp Biol Med. 2015;158:726–728. doi: 10.1007/s10517-015-2847-3. [DOI] [PubMed] [Google Scholar]
- 10.Beretta G, Artali R, Regazzoni L, Panigati M, Facino RM. Glycyl-histidyl-lysine (GHK) Is a quencher of α,β-4-Hydroxy-trans-2-nonenal: a comparison with carnosine. Insights into the mechanism of reaction by electrospray ionization mass spectrometry, 1H NMR, and computational techniques. Chem Res Toxicol. 2007;20:1309–1314. doi: 10.1021/tx700185s. [DOI] [PubMed] [Google Scholar]
- 11.Beretta G, Arlandini E, Artali R, Anton JM, Maffei Facino R. Acrolein sequestering ability of the endogenous tripeptide glycyl-histidyl-lysine (GHK): characterization of conjugation products by ESI-MSn and theoretical calculations. J Pharm Biomed Anal. 2008;47:596–602. doi: 10.1016/j.jpba.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Venkataraman S, Schafer FQ, Buettner GR. Detection of lipid radicals using EPR. Antioxid Redox Singal. 2004;6:631–638. doi: 10.1089/152308604773934396. [DOI] [PubMed] [Google Scholar]
- 13.Decker EA, Livisay SA, Zhou S. A re-evaluation of the antioxidant activity of purified carnosine. Biochemistry (Moscow) 2000;65:901–906. [PubMed] [Google Scholar]
- 14.Lluis JM, Morales A, Blasco C, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. J Biol Chem. 2005;280:3224–3232. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- 15.Kohda T, Sakuma S, Fujimoto Y. Vitamin E-like molecules potentiate the curcumin-induced suppression of Caco-2 cell proliferation. Int J Pharmacol Res. 2016;6:41–46. [Google Scholar]
- 16.Lasram MM, Dhouib IB, Annabi A, El Fazaa S, Gharbi N. A review on the possible molecular mechanism of action of N-acetylcysteine against insulin resistance and type-2 diabetes development. Clin Biochem. 2015;48:1200–1208. doi: 10.1016/j.clinbiochem.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Duffy SJ, Biegelsen ES, Holbrook M, Russell JD, Gokce N, Keaney JF Jr, Vita JA. Iron chelation improves endothelial function in patients with coronary artery disease. Circulation. 2001;103:2799–2804. doi: 10.1161/01.cir.103.23.2799. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa S. Copper uptake is required for pyrrolidine dithiocarbamate-mediated oxidation and protein level increase of p53 in cellsEstimation of relative reaction rate of hydroxy radical with poly-hydroxy benzenes: ESR spin trapping combined with UV-A photolysis. Anal Sci. 2013;29:377–380. doi: 10.2116/analsci.29.377. [DOI] [PubMed] [Google Scholar]
- 19.Buettner GR. The spin trapping of superoxide and hydroxyl free radicals with DMPO (5,5-Dimethylpyrroline-N-oxide): more about iron. Free Rad Res Commun. 1993;19:S79–S87. doi: 10.3109/10715769309056s79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ranguelova K, Mason RP. The fidelity of spin trapping with DMPO in biological systems. Magn Reson Chem. 2011;49:152–158. doi: 10.1002/mrc.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panasenko OM, Osipov AN, Chekanov AV, Arnhold J, Sergienko VI. Peroxyl radical is produced upon the interaction of hypochlorite with tert-butyl hydroperoxide. Biochemistry (Moscow) 2002;67:1061–1070. doi: 10.1023/a:1019962519941. [DOI] [PubMed] [Google Scholar]
- 22.Panasenko OM, Chekanov AV, Arnhold J, Sergienko VI, Osipov AN, Vladimirov YA. Generation of free radicals during decomposition of hydroperoxide in the presence of myeloperoxidase or activated neutrophils. Biochemistry (Moscow) 2005;70:998–1004. doi: 10.1007/s10541-005-0215-z. [DOI] [PubMed] [Google Scholar]
- 23.Barclay LR, Artz JD, Mowat JJ. Partitioning and antioxidant action of the water-soluble antioxidant, Trolox, between the aqueous and lipid phases of phosphatidylcholine membranes: 14C tracer and product studies. Biochim Biophys Acta. 1995;1237:77–85. doi: 10.1016/0005-2736(95)00071-a. [DOI] [PubMed] [Google Scholar]
- 24.Stefek M, Kyselova Z, Rackova L, Krizanova L. Oxidative modification of rat eye lens proteins by peroxyl radicals in vitro: protection by the chain-breaking antioxidants stobadine and Trolox. Biochim Biophys Acta. 2005;1741:183–190. doi: 10.1016/j.bbadis.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Pickart L, Thaler MM, Millard M. Effect of transition metals on recovery from plasma of the growth-modulating tripeptide glycylhistidyllysine. J Chromatogr. 1979;175:65–73. doi: 10.1016/s0021-9673(00)86403-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.