Short abstract
Background
Diabetic peripheral neuropathy is a common long-term complication of diabetes. Accumulating evidence suggests that vascular impairment plays important roles in the pathogenesis of diabetic peripheral neuropathy, while the mechanism remains unclear. We recently reported that transient receptor potential ankyrin 1 (TRPA1) is sensitized by hypoxia, which can contribute to cold hypersensitivity. In this study, we investigated the involvement of TRPA1 and vascular impairment in painful diabetic peripheral neuropathy using streptozotocin-induced diabetic model mice.
Results
Streptozotocin-induced diabetic model mice showed mechanical and cold hypersensitivity with a peak at two weeks after the streptozotocin administration, which were likely to be paralleled with the decrease in the skin blood flow of the hindpaw. Streptozotocin-induced cold hypersensitivity was significantly inhibited by an antagonist HC-030031 (100 mg/kg) or deficiency for TRPA1, whereas mechanical hypersensitivity was unaltered. Consistent with these results, the nocifensive behaviors evoked by an intraplantar injection of the TRPA1 agonist allyl isothiocyanate (AITC) were enhanced two weeks after the streptozotocin administration. Both streptozotocin-induced cold hypersensitivity and the enhanced AITC-evoked nocifensive behaviors were significantly inhibited by a vasodilator, tadalafil (10 mg/kg), with recovery of the decreased skin blood flow. Similarly, in a mouse model of hindlimb ischemia induced by the ligation of the external iliac artery, AITC-evoked nocifensive behaviors were significantly enhanced three and seven days after the ischemic operation, whereas mechanical hypersensitivity was unaltered in TRPA1-knockout mice. However, no difference was observed between wild-type and TRPA1-knockout mice in the hyposensitivity for current or mechanical stimulation or the deceased density of intraepidermal nerve fibers eight weeks after the streptozotocin administration.
Conclusion
These results suggest that TRPA1 sensitization during diabetic vascular impairment causes cold, but not mechanical, hypersensitivity in the early painful phase of diabetic peripheral neuropathy. However, TRPA1 may play little or no role in the progression of diabetic peripheral neuropathy.
Keywords: Diabetic peripheral neuropathy, TRPA1, cold hypersensitivity, vascular impairment, ischemia
Introduction
Diabetic peripheral neuropathy (DPN) is a common long-term complication of diabetes that affects up to 50% of patients with diabetes.1 Bilateral and symmetrical damage to peripheral nerves progresses with a distal to proximal gradient. The early phase of DPN is characterized by positive sensory symptoms, such as paresthesia, dysesthesia, and pain. The prevalence of painful DPN is estimated to be 10% to 25% of patients with diabetes.2,3 Further DPN progression leads to negative sensory symptoms, including loss of sensation, proprioception, temperature discrimination, and pain.4 The recommended clinical treatment is control of blood glucose and improved lifestyle, which only delay the onset and slow the progression.4 For painful DPN, treatment with anticonvulsants, antidepressants, opioids, and topical agents is recommended; however, these analgesic approaches are only partially successful or often ineffective.2,5
The development and progression of DPN is caused by hyperglycemia-induced damage to peripheral nerves through the polyol pathway, hexosamine pathway, inappropriate activation of protein kinase C, and accumulation of advanced glycation end products in peripheral nerves, which collectively lead to mitochondrial dysfunction, oxidative stress, and inflammation.6 Accumulating evidence from human and animal DPN studies suggests that vascular impairment and resultant nerve ischemia are closely related to the pathogenesis of DPN.7,8 Endothelial dysfunction induced by decreased vasodilators, such as nitric oxide and prostacyclin, and increased vasoconstrictors, such as endothelin-1, in diabetes results in both macrovascular and microvascular damages.7,9 Endoneurial hypoxia caused by decreased nerve blood flow alters nerve function10,11 and leads to nerve degeneration and loss of nerve fibers, especially in diabetic nerves with increased vulnerability to ischemia.7,12 In patients with diabetes, microvascular dysfunction is regarded as one of the pathologic hallmarks of DPN13 and is related to the severity of painful DPN.14 Furthermore, in experimental diabetic animal models, a variety of vasodilator agents ameliorate vascular impairment, neural dysfunction, nerve degeneration, loss of sensitivity, and neuropathic pain.15–19
Transient receptor potential ankyrin 1 (TRPA1), a nonselective cation channel, is highly expressed in a subset of nociceptive C fibers and acts as a polymodal nociceptor.20 TRPA1 is activated by a large number of irritants and oxidative stimuli, such as reactive oxygen species (ROS) and hyperoxia, through reversible covalent or oxidative modification of cysteine residues in the N-terminal region.21–23 We previously reported that TRPA1 is also activated or sensitized by hypoxia through another mechanism: a decrease in oxygen concentration inhibits the activity of prolyl hydroxylases (PHDs) and relieves TRPA1 from the PHD-dependent hydroxylation of a proline residue located within the N-terminal ankyrin repeat domain.23,24 PHD inhibition increases the sensitivity of TRPA1 to ROS, which endows or enhances the cold sensitivity of TRPA1 by detecting cold-evoked ROS production.25,26 Given these findings, we propose that hypoxia-induced TRPA1 sensitization contributes to the painful aspect of peripheral neuropathy, especially to cold hypersensitivity.24–28
A body of evidence suggests that TRPA1 is involved in painful DPN.29,30 Methylglyoxal, a reactive dicarbonyl compound that is an intermediate of glycolysis, mediates diabetic neuropathic pain by stimulating TRPA1 in primary afferent sensory neurons.31,32 In the present study, we examined the roles of TRPA1 in DPN by focusing on the vascular impairment in a streptozotocin (STZ)-induced diabetic mouse model, which exerts mechanical and cold hypersensitivity33,34 like patients with painful DPN.3 We show that TRPA1 sensitized by vascular impairment is responsible for the cold hypersensitivity observed in the early painful phase of DPN.
Materials and methods
Animals
This study was carried out in strict accordance with the ethical guidelines recommended by the Kyoto University Animal Research Committee. The protocol was approved by the Kyoto University Animal Research Committee (permit number, 13–38). All efforts were made to minimize the number of animals used and to limit experimentation that was necessary to produce reliable scientific information. The C57BL/6J mice aged six to eight weeks were purchased from Japan SLC (Shizuoka, Japan). The Trpa1+/+ (wild-type (WT)) and Trpa1−/− (TRPA1-knockout (KO)) mouse lines were bred from heterozygous mice with a C57BL/6 × 129 S1 background that were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mouse lines were backcrossed to C57BL/6J mice for at least 10 generations and genotyped by genomic polymerase chain reaction (PCR) using primers 5′-TCATCTGGGCAACAATGTCACCTGCT-3′ and 5′-TCCTGCAAGGGTGATTGCGTTGTCTA-3′. Male WT and TRPA1-KO mice aged six to eight weeks were used for experiments. All mice were housed under constant ambient temperature (24 ± 1°C) and humidity (55% ± 10%), with alternate 12 h light/dark cycles from 8.00 a.m. to 8.00 p.m. Food and water were freely available.
Drugs and reagents
STZ (Wako Pure Chemical Industries, Osaka, Japan) was dissolved in sterile saline. HC-030031 (Haoyuan Chemexpress, Shanghai, China) was freshly suspended in 0.5% methylcellulose (Wako) and injected intraperitoneally 30 min before behavioral testing. Allyl isothiocyanate (AITC; Wako) was dissolved in corn oil (Sigma-Aldrich, St. Louis, MO, USA). Tadalafil (Sigma-Aldrich) was dissolved in 10% dimethyl sulfoxide/20% polyethylene glycol 400 in distilled water and injected intraperitoneally 1 h before behavioral testing.
STZ-induced diabetic mouse model
Diabetes was induced in mice by injecting them with STZ (50 mg/kg, intraperitoneal (i.p.)) for seven consecutive days. Blood was sampled from the tail vein, and the blood glucose level was measured using an Accu-Check ST meter (Roche Diagnostics, Indianapolis, IN, USA). Mice were deemed diabetic when the casual blood glucose concentration exceeded 250 mg/dL.
Mouse model of hindlimb ischemia
Mice were anesthetized with pentobarbital (64.8 mg/kg, i.p.), and the bilateral lower abdomen area was shaved. An incision was made along the thigh, extending from the inguinal ligament to the abdomen. The external iliac artery and vein were exposed and tightly ligated with 7–0 silk thread (Alfresa Pharma Corporation, Osaka, Japan), which resulted in complete occlusion of the lumen of the blood vessel. The wound was closed by suturing the skin layer. Sham-operated mice received an identical surgery, except that their external iliac artery and vein were not ligated.
Measurement of skin blood flow in the hindpaw
The endoneurial blood flow within the sciatic nerve has often been investigated to resolve the relationship between diabetes-induced vascular impairment and neural dysfunction or degeneration.8 However, because the aim of the present study was to examine the effects of hypoxia around TRPA1 expressed on the peripheral nerve terminal of primary sensory neurons, we measured the decrease in the hindpaw skin blood flow of diabetic mice.14,35 Mice were anesthetized with pentobarbital (64.8 mg/kg, i.p.). Each animal was placed in the prone position on a heating pad maintained at 36.5°C by an animal blanket controller (Nihon-Koden, Tokyo, Japan), and the right hindpaw was secured in the desired position. The skin blood flow of the plantar surface of the hindpaw was measured by scanning the region of interest using a laser speckle blood flow analyzing system (OMEGA ZONE, Omega Wave Co., Tokyo, Japan). The values were normalized to that in the control in each experiment.
Cold-plate test
Cold sensitivity was measured with a hot/cold-plate analgesimeter (Ugo Basile, Milan, Italy) as previously described.27 Mice were allowed to acclimate to the testing apparatus for 1 h, after which they were individually placed on the center of a cold plate maintained at 5°C in a transparent Plexiglas cylinder. Escape behaviors were observed for 60 s and graded using the following scoring system: 0, no response; 1, moderate effort to avoid cold, such as lifting a hindpaw or walking backward; or 2, vigorous effort to escape cold, such as jumping. The scores recorded within a 60 s period were summed.
von Frey filament test
Mechanical sensitivity was assessed by measuring the paw withdrawal threshold using calibrated von Frey filaments as previously described with slight modifications.36,37 Mice were acclimatized on a metal mesh floor in small cylinders for 1 h. For the up-down method, mechanical sensitivity was evaluated using seven calibrated von Frey filaments (0.008, 0.02, 0.04, 0.07, 0.16, 0.4, and 1.0 g) that were applied to the plantar surface of the hindpaw until the filament bent slightly for a few seconds. The first applied stimulus was always the 0.16 g filament. When a mouse demonstrated a positive response, such as flicking or lifting the paw, the next lower weight filament was applied. When a mouse demonstrated a negative response (i.e., no movement), the next higher weight filament was applied. After the first change in response, four additional responses were observed, and the 50% paw withdrawal threshold was calculated.37
TRPA1 agonist-evoked nocifensive behaviors
TRPA1 agonist-evoked nocifensive behaviors were measured as previously described.27 The mice were allowed to acclimate in a clear acrylic cylinder for at least 40 min, after which AITC (20 μL of 0.05%) was subcutaneously injected into the plantar surface of the left hindpaw. AITC-evoked nocifensive behaviors were measured for 5 min as the duration of consecutive licking behavior in STZ-induced diabetic mice and licking or lifting behavior in the mice subjected to the hindlimb ischemic model.
Measurement of current perception threshold
For measurement of paw withdrawal responses to transcutaneous current stimuli, sine-wave pulses were produced by a Neurometer CPT/C (Neurotron Inc., Baltimore, MD, USA), a device clinically used for measuring perception and pain thresholds.38,39 Each mouse was kept in a Ballmann cage (Natsume, Tokyo, Japan), and the hair of the hindpaw was shaved. An electrode for stimulation was attached to the plantar surface, and transcutaneous current stimuli comprising three types of sine-wave pulses (5, 250, and 2000 Hz) were separately applied. The intensity of each stimulation was gradually increased, and the intensity (mA) eliciting a paw withdrawal response was determined. Three consecutive measurements were conducted at each frequency, and the average intensity was defined as the current perception threshold.
Real-time PCR
For assessing mRNA expression levels, real-time PCR analyses were performed two weeks after STZ administration. Total RNAs were extracted from the L4 dorsal root ganglion (DRG) using NucleoSpin RNA (TaKaRa Bio Inc., Shiga, Japan), and the extracted RNAs were reverse transcribed (RT) using the ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). Real-time quantitative RT-PCR was performed using a StepOne Real-Time PCR System (Life Technologies, Carlsbad, CA, USA) and Thunderbird SYBR qPCR Mix (Toyobo). Each PCR amplification consisted of heat activation for 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. The oligonucleotide primers used for RT-PCR were as follows: 5′-GCA ATT ATT CCC CAT GAA CG-3′ and 5′-GGC CTC ACT AAA CCA TCC AA-3′ for the 18S ribosomal RNA gene (18S rRNA); and 5′-TCC GGT CGA TCT CAG CAA TG-3′ and 5′-GGC AAT GTG GAG CAA TAG CG-3′ for TRPA1. The mRNA expression levels were normalized to that of 18S rRNA, which was measured in parallel with each sample. The mRNA expression level of each gene was expressed relative to the vehicle-treated group.
Immunohistochemistry
Mice were deeply anesthetized with sodium pentobarbital and perfused through the ascending aorta with phosphate-buffered saline (PBS) followed by 4% (W/V) paraformaldehyde in phosphate buffer. The right hindpaw tissues, including the skin and underlying muscle, were removed and embedded in paraffin. Paraffin-embedded tissues were cut into 5 μm sections and treated with 3% bovine serum albumin for 1 h at room temperature. After washing with PBS, the sections were incubated with a rabbit polyclonal anti-protein gene product 9.5 (PGP9.5) antibody (1:600, Ultraclone Ltd, Cambridge, UK) at 4°C overnight. The sections were washed three times in PBS and incubated for 1 h with biotinylated anti-rabbit IgG antibody (Vector Laboratories, Burlingame, CA, USA). The sections were then incubated for 2 h with avidin–biotin complex (Vectastain ABC Standard Kit, Vector Laboratories) and processed in a standard 3,3′-diaminobenzidine tetrahydrochloride (Nacalai Tesque, Kyoto, Japan) reaction. The tissue sections were then stained with hematoxylin (Wako) and coverslipped with Entellan® new (Merck, Darmstadt, Germany). Histopathological examination was performed with a light microscope (BX-53F, Olympus, Tokyo, Japan). For quantification of intraepidermal nerve fibers (IENFs), the number of PGP9.5-immunopositive nerve fibers in the plantar skin section was counted in at least five slices per animal following the estimation of immunolabeled nerve fiber profiles. The IENF density was calculated as the number of PGP9.5-immunopositive nerve fibers per length of epidermis (number/mm).
Statistical analysis
The data were analyzed using GraphPad Prism and are presented as means ± SEM. Differences between two groups were compared using Student’s t test. Data with more than two groups were compared using one-way or two-way analysis of variance (ANOVA), followed by Bonferroni or Sidak post hoc tests. Time-course data were analyzed by two-way ANOVA for repeated measures, followed by Bonferroni post hoc tests. In all cases, differences of P < 0.05 were considered statistically significant.
Results
Changes in blood glucose level, body weight, and skin blood flow in STZ-induced diabetic mice
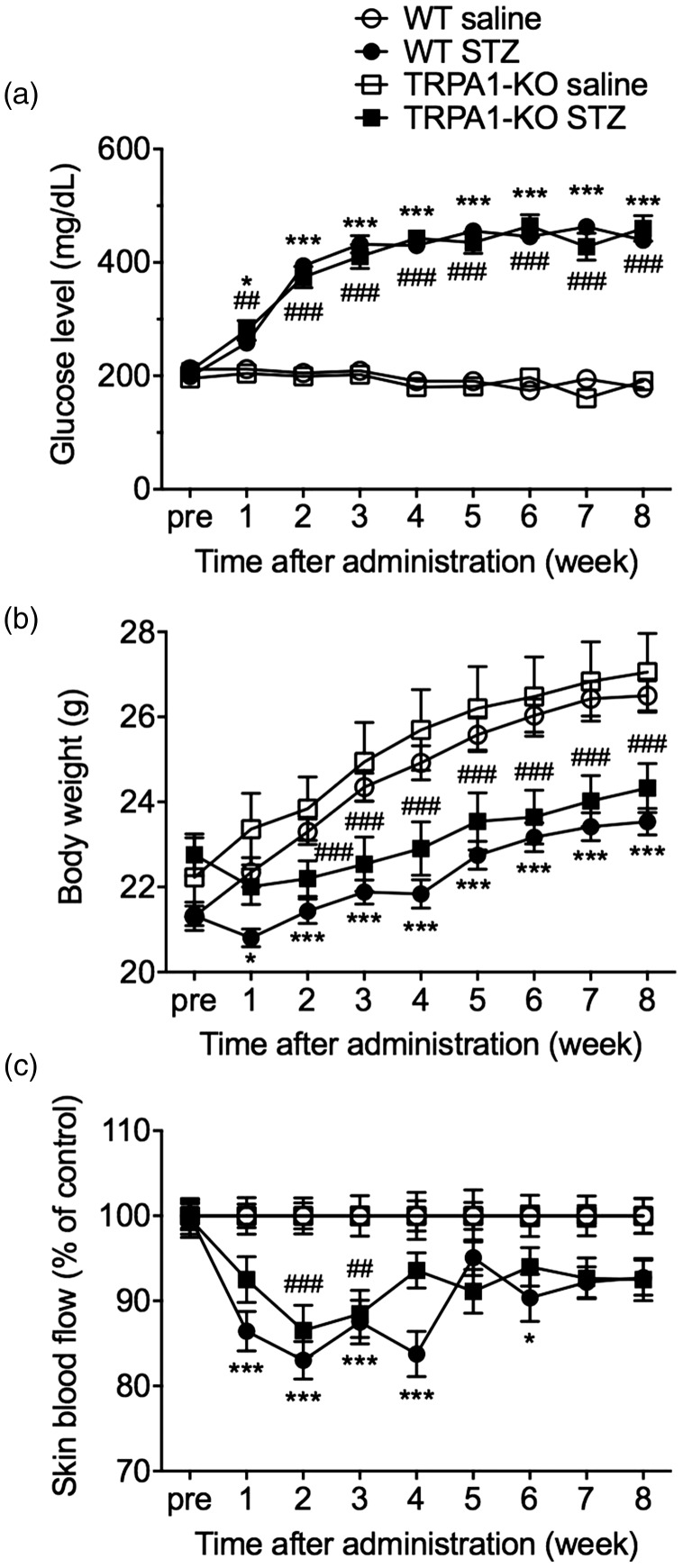
The i.p. administration of STZ (50 mg/kg) for seven consecutive days significantly increased casual blood glucose levels in both WT (STZ × time interaction: F(8, 304) = 71.50, P < 0.001) and TRPA1-KO mice (STZ × time interaction: F(8, 208) = 44.73, P < 0.001) and significantly decreased body weight in both WT (STZ × time interaction: F(8, 304) = 18.66, P < 0.001) and TRPA1-KO mice (STZ × time interaction: F(8, 208) = 14.4, P < 0.001), compared with the saline-treated nondiabetic groups. There were no differences in the increased blood glucose level (genotype: F(1, 34) = 0.01, P = 0.92) and the decreased body weight (genotype: F(1, 34) = 2.60, P = 0.12) between WT-STZ and TRPA1-KO-STZ mice (Figure 1a and b).
Figure 1.
Changes in blood glucose level, body weight, and skin blood flow in mice with STZ-induced diabetes. WT or TRPA1-KO mice were administered with saline or STZ (50 mg/kg, i.p.) for seven consecutive days. The casual blood glucose levels (a), body weight (b), and skin blood flow (c) were measured before and weekly after the administrations. The skin blood flow of the hindpaw was measured using laser Doppler flowmetry and expressed as the percentage of each saline-treated group on the same day. Data are expressed as means ± SEM. n = 14–22. *P < 0.05, ***P < 0.001 compared with the WT saline group; ##P < 0.01, ###P < 0.001 compared with the TRPA1-KO saline group.
KO: knockout; STZ: streptozotocin; TRPA1: transient receptor potential ankyrin 1; WT: wild-type.
To detect STZ-induced peripheral vascular impairment, we measured skin blood flow in the hindpaw. The STZ administration significantly reduced skin blood flow in both WT (STZ × time interaction: F(8, 304) = 5.103, P < 0.001) and TRPA1-KO mice (STZ × time interaction: F(8, 208) = 1.99, P < 0.05), compared with saline-treated nondiabetic mice. A peak reduction was observed two weeks after STZ administration. However, there was no significant difference in the reduced skin blood flow between the WT-STZ and TRPA1-KO-STZ groups (genotype: F(1, 34) = 0.90, P = 0.35; Figure 1c).
Involvement of TRPA1 in the early painful phase of DPN
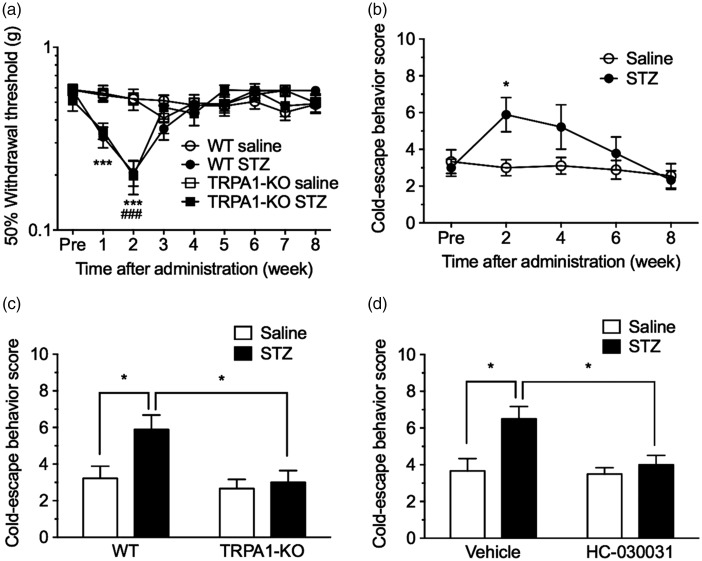
We assessed behavioral sensitivity to mechanical and cold stimuli using von Frey filament and cold-plate tests, respectively. Compared with the saline-treated nondiabetic groups, STZ administration significantly decreased the 50% withdrawal threshold to mechanical stimulation with von Frey filaments at one to two weeks in WT mice and at two weeks in TRPA1-KO mice, with no difference between these two genotypes (genotype: F(1, 28) = 0.05, P = 0.82). The STZ-induced mechanical hypersensitivity returned to control levels within three weeks after STZ administration in both genotypes (Figure 2a).
Figure 2.
Involvement of TRPA1 in the early painful phase of DPN. WT or TRPA1-KO mice were administered with saline or STZ (50 mg/kg, i.p.) for seven consecutive days. (a) The 50% withdrawal thresholds to mechanical stimulation with von Frey filaments were measured before and weekly after the administrations. n = 9–21. ***P < 0.001 compared with the WT saline group; ###P < 0.001 compared with the TRPA1-KO saline group. (b to d) Cold sensitivities were measured using the cold-plate test (5°C). (b) Cold-escape behavior scores in saline- or STZ-treated WT mice were measured before and biweekly after the administrations. n = 9. *P < 0.05 compared with the saline-treated group. (c) Effect of TRPA1-KO on cold hypersensitivity in mice with STZ-induced diabetes. Cold-escape behavior scores in WT and TRPA1-KO mice were determined two weeks after STZ administration. n = 9. *P < 0.05. (d) Effect of the selective TRPA1 antagonist HC-030031 on cold hypersensitivity in mice with STZ-induced diabetes. Saline- or STZ-treated mice two weeks after the treatment were administered vehicle or HC-030031 (100 mg/kg, i.p.) 30 min before the cold-plate test, and cold-escape behavior scores were determined. n = 6. *P < 0.05. Data are expressed as means ± SEM.
KO: knockout; STZ: streptozotocin; TRPA1: transient receptor potential ankyrin 1; WT: wild-type.
Escape behavior scores in response to cold stimulation in STZ-treated mice were significantly increased and peaked two weeks after STZ administration (Figure 2b). These increased cold-escape behavior scores returned to controls level within six to eight weeks. In TRPA1-KO mice, increased cold-escape behavior scores two weeks after STZ administration were significantly attenuated compared with those in WT mice (genotype: F(1, 32) = 6.86, P < 0.05; Figure 2c). Consistent with this result, administration of the selective TRPA1 antagonist HC-030031 (100 mg/kg, i.p.) 30 min before the cold-plate test significantly attenuated increased cold-escape behavior scores two weeks after STZ administration (HC-030031: F(1, 20) = 5.56, P < 0.05; Figure 2d). However, neither TRPA1-KO nor HC-030031 changed cold-escape behavior scores in the saline-treated nondiabetic mice.
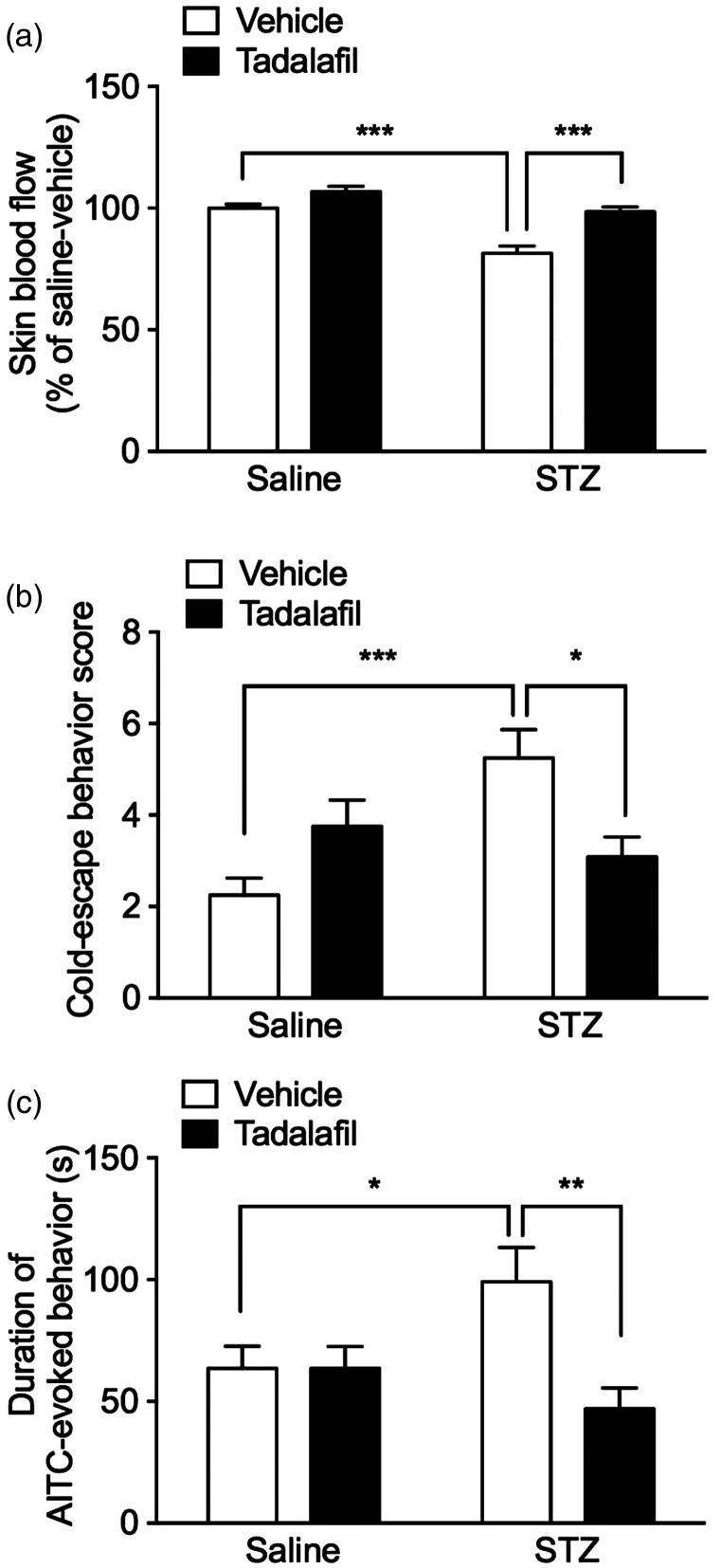
STZ-induced cold hypersensitivity and TRPA1 sensitization are reversed by tadalafil
To investigate the relationship between the STZ-induced vascular impairment and cold hypersensitivity, we examined the effects of a phosphodiesterase-5 (PDE-5) inhibitor, tadalafil, which is a vasodilator. Administration of tadalafil (10 mg/kg, i.p.) 1 h before the tests significantly reversed the reduced skin blood flow at two weeks after STZ administration compared with that in vehicle-injected diabetic mice (tadalafil: F(1, 20) = 28,1, P ≤ 0.0001), whereas it had no effect on basal skin blood flow in saline-treated nondiabetic mice (Figure 3a). Furthermore, administration of tadalafil reversed the increased cold-escape behavior scores at two weeks after STZ administration (Figure 3b). Although two-way ANOVA analysis showed no significant difference (tadalafil: F(1, 44) = 0.426, P = 0.517), Bonferroni’s multiple comparison test indicated that tadalafil significantly inhibited it, compared with that in vehicle-injected diabetic mice. On the other hand, tadalafil tended to increase the cold-escape behaviors in saline-injected control animals, while there is no significant difference between vehicle and tadalafil injection.
Figure 3.
Effects of tadalafil on STZ-induced cold hypersensitivity and TRPA1 sensitization. Mice were administered with saline or STZ (50 mg/kg, i.p.) for seven consecutive days. Two weeks after the administrations, the PDE-5 inhibitor tadalafil (10 mg/kg, i.p.), which is a vasodilator, or its vehicle was administered 1 h before the behavioral tests. (a) Skin blood flow of the hindpaw was measured and expressed as the percentage of the saline-treated nondiabetic group injected with vehicle. n = 6. (b) Cold-escape behavior scores for the cold-plate test. n = 12. (c) Duration(s) of nocifensive behavior (licking) evoked by intraplantar injection of 0.05% AITC was measured for 5 min. n = 11–13. Data are expressed as means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
AITC: allyl isothiocyanate; STZ: streptozotocin.
To investigate the change in TRPA1 responsiveness in STZ-induced diabetic mice, we measured the duration of licking, a nocifensive behavior, evoked by intraplantar injection of a TRPA1 agonist, AITC. In STZ-induced diabetic mice, the duration of the AITC-evoked nocifensive behavior was significantly prolonged at two weeks after STZ administration, compared with that in saline-treated nondiabetic mice. The administration of tadalafil (10 mg/kg, i.p.) 1 h before the tests significantly inhibited the prolonged duration of AITC-evoked nocifensive behavior (tadalafil: F(1, 44) = 5.93, P ≤ 0.05; Figure 3c). However, TRPA1 mRNA expression levels in the DRG were not changed two weeks after STZ administration (Supplementary Figure 1).
TRPA1 sensitization in a mouse model of hindlimb ischemia
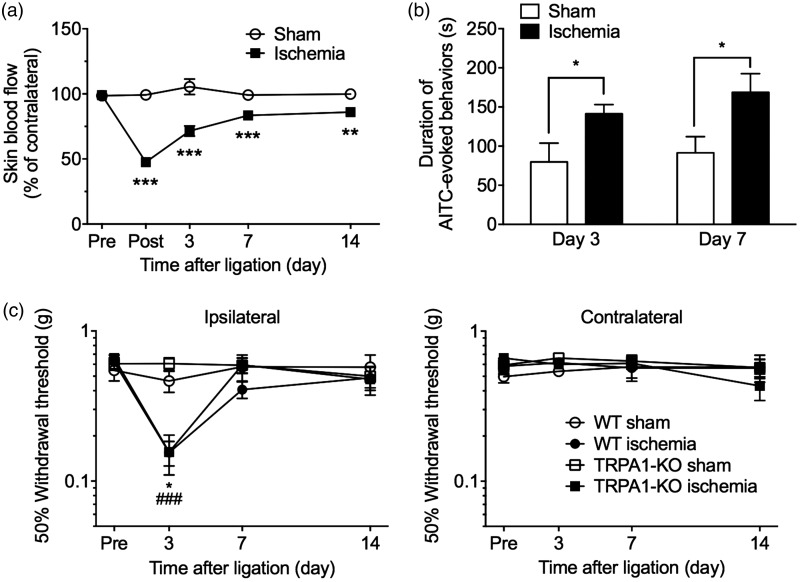
To further investigate the relationship between peripheral vascular impairment and TRPA1 sensitization, we examined AITC-evoked paw licking and lifting in a mouse model of hindlimb ischemia. Tight ligation of the external iliac artery and vein significantly reduced the ipsilateral hindpaw skin blood flow, which peaked just after the ligation and gradually recovered (operation × time interaction: F(4, 148) = 26.3, P < 0.001). Compared with that in sham-operated mice, significant reductions in skin blood flow lasted at least 14 days after the ligation (Figure 4a). In mice with hindlimb ischemia, the duration of the AITC-evoked nocifensive behavior was significantly prolonged three and seven days after the ligation, compared with that in sham-operated mice (Figure 4b).
Figure 4.
TRPA1 sensitization in mice subjected to hindlimb ischemia. To induce hindlimb ischemia in mice, the external iliac artery and vein were tightly ligated. (a) Skin blood flow in the ipsilateral and contralateral hindpaws was measured in sham-operated or hindlimb ischemic mice before (pre); just after (post); and 3, 7, and 14 days after the ligation. The values in the ipsilateral hindpaw are expressed as a percentage of the contralateral hindpaw. n = 19–20. **P < 0.01, ***P < 0.001 compared with the sham-operated group. (b) The duration(s) of nocifensive behavior (licking or lifting) evoked by intraplantar injection of 0.05% AITC was measured for 5 min at three (n = 4–8) and seven days (n = 4–5) after the sham or hindlimb ischemic ligation. (c) Sham or hindlimb ischemic ligation was performed in WT or TRPA1-KO mice. The 50% withdrawal thresholds to mechanical stimulation with von Frey filaments in the ipsilateral (left panel) and contralateral (right panel) hindpaw were measured before (pre) and 3, 7, and 14 days after ligation. n = 3–5. *P < 0.05 compared with the sham-operated WT group; ###P < 0.001 compared with the sham-operated TRPA1-KO group. Data are expressed as means ± SEM.
AITC: allyl isothiocyanate; KO: knockout; TRPA1: transient receptor potential ankyrin 1; WT: wild-type.
Compared with that in sham-operated mice, the 50% withdrawal threshold to mechanical stimulation in the ipsilateral hindpaw was significantly decreased three days after ligation in both WT and TRPA1-KO ischemic mice. This decrease recovered within seven days after the ligation. There was no difference in mechanical hypersensitivity between the two genotypes (genotype: F(1, 7) = 0.63, P = 0.45). In the contralateral hindpaw, the 50% withdrawal threshold was not changed in either group (Figure 4c).
TRPA1 is not involved in the late phase of STZ-induced DPN
Eight weeks after the STZ administration, the duration of the AITC-evoked nocifensive behaviors was not changed from that in saline-treated nondiabetic mice (Supplementary Figure 2).
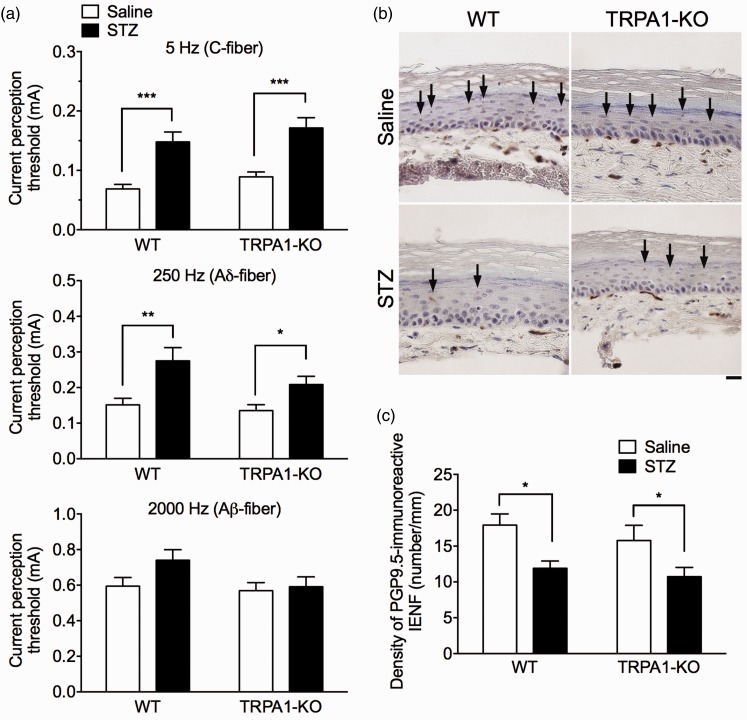
To detect hyposensitivity in the late phase of STZ-induced DPN, we measured current perception thresholds to sine-wave pulses of various frequencies (5, 250, and 2,000 Hz) produced by a Neurometer, which primarily stimulates C-, Aδ-, and Aβ-fibers, respectively, in saline- or STZ-treated mice eight weeks after STZ administration. The STZ administration significantly increased the current perception thresholds for 5 Hz and 250 Hz stimuli (STZ: 5 Hz, F(1, 50) = 36.74, P < 0.001; 250 Hz, F(1, 50) = 14.86, P < 0.001), whereas it had no effect on the threshold for 2,000 Hz stimulation (STZ: 2,000 Hz, F(1, 50) = 2.54, P = 0.12) in both WT and TRPA1-KO mice. There were no significant differences in the current perception thresholds at each current stimulation between the genotypes (genotype: 5 Hz, F(1, 50) = 2.70, P = 0.11; 250 Hz, F(1, 50) = 2.65, P = 0.11; 2,000 Hz, F(1, 50) = 2.76, P = 0.10; Figure 5a).
Figure 5.
Hyposensitivity and reduced IENFs in the late phase of STZ-induced DPN in TRPA1-KO mice. WT or TRPA1-KO mice were injected with saline or STZ (50 mg/kg, i.p.) for seven consecutive days. (a) Eight weeks after the administrations, the current perception thresholds (mA) to the sine-wave pulses of various frequencies at 5 (upper panel), 250 (middle panel), and 2000 Hz (lower panel) were measured. n = 13–14. *P < 0.05, **P < 0.01, ***P < 0.001. (b) Representative photographs of PGP9.5-immunoreactive staining of IENFs in the plantar skin of the hindpaw of saline- or STZ-treated WT or TRPA1-KO mice eight weeks after STZ administration. Scale bar = 20 µm. Arrows indicate PGP9.5-positive IENFs. (c) The densities of PGP9.5-immunoreactive IENFs (number/mm) were quantified. n = 8–9. *P < 0.05. Data are expressed as means ± SEM.
IENFs: intraepidermal nerve fibers; KO: knockout; PGP9.5: protein gene product 9.5; STZ: streptozotocin; TRPA1: transient receptor potential ankyrin 1; WT: wild-type.
Because we were unable to detect STZ-induced hyposensitivity to the von Frey filaments using the up-down method (Figure 2a), we applied a calibrated von Frey filament (0.4 g) to the STZ-induced diabetic mice eight weeks after STZ administration and measured paw withdrawal response scores to detect mechanical hyposensitivity. The STZ administration significantly decreased the scores for mechanical stimulation in both WT and TRPA1-KO mice compared with those in saline-treated nondiabetic mice (STZ: F(1, 66) = 16.46, P ≤ 0.001). However, there was no difference in mechanical hyposensitivity between the two genotypes (genotype: F(1, 66) = 0.13, P = 0.72; Supplementary Figure 3).
Assessment of IENF density in the skin is a reliable method for measuring DPN in both humans with diabetes and rodent diabetic models.40,41 We performed an immunohistochemical analysis for PGP9.5-immunoreactive IENFs in the hindpaw plantar skin of saline- or STZ-treated mice eight weeks after the treatment. STZ administration significantly decreased the density of PGP9.5-immunoreactive IENFs (STZ: F(1, 30) = 13.36, P < 0.001) in both WT and TRPA1-KO mice; however, there was no difference in the density between the two genotypes (genotype: F(1, 30) = 1.21, P = 0.28; Figure 5b and c).
Discussion
In the present study using a STZ-induced mouse model of diabetes, we provide evidence that TRPA1 is sensitized by diabetic vascular impairment, which contributes to the cold hypersensitivity observed in the early painful phase of DPN. However, the involvement of TRPA1 in the hyposensitivity and in the reduced IENF density observed in the late phase of DPN was limited.
The STZ-induced diabetic mouse model, which is an often-used model of type 1 diabetes by damaging pancreatic β cells, shows various pathologies of DPN.42 TRPA1 is reported to be associated with glucose homeostasis, such as secretion of insulin, glucagon-like peptide 1, and ghrelin.43 However, we showed that neither the blood glucose increase nor the body weight loss differed between WT and TRPA1-KO mice that were treated with STZ, consistent with a previous report using TRPA1-KO mice.44 This finding enabled us to investigate the contribution of TRPA1 deficiency to DPN without affecting STZ-induced diabetes. In addition, the present results showed that TRPA1 deficiency had no effect on the decreased hindpaw skin blood flow in diabetic mice, suggesting little role for TRPA1 in diabetes-induced vascular impairment. TRPA1 is responsible for vascular responses to cold exposure, and TRPA1 stimulation induces both vasoconstriction (by releasing noradrenaline and increasing ROS) and subsequent vasodilation (by releasing sensory nerve-derived neuropeptides and nitric oxide).45,46 In humans with diabetes and in animal models of the disease, the skin rewarming rate after cold exposure is commonly delayed, which is useful for early diagnosis of DPN.41,47 Although this phenomenon is considered to be associated with microvascular dysfunction,41 the involvement of TRPA1 has not been clarified.
Cold hypersensitivity is observed in STZ-induced diabetic rats48 and mice33,34 as well as in patients with DPN.3 In the present study, cold hypersensitivity was observed in the early phase (at two weeks) of STZ-induced DPN, which shows a time course similar to that previously reported.48 This time course is likely paralleled by peripheral vascular impairment, suggesting a possible association between them. The present study using TRPA1-KO mice and a TRPA1 antagonist revealed that STZ-induced cold hypersensitivity is mediated through TRPA1. Accumulating evidence suggests that TRPA1 is responsible for cold hypersensitivity in various rodent pain models.27,49–52 Although we cannot fully exclude the possibility of the involvement of TRP melastatin 8, a cold-sensitive TRP channel,53,54 the present results showed that cold hypersensitivity is accompanied by TRPA1 sensitization in the early phase of STZ-induced DPN. Consistent with a previous finding,53 the TRPA1 mRNA expression level in the DRG was not changed in STZ-induced diabetic mice in the present study, suggesting that the enhanced responsiveness of TRPA1 is caused by functional sensitization rather than by upregulation. It is noted that STZ directly stimulates TRPA1.44 However, STZ administered systemically is unstable; the half-life of STZ (200 mg/kg) after an intravenous injection is approximately 5 min, and it is completely eliminated from the blood by 2 h.55 In the present study, cold hypersensitivity was observed one week after the last administration of a relatively low dose (50 mg/kg) of STZ. Thus, direct activation of TRPA1 by STZ is unlikely to be responsible for the cold hypersensitivity observed in the present study.
The key finding of the present study is that both the TRPA1-mediated cold hypersensitivity and the enhanced AITC-evoked nocifensive behaviors were ameliorated along with the recovery of the decreased skin blood flow by tadalafil. These data suggest that diabetic vascular impairment induces TRPA1 sensitization, resulting in cold hypersensitivity in the early phase of DPN. This hypothesis is further supported by the present findings using the hindlimb ischemia model, which has been widely used to study peripheral arterial disease,56 and is reported to induce cold hypersensitivity.57 These results suggest that TRPA1 sensitization could be induced by diabetic vascular impairment rather than by other factors, such as metabolic changes or peripheral nerve damage. We previously reported that hypoxia induces TRPA1 sensitization through the inhibition of PHD-dependent hydroxylation of a TRPA1 proline residue, leading to transient hindlimb ischemic/reperfusion-evoked spontaneous licking behavior through ROS-evoked activation of TRPA1.24 Although the cold sensitivity of TRPA1 is controversial,21,58 we recently demonstrated that TRPA1 sensitization via PHD inhibition induces cold hypersensitivity by detecting cold-evoked ROS production.25,26 Taken together with our present results, it is conceivable that the same mechanism, that is, PHD inhibition-mediated TRPA1 sensitization, may underlie the cold hypersensitivity observed in the early phase of DPN. However, we cannot exclude the possibility that tadalafil directly acts on primary afferents and suppresses TRPA1 sensitization. To obtain direct evidence that TRPA1 sensitization is caused by diabetic vascular impairment through inhibition of PHD and hydroxylation of a TRPA1 proline residue, further investigations will be needed.
By contrast, TRPA1 deficiency had no effect on the mechanical hypersensitivity observed in the early phase of STZ-induced DPN or in the mouse model of hindlimb ischemia. It is reported that the STZ-induced DPN rodent model shows both hypersensitivity and hyposensitivity to mechanical stimulation by von Frey filaments. However, the time course of either mechanical hypersensitivity or hyposensitivity is controversial and may depend on several factors, including the STZ dose, rodent species and strain, and method for detecting mechanical sensitivity.59 Typically, in mice, mechanical hypersensitivity is induced one to four weeks after the STZ administration,60,61 but then mechanical hyposensitivity is induced after longer periods of DPN.62,63 Mechanical hypersensitivity in the early phase of DPN appears to parallel the time course of STZ-induced peripheral vascular impairment. Taken together with the present finding that hindlimb ischemia also induced transient mechanical hypersensitivity, peripheral vascular impairment may play a role in mechanical hypersensitivity through TRPA1-independent mechanisms. However, further investigations will be needed to determine the association between them. In contrast to the present data, it was reported that TRPA1 antagonists inhibit the mechanical hypersensitivity observed in the painful DPN models.29,30 Accumulating evidence suggests that TRPA1 plays roles in mechanical hypersensitivity in other pain models,52,64,65 although the results of some studies are inconsistent with this evidence.49,66 Although we cannot explain these discrepant results, complex mechanisms through TRPA1-independent pathways (e.g., TRPA1 nonexpressing large myelinated fibers) may underlie the mechanical hypersensitivity observed in painful DPN.
Consistent with previous findings,62,67 we observed a current perception threshold increase, an IENF density decrease and mechanical hyposensitivity in the late stage of DPN (eight weeks after STZ administration). However, TRPA1 deficiency had no effects on these phenomena, suggesting little role for TRPA1 in the progression of DPN. By contrast, repeated injections of a TRPA1 antagonist in a STZ-induced diabetic rat model were shown to inhibit the decreased number of IENFs observed four weeks after STZ administration.68 Although we cannot fully explain the discrepancy between TRPA1 gene deficiency and TRPA1 antagonist, sustained activation of TRPA1 by endogenous agonists, such as methylglyoxal, generated in diabetes31,32 may have little effect on the degeneration of primary afferent nerves. However, accumulating evidence suggests that vasodilators can prevent the progression of DPN, including neural dysfunction, nerve degeneration, and hyposensitivity.15–19 Thus, these effects are likely to be caused by amelioration of the diabetic vascular impairment, but probably not through inhibition of TRPA1 sensitization/activation.
In conclusion, the present study determined that cold hypersensitivity in the early phase of DPN is mediated through the TRPA1 sensitization during diabetic vascular impairment in the STZ-induced diabetic mouse model. It is possible that diabetic-related endogenous TRPA1 agonists, such as methylglyoxal,31,32 can activate the sensitized TRPA1, which may then more potently cause spontaneous pain. We propose that the therapeutic strategy of targeting TRPA1 for treating some aspects of painful DPN, as well as various peripheral ischemic diseases, such as peripheral arterial occlusive disease, may be warranted, although the involvement of TRPA1 in the progression of DPN may be limited.
Supplemental Material
Supplemental material, Supplementary figures for TRPA1 sensitization during diabetic vascular impairment contributes to cold hypersensitivity in a mouse model of painful diabetic peripheral neuropathy by Haruka Hiyama, Yuichi Yano, Kanako So, Satoshi Imai, Kazuki Nagayasu, Hisashi Shirakawa, Takayuki Nakagawa and Shuji Kaneko in Molecular Pain
Author Contributions
HH, TN, and SK designed the project. HH and YY conducted the experiments. HH, YY, KS, and TN analyzed the data. KS, SI, KN, and HS helped design the study and provided technical advices. HH and TN wrote the manuscript, and SK finalized the manuscript.
Declaration of Conflicting Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI) from the Japanese Society for the Promotion of Science (Grants-in-Aid for Scientific Research (B) to TN (17H04008), Challenging Exploratory Research to TN (17K19722), and Scientific Research on Innovative Area “Thermal Biology” to TN (16H01386 and 18H04696)) and by a grant from The Naito Foundation to TN.
Supplemental Material
Supplementary material is available for this article online.
References
- 1.Tesfaye S, Boulton AJM, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010; 33: 2285–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ziegler D, Fonseca V. From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complications 2015; 29: 146–156. [DOI] [PubMed] [Google Scholar]
- 3.Scholz J, Rathmell JP, David WS, Chad DA, Broderick AC, Perros SG, Shin NS, Wells JL, Davis JB, DiMaggio CJ, Wang S, Tate SN. A standardized clinical evaluation of phenotypic diversity in diabetic polyneuropathy. Pain 2016; 157: 2297–2308. [DOI] [PubMed] [Google Scholar]
- 4.Pop-Busui R, Boulton AJM, Feldman EL, Bril V, Freeman R, Malik RA, Sosenko JM, Ziegler D. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: an update. Am Fam Physician 2016; 94: 227–234. [PubMed] [Google Scholar]
- 6.Feldman EL, Nave K-A, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 2017; 93: 1296–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nukada H. Ischemia and diabetic neuropathy. Handb Clin Neurol 2014; 126: 469–487. [DOI] [PubMed] [Google Scholar]
- 8.Yorek MA. Vascular impairment of epineurial arterioles of the sciatic nerve: implications for diabetic peripheral neuropathy. Rev Diabet Stud 2015; 12: 13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron N, Eaton SEM, Cotter MA, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001; 44: 1973–1988. [DOI] [PubMed] [Google Scholar]
- 10.Cameron NE, Cotter MA, Low PA. Nerve blood flow in early experimental diabetes in rats: relation to conduction deficits. Am J Physiol 1991; 261: E1–E8. [DOI] [PubMed] [Google Scholar]
- 11.Coppey LJ, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Slowing of motor nerve conduction velocity in streptozotocin-induced diabetic rats is preceded by impaired vasodilation in arterioles that overlie the sciatic nerve. Int J Exp Diabetes Res 2000; 1: 131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zochodne DW, Cheng C, Sun H. Diabetes increases sciatic nerve susceptibility to endothelin-induced ischemia. Diabetes 1996; 45: 627–632. [DOI] [PubMed] [Google Scholar]
- 13.Charles M, Soedamah-Muthu SS, Tesfaye S, Fuller JH, Arezzo JC, Chaturvedi N, Witte D R. Low peripheral nerve conduction velocities and amplitudes are strongly related to diabetic microvascular complications in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care 2010; 33: 2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quattrini C, Harris ND, Malik RA, Tesfaye S. Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care 2007; 30: 655–659. [DOI] [PubMed] [Google Scholar]
- 15.Cameron NE, Cotter MA, Robertson S. Angiotensin converting enzyme inhibition prevents development of muscle and nerve dysfunction and stimulates angiogenesis in streptozotocin-diabetic rats. Diabetologia 1992; 35: 12–18. [DOI] [PubMed] [Google Scholar]
- 16.Hotta N, Koh N, Sakakibara F, Nakamura J, Hamada Y, Hara T, Mori K, Nakashima E, Naruse K, Fukasawa H, Kakuta H, Sakamoto N. Effects of beraprost sodium and insulin on the electroretinogram, nerve conduction, and nerve blood flow in rats with streptozotocin-induced diabetes. Diabetes 1996; 45: 361–366. [DOI] [PubMed] [Google Scholar]
- 17.Kihara M, Schmelzer JD, Low PA. Effect of cilostazol on experimental diabetic neuropathy in the rat. Diabetologia 1995; 38: 914–918. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Chopp M, Szalad A, Jia L, Lu X, Lu M, Zhang L, Zhang Y, Zhang R, Zhang ZG. Sildenafil ameliorates long term peripheral neuropathy in type II diabetic mice. PLoS One 2015; 10: e0118134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Chopp M, Szalad A, Lu X, Jia L, Lu M, Zhang RL, Zhang ZG. Tadalafil promotes the recovery of peripheral neuropathy in type II diabetic mice. PLoS One 2016; 11: e0159665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viana F. TRPA1 channels: molecular sentinels of cellular stress and tissue damage. J Physiol (Lond) 2016; 594: 4151–4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordt S-E, Bautista DM, Chuang H-H, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427: 260–265. [DOI] [PubMed] [Google Scholar]
- 22.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007; 445: 541–545. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi N, Kuwaki T, Kiyonaka S, Numata T, Kozai D, Mizuno Y, Yamamoto S, Naito S, Knevels E, Carmeliet P, Oga T, Kaneko S, Suga S, Nokami T, Yoshida J-I, Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol 2011; 7: 701–711. [DOI] [PubMed] [Google Scholar]
- 24.So K, Tei Y, Zhao M, Miyake T, Hiyama H, Shirakawa H, Imai S, Mori Y, Nakagawa T, Matsubara K, Kaneko S. Hypoxia-induced sensitization of TRPA1 in painful dysesthesia evoked by transient hindlimb ischemia/reperfusion in mice. Sci Rep 2016; 6: 23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyake T, Nakamura S, Zhao M, So K, Inoue K, Numata T, Takahashi N, Shirakawa H, Mori Y, Nakagawa T, Kaneko S. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat Commun 2016; 7: 12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyake T, Nakamura S, Meng Z, Hamano S, Inoue K, Numata T, Takahashi N, Nagayasu K, Shirakawa H, Mori Y, Nakagawa T, Kaneko S. Distinct mechanism of cysteine oxidation-dependent activation and cold sensitization of human transient receptor potential ankyrin 1 channel by high and low oxaliplatin. Front Physiol 2017; 8: 878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain 2012; 8: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakagawa T, Kaneko S. Roles of transient receptor potential ankyrin 1 in oxaliplatin-induced peripheral neuropathy. Biol Pharm Bull 2017; 40: 947–953. [DOI] [PubMed] [Google Scholar]
- 29.Wei H, Hämäläinen MM, Saarnilehto M, Koivisto A, Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology 2009; 111: 147–154. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Kobayashi K, Kogure Y, Yamanaka H, Yamamoto S, Yagi H, Noguchi K, Dai Y. Negative regulation of TRPA1 by AMPK in primary sensory neurons as a potential mechanism of painful diabetic neuropathy. Diabetes 2018; 67: 98–109. [DOI] [PubMed] [Google Scholar]
- 31.Andersson DA, Gentry C, Light E, Vastani N, Vallortigara J, Bierhaus A, Fleming T, Bevan S. Methylglyoxal evokes pain by stimulating TRPA1. PLoS One 2013; 8: e77986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Q, Chen Y, Gong N, Wang Y-X. Methylglyoxal mediates streptozotocin-induced diabetic neuropathic pain via activation of the peripheral TRPA1 and Nav1.8 channels. Metabolism 2016; 65: 463–474. [DOI] [PubMed] [Google Scholar]
- 33.Zychowska M, Rojewska E, Piotrowska A, Kreiner G, Mika J. Microglial inhibition influences XCL1/XCR1 expression and causes analgesic effects in a mouse model of diabetic neuropathy. Anesthesiology 2016; 125: 573–589. [DOI] [PubMed] [Google Scholar]
- 34.Rojewska E, Zychowska M, Piotrowska A, Kreiner G, Nalepa I, Mika J. Involvement of macrophage inflammatory protein-1 family members in the development of diabetic neuropathy and their contribution to effectiveness of morphine. Front Immunol 2018; 9: 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monafo WW, Eliasson SG, Shimazaki S, Sugimoto H. Regional blood flow in resting and stimulated sciatic nerve of diabetic rats. Exp Neurol 1988; 99: 607–614. [DOI] [PubMed] [Google Scholar]
- 36.Callahan BL, Gil ASC, Levesque A, Mogil JS. Modulation of mechanical and thermal nociceptive sensitivity in the laboratory mouse by behavioral state. J Pain 2008; 9: 174–184. [DOI] [PubMed] [Google Scholar]
- 37.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 38.Masson EA, Veves A, Fernando D, Boulton AJM. Current perception threshold: a new, quick, and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia 1989; 32: 724–728. [DOI] [PubMed] [Google Scholar]
- 39.Kiso T, Nagakura Y, Toya T, Matsumoto N, Tamura S, Ito H, Okada M, Yamaguchi T. Neurometer measurement of current stimulus threshold in rats. J Pharmacol Exp Ther 2001; 297: 352–356. [PubMed] [Google Scholar]
- 40.Narayanaswamy H, Facer P, Misra VP, Timmers M, Byttebier G, Meert T, Anand P. A longitudinal study of sensory biomarkers of progression in patients with diabetic peripheral neuropathy using skin biopsies. J Clin Neurosci 2012; 19: 1490–1496. [DOI] [PubMed] [Google Scholar]
- 41.Kambiz S van Neck JW Cosgun SG van Velzen MH Janssen JA Avazverdi N Hovius SE, andWalbeehm ET.. An early diagnostic tool for diabetic peripheral neuropathy in rats. PLoS One 2015; 10: e0126892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao F, Zheng ZM. Animal models of diabetic neuropathic pain. Exp Clin Endocrinol Diabetes 2014; 122: 100–106. [DOI] [PubMed] [Google Scholar]
- 43.Derbenev AV, Zsombok A. Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin Immunopathol 2016; 38: 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andersson DA, Filipović MR, Gentry C, Eberhardt M, Vastani N, Leffler A, Reeh P, Bevan S. Streptozotocin stimulates the ion channel TRPA1 directly: involvement of peroxynitrite. J Biol Chem 2015; 290: 15185–15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aubdool AA, Graepel R, Kodji X, Alawi KM, Bodkin JV, Srivastava S, Gentry C, Heads R, Grant AD, Fernandes ES, Bevan S, Brain SD. TRPA1 is essential for the vascular response to environmental cold exposure. Nat Commun 2014; 5: 5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aubdool AA, Kodji X, Abdul-Kader N, Heads R, Fernandes ES, Bevan S, Brain SD. TRPA1 activation leads to neurogenic vasodilatation: involvement of reactive oxygen nitrogen species in addition to CGRP and NO. Br J Pharmacol 2016; 173: 2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Balbinot LF, Canani LH, Robinson CC, Achaval M, Zaro MA. Plantar thermography is useful in the early diagnosis of diabetic neuropathy. Clinics (Sao Paulo) 2012; 67: 1419–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boric M, Skopljanac I, Ferhatovic L, Jelicic Kadic A, Banozic A, Puljak L. Reduced epidermal thickness, nerve degeneration and increased pain-related behavior in rats with diabetes type 1 and 2. J Chem Neuroanat 2013; 53: 33–40. [DOI] [PubMed] [Google Scholar]
- 49.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 2005; 115: 2393–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci 2010; 30: 15165–15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.da Costa DSM, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 2010; 148: 431–437. [DOI] [PubMed] [Google Scholar]
- 52.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, Mancini F, Pedretti P, Nilius B, Patacchini R, Geppetti P. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain 2011; 152: 1621–1631. [DOI] [PubMed] [Google Scholar]
- 53.Nam JS, Cheong YS, Karm MH, Ahn HS, Sim JH, Kim JS, Choi SS, Leem JG. Effects of nefopam on streptozotocin-induced diabetic neuropathic pain in rats. Korean J Pain 2014; 27: 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciobanu AC, Selescu T, Gasler I, Soltuzu L, Babes A. Glycolytic metabolite methylglyoxal inhibits cold and menthol activation of the transient receptor potential melastatin type 8 channel. J Neurosci Res 2016; 94: 282–294. [DOI] [PubMed] [Google Scholar]
- 55.Schein PS, Loftus S. Streptozotocin: depression of mouse liver pyridine nucleotides. Cancer Res 1968; 28: 1501–1506. [PubMed] [Google Scholar]
- 56.Matsui R, Watanabe Y, Murdoch CE. Redox regulation of ischemic limb neovascularization – what we have learned from animal studies. Redox Biol 2017; 12: 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao J-X, Blakeman KH, Yu W, Hultenby K, Xu X-J, Wiesenfeld-Hallin Z. Development of a mouse model of neuropathic pain following photochemically induced ischemia in the sciatic nerve. Exp Neurol 2000; 163: 231–238. [DOI] [PubMed] [Google Scholar]
- 58.Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 2013; 4: 2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics 2009; 6: 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Latham JR, Pathirathna S, Jagodic MM, Choe WJ, Levin ME, Nelson MT, Lee WY, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V. Selective T-type calcium channel blockade alleviates hyperalgesia in ob/ob mice. Diabetes 2009; 58: 2656–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang YP, Eber A, Yuan Y, Yang Z, Rodriguez Y, Levitt RC, Takacs P, Candiotti KA. Prophylactic and antinociceptive effects of coenzyme Q10 on diabetic neuropathic pain in a mouse model of type 1 diabetes. Anesthesiology 2013; 118: 945–954. [DOI] [PubMed] [Google Scholar]
- 62.Lennertz RC, Medler KA, Bain JL, Wright DE, Stucky CL. Impaired sensory nerve function and axon morphology in mice with diabetic neuropathy. J Neurophysiol 2011; 106: 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hyposensitivity in mice. J Pain 2003; 4: 493–504. [DOI] [PubMed] [Google Scholar]
- 64.Kwan KY, Glazer JM, Corey DP. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci 2009; 29: 4808–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity. J Physiol 2011; 589: 3575–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehto SG, Weyer AD, Youngblood BD, Zhang M, Yin R, Wang W, Teffera Y, Cooke M, Stucky CL, Schenkel L, Geuns-Meyer S, Moyer BD, Wild KD, Gavva NR. Selective antagonism of TRPA1 produces limited efficacy in models of inflammatory- and neuropathic-induced mechanical hypersensitivity in rats. Mol Pain 2016; 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shibata T, Naruse K, Kamiya H, Kozakae M, Kondo M, Yasuda Y, Nakamura N, Ota K, Tosaki T, Matsuki T, Nakashima E, Hamada Y, Oiso Y, Nakamura J. Transplantation of bone marrow-derived mesenchymal stem cells improves diabetic polyneuropathy in rats. Diabetes 2008; 57: 3099–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koivisto A, Hukkanen M, Saarnilehto M, Chapman H, Kuokkanen K, Wei H, Viisanen H, Åkerman KE, Lindstedt K, Pertovaara A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol Res 2012; 65: 149–158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary figures for TRPA1 sensitization during diabetic vascular impairment contributes to cold hypersensitivity in a mouse model of painful diabetic peripheral neuropathy by Haruka Hiyama, Yuichi Yano, Kanako So, Satoshi Imai, Kazuki Nagayasu, Hisashi Shirakawa, Takayuki Nakagawa and Shuji Kaneko in Molecular Pain