Short abstract
Painful neuropathy is a frequent comorbidity in diabetes. Zucker diabetic fatty (fa/fa) rats develop type 2 diabetes spontaneously with aging and show nociceptive hypersensitivity at the age of 13 weeks. In preclinical and clinical studies, the treatment of diabetic neuropathy is challenging, but complementary medicine such as transcutaneous auricular vagus nerve stimulation (taVNS) appears beneficial to the relief of neuropathic pain. However, the mechanism behind the effectiveness of taVNS remains unclear. In this study, we show that daily 30-min taVNS (2/15 Hz, 2 mA) for consecutive 27 days effectively inhibited the development of nociceptive hypersensitivity in Zucker diabetic fatty rats as detected by thermal hyperalgesia and mechanical allodynia in hindpaw. We also demonstrated that this beneficial effect in nociceptive behavior is related to an elevated serotonin (5-HT) plasma concentration and an upregulated expression of 5-HT receptor type 1A (5-HT1AR) in hypothalamus. We conclude that daily 30-min taVNS sessions lessen diabetic neuropathy development by enhancing serotonergic function in genetically diabetes prone individuals.
Perspective
This article presents taVNS as a new approach to inhibit the development of diabetic neuropathy in genetically prone individuals. This approach could potentially help clinicians who seek to avoid the complication of neuropathic pain in diabetic patient or to relieve the pain if there was one.
Keywords: Auricular vagus nerve stimulation, serotonin, melatonin, serotonin receptor type 1A, melatonin receptor type 1
Introduction
Painful neuropathy is a frequent comorbidity in diabetes. Despite the high incidence of diabetes, current prophylactic or acute treatment of diabetic neuropathy is unsatisfactory especially in long-term management.1 Various treatments have been developed over the last decades for this pain condition, including invasive, noninvasive, and alternative therapies.2–5 Among these treatments, acupuncture was found to significantly decrease neuropathy-associated pain.5,6 Multiple mechanisms could be involved in this effect of acupuncture. These include increased nitric oxide or improved melatonergic function involving transcutaneous auricular vagus nerve stimulation (taVNS) triggered melatonin (MEL) release and/or upregulated melatonin receptor type 1 (MT1) expression.7–9 In preclinical and clinical studies, taVNS was beneficial in treating other MEL-deficient diseases including hyperglycemia, hypertension, depression, epilepsy, insomnia, and inflammation.7,9–11 The neurophysiological basis for enhanced melatonergic function from this stimulation may be due to the taVNS signal being delivered into hypothalamus via the vagus nerve branch12–15 which subsequently modulates the pineal secretion of MEL. MEL is known to have beneficial effects in the above diseases. It upregulates MT1 expression, a process which seems to be ligand dependent.7,9,10,16
The central serotonergic system has also been implicated in pain.17–23 In the central nervous system (CNS), serotonin (5-HT) is a monoamine neurotransmitter in the descending inhibitory system.23,24 The 5-HT receptors are a group of G protein-coupled receptors and ligand-gated ion channels found in the central and peripheral nervous systems.25 It is now clear that 5-HT receptor type 1A (5-HT1AR) plays a role in nociception control.17,19,20,22,23,26–28 They are expressed throughout the CNS and at high levels in pain-related CNS regions.
The Zucker diabetic fatty (ZDF; fa/fa) rats are genetically leptin receptor expression defective and develop type 2 diabetes and neuropathy automatically. Without interference, the ZDF rats will be hypersensitive to thermal and mechanical stimulations at 13 weeks of age,29 a progression frequently seen in diabetic patients. It is not clear whether taVNS enhances the serotonergic system as it does in melatonergic systems in genetically diabetic prone individuals. In this study, we report that taVNS in ZDF rats elevates serotonergic function and halts the progress of diabetic neuropathy.
Materials and methods
Animal model
The experimental protocol was approved by the Institutional Animal Care and Use Committee in China Academy of Chinese Medical Sciences. Principles of laboratory animal care were followed. Five-week-old male ZDF (fa/fa, n = 14) rats and Zucker lean (ZL, +/fa, n = 13) littermates were purchased from Vital River Laboratories International Inc. (Beijing, China). Animals were housed under controlled temperature (21°C ± 2°C), relative humidity (50% ± 10%), and artificial light (12-h light/dark cycle, lights on at 7 a.m.). The health condition of animals was inspected daily. Littermates from the same or foster mother were housed in one large cage with distilled water and a standard rat diet pellets available ad libitum except during behavior testing or electroacupuncture (EA) sessions. Rats entered the experimental procedure at eight weeks of age, divided into ZL and ZDF groups according to the rat’s size, appearance, and genotype. The ZDF rats were further divided into three subgroups randomly, the Naïve, taVNS treated, and auricular margin electroacupuncture (AMEA) treated. The four groups included the following: N-ZL, N-ZDF, ZDF-taVNS, and ZDF-AMEA, n = 4–6 in each group. The number of rats was calculated using power analysis (.8), considering the data variation of nociceptive behavior and of plasma 5-HT levels in rats. The investigators were not blinded to the group allocation during the experiment because treatment procedures, for example, the taVNS, must be kept in the same group, and the tests used in this study are all objective. To minimize a possible confounding effect from gender and age differences on the levels of endogenous MEL, 5-HT, and other possible hormones, only male ZDF rats of the same age were used.
Behavioral tests and sampling time points
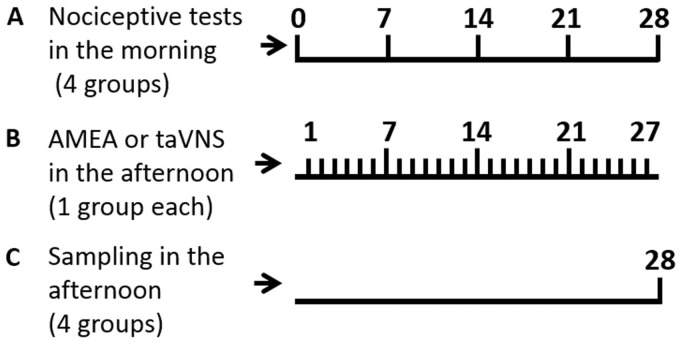
Animals were habituated to the test environment daily (a 60-min session) for two days before baseline testing. The testing procedure for thermal hyperalgesia was performed according to a previously published method.30 Temperature was set to have the baseline latency at 10 to 12 s and the cutoff of 20 s. Mechanical allodynia was examined by applying a von Frey filament to the plantar surface of each hindpaw.31 The cutoff force was 20 g. Thermal hyperalgesia and mechanical allodynia were tested at designated time points, all conducted between 9:00 a.m. and 1:00 p.m. When both thermal and mechanical nociceptive thresholds were statistically different among the groups at a time point, the taVNS or the AMEA session was canceled at the point, and animals were sacrificed and sampled in the afternoon (Figure 1).
Figure 1.
Time points of experimental sessions arrange. (a) Weekly nociceptive behavior tests in all four groups. (b) Daily AMEA or taVNS in ZDF rats on day 1 to 27. (c) Sampling on day 28. AMEA: auricular margin electroacupuncture; taVNS: transcutaneous auricular vagus nerve stimulation.
Noninvasive taVNS
All the time points recorded in this study are in accordance with the taVNS occurrences, that is, the first taVNS session occurs on day 1, the seventh recorded as day 7, and so on. Rats were exposed to taVNS for 27 consecutive days daily in the afternoon. The taVNS was applied on both ears, and the auricular margin was used as the sham stimulation.
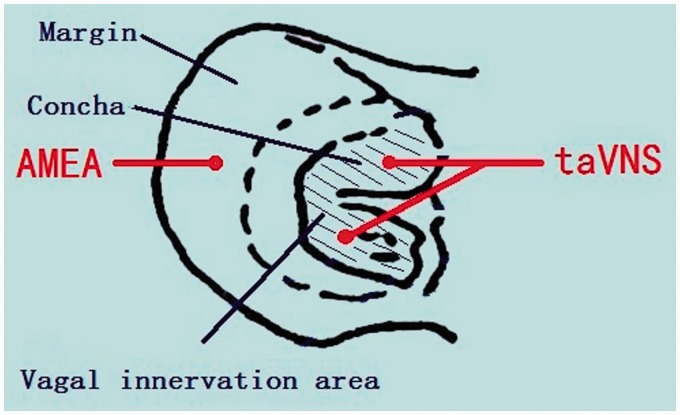
For taVNS procedure, under 2% isoflurane anesthesia, two oppositely charged magnetic electrodes (+/−) were placed over the auricular concha region, inside and outside respectively, of each ear. A session of 30-min transcutaneous electrical stimulation at frequencies of 2/15 Hz (2 and 15 Hz, switched every second), pulse-width 67 to 500 ms, and an intensity of 2 mA was applied via an electrical stimulator (HANS-100, Nanjing, China). The procedures were performed in the afternoon and at least 2 h after the behavioral tests at the designated time points. The EA condition at auricular margin was the same parameters as in taVNS (Figure 2).7,9,10
Figure 2.
Diagram of a rat’s ear and the taVNS or AMEA location. Shadowed area indicates the area innervated by vagus nerve. The taVNS administrated in this area. AMEA: auricular margin electroacupuncture; taVNS: transcutaneous auricular vagus nerve stimulation.
Collection of plasma and ELISA
Blood samples were collected from the right atrium upon endpoint sampling, centrifuged for 10 min at 110 g in cold room and plasma collected. All plasma samples were wrapped in foil and stored at −80°C until use. The concentration of plasma 5-HT was evaluated using enzyme linked immunosorbent assay (ELISA) kits purchased from R&D System (Beijing, China) and analyzed by Huanya Biomedicine Technology Co., Ltd (Beijing, China). The results were read using a microplate reader (Multiskan MK3, Thermo Scientific, Beijing, China) at 450 nm.
Immunohistochemical staining
The expression level of 5-HT1AR was detected by immunofluorescence staining and Western blotting. For immunofluorescence staining, naïve rats (ZDF, n = 2; ZL, n = 2) were euthanized with sodium pentobarbital (60 mg/kg, intraperitoneally) and transcardially perfused with 200 mL cold saline followed by 400 mL cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.35). Tissues of brain were dissected, postfixed for 2 h, and kept in 30% sucrose in 0.1 M phosphate buffer at 4°C until they sank to the bottom. Tissues were then cut using a cryostat (30 µm) and mounted onto microscope slides. Immunofluorescence staining was used to detect the qualitative expression of 5-HT1AR (mouse monoclonal anti-5-HT1AR antibody (MAB11041), clone 19A9.2, 1:500; Millipore, Chicago, IL) and its colocalization with MT1 (Rabbit polyclonal anti-MT1 antibody, 1:500; Abbiotec, San Diego, CA). Briefly, sections were washed (3 × 10 min), blocked with 3% donkey serum in 0.1 M phosphate-buffered saline containing 0.3% Triton for 1 h at room temperature (RT), and incubated overnight at 4°C with the 5-HT1AR primary antibody. After rinsing (3 × 10 min), sections were incubated for 1 h with a corresponding FITC- or Cy3-conjugated secondary antibody (donkey polyclonal, 1:800; RT, Jackson ImmunoResearch, West Grove, PA). For double immunolabeling of 5-HT and MT1, the 5-HT primary antibody was added after the completion of staining for the MT1 primary antibody. For controls, the primary antibody was omitted. Slides were coverslipped with Vectashield (Vector Laboratories, Burlingame, CA) and read using a LEXT OLS4000 3D Laser Measuring Confocal Microscope (Olympus, Center Valley, PA) and recorded using a digital camera.
Western blot analyses
For Western blotting session, ZDF (naïve, four weeks after the AMEA or taVNS, n = 2 each) and ZL rats (naive, n = 2) were beheaded following sodium pentobarbital (60 mg/kg, intraperitoneally) anesthesia. Fresh brain tissues were collected and saved at −80°C until use. The samples were homogenized in sodium dodecyl (lauryl) sulfate (SDS) buffer containing a mixture of proteinase inhibitors (Sigma, St. Louis, MO). Protein samples were separated on an SDS polyacrylamide electrophoresis gel (4%–15% gradient; Bio-Rad, Hercules, CA) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked with 3% milk and incubated overnight at 4°C with a primary antibody to 5-HT (46 kD, mouse monoclonal anti-5-HT antibody [MAB11041], anti-5-HT receptor 1A, clone 19A9.2, 1:1000; Millipore, Chicago, IL). After rinsing with phosphate-buffered saline three times for 10 min, the membranes were incubated with horseradish peroxidase–conjugated secondary antibody (1:10,000; Abcam, RT) for 1 h. The blots were visualized in enhanced chemiluminescence solution (NEN, Boston, MA) for 1 min and exposed on hyperfilms (Amersham Biosciences, Beijing, China) for 1 to 10 min. The membranes were then incubated in a stripping buffer (67.5 mM Tris, pH 6.8, 2% SDS, and 0.7% b-mercaptoethanol) for 30 min at 50°C and reprobed with a polyclonal rabbit anti-glyceraldehyde 3-phosphate dehydrogenase antibody (1:20,000; Beijing TDY Biotec, Beijing, China) as a loading control. The Western blot analysis was done in triplicate. The density and size of the bands was measured with a computer-assisted imaging analysis system and normalized against loading controls.
Statistical analysis
The raw data from both thermal hyperalgesia (withdrawal latency in seconds) and mechanical allodynia (threshold bending force in grams) tests were analyzed using repeated measure two-way analysis of variance to detect overall differences among treatment groups (SigmaPlot version 11.0 for Windows; San Jose, CA). When significant effects were observed, the Holm–Sidak tests were performed to determine sources of differences. Data from enzyme-linked immunosorbent assay and Western blot analyses were analyzed using the Student’s t test to detect differences between treatment groups. The data are presented as mean ± standard error. Differences were considered statistically significant at the level of α = 0.05.
Results
Natural development of mechanical and thermal nociception in ZDF rats was inhibited by auricular electrostimulation with taVNS showed stronger effect
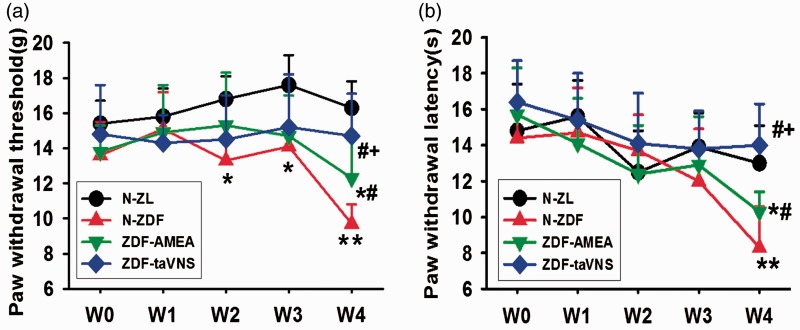
The paw withdrawal threshold to mechanical stimulation was not statistically different between naïve ZDF and ZL rats at the beginning (w0) and at experimental week 1 (w1). However, at experimental weeks 2 to 4 (w2, w3, and w4), while it remained the same level in taVNS-treated ZDF rats, the mechanical withdrawal thresholds declined in naïve ZDF rats to a level statistically lower than in naïve ZL rats. At the last time point (w4, 13 weeks of age), the withdrawal threshold in naïve ZL and taVNS-treated ZDF rats was significantly higher than that in naïve or sham taVNS (the AMEA)-treated ZDF rats. Also at w4, the taVNS-treated ZDF rats and naïve ZL rats showed comparable mechanical withdrawal threshold. Even between the taVNS- and the sham taVNS-treated ZDF rats, there was a statistical difference between the mechanical withdrawal thresholds (Figure 3(a)).
Figure 3.
Nociceptive thretholds in naïve ZL and ZDF rats and in ZDF rats treated with auricular electrostimulation. (a) Paw withdrawal threshold (g) to mechanical stimulation during the four weeks of experiment. (b) Paw withdrawal latency (s) to thermal stimulation during the four weeks of experiment. Data represent mean ± SD (n = 4–6). W: time in weeks; N-ZL: naïve Zucker lean rats; N-ZDF: naïve ZDF rats; ZDF-AMEA: ZDF rats treated with auricular margin electroacupuncture; ZDF-taVNS: ZDF rats treated with auricular concha electroacupuncture. *P < 0.05, **P < 0.01 vs. N-ZL; #P < 0.05 vs. N-ZDF, and +P < 0.05 vs. ZDF-AMEA.
Under the same experimental conditions and within the same batch of animals, there was a slightly differential development trends between mechanical and thermal nociception thresholds. There was no statistical thermal hyperalgesia latency difference at time points w0–w3 when compared among groups, in contrasting to the significant differences of mechanical thresholds at w2–w3. However, at w4 of the experiment, the same vision as in the mechanical thresholds appeared in thermal withdrawal latency, such that in both naïve and sham taVNS-treated ZDF rats, the latencies were significantly lower than in both naïve ZL and taVNS-treated ZDF rats, and the thermal withdrawal latencies were statistically different between the taVNS-treated and AMEA-treated ZDF rats, with the taVNS-treated rats showing less thermal stimulation sensitivity (Figure 3(b)).
Auricular stimulations improve serotonergic function through elevated plasma 5-HT concentration and upregulated central 5-HT1AR expression
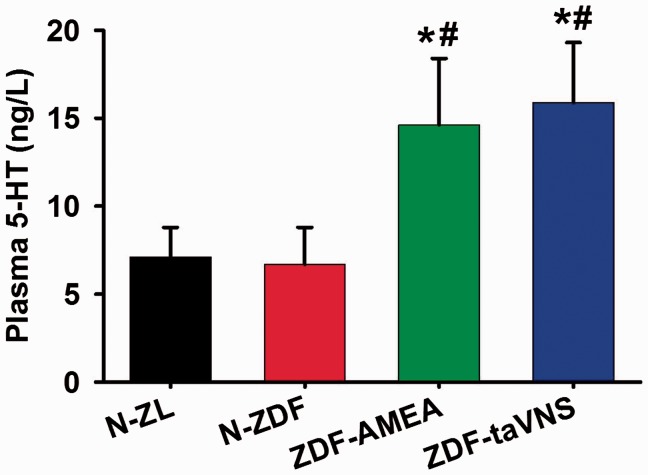
The plasma concentrations of 5-HT as detected by ELISA upon sacrifice (about 23 h after the last taVNS or AMEA session) showed that, versus naïve ZL and ZDF rats, both taVNS- and AMEA-treated ZDF rats showed an elevated plasma 5-HT concentration, while there was no statistical difference between naïve ZL and ZDF or between taVNS- and AMEA-treated ZDF rats (Figure 4).
Figure 4.
Plasma concentration of 5-HT on experimental day 28. Data represent mean ± SD (n = 4–6). N-ZL: naïve Zucker lean rats; N-ZDF: naïve ZDF rats; ZDF-AMEA: ZDF rats treated with auricular margin electroacupuncture; ZDF-taVNS: ZDF rats treated with auricular concha electroacupuncture; 5-HT: serotonin. *P < 0.01 vs. N-ZL; #P < 0.01 vs. N-ZDF.
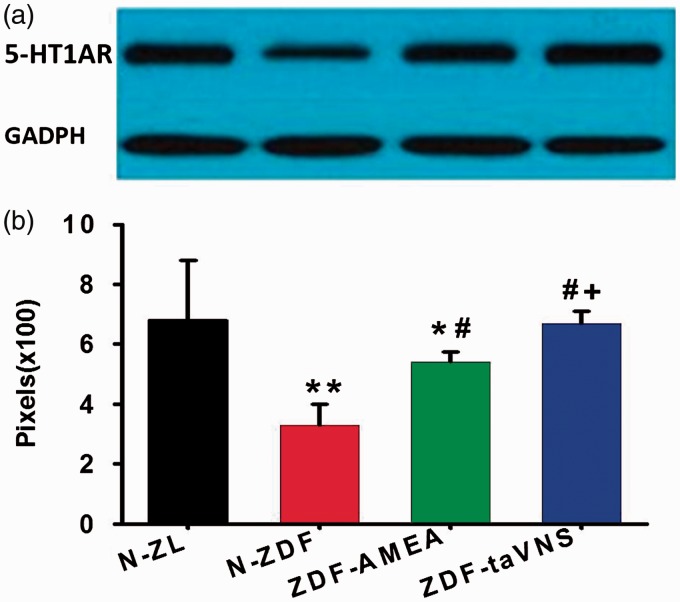
As quantified by Western blotting, the protein expression level of 5-HT1AR in the hypothalamus of naïve ZDF was significantly lower than that in naïve ZL rats. On the other hand, the expression of 5-HT1AR in the hypothalamus of both taVNS- and AMEA-treated ZDF rats was significantly higher than that in the hypothalamus of naïve ZDF rats, with animals in taVNS-treated group showing comparable 5-HT1AR levels to that in naïve ZL rats. There was also a statistical difference between the 5-HT1AR expression levels in rats treated with taVNS and AMEA (Figure 5).
Figure 5.
Western blots of 5-HT1AR in the hypothalamus of each experimental group. (a) A typical blot showing the expression level of 5-HT1AR. (b) Relative density of the blot in pixels. Each bar represents the mean ± SD of six blots from two animals. N-ZL: naïve Zucker lean rats; N-ZDF: naïve ZDF rats; ZDF-AMEA: ZDF rats treated with auricular margin electroacupuncture; ZDF-taVNS: ZDF rats treated with auricular concha electroacupuncture; 5-HT1AR: 5-HT receptor type 1A; GAPDH: glyceraldehyde 3-phosphate dehydrogenase. *P < 0.05, **P < 0.01 vs. N-ZL; #P < 0.01 vs. N-ZDF; +P < 0.05 vs. ZDF-AMEA.
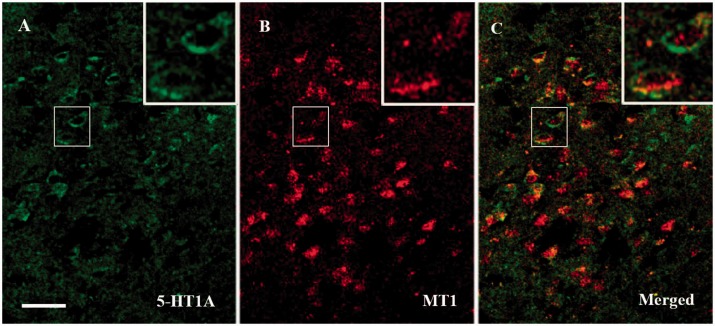
Colocalization of central 5-HT1AR and MT1 receptors
Morphologically, there were both 5-HT1AR and MT1 immunopositive cells localized in the ventromedial hypothalamic nucleus of ZDF rats. While the 5-HT1AR was predominantly expressed in the cell membrane, MT1 was expressed mainly in the cytoplasm. The MT1 immunopositive cells outnumbered the 5-HT1AR positive cells by approximately 5:4. Around 85% of 5-HT1AR positive cells expressed MT1, and vice versa (Figure 6).
Figure 6.
Distribution of MT1 and 5-HT1AR immunopositive cells in the hypothalamus of ZDF rats. (a) 5-HT1AR reactive cells (green). (b) MT1 reactive cells (red). (c) Colocalization of MT1 and 5-HT1AR positive cells (yellow). 5-HT1AR: 5-HT receptor type 1A; MT1: melatonin receptor type 1. Bar, 50 µm.
Discussion
In this study, we demonstrated that repeated auricular electronic stimulations, which excite the parasympathetic nervous system through triggering vagal terminal branches in the area, elevate the circulating 5-HT concentration, upregulate the expression of central 5-HT1AR, and halt the development of nociceptive sensitivity that has been demonstrated to be established at 13 weeks of age in the genetically diabetes prone ZDF rats.
Previously, we reported that taVNS triggered extrapineal MEL release, elevated the circulating concentration of MEL, and upregulated the central expression of MT1 in ZDF rats.7,9 taVNS in this study promoted serotonergic function.
5-HT is primarily found in the gastrointestinal tract, blood platelets, and the CNS of animals.32 Like the high content of MEL in gastrointestinal tract,33 approximately 90% of the human body’s total 5-HT is located in the enterochromaffin cells in the gastrointestinal tract which is secreted luminally and basolaterally. Only 10% of 5-HT is synthesized in serotonergic neurons of the CNS.32
Consistent with the elevated plasma 5-HT concentration, we also observed an upregulated expression of 5-HT1AR in the hypothalamus, brain area that mediate autonomic nervous system function, of both taVNS- and AMEA-treated ZDF rats, with taVNS being more effective. Although central 5-HT receptors mediate both excitatory and inhibitory neurotransmission and 5-HT in peripheral blood may be pronociceptive, the upregulated central 5-HT1ARs play a stronger role in the descending inhibitory pathway.17,19,20,27,34 It has been reported that (1) EA activates 5-HT neurons in the nucleus raphe magus, a relay of the descending inhibitory system,35,36 (2) 5-HT1AR antagonist blocks 2 Hz EA analgesia, and (3) 10 Hz EA inhibits thermal hyperalgesia through spinal 5-HT1A but not 5-HT2C receptor.37–39 Considering the wide spread of central 5-HT1AR positive neurons,40 EA might produce its beneficial effects through multiple brain areas.
Since both 5-HT and MEL (1) are biochemically derive from tryptophan, (2) are mainly distributed in the gastrointestinal tract, and (3) can be elevated by daily taVNS sessions, there may be a shared mechanism in the stimulated secretion of each. Physiologically, MEL functions as the mediator of photoperiodic information to the CNS in vertebrates and allows central circadian regulation of numerous physiological homeostatic mechanisms.41 Thus, the taVNS-induced 5-HT secretion may be the results of the secretion of MEL, or both released at the same time. Either way, it is possible that the activation of the parasympathetic or inhibitory of the sympathetic system upon or following vagal stimulation was a contributor.42–44 The high colocalization of receptors MT1 and 5-HT1A in the ventromedial hypothalamus nucleus might be another clue for the relationship.
Despite the exact mechanism, our results suggest a functional and manipulable approach to improve serotonergic and melatonergic functions through multiple taVNS sessions. These findings may hold promise for prophylactic or acute treatment off a variety of serotonergic and melatonergic dysfunction conditions such as diabetic neuropathy.
Conclusion
Multiple auricular electro vagal stimulation prevents the development of thermal hyperalgesia and mechanical allodynia in ZDF rats. This effect may be achieved via increasing plasma 5-HT concentration and upregulating expression of 5-HT1AR in hypothalamus.
Acknowledgments
The authors thank Drs. William Xu (University of Iowa Hospital and Clinics), Wancai Yang, Xing Wang, and Xinhua Cai (Xinxiang Medical College) and members of their laboratories for generous assistance.
Author Contributions
Conceived and designed the experiments: SW and PR. Performed the experiments: SL, XZ, JZ, CS, and SW. Analyzed the data: XZ, SL, and SW. Contributed reagents/materials/analysis tools: SW. Wrote the paper: SW, MB, and PR.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers: 81571085, 81473780, and 81271243); Sino-German Center for Research Promotion Foundation (grant number: GZ 1236); The High-level Leading Talent Introduction Program of GDAS (grant number: 2016GDASRC-0205). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chong MS, Bajwa ZH. Diagnose and treatment of neuropathic pain. J Pain Symptom Manage 2003; 25: S4–S11. [DOI] [PubMed] [Google Scholar]
- 2.Brunelli B, Gorson KC. The use of complementary and alternative medicines by patients with peripheral neuropathy. J Neurol Sci 2004; 218: 59–66. [DOI] [PubMed] [Google Scholar]
- 3.Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception – an experimental study. Brain Stimul 2013; 6: 202–209. [DOI] [PubMed] [Google Scholar]
- 4.Mercante B, Deriu F, Rangon CM. Auricular neuromodulation: the emerging concept beyond the stimulation of vagus and trigeminal nerves. Medicines (Basel) 2018; 5: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seo S Y, Lee K-B, Shin J-S, Lee J, Kim M-R, Ha I-H, Ko Y, Lee Y J. Effectiveness of acupuncture and electroacupuncture for chronic neck pain: a systematic review and meta-analysis. Am J Chin Med 2017; 45: 1573–1595. [DOI] [PubMed] [Google Scholar]
- 6.Abuaisha BB, Costanzi JB, Boulton AJM. Acupuncture for the treatment of chronic painful peripheral diabetic neuropathy: a long term study. Diabetes Res Clin Pract 1998; 39: 115–121. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Zhai X, Rong P, McCabe M F, Zhao J, Ben H, Wang X, Wang S. Transcutaneous auricular vagus nerve stimulation triggers melatonin secretion and is antidepressive in Zucker diabetic fatty rats. PLoS One 2014; 9: e111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rong PJ, Ma SX. Electroacupuncture Zusanli (ST36) on release of nitric oxide in the gracile nucleus and improvement of sensory neuropathies in Zucker diabetic fatty rats. Evid Based Complement Alternat Med 2011; 2011: 13454500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang S, Zhai X, Li S, McCabe MF, Wang X, Rong P. Transcutaneous vagus nerve stimulation induces tidal melatonin secretion and has an antidiabetic effect in Zucker fatty rats. PLoS One 2015; 10: e0124195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Zhai X, Rong P, McCabe MF, Wang X, Zhao J, Ben H, Wang S. Therapeutic effect of vagus nerve stimulation on depressive-like behavior, hyperglycemia and insulin receptor expression in Zucker fatty rats. PLoS One 2014; 9: e112066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo M, Qu X, Li S, Zhao J, Zhao Y, Jiao Y, Rong P. Transcutaneous vagus nerve stimulation for primary insomnia and affective disorder: a report of 35 cases. Zhongguo Zhen Jiu 2017; 37: 269–273. [DOI] [PubMed] [Google Scholar]
- 12.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul 2015; 8: 624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 2007; 114: 1485–1493. [DOI] [PubMed] [Google Scholar]
- 14.Kraus T, Kiess O, Hösl K, Terekhin P, Kornhuber J, Forster C. CNS BOLD fMRI effects of sham-controlled transcutaneous electrical nerve stimulation in the left outer auditory canal – a pilot study. Brain Stimul 2013; 6: 798–804. [DOI] [PubMed] [Google Scholar]
- 15.Yakunina N, Kim SS, Nam EC. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation 2017; 20: 290–300. [DOI] [PubMed] [Google Scholar]
- 16.Cecon E, Oishi A, Jockers R. Melatonin receptors: molecular pharmacology and signalling in the context of system bias. Br J Pharmacol. Epub ahead of print 13 July 2017. DOI: 10.1111/bph.13950. [DOI] [PMC free article] [PubMed]
- 17.Bardin L. The complex role of serotonin and 5-HT receptors in chronic pain. Behav Pharmacol 2011; 22: 390–404. [DOI] [PubMed] [Google Scholar]
- 18.Calejesan AA, Ch’ang MH, Zhuo M. Spinal serotonergic receptors mediate facilitation of a nociceptive reflex by subcutaneous formalin injection into the hindpaw in rats. Brain Res 1998; 798: 46–54. [DOI] [PubMed] [Google Scholar]
- 19.Daval G, Vergé D, Basbaum AI, Bourgoin S, Hamon M. Autoradiographic evidence of serotonin1 binding sites on primary afferent fibres in the dorsal horn of the rat spinal cord. Neurosci Lett 1987; 83: 71–76. [DOI] [PubMed] [Google Scholar]
- 20.Perrin FE, Gerber YN, Teigell M, Lonjon N, Boniface G, Bauchet L, Rodriguez JJ, Hugnot JP, Privat AM. Anatomical study of serotonergic innervation and 5-HT(1A) receptor in the human spinal cord. Cell Death Dis 2011; 2: e218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sałat K, Kołaczkowski M, Furgała A, Rojek A, Śniecikowska J, Varney MA, Newman-Tancredi A. Antinociceptive, antiallodynic and antihyperalgesic effects of the 5-HT(1A) receptor selective agonist, NLX-112 in mouse models of pain. Neuropharmacology 2017; 125: 181–188. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Yang Z, Gao X, Wu G. The role of 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptors in modulating spinal nociceptive transmission in normal and carrageenan-injected rats. Pain 2001; 92: 201–211. [DOI] [PubMed] [Google Scholar]
- 23.Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res 1991; 550: 35–48. [DOI] [PubMed] [Google Scholar]
- 24.Yaksh TL, Wilson PR. Spinal serotonin terminal system mediates antinociception. J Pharmacol Exp Ther 1979; 208: 446–543. [PubMed] [Google Scholar]
- 25.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev 1994; 46: 157–203. [PubMed] [Google Scholar]
- 26.Bardin L, Tarayre JP, Malfetes N, Koek W, Colpaert FC. Profound, non-opioid analgesia produced by the high-efficacy 5-HT(1A) agonist F 13640 in the formalin model of tonic nociceptive pain. Pharmacology 2003; 67: 182–194. [DOI] [PubMed] [Google Scholar]
- 27.Di Cesare Mannelli L, Ghelardini C, Micheli L, Del Bello F, Giannella M, Piergentili A, Pigini M, Quaglia W. Synergic stimulation of serotonin 5-HT(1A) receptor and α(2)-adrenoceptors for neuropathic pain relief: preclinical effects of 2-substituted imidazoline derivatives. Eur J Pharmacol 2017; 810: 128–133. [DOI] [PubMed] [Google Scholar]
- 28.Kalipatnapu S, Chattopadhyay A. Membrane organization and function of the serotonin(1A) receptor. Cell Mol Neurobiol 2007; 27: 1097–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai X, Sun C, Rong P, Li S, McCabe MF, Wang X, Mao J, Wang S. A correlative relationship between chronic pain and insulin resistance in Zucker fatty rats: role of downregulation of insulin receptors. J Pain 2016; 17: 404–413. [DOI] [PubMed] [Google Scholar]
- 30.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988; 32: 77–88. [DOI] [PubMed] [Google Scholar]
- 31.Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain 1994; 57: 375–382. [DOI] [PubMed] [Google Scholar]
- 32.Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 2009; 60: 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci 2002; 47: 2336–2348. [DOI] [PubMed] [Google Scholar]
- 34.Chen J, Lariviere WR. The nociceptive and anti-nociceptive effects of bee venom injection and therapy: a double-edged sword. Prog Neurobiol 2010; 92: 151–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li A, Wang Y, Xin J, Lao L, Ren K, Berman B M, Zhang R-X. Electroacupuncture suppresses hyperalgesia and spinal Fos expression by activating the descending inhibitory system. Brain Res 2007; 1186: 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Zhu B, Zhang SX. Relationship between electroacupuncture analgesia and descending pain inhibitory mechanism of nucleus raphe magnus. Pain 1986; 24: 383–396. [DOI] [PubMed] [Google Scholar]
- 37.Silva JR, Silva ML, Prado WA. Analgesia induced by 2- or 100-Hz electroacupuncture in the rat tail-flick test depends on the activation of different descending pain inhibitory mechanisms. J Pain 2011; 12: 51–60. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhang RX, Zhang M, Shen XY, Li A, Xin J, Ren K, Berman BM, Tan M, Lao L. Electroacupuncture inhibition of hyperalgesia in an inflammatory pain rat model: involvement of distinct spinal serotonin and norepinephrine receptor subtypes. Br J Anaesth 2012; 109: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Li A, Xin J, Lao L, Ren K, Berman B M, Tan M, Zhang R-X. Involvement of spinal serotonin receptors in electroacupuncture anti-hyperalgesia in an inflammatory pain rat model. Neurochem Res 2011; 36: 1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito H, Okubo Y, Halldin C, Farde L. Localization of 5-HT1A receptors in the living human brain using [carbonyl-11C]WAY-100635: PET with anatomic standardization technique. J Nucl Med 1999; 9: 235–109. [PubMed] [Google Scholar]
- 41.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev 2010; 62: 343–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clancy JA, Mary DA, Witte KK, Greenwood JP, Deuchars SA, Deuchars J. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 2014; 7: 871–877. [DOI] [PubMed] [Google Scholar]
- 43.Deuchars SA, Lall VK, Clancy J, Mahadi M, Murray A, Peers L, Deuchars J. Mechanisms underpinning sympathetic nervous activity and its modulation using transcutaneous vagus nerve stimulation. Exp Physiol 2018; 103: 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao YX, He W, Jing XH, Liu JL, Rong PJ, Ben H, Liu K, Zhu B. Transcutaneous auricular vagus nerve stimulation protects endotoxemic rat from lipopolysaccharide-induced inflammation. Evid Based Complement Alternat Med 2012; 2012: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]