Abstract
Background:
The underlying cause of glenohumeral arthritis is poorly understood. Glenohumeral arthrosis patterns have been classified and described, and differential contact stresses within the joint have been implicated as a cause of joint degeneration, but the intrinsic cause of degeneration patterns in the glenohumeral joint (GHJ) remains largely unknown.
Purpose/Hypothesis:
The purpose of this study was to assess morphological and mechanical differences in articular cartilage (AC) and subchondral bone (SCB) of the glenoid and humeral head in matched cadaveric specimens. We hypothesized that there would be significant zone-dependent differences between the intrinsic characteristics (AC thickness, SCB thickness, compressive forces) of the glenoid and humeral head.
Study Design:
Descriptive laboratory study.
Methods:
Ten human cadaveric GHJs (mean age, 60.2 years) were dissected to expose articular surfaces to facilitate biomechanical testing. A 2-mm and 6-mm osteochondral plug was harvested at 5 zones (central, anterior, posterior, inferior, superior) on the glenoid and humeral head (N = 200 plugs). Each 2-mm core was histologically sectioned and stained with hematoxylin and eosin. AC thickness measurements were taken using light microscopy. The 6-mm plugs were imaged using micro–computed tomography to measure SCB thickness. After imaging, AC specimens were removed from the SCB and tested in confined compression. The compressive aggregate modulus (HA0), compressive stiffening coefficient (β), and compressive modulus at 16% strain (HA0.16) and at 50% strain (HA0.50) were calculated.
Results:
The overall AC thickness was significantly greater on the glenoid. The glenoid also had significantly thicker AC at the inferior, posterior, and superior zones as well as significantly higher SCB thickness overall and significantly greater SCB thickness at the anterior and central zones. The glenoid had significantly greater overall HA0.50 and HA0.50 values at the superior zone and had a significantly greater overall compressive stiffening coefficient (β).
Conclusion:
The glenoid had thicker AC, thicker SCB, and greater compressive stiffness at high strain.
Clinical Relevance:
These intrinsic differences may help better elucidate the cause of differential degeneration patterns between the glenoid and humeral head.
Keywords: glenoid, humerus, cartilage, subchondral, differential, arthrosis
In the United States alone, an estimated 51,638 shoulder arthroplasty procedures were performed in 2011 for symptomatic shoulder arthritis.1,13 This growing number of people with end-stage arthritis accounts for only a small percentage of patients with symptomatic glenohumeral joint (GHJ) degeneration. The underlying cause of this increasingly prevalent abnormality is poorly understood. Early clinical observations of specific arthrosis patterns in the humeral head12 and glenoid2,17 have been classified and described, and differential contact stresses within the joint have been implicated as a proposed cause of joint degeneration.14–16 The most commonly reported GHJ arthrosis patterns have been associated with anatomic characteristics; namely, erosion of the posterior aspect of the glenoid surface, posterior subluxation of the humeral head, glenoid retroversion, and increased patient age.16 It has also been reported that thickened subchondral bone (SCB) alone can be an early sign of osteoarthritis. Despite this, the intrinsic cause of degeneration patterns in the GHJ remains largely unknown and understudied.
Previous studies have examined the morphological properties of articular cartilage (AC) and SCB of the GHJ, trying to elucidate wear patterns. AC thickness has been measured in vivo and in vitro using modalities such as fixed distance photography,3 computed tomography (CT), and magnetic resonance imaging (MRI).8,18 These studies reported thicker cartilage centrally on the humerus and thinner cartilage in the central glenoid but also reported the heterogeneity of measurements that were dependent on anatomic regions.3,8,18,19 Other studies concluded that the mismatch or morphological differences between GHJ components may contribute to joint degeneration by allowing for excessive translation in the joint space.5,11,12 None of the aforementioned studies measured numerous properties of the glenoid and humeral head from matched cadaveric specimens, making generalizable conclusions difficult.
To date, the cause of the differential patterns of degeneration between the glenoid and humeral head remains largely unknown. More extensive characterization of the mechanical and morphological properties of AC and SCB of the GHJ can further our understanding of the intrinsic properties of the native joint and ultimately the degenerate joint. To this end, the purpose of this study was to evaluate AC thickness, SCB thickness, and mechanical properties of AC in various anatomic zones of the GHJ. We anticipated finding zonal differences between the above material properties as they apply to the articulating surfaces of the GHJ, which will foster continued exploration of the intrinsic causes contributing to the formation of GHJ arthrosis.
Methods
Specimens
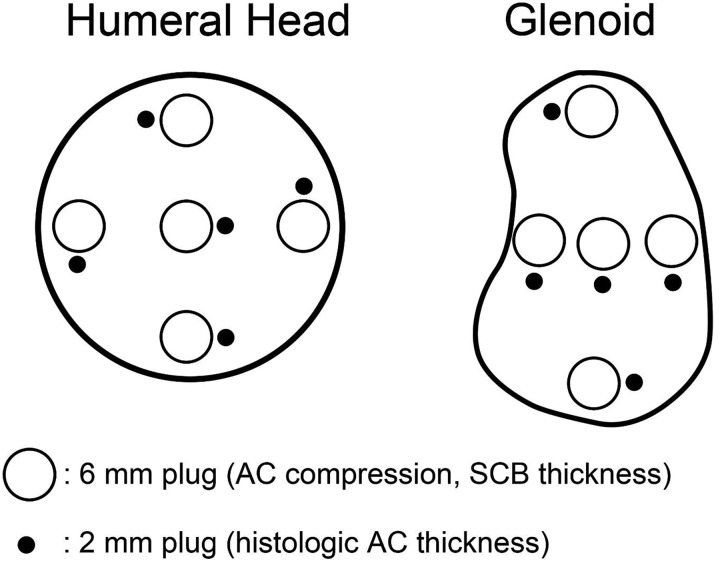
Ten fresh-frozen human cadaveric GHJs (mean age, 60.2 ± 11.8 years [range, 47-90 years]) were dissected to expose the articular surface (Table 1). The cadaveric shoulder specimens were graciously donated to the Department of Orthopaedic Surgery at William Beaumont Hospital for the purposes of this study. No gross evidence of cartilage degeneration or osteoarthritis was found on the surfaces of the glenoid or humeral head. While frozen at –20°C, a 2-mm osteochondral cylinder and a 6-mm osteochondral cylinder were harvested at each of 5 intra-articular zones (central, anterior, posterior, inferior, superior) on both the glenoid and humeral head using a sharp cylindrical punch (Figure 1). The 2-mm cylindrical samples were thawed at room temperature, rinsed 3 times in sterile phosphate-buffered saline (PBS), and fixed in 10% neutral-buffered formalin for 48 hours. The 6-mm cylindrical specimens remained frozen until further evaluation.
TABLE 1.
Characteristics of Cadaveric Specimens
| Specimen | Sex | Age, y |
|---|---|---|
| 1 | Female | 90 |
| 2 | Male | 59 |
| 3 | Male | 47 |
| 4 | Male | 49 |
| 5 | Male | 57 |
| 6 | Male | 63 |
| 7 | Male | 55 |
| 8 | Male | 63 |
| 9 | Male | 58 |
| 10 | Male | 61 |
Figure 1.
Harvest locations of 6-mm and 2-mm osteochondral plugs used in this study. AC, articular cartilage; SCB, subchondral bone.
Histological AC Thickness
After fixation, each specimen was embedded in paraffin, and 3 equally spaced 5-µm sections (200-µm spacing) were stained with hematoxylin and eosin (Figure 2). Sections were imaged using a light microscope (Eclipse 90i; Nikon) at 10× magnification. AC thickness measurements were performed on commercially available imaging software (NIS-Elements; Nikon) by a blinded researcher. Seven measurements of AC thickness were taken from each section (1 midline, 3 left, 3 right) (Figure 2), and these measurements were averaged to obtain a mean thickness for each section. Section thicknesses were averaged to obtain a mean sample thickness.
Figure 2.

Histological measurement of articular cartilage thickness. Seven measurements (1 midline, 3 right, 3 left) were taken on each section.
Computed Tomographic SCB Thickness
To measure SCB thickness, all 6-mm osteochondral specimens were imaged using micro–CT (µCT) (Triumph Trimodality; Gamma Medica). Imaging was performed while specimens remained frozen to limit the freeze thaw–induced degradation of AC. Raw images were converted to DICOM format and reoriented using a 3-plane DICOM viewer (OsiriX; Pixmeo). Reorientation ensured that all measurements were normal to the SCB surface. Five measurements of SCB thickness were obtained on each of 15 slices centered about the center of the specimen using calibrated imaging software (OsiriX) (Figure 3). All measurements were performed in a blinded fashion, and the measurements were averaged to obtain the mean SCB thickness of each cylindrical specimen.
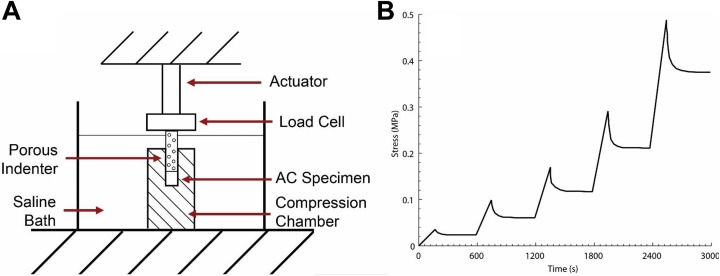
Figure 3.
Schematic representation of confined compression testing of articular cartilage (AC). (A) Confined compression testing was performed using a porous indenter in a saline-filled custom testing chamber. (B) Stress relaxation testing was performed using 5 sequential ramps in increments of 10% strain applied at 0.25 µm/s.
Confined Compression of AC
After µCT, the 6-mm cylindrical samples were thawed to room temperature and equilibrated in a sterile PBS bath for 4 hours. AC was separated from SCB using a sharp blade and mounted in a confined compression chamber in a sterile PBS bath at 22°C (Figure 3). Using a porous indenter, a 0.1-N tare load was first applied for 600 seconds. Stress relaxation testing was then performed by subjecting each cartilage sample to 5 sequential ramps in increments of 10% strain applied at 0.25 µm/s.9 Each ramp was held for 600 seconds.
The compressive aggregate modulus is a biomechanical term and is calculated from confined compression testing illustrated above. It is used in describing the stiffness of cartilage (stress vs strain behavior). It takes the place of the Young modulus (in terms of material characterization), which is a well-known proportionality constant that describes the linear elasticity of a given material. Unlike the majority of materials, however, cartilage experiences nonlinear stress-strain forces because of its high water content and dynamic material composition, making it more amenable to the compressive aggregate modulus.10 Similar to the Young modulus, the higher the compressive aggregate modulus, the less the cartilage deforms under a given load. The compressive aggregate modulus at 0% strain (HA0) and compressive stiffening coefficient (β) were calculated by curve-fitting the equilibrium stress-stretch response to the inhomogeneous finite deformation biphasic model, described by Equation 1 18:
| (1) |
The compressive modulus (HA) at any strain can then be calculated by differentiating Equation 1, obtaining Equation 2:
| (2) |
The compressive modulus at 16% strain (HA0.16) and 50% strain (HA0.50) were calculated using Equation 2. HA0.16 was chosen based on previous literature,17 and we chose to analyze HA0.50 because this was the point immediately before failures were observed in our specimens.
Statistical Analysis
All statistical analyses were performed in SPSS (v 22; IBM). Differences in AC thickness, SCB thickness, HA0, HA0.16, HA0.50, and β of the humerus and glenoid as a function of the anatomic zone (anterior, posterior, central, superior, inferior) were assessed using 1-way analysis of variance, with α = 0.05. To assess differences in these variables between the humerus and glenoid at each zone, paired t tests were performed. Correlations were calculated using the Pearson product-moment correlation coefficient. P values <.05 were considered statistically significant.
Results
AC Thickness
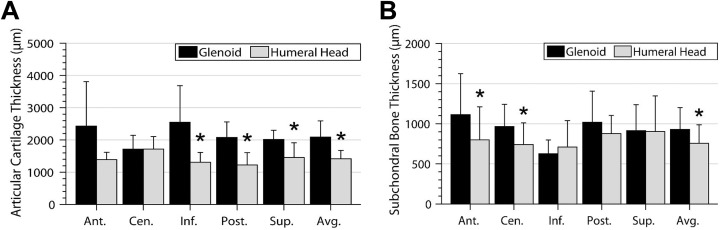
The results of AC thickness measurements of the GHJ, stratified by anatomic zone, are shown in Figure 4A. The overall AC thickness was significantly higher on the glenoid compared with the humeral head, particularly at the inferior, posterior, and superior zones. No significant differences in AC thickness were observed at the anterior or central zones.
Figure 4.
(A) Articular cartilage thickness and (B) subchondral bone thickness of the glenoid and humeral head stratified by zone. *Significant differences between the glenoid and humeral head. Ant, anterior; Cen, central; Inf, inferior; Post, posterior; Sup, superior.
On the glenoid surface, AC was significantly thinner at the central zone compared with the inferior zone. On the humeral head, AC at the central zone was significantly thicker compared with AC at the anterior, inferior, and posterior zones.
SCB Thickness
The results of SCB thickness measurements, stratified by anatomic zone, are shown in Figure 4B. The glenoid had significantly higher overall SCB thickness compared with the humeral head. Furthermore, the glenoid had significantly thicker SCB thickness at the anterior zone (1114.1 ± 511 µm vs 800.3 ± 409 µm, respectively; P = .022) and central zone (964.9 ± 276 µm vs 739.5 ± 271 µm, respectively; P = .033) compared with the humeral head.
Zone-dependent differences in SCB thickness were only observed on the glenoid. SCB thickness at the inferior zone was significantly lower compared with the anterior zone (P = .003), central zone (P = .037), and posterior zone (P = .016).
There were no significant correlations between SCB thickness and AC thickness at any individual zone of the glenoid (anterior: r = 0.440, P = .497; central: r = –0.04, P = .990; inferior: r = 0.174, P = .631; posterior: r = –0.01, P = .978; superior: r = –0.462, P = .125) or humeral head (anterior: r = 0.673, P = .067; central: r = 0.018, P = .960; inferior: r = –0.316, P = .374; posterior: r = 0.214, P = .580; superior: r = 0.295, P = .441). Furthermore, there were no significant correlations between overall SCB thickness and overall AC thickness of the glenoid (r = 0.358, P = .309) or humeral head (r = 0.168, P = .644).
Biomechanical Properties of AC
There were no significant differences in overall HA0, zone-dependent HA0, average HA0.16, or zone-dependent HA0.16 values between the glenoid and humerus (Table 2). The glenoid had significantly higher overall HA0.50 (4.796 ± 1.720 MPa vs 3.105 ± 1.280 MPa, respectively; P = .035) and HA0.50 values at the superior zone (5.111 ± 2.460 MPa vs 2.813 ± 1.080 MPa, respectively; P = .004) compared with the humeral head. No significant differences in HA0, HA0.16, or HA0.50 values were observed between the individual zones of the glenoid or between the individual zones of the humeral head.
TABLE 2.
Compressive Mechanical Properties of Articular Cartilage of the Glenoid and Humeral Heada
| HA0, MPa | HA0.16, MPa | HA0.50, MPa | β | |
|---|---|---|---|---|
| Glenoid | ||||
| Anterior | 0.229 ± 0.172 | 0.351 ± 0.230 | 4.974 ± 2.660 | 2.002 ± 0.906b |
| Central | 0.352 ± 0.432 | 0.534 ± 0.655 | 5.945 ± 7.050 | 1.458 ± 0.664b |
| Inferior | 0.295 ± 0.265 | 0.460 ± 0.447 | 2.248 ± 1.900 | 1.349 ± 1.170 |
| Posterior | 0.328 ± 0.211 | 0.488 ± 0.301 | 5.057 ± 2.960b | 1.560 ± 0.654 |
| Superior | 0.367 ± 0.173 | 0.543 ± 0.244 | 5.111 ± 2.460b | 1.393 ± 0.450b |
| Overall | 0.319 ± 0.118 | 0.482 ± 0.184 | 4.796 ± 1.720b | 1.542 ± 0.369b |
| Humeral head | ||||
| Anterior | 0.279 ± 0.210 | 0.400 ± 0.309 | 2.930 ± 2.620 | 1.047 ± 0.341b |
| Central | 0.370 ± 0.181 | 0.516 ± 0.261 | 3.184 ± 2.130 | 0.910 ± 0.248b |
| Inferior | 0.303 ± 0.130 | 0.436 ± 0.182 | 3.701 ± 1.860 | 1.251 ± 0.785 |
| Posterior | 0.320 ± 0.168 | 0.449 ± 0.228 | 2.909 ± 1.300b | 0.968 ± 0.357 |
| Superior | 0.318 ± 0.100 | 0.445 ± 0.140 | 2.813 ± 1.080b | 0.978 ± 0.226b |
| Overall | 0.316 ± 0.119 | 0.447 ± 0.170 | 3.105 ± 1.280b | 1.041 ± 0.285b |
aData are presented as mean ± SD.
bStatistically significant differences between the glenoid and humeral head.
The glenoid exhibited a significantly higher overall compressive stiffening coefficient (β) (1.542 ± 0.369 vs 1.041 ± 0.285, respectively; P = .019) compared with the humeral head. Furthermore, the glenoid had a significantly higher compressive stiffening coefficient (β) at the anterior zone (2.002 ± 0.906 vs 1.047 ± 0.341, respectively; P = .043), central zone (1.458 ± 0.664 vs 0.910 ± 0.248, respectively; P = .038), and superior zone (1.393 ± 0.450 vs 0.978 ± 0.226, respectively; P = .018) compared with the humeral head. No differences in the compressive stiffening coefficient (β) were observed between the individual zones of the glenoid or between the individual zones of the humeral head.
Discussion
Intrinsic differences in the properties of AC and SCB of the GHJ may be key contributors to clinically observed arthrosis patterns. However, to date, it is largely unknown which intrinsic properties vary between the glenoid and humeral head. Furthermore, it is unknown whether the differences are largely in morphological properties, biomechanical properties, or both. The purpose of this study was to quantify zonal differences in AC thickness, SCB thickness, and compressive properties of AC between the glenoid and humeral head in a set of matched specimens. Our data indicate that the glenoid had significantly thicker AC and SCB compared with the humeral head. We also found that the glenoid had a significantly higher compressive stiffening coefficient and aggregate compressive modulus at 50% stretch. There were statistically significant differences in the thickness of AC and SCB and the biomechanical properties between the glenoid and humeral head specimens. Our findings are in agreement with the original hypothesis, demonstrating that intrinsic differences do exist between the glenoid surface and humeral head in terms of AC thickness, SCB thickness, and elasticity.
Zonal variations in GHJ AC thickness have been partially investigated in previous studies. Fox et al3 performed bulk sectioning of 16 cadaveric humeral heads to obtain digital photography–based measurements of AC at various anatomic locations. They found cartilage at the central aspect to be thickest (mean thickness, 1.21 mm) and the thinnest at the circumferential periphery. Central cartilage was significantly thicker than that at most other anatomic zones, which is a finding that corroborates our results. In another study, Graichen et al7 utilized quantitative MRI and A-mode ultrasound to obtain measurements of 8 humeral heads and 8 glenoids, and they measured humeral head cartilage at a mean of 1.2 mm and glenoid cartilage at a mean of 1.7 mm. Furthermore, they measured the thickest cartilage at the central aspect of the humeral head and the anterior and inferior aspects of the glenoid, both of which are findings that agree with our results. Aside from stating the location of thickest cartilage, no additional zone-dependent measurements were given in their study, and comparisons to zone-dependent measurements in our study are therefore not possible. Overall cartilage thickness was markedly higher in Graichen et al’s7 study, and we attribute this to the difference in the mean age of their cadaveric specimens (50.6 years) compared with our cadaveric specimens (60.2 years) in addition to errors in measurement in both studies.
Recently, Zumstein et al19 utilized CT to obtain cartilage thickness measurements in 9 cadaveric specimens. In their study, humeral cartilage was thickest at the central and superior aspects, which agrees partially with the findings in our study. Furthermore, glenoid cartilage was thickest at the inferior and anterior aspects in their study, which is a finding consistent with our data set. The mean cartilage thickness of the glenoid was comparable between our data set (2.08 mm) and theirs (1.93 mm). However, there is a marked difference in the mean humeral head cartilage thickness between our study (1.42 mm) and their study (1.74 mm), which may be attributed to the difference in age between the cadaveric specimens (mean, 60.2 vs 41 years) in addition to differences in measurement modality, technique, and resolution.
Little information exists regarding SCB thickness as a function of the anatomic zone in the GHJ. Mimar et al11 utilized µCT to measure SCB thickness in 19 cadaveric glenoids with a mean age of 82 years. Their findings indicate anterior glenoid bone to be thickest at 1.15 mm, which is a finding similar to our result of 1.11 mm. Thinnest glenoid bone was measured at the inferior glenoid at 0.88 mm by Mimar et al,11 and while we also found that the inferior glenoid had the thinnest SCB, our measurement of 0.62 mm is lower, which can be attributed to slight differences in the definition of anatomic locations in addition to specimen age. Mimar et al11 did not investigate SCB of the humeral head, and comparisons with our humeral head measurements are therefore not possible. Frich and Jensen4 indicated mean SCB plate thickness to range between 1.2 and 2.9 mm, with a mean of 1.9 mm, which are values markedly higher than those reported by previous authors and those in the present study. Thickness at varying anatomic zones was not provided by Frich and Jensen,4 and accurate comparisons cannot be made with our study. In another study by Frich et al,5 SCB of the humeral head was measured at predefined anatomic zones very similar to the definitions used in our study. The average SCB of the humeral head was found to be 0.60 mm, slightly lower than the 0.75 mm measured in our study. Furthermore, Frich et al5 indicated that the thickest SCB was found at the central aspect, and although we did not find significant differences in SCB thickness as a function of the anatomic location, the superior humeral head had the thickest SCB in our data set.
A previously hypothesized reason for differential arthrosis patterns of the glenoid and humeral head is intrinsic differences in the mechanical properties of AC.9 GHJ cartilage has been shown to exhibit tensile anisotropy and depth-dependent inhomogeneity,8 but intrinsic differences in mechanical properties between the glenoid and humeral head, if any, remain largely unknown. Our data set partially supports this theory, as we found differences in material constants of AC between the glenoid and humeral head. We found that the glenoid had a significantly higher overall HA0.50; higher HA0.50 at the superior zone; higher overall compressive stiffening coefficient (β); and higher compressive stiffening coefficient at the anterior, central, and superior zones. However, we found no differences in HA0 or HA0.16 values between the glenoid and humeral head, which is a finding consistent with a similar previous study by Huang et al,9 who performed tensile and compressive testing of various zones of cartilage of the glenoid and humeral head. At present, it remains unclear whether the differences observed in our data set are of sufficient magnitude to affect downstream tissue degeneration. However, as the material properties of AC dictate the tissue’s ability to withstand biomechanical loading, any differences in material properties between the 2 articulating surfaces of the GHJ may affect the onset and progression of arthrosis within the joint.
We find it intriguing that the glenoid specimens showed overall thicker AC, thicker SCB, higher compressive stiffening coefficients (β), and higher compressive aggregate modulus values at HA0.50 when compared with their humeral head counterparts. Conversely, given that the most common patterns of arthrosis in the GHJ occur on the posterior aspect of the glenoid surface, we would expect AC to be thinner on the glenoid surface compared with the humeral head. While thickening of SCB and thinning of AC are known processes in the formation of joint arthroses, in this study, the glenoid had both thicker AC and thicker SCB. Pronounced SCB thickness is often associated with the pathogenesis of arthrosis because thicker bone results in higher load transfers onto the overlying cartilage, although the exact biomechanical relationship of SCB and AC is still largely undefined.6 This relationship did not appear to hold true in this study.
Additionally, the tensile properties (compressive aggregate modulus and compressive stiffening coefficient) favored the glenoid surface in this study, possibly because this surface takes on more mechanical loading given the innate biomechanics of the GHJ. We also noted variability in the compressive modulus between specific zones of the glenoid compared with the humeral head, favoring the central and superior zones of the glenoid. These subtle differences in tensile properties between the glenoid and humeral head and between different amounts of applied strain (HA0, HA0.16, HA0.50) demonstrate the complexity of this joint and the need for further investigation. These findings, namely, stronger tensile properties in the superior and central zones of the glenoid, may offer some theoretical explanation for the posterior glenoid erosion described in the literature.16
We were correct in anticipating zonal differences in the biomechanical properties between the glenoid and humeral head. Although there were no statistically significant differences in the tensile forces within the zones of the glenoid or humeral head individually, there were statistical differences between the 2 components overall. The explanation for why these differences exist has yet to be fully elucidated. This study contributes to our understanding and classification of the intrinsic biomechanical properties of the GHJ. Furthermore, continued exploration of the biomechanical properties within the GHJ may allow for more precise prosthesis designs and a better understanding of the pathogenesis of osteoarthritis in the future.
Our study has several limitations that warrant discussion. First, our results are from fresh-frozen cadaveric specimens, and all limitations associated with cadaveric samples should be considered. Furthermore, we used a total of 10 cadaveric specimens, and our results may not be fully generalizable. However, our analysis was based on matched glenoid and humeral head samples, providing a greater degree of accuracy by partially eliminating interspecimen variability. Another potential limitation is that the mean age of our specimens was 60.2 years. However, this was in part the consequence of 1 specimen being 90 years old. With the removal of that specimen, the mean specimen age was 57 years, with a range of 16 years as opposed to 40 years. While we did not observe any gross evidence of cartilage delamination, wear, or degradation, age-related tissue degeneration cannot be fully ruled out. Last, although all attempts were made to harvest osteochondral plugs from the same anatomic zone in each specimen, small errors in harvest location could introduce variability into our data set.
Conclusion
This study assessed the morphological and mechanical differences of AC and SCB of the glenoid and humeral head in a set of matched cadaveric specimens. We found that the glenoid had thicker AC, thicker SCB, and slight differences in compressive material properties compared with the humeral head. These findings may help to elucidate the cause of age-related GHJ degeneration. As differential arthrosis patterns are clinically observed in the GHJ, further investigation into the intrinsic differences in tissue morphology and mechanical properties can provide crucial information for future treatment strategies and prosthesis designs.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: B.N.L. has received educational support from DePuy Synthes and has received hospitality payments from Zimmer Biomet. A.L.G. has received educational support from Arthrex, Pinnacle, Supreme Orthopedic Systems, and Zimmer and has received hospitality payments from DePuy, Smith & Nephew, and Stryker. J.B. is a consultant for Biomet Orthopedics, DePuy, Smith & Nephew, and Vericel; has received educational support from Arthrex; and has received hospitality payments from Aesculap Biologics and Pinnacle. J.G. is a consultant for DePuy and Smith & Nephew and has received hospitality payments from Aesculap Biologics.
Ethical approval was not sought for the present study.
References
- 1. Ateshian GA, Kim JJ, Grelsamer RP, Mow VC, Warden WH. Finite deformation of biphasic material properties of bovine articular cartilage from confined cartilage compression experiments. J Biomech. 1997;30(11-12):1157–1164. [DOI] [PubMed] [Google Scholar]
- 2. DePalma AF, White JB, Callery G. Degenerative lesions of the shoulder joint at various age groups which are compatible with good function. Instr Course Lect. 1950;7:168–180. [Google Scholar]
- 3. Fox JA, Cole BJ, Romeo AA, et al. Articular cartilage thickness of the humeral head: an anatomic study. Orthopedics. 2008;31(3):216. [DOI] [PubMed] [Google Scholar]
- 4. Frich LH, Jensen NC. Bone properties of the humeral head and resistance to screw cutout. Int J Shoulder Surg. 2014;8(1):21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frich LH, Odgaard A, Dalstra M. Glenoid bone architecture. J Shoulder Elbow Surg. 1998;7(4):356–361. [DOI] [PubMed] [Google Scholar]
- 6. Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann NY Acad Sci. 2010;1192:230–237. [DOI] [PubMed] [Google Scholar]
- 7. Graichen H, Jakob J, von Eisenhart-Rothe R, Englmeier KH, Reiser M, Eckstein F. Validation of cartilage volume and thickness measurements in the human shoulder with quantitative magnetic resonance imaging. Osteoarthritis Cartilage. 2003;11(7):475–482. [DOI] [PubMed] [Google Scholar]
- 8. Hodler J, Loredo RA, Longo C, Trudell D, Yu J, Resnick D. Assessment of articular cartilage thickness of the humeral head: MR-anatomic correlation in cadavers. AJR Am J Roentgenol. 1995;165(3):615–620. [DOI] [PubMed] [Google Scholar]
- 9. Huang C-Y, Stankiewicz A, Ateshian G, Mow VC. Anisotropy, inhomogeneity, and tension-compression nonlinearity of human glenohumeral cartilage in finite deformation. J Biomech. 2013;38(4):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu XL, Mow VC. Biomechanics of articular cartilage and determination of material properties. Med Sci Sports Exerc. 2008;40(2):193–199. [DOI] [PubMed] [Google Scholar]
- 11. Mimar R, Limb D, Orth FE, Hall RM. Evaluation of the mechanical and architectural properties of glenoid bone. J Shoulder Elbow Surg. 2018;17(2):336–341. [DOI] [PubMed] [Google Scholar]
- 12. Neer CS. Degenerative lesions of the proximal humeral articular surface. Clin Orthop Relat Res. 1961;20:116–125. [PubMed] [Google Scholar]
- 13. Schairer WW, Nwachukwu BU, Lyman S, Craig EV, Gulotta LV. National utilization of reverse total shoulder arthroplasty in the United States. J Shoulder Elbow Surg. 2018;24(1):91–97. [DOI] [PubMed] [Google Scholar]
- 14. Simon P, Gupta A, Pappou I, et al. Glenoid subchondral bone density distribution in male total shoulder arthroplasty subjects with eccentric and concentric wear. J Shoulder Elbow Surg. 2018;24(3):416–424. [DOI] [PubMed] [Google Scholar]
- 15. Soslowsky L, Flatow E, Bigliani L, Mow V. Articular geometry of the glenohumeral joint. Clin Orthop Relat Res. 1992;(285):181–190. [PubMed] [Google Scholar]
- 16. Soslowsky LJ, Flatow EL, Bigliani LU, Pawluk RJ, Ateshian GA, Mow VC. Quantitation of in situ contact areas at the glenohumeral joint: a biomechanical study. J Orthop Res. 1992;10(4):524–534. [DOI] [PubMed] [Google Scholar]
- 17. Walch G, Badet R, Boulahia A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty. 1999;14(6):756–760. [DOI] [PubMed] [Google Scholar]
- 18. Yeh L, Kwak S, Kim Y, et al. Evaluation of articular cartilage thickness of the humeral head and the glenoid fossa by MR arthrography: anatomic correlation in cadavers. Skeletal Radiol. 1998;27(9):500–504. [DOI] [PubMed] [Google Scholar]
- 19. Zumstein V, Kraljevi M, Hoechel S, Conzen A, Nowakowski AM, Müller-Gerbl M. The glenohumeral joint: a mismatching system? A morphological analysis of the cartilaginous and osseous curvature of the humeral head and the glenoid cavity. J Orthop Surg Res. 2014;9(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]