Significance
Memory consists of conscious and unconscious systems with different brain substrates. Eye movements are affected by past experience, but it is often unclear what kind of memory is involved. We examined the preferential viewing effect, whereby in multiple-choice tests of recognition memory, more viewing is directed toward an item that is about to be selected when the choice is correct than when the choice is incorrect. The magnitude of this effect was correlated with measures of conscious, declarative memory, was absent when declarative memory was minimized, and was diminished in memory-impaired patients with medial temporal lobe lesions. We propose that this eye movement effect is a phenomenon of conscious memory, supporting the link between conscious memory and medial temporal lobe function.
Keywords: hippocampus, amnesia, awareness, recognition memory
Abstract
When individuals select the recently studied (and familiar) item in a multiple-choice memory test, they direct a greater proportion of viewing time toward the to-be-selected item when their choice is correct than when their choice is incorrect. Thus, for both correct and incorrect choices, individuals indicate that the chosen item is old, but viewing time nevertheless distinguishes between old and new items. What kind of memory supports this preferential viewing effect? We recorded eye movements while participants made three-alternative, forced-choice recognition memory judgments for scenes. In experiment 1 (n = 30), the magnitude of the preferential viewing effect was strongly correlated with measures of conscious, declarative memory: recognition accuracy as well as the difference in confidence ratings and in response times for correct and incorrect choices. In four analyses that minimized the contribution of declarative memory in order to detect a possible contribution from other processes, the preferential viewing effect was absent. In experiment 2, five memory-impaired patients with medial temporal lobe lesions exhibited a diminished preferential viewing effect. These patients also exhibited poor recognition accuracy and reduced differences in confidence ratings and response times for correct and incorrect choices. We propose that the preferential viewing effect is a phenomenon of conscious, declarative memory and is dependent on the medial temporal lobe. The findings support the link between medial temporal lobe function and declarative memory. When the effects of experience depend on the medial temporal lobe, the effects reflect conscious memory.
Memory is not a unitary faculty of the mind but is composed of different systems that depend on different brain structures (1–3). The medial temporal lobe (MTL) supports the ability to learn about facts and events (declarative memory), and the acquired knowledge is thought to be accessible to conscious awareness (4, 5). In contrast, other brain regions support a collection of abilities (nondeclarative memory), including skills, habits, and priming (6–8). Nondeclarative memory is expressed through performance without requiring any conscious memory content and often without awareness that memory is being used.
Useful methods for studying declarative and nondeclarative memory have come from the analysis of eye movements, which can differ as a result of recent experience (9). In some circumstances, it is unclear what kind of memory is expressed by eye movements. For example, in one task, participants were asked to select the old item when it was presented together with two novel items (10). As participants made their selection, they tended to gaze at the item they were about to select. Interestingly, participants directed even more viewing toward the to-be-selected item when their choice was going to be correct (the target) than when their choice was going to be incorrect (a foil). Thus, for both correct and incorrect choices, individuals judged the selected item to be “old,” but viewing time nevertheless differentiated between old and new items. Similar findings have been reported in other tasks (11, 12). In these cases, eye movements appeared to reveal information in memory beyond what was reflected in overt behavioral choices. Accordingly, what we here term a preferential viewing effect might reflect automatic, unconscious memory. In other studies, different measures of preferential viewing were correlated with hippocampal activity and were diminished after MTL lesions (13, 14). Thus, if the viewing effect does reflect unconscious memory, it would appear to provide an exception to the supposed link between MTL-dependent memory and conscious, aware memory (15). Moreover, whether participants have conscious knowledge about what they have learned might not be a useful distinction for understanding the function of the MTL (16).
Yet, this preferential viewing effect might reflect conscious, declarative memory. Even though individuals judge the selected item to be old on both correct and incorrect trials, what individuals consciously know about the test item need not be the same in the two cases. We reasoned that viewing time might differ for to-be-selected correct and incorrect items in the same way that other measures of declarative memory distinguish between correct and incorrect choices. For example, when individuals incorrectly designate a foil as old in a recognition memory test, confidence ratings are typically lower and response times are typically slower than when a target is correctly designated as old (17, 18). If the preferential viewing effect reflects declarative memory, then its magnitude (i.e., the difference in viewing time for to-be-selected targets vs. foils) should correlate with measures of declarative memory.
In two experiments, we recorded eye movements while participants made three-alternative, forced-choice, recognition memory judgments for scenes (Fig. 1). We first asked whether the preferential viewing effect reflects conscious, declarative memory or unconscious memory (experiment 1), and then whether this viewing effect depends on the MTL (experiment 2).
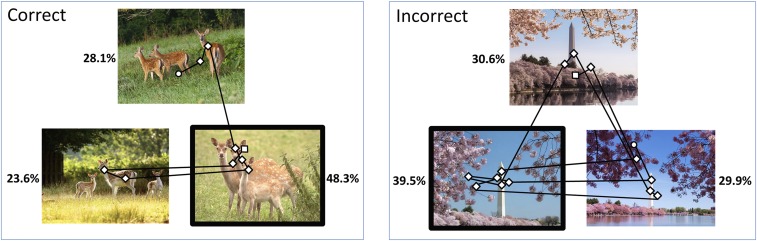
Fig. 1.
Two sample test trials. Two hundred scenes were studied for 0.5 s each, and memory was tested after a delay of 30 min (experiment 1). Eye movement traces (black lines) and fixations (diamonds) as a participant decided which of three scenes was the one presented earlier. Data are for the period from presentation of the scenes to the memory judgment. The white circle represents the first fixation, and the white square represents the last fixation. The black box indicates the scene that was selected on each trial. (Left) Correct choice. (Right) Incorrect choice. Percentages indicate the percent time viewing each scene. These trials illustrate the preferential viewing effect, whereby participants direct more viewing toward a to-be-selected scene when the choice is correct than when the choice is incorrect (in this case, 48.3% vs. 39.5%).
Results
Experiment 1.
Participants exhibited good recognition memory for the scenes (accuracy, 58.1 ± 1.8% correct; t[29] = 13.90; P < 0.001, one-sample t test; chance, 33%). Eye movements in response to the test scenes were examined to determine how memory for the previously studied scenes affected viewing. First, we examined viewing time for the studied scenes (targets) and the foil scenes (i.e., the mean percent time viewing the two foil scenes on each trial), independent of the scene selected. Percent time viewing the targets (40.7 ± 1.1%) was greater than chance (t[29] = 10.52; P < 0.001, one-sample t test), and percent time viewing each foil (29.6 ± 1.1%) was lower than chance (t[29] = 10.51; P < 0.001, one-sample t test). These measures indicate the tendency of participants to look at the old scene (when they remembered it), together with the natural tendency to look at the scene that is about to be selected.
We next examined viewing time as a function of which scene was selected, the target or a foil. Specifically, we compared the percent time viewing the target on trials when the target was selected (correct choices) with the percent time viewing a foil on trials when a foil was selected (incorrect choices). Percent time viewing a to-be-selected target for the time from presentation of the scenes to the memory judgment was greater than the percent time viewing a to-be-selected foil (49.7 ± 0.5% vs 44.4 ± 0.7%; t[29] = 8.78; P < 0.001, paired t test) (Fig. 2A). This finding demonstrates a preferential viewing effect, that is greater percent time viewing to-be-selected targets than to-be-selected foils. This effect was evident in each of the three 0.5-s time bins immediately preceding the memory judgment (t[29] > 2.29; P < 0.030, paired t test) (Fig. 2B).
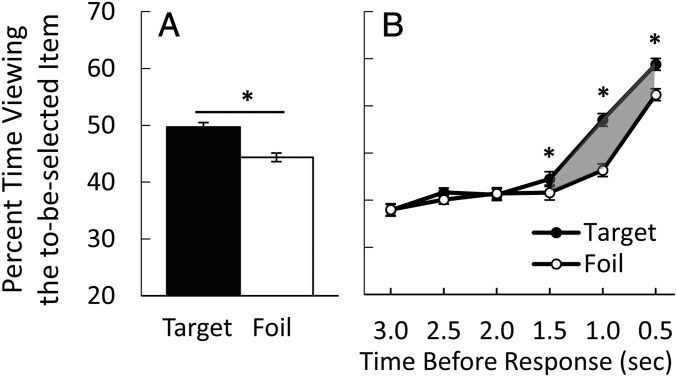
Fig. 2.
The preferential viewing effect in young adults (n = 30). (A) Percent time viewing the target (on trials when the target was selected), and percent time viewing the foil (on trials when a foil was selected) during the time from presentation of the scenes to the memory judgment (experiment 1). The percent time viewing the to-be-selected scene was greater when the choice was correct (target) than when the choice was incorrect (foil) (i.e., a preferential viewing effect). (B) The percent time viewing the to-be-selected scene in 0.5-s time bins for the 3.0 s immediately preceding the memory judgment. The preferential viewing effect was evident in each time bin during the 1.5 s before the memory judgment (shaded area in B). *P < 0.030. Error bars denote SE.
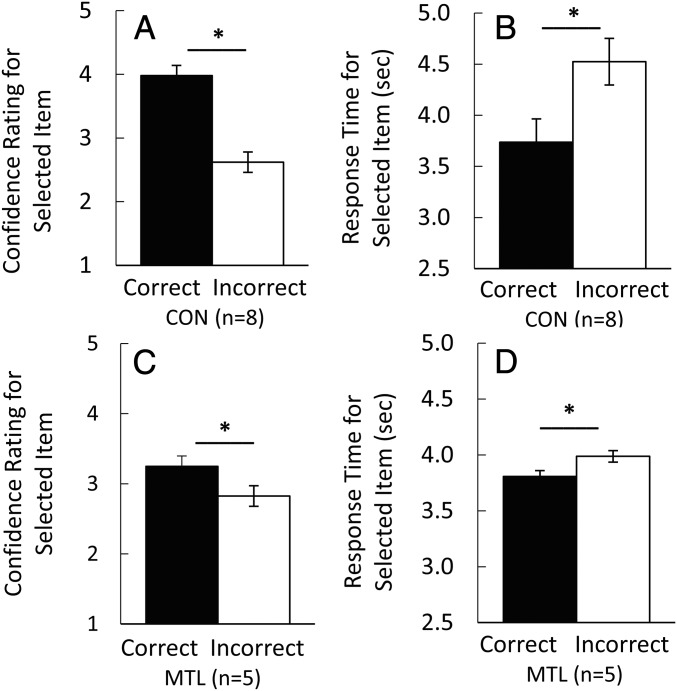
The preferential viewing effect just described shows that the viewing of a to-be-selected item differed depending on whether the selection was correct or incorrect. Correct and incorrect choices also differed with respect to how much confidence participants expressed in their choices and with respect to the speed at which choices were made (Fig. 3). Specifically, confidence ratings were higher and response times were faster when choices were correct than when choices were incorrect (3.5 ± 0.1 vs. 2.4 ± 0.1 for confidence ratings; t[29] = 14.94; P < 0.001, paired t test and 3.4 ± 0.1 s vs. 4.0 ± 0.1 s for response times; t[29] = 8.43; P < 0.001, paired t test). Thus, although participants indicated for both their correct and incorrect choices that they judged the selected scene to be the one previously studied, their behavior differed for the two kinds of choices. They looked more at the to-be-selected scene on correct trials, were more confident on correct trials, and responded faster on correct trials.
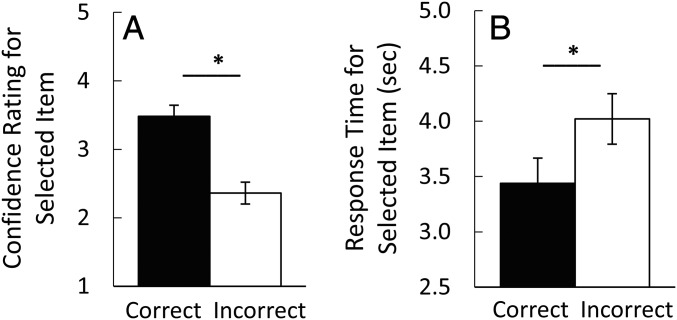
Fig. 3.
Confidence ratings and response times associated with a correct choice of the target or an incorrect choice of one of the two foils (experiment 1). When participants correctly selected the target, their confidence ratings were higher (A) and their response times were faster (B) than when they incorrectly selected a foil. *P < 0.001. Error bars denote SE.
To explore what kind of memory supports the preferential viewing effect, we first calculated correlations between the magnitude of the preferential viewing effect and three other measures associated with declarative memory: (i) the difference in confidence ratings for correct vs. incorrect trials, (ii) the difference in response times for correct vs. incorrect trials, and (iii) accuracy (percent correct scores). Across the 30 participants, the magnitude of the preferential viewing effect was positively correlated with the difference in confidence ratings (r = 0.61; P < 0.001; Fig. 4A), the difference in response times (r = 0.65; P < 0.001; Fig. 4B), and overall accuracy (r = 0.70; P < 0.001; Fig. 4C; Pearson’s correlation for all three measures). These findings show that participants who exhibited a greater preferential viewing effect also had better memory for the scenes (higher accuracy), as well as larger differences in both confidence ratings and response times for correct vs. incorrect choices.
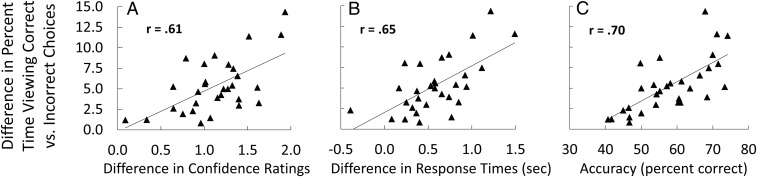
Fig. 4.
Association between the size of the preferential viewing effect and other behavioral measures. The difference between the percent time viewing the target (on trials when the target was correctly selected) and the foil (on trials when a foil was incorrectly selected) was calculated for each participant during the period preceding the memory judgment (experiment 1). Across participants, these difference scores were positively correlated with the difference in confidence ratings associated with correct and incorrect choices (A), the difference in response times associated with correct and incorrect choices (B), and the accuracy of the behavioral choice (C). Each data point shows the score of one participant (n = 30).
We next asked whether the preferential viewing effect can still be detected when the role of declarative memory is minimized. First, based on the regression lines in Fig. 4, we calculated the magnitude of the preferential viewing effect (i.e., the value on the y-axis) when the difference in confidence ratings for correct and incorrect choices reached 0 (Fig. 4A), when the difference in response times for correct and incorrect choices reached 0 (Fig. 4B), and also when accuracy reached chance level (33% correct; Fig. 4C). In each of these conditions, the magnitude of the preferential viewing effect was absent or very small (−0.2%, 2.0%, and −0.5%, respectively).
We next carried out a repeated-measures analysis of covariance (ANCOVA) to determine whether a difference between the percent time viewing the selected targets (correct) vs. percent time viewing selected foils (incorrect) remained after the contributions of confidence ratings and response times were removed. The within-subject dependent variable was the mean percent time viewing the selected items (one value for correct choices and one value for incorrect choices for each participant). Confidence ratings and response times for the correct and incorrect choices were covariates. After accounting for the effects of the covariates, there was no longer a difference between the percent time viewing the selected targets vs. the selected foils (P = 0.808); that is, the preferential viewing effect was absent.
We then calculated the magnitude of the preferential viewing effect for a subset of trials when declarative memory was minimal. Specifically, we selected trials in which participants indicated that they were guessing about which scene was the target (confidence level, 1; 13.6 ± 1.7 correct trials/participant and 23.8 ± 3.0 incorrect trials/participant). For this subset of trials, response times were similar for correct and incorrect trials (4.1 ± 0.2 s vs. 4.2 ± 0.1 s), accuracy was near chance (36.8 ± 1.6%), and the preferential viewing effect was absent [percent time viewing the selected target (correct), 42.7 ± 0.6%; percent time viewing the selected foil (incorrect), 42.7 ± 0.7%].
Finally, we considered trials in which a foil was selected rather than the target (incorrect trials). For these trials, we compared percent viewing time for the target and percent viewing time for the foil that was not selected. In this way, we asked whether viewing behavior might reveal knowledge about the target, even though the choice was incorrect on these trials and little declarative memory was presumably available. In this circumstance, there was no evidence that participants had knowledge about the target. Across all participants, the percent time viewing the target was virtually the same as the percent time viewing the nonselected foil (27.9 ± 0.3% vs. 27.7 ± 0.3%; t[29] = 0.49; P = 0.629, paired t test).
Experiment 2.
Patients were impaired in remembering the studied scenes (accuracy, 47.0 ± 4.7% correct for patients vs. 74.9 ± 4.9% correct for controls; t[11] = 3.84; P = 0.003, independent t test; Fig. 5). The patient with large MTL lesions had the lowest score (38.2% correct, not significantly above chance, P = 0.308, binomial probability). Patients also differed from controls with respect to confidence ratings and response times (Fig. 6). Specifically, controls had higher confidence ratings and faster response times when they were correct than when they were incorrect (4.0 ± 0.2 vs. 2.6 ± 0.2 for confidence ratings, t[7] = 8.51; P < 0.001, paired t test and 3.7 ± 0.2 s vs. 4.5 ± 0.2 s for response times, t[7] = 3.97; P = 0.005, paired t test; Fig. 6 A and B). Patients exhibited these same two effects but less strongly than controls (t[11] > 2.36; P < 0.037, independent t tests for the difference scores). For the patients, confidence ratings were 3.3 ± 0.2 and 2.8 ± 0.2 for correct and incorrect choices (t[4] = 2.90; P = 0.044, paired t test; Fig. 6C). Response times were 3.8 ± 0.1 s for correct choices and 4.0 ± 0.1 s for incorrect choices (t[4] = 3.48; P = 0.025, paired t test; Fig. 6D).
Fig. 5.
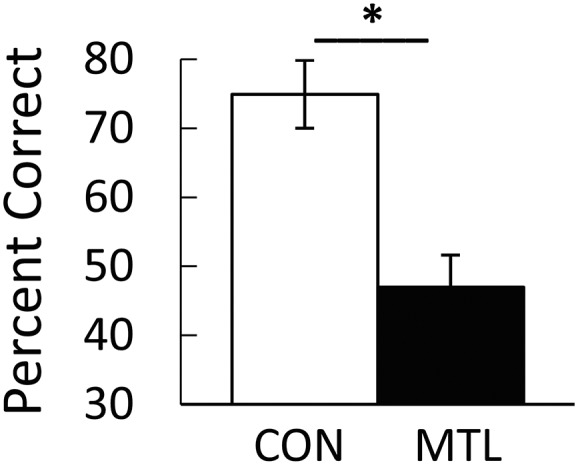
Performance of patients with MTL damage (MTL; n = 5) and controls (CON; n = 8) on a three-alternative test of recognition memory. One hundred scenes were studied for 1.5 s each, and memory was tested after a delay of 3 min (experiment 2). The patients were impaired at identifying the studied scene relative to controls. *P = 0.003. Error bar denotes SE.
Fig. 6.
Confidence ratings and response times associated with a correct choice of the target or an incorrect choice of one of the two foils in patients with MTL damage and controls. When participants correctly selected the target, their confidence ratings were higher (A and C) and their response times were faster (B and D) than when they incorrectly selected a foil (experiment 2). The difference in confidence ratings and the difference in response times for correct vs. incorrect choices was smaller for MTL patients than for controls (CON). *P < 0.050. Error bars denote SE.
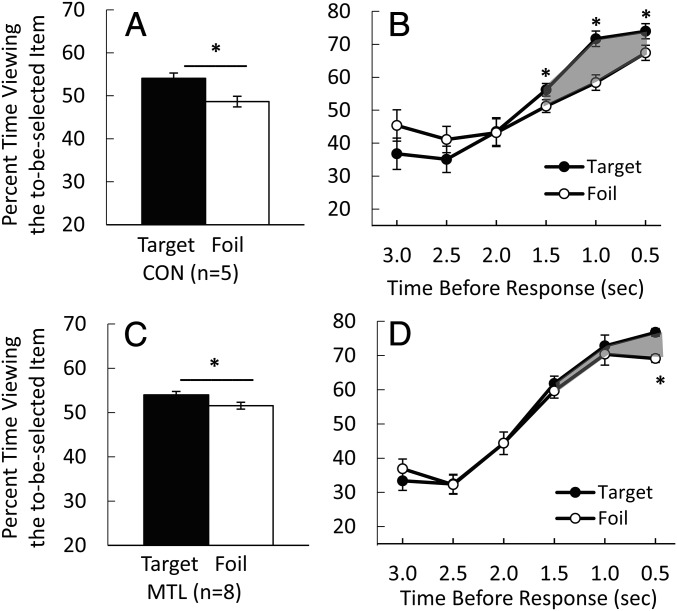
Importantly, the patients differed from controls with respect to the magnitude and duration of the preferential viewing effect (Fig. 7). First, for the time from presentation of the scenes to the memory judgment (Fig. 7 A and C), the preferential viewing effect was evident in both groups (5.4 ± 1.2% for controls; t[7] = 4.38; P = 0.003 vs. 2.4 ± 0.8% for patients; t[4] = 3.66; P = 0.034), but the effect was marginally smaller in patients than in controls (t[11] = 2.04; P = 0.067, independent t test). In addition, the small but significant preferential viewing effect in patients (2.4%) was undetectable in conditions where the contribution of declarative memory was minimized (0.8% when patients indicated they were guessing or −1.4% when viewing behavior during incorrect trials was analyzed as in experiment 1).
Fig. 7.
The preferential viewing effect in patients with MTL damage and controls. (A and C) During the time from presentation of the scenes to the memory judgment, the percent time viewing the to-be-selected scene was greater when the choice was correct (target) than when the choice was incorrect (foil) (experiment 2). This effect was marginally smaller in the patients than in the controls (CON) (P = 0.067). (B and D) Percent time viewing the to-be-selected scene in 0.5-s time bins for only the 3.0 s before the memory judgment. For controls, the preferential viewing effect was evident 1.5 s before the memory judgment (B). For patients, the preferential viewing effect was evident only 0.5 s before the memory judgment (D). During the 1.5 s immediately preceding the memory judgment (shaded areas in B and D), the preferential viewing effect was smaller (P = 0.029) in the patients than in the controls. *P < 0.033. Error bars denote SE.
Second, the controls exhibited a preferential viewing effect in each of the three time bins immediately preceding the memory judgment (t[7] > 2.62; P < 0.033, paired t test; Fig. 7B). However, the patients exhibited this effect only in the single 0.5-s time bin immediately preceding the memory judgment (t[4] = 8.51; P = 0.001, paired t test; Fig. 7D). Third, we examined the preferential viewing effect during the 1.5-s period immediately before the memory judgment, because in experiment 1 (Fig. 2B), the effect was most pronounced (and was statistically significant) during this period. In this time period, the preferential viewing effect was significant for the controls (t[7] = 8.37; P < 0.001, paired t test), not significant for the patients (t[4] = 2.23; P = 0.090, paired t test), and smaller for the patients than for the controls (compare the shaded areas in Fig. 7 B and D; 4.0 ± 1.8% vs. 8.9 ± 1.1%; t[11] = 2.51; P = 0.029, independent t test).
Discussion
In two experiments, participants saw three similar scenes and selected the scene that had been presented previously. In experiment 1, participants exhibited a preferential viewing effect that reflected the old-new status of the scenes; that is, preceding the memory judgment, participants directed more viewing toward a to-be-selected target than toward a to-be-selected foil (Fig. 2). In addition, participants’ confidence ratings were higher and their response times faster when they selected a target than when they selected a foil (Fig. 3). This difference in confidence ratings and response times for correct and incorrect trials, as well as overall accuracy scores, positively correlated with the magnitude of the preferential viewing effect (Fig. 4). In experiment 2, participants with MTL lesions exhibited poor recognition memory (Fig. 5) and reduced differences in confidence ratings and response times for correct and incorrect choices (Fig. 6). Importantly, the magnitude and duration of the preferential viewing effect were also reduced in the patients relative to controls (Fig. 7). We suggest that the preferential viewing effect reflects conscious, declarative memory and depends on the integrity of the MTL.
A different study of eye movements and face-scene associative memory (14) reported a similar finding in memory-impaired patients. Healthy participants spent a greater percent time viewing a target, when a target was selected, than they spent viewing a foil when a foil was selected (from displays where targets were sometimes present and sometimes absent). Patients with MTL lesions performed at chance on the associative memory test and did not exhibit the eye-movement effect.
Earlier studies have also described differences in confidence ratings (12) or response times (10, 11) in conjunction with differences in viewing time for correct and incorrect choices [refs. 10 (Fig. 2B, Left), 11 (Fig. 3B), and 12 (Fig. 3)]. However, these studies either did not identify whether any of these behavioral differences were associated with the magnitude of the preferential viewing effect or included viewing time from after the behavioral decision to demonstrate an association. Our study found that confidence ratings, response times for the behavioral decision, and recognition accuracy all strongly predicted the magnitude of the preferential viewing effect when all of the viewing data were collected before the memory judgment.
We considered the possibility that the preferential viewing effect might also be supported by other processes, including nondeclarative (unconscious) forms of memory operating independently of the MTL. We explored this idea in four analyses that asked whether viewing behavior could reveal knowledge about the target when the contribution of declarative memory was minimized. First, when the difference in confidence ratings and response times for correct and incorrect responses reached zero, the preferential viewing effect became negligible (Fig. 4 A and B). Similarly, we calculated that when recognition memory accuracy reached chance (33%), the preferential viewing effect was absent (Fig. 4C). Second, when the effects of confidence ratings and response times were removed using ANCOVA, no residual preferential viewing effect could be detected. Third, for a subset of correct and incorrect trials in which participants indicated that they were guessing, response times were similar, and accuracy was near chance level, no preferential viewing effect could be detected. Fourth, in incorrect trials when declarative memory about the correct scene was presumably minimal, there was no evidence in participants’ viewing behavior to indicate that they had knowledge about the target. Thus, we could find no evidence that processes independent of declarative memory support the preferential viewing effect.
An earlier study reached a different conclusion about the nature of eye movements in memory tests (11)—specifically, that eye movements can reveal information not available to conscious memory. In that study, participants were asked to make a recognition decision when presented with target-present displays and also to make a decision when presented with target-absent displays. Participants were then asked in both cases to endorse (or not endorse) the selected item as having appeared on the study list. That study reported that increasing task difficulty increased the frequency with which participants falsely endorsed items in the target-absent displays, but that the eye movements preceding the recognition decision were impervious to this manipulation. Specifically, viewing time for the foils that were about to be selected from the target-absent displays was reported to be similar when the task was easy and when it was difficult. In this way, eye movements appeared to represent past experience better than overt behavior. However, according to figure 2B in that report (11), the viewing time for the selected foils, rather than being impervious to task difficulty, was marginally different in the two task conditions in some time bins (P = 0.07), in parallel with the effect of task difficulty on false endorsements. In addition, we suggest that the appropriate comparison would be a contrast between viewing time for the foils that were not only selected in the easy and difficult task conditions but also endorsed as familiar.
Another study used neuroimaging to examine face-scene associative memory in a three-alternative, forced-choice test (13). The percent time viewing the target was calculated for error trials (i.e., trials in which the target was not selected). Error trials in which there was more viewing of the target were associated with higher bilateral hippocampal activity compared with error trials in which there was less viewing of the target. This activity was interpreted as reflecting unconscious, unaware memory, because overt recognition had failed. Nonetheless, even when recognition fails, error trials may still reveal information about which stimulus is the target. For example, confidence ratings and response times may differ between error trials in which there was more viewing of the target and error trials in which there was less viewing of the target. We tested this prediction in our own data for the 50% of error trials in which the target was viewed the most (mean, 34.6 ± 1.2% of the time) and the 50% of error trials in which the target was viewed the least (mean, 20.6 ± 0.6% of the time). These two kinds of error trials were associated with markedly different confidence ratings and response times (t[29] > 3.20, P < 0.003, paired t test). Thus, we suggest that the finding of hippocampal activity can be understood as reflecting not memory without awareness, but rather conscious knowledge about the accuracy of the behavioral decision.
It is worth mentioning that the MTL patients exhibited some residual recognition memory for the scenes (Fig. 5), consistent with the extensive literature showing that memory impairment is seldom absolute after MTL lesions (19). Similarly, the preferential viewing effect was impaired in the patients but was not entirely absent (experiment 2). Thus, the finding that an impaired but still detectable preferential viewing effect was observed in the MTL patients conforms with findings obtained using more typical measures of memory. Also noteworthy is that when the role of declarative memory was minimized by examining trials when the patients were guessing or when they were incorrect, the preferential viewing effect was absent.
Earlier reports of other experience-dependent eye movement effects are consistent with this link between MTL function and conscious memory: the visual paired-comparison task (i.e., when a novel scene and a repeated scene are presented together and a memory test is not expected, healthy participants spend more time viewing the novel scene; refs. 20 and 21); and the repetition effect (i.e., during an old-new recognition memory test, with the expectation that memory will be tested, healthy participants explore the novel scenes more than the repeated scenes; refs. 22 and 23). These eye movement effects were correlated with measures of declarative memory (visual paired-comparison) or were observed only when participants were aware of which scenes were old and which were new (repetition effect). MTL patients were less aware than controls of the old/new status of the scenes and did not exhibit these two eye movement effects. Two other studies of the repetition effect also reported results consistent with the association between MTL function and conscious memory by showing that in the case of unaware memory, eye movement effects are independent of the MTL (23, 24); that is, when there was no expectation of memory testing, healthy participants exhibited the repetition effect regardless whether they were correct or incorrect in their memory judgments, and MTL patients also exhibited the repetition effect.
One early study of eye movements has sometimes been taken as running counter to the link between aware memory and MTL function (9). Participants exhibited a manipulation effect (looking disproportionately at the altered region of a scene) when they were reportedly unaware of the manipulation. In follow-up work, a patient with hippocampal lesions did not exhibit this effect (25). However, the finding of unawareness was reported in only a small group of healthy participants (n = 12) and was significant for only one of three eye movement measures. Note too that three other studies have investigated the manipulation effect using different methods. In all three studies, the manipulation effect was observed for each of three eye movement measures but only when participants were aware of the manipulation [refs. 22 (experiment 2, n = 51) and 26 (experiment 1, n = 20; experiment 2, n = 20)]. Thus, if there are conditions under which a manipulation effect can occur in unaware participants (such as under the conditions unique to ref. 9), it would be useful to document the effect with a large number of participants and with multiple measures of eye movements.
Based on the available data, we propose that when experience-dependent behavior is dependent on the MTL, the behavior reflects conscious (aware) memory. Similarly, we propose that when experience-dependent behavior is independent of the MTL, the behavior reflects unaware memory.
Materials and Methods
Experiment 1.
Participants.
Thirty undergraduates from the University of California San Diego participated for course credit (nine males; mean age, 21.4 ± 0.4 y). All procedures in experiments 1 and 2 were approved by the Institutional Review Board at the VA San Diego Healthcare System, and participants gave written informed consent before participation.
Materials and procedure.
The stimuli were 600 color photos of indoor or outdoor scenes. The study list consisted of 200 scenes, and the recognition memory test consisted of the 200 study items, each presented together with two similar scenes (Fig. 1). Each of the three similar scenes served as the study scene for one third of the participants. Items in both the study and test lists were presented in a random order for each participant, and during the test, the studied scene appeared equally often in the three positions (top, left, and right).
Each study scene was presented in the center of a computer screen for 1.0 s with a 0.5-s gap between scenes (visual angle for each scene, 12.4° × 9.3°). Participants were instructed to remember the scenes for a later memory test. At 30 min after presentation of the study list, memory was tested by presenting a target scene and two similar foil scenes for 7 s. The size of each test scene was the same as the size of the study scene. Participants were instructed to indicate by key press the previously studied scene. The three test scenes remained on the screen for the full 7 s, but participants were asked to respond as soon as they had made their decision. After the 7 s, participants rated their confidence on a scale of 1–5 (1, pure guess; 5, very sure). The next trial was initiated by the experimenter when gaze was directed at the center of the screen. The intertrial interval was typically 1–2 s. A break was provided after each block of 50 trials. Participants completed three practice trials (with additional sets of scenes) before the experiment to become familiar with the format of the recognition memory test.
Apparatus.
Eye movements were recorded during testing by tracking pupillary position and corneal reflection. SI Appendix provides additional information.
Experiment 2.
Participants.
Five memory-impaired patients participated, four with bilateral lesions thought to be limited to the hippocampus (CA fields, dentate gyrus, and subicular complex) and one with larger MTL lesions (Table 1). Information on etiology, quantitative measures of lesions, and magnetic resonance images from each patient is provided in SI Appendix. Eight healthy controls (two females) also participated (mean age, 60.5 ± 4.0 y; mean education, 14.3 ± 0.8 y).
Table 1.
Characteristics of memory-impaired patients
| Patient | Age, y | Education, y | WAIS-III IQ | WMS-R score | ||||
| Attention | Verbal | Visual | General | Delay | ||||
| D.A. | 34 | 12 | 95 | 104 | 90 | 91 | 90 | 56 |
| K.E. | 75 | 13.5 | 108 | 114 | 64 | 84 | 72 | 55 |
| L.J. | 79 | 12 | 101 | 105 | 83 | 60 | 69 | <50 |
| G.W. | 58 | 12 | 108 | 105 | 65 | 86 | 70 | <50 |
| G.P. | 71 | 16 | 98 | 102 | 79 | 62 | 66 | 50 |
The WMS-R does not provide numerical scores for individuals who score <50. The IQ score for patient D.A. is from the WAIS-IV. WAIS-III, Wechsler Adult Intelligence Scale-III; WMS-R, Wechsler Memory Scale-Revised.
Materials and procedure.
The apparatus, materials, and procedure were the same as in experiment 1 with modifications to accommodate the older participants in experiment 2. The study list consisted of 100 scenes (not 200), thereby allowing for two separate 100-item tests. Each scene was studied for 1.5 s (not 1.0 s). The study-test interval was approximately 3 min (not 30 min). Finally, before the experiment, participants completed one practice trial, and during the study–test interval they completed two additional practice trials. All participants were tested on two separate occasions, once with each 100-item test, at a mean interval of 102 d between tests.
Data analysis (experiments 1 and 2).
We calculated the viewing time for each scene in a test display by summing the duration of the fixations directed toward each scene for the time from presentation of the scene to the memory judgment. For each trial, we then calculated the percent viewing time for each scene relative to the total viewing time directed toward all three scenes. To examine the time course of the preferential viewing effect, percent viewing time was calculated separately for six 500-ms time bins spanning 3.0 s before the memory judgment.
Trials were eliminated if participants did not respond within the 7 s in which the test scenes were displayed (1.7% of trials/participant in experiment 1; 4.6% of trials/participant in experiment 2). Trials were also eliminated due to occasional technical difficulties in tracking the eyes that resulted in abnormally low viewing times for the test items (1.7% of trials/participant in experiment 1; 1.4% of trials/participant in experiment 2). Low viewing times in each experiment were defined as times >3 SD below the mean viewing time for the 7-s test period across all trials.
Supplementary Material
Acknowledgments
We thank Isabel Asp, Jennifer Frascino, Megan Tsui, and Grace Woo for assistance. This work was supported by the Medical Research Service of the Department of Veterans Affairs (5IK6CX001644), Awards I01CX000359 (to L.R.S.) and I01CX001375 (to C.N.S.), National Institute of Mental Health Grant 24600 (to L.R.S.), and National Institute of Health Training Grant 5T32AG000216 (to Z.J.U.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803791115/-/DCSupplemental.
References
- 1.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford Univ Press; New York: 2001. [Google Scholar]
- 2.Schacter DL, Tulving E. What are the memory systems of 1994? In: Schacter DL, Tulving E, editors. Memory Systems 1994. MIT Press; Cambridge, MA: 1994. pp. 1–38. [Google Scholar]
- 3.Squire LR. The neuropsychology of human memory. Annu Rev Neurosci. 1982;5:241–273. doi: 10.1146/annurev.ne.05.030182.001325. [DOI] [PubMed] [Google Scholar]
- 4.Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 5.Eichenbaum H. Declarative memory: Insights from cognitive neurobiology. Annu Rev Psychol. 1997;48:547–572. doi: 10.1146/annurev.psych.48.1.547. [DOI] [PubMed] [Google Scholar]
- 6.Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- 7.Gabrieli JDE. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- 8.Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 9.Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- 10.Chen HC, Lee YS. The eye movement measure of memory and its relationship with explicit measures. Conscious Cogn. 2015;33:354–363. doi: 10.1016/j.concog.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Hannula DE, Baym CL, Warren DE, Cohen NJ. The eyes know: Eye movements as a veridical index of memory. Psychol Sci. 2012;23:278–287. doi: 10.1177/0956797611429799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chua EF, Hannula DE, Ranganath C. Distinguishing highly confident accurate and inaccurate memory: Insights about relevant and irrelevant influences on memory confidence. Memory. 2012;20:48–62. doi: 10.1080/09658211.2011.633919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hannula DE, Ranganath C. The eyes have it: Hippocampal activity predicts expression of memory in eye movements. Neuron. 2009;63:592–599. doi: 10.1016/j.neuron.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid-onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J Cogn Neurosci. 2007;19:1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- 15.Hannula DE, Greene AJ. The hippocampus reevaluated in unconscious learning and memory: At a tipping point? Front Hum Neurosci. 2012;6:80. doi: 10.3389/fnhum.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henke K. A model for memory systems based on processing modes rather than consciousness. Nat Rev Neurosci. 2010;11:523–532. doi: 10.1038/nrn2850. [DOI] [PubMed] [Google Scholar]
- 17.Norman DA, Wickelgren WA. Strength theory of decision rules and latency in retrieval from short-term memory. J Math Psychol. 1969;6:192–208. [Google Scholar]
- 18.Mickes L, Hwe V, Wais PE, Wixted JT. Strong memories are hard to scale. J Exp Psychol Gen. 2011;140:239–257. doi: 10.1037/a0023007. [DOI] [PubMed] [Google Scholar]
- 19.Reed JM, Hamann SB, Stefanacci L, Squire LR. When amnesic patients perform well on recognition memory tests. Behav Neurosci. 1997;111:1163–1170. doi: 10.1037//0735-7044.111.6.1163. [DOI] [PubMed] [Google Scholar]
- 20.Manns JR, Stark CE, Squire LR. The visual paired-comparison task as a measure of declarative memory. Proc Natl Acad Sci USA. 2000;97:12375–12379. doi: 10.1073/pnas.220398097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKee RD, Squire LR. On the development of declarative memory. J Exp Psychol Learn Mem Cogn. 1993;19:397–404. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- 22.Smith CN, Squire LR. Experience-dependent eye movements reflect hippocampus-dependent (aware) memory. J Neurosci. 2008;28:12825–12833. doi: 10.1523/JNEUROSCI.4542-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith CN, Squire LR. When eye movements express memory for old and new scenes in the absence of awareness and independent of hippocampus. Learn Mem. 2017;24:95–103. doi: 10.1101/lm.043851.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olsen RK, et al. The relationship between eye movements and subsequent recognition: Evidence from individual differences and amnesia. Cortex. 2016;85:182–193. doi: 10.1016/j.cortex.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Ryan JD, Cohen NJ. Processing and short-term retention of relational information in amnesia. Neuropsychologia. 2004;42:497–511. doi: 10.1016/j.neuropsychologia.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Smith CN, Hopkins RO, Squire LR. Experience-dependent eye movements, awareness, and hippocampus-dependent memory. J Neurosci. 2006;26:11304–11312. doi: 10.1523/JNEUROSCI.3071-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.