Significance
Ice-binding proteins (IBPs) can be considered a prominent example of macromolecules affecting ice growth kinetics. Until now, two groups of IBPs have been described based on their activity as ice growth inhibitors: moderate and hyperactive IBPs. The mechanism underlying these activity differences has not been clarified yet. Although it is commonly believed that hyperactivity is related to growth inhibition of the basal faces of ice crystals, we show that a moderate IBP can also attach to the basal faces and inhibit their growth. Our observations clearly indicate that this moderate IBP occupies a separate position in the classification of IBPs and contribute to our understanding of interaction between macromolecules and ice and more generally, between macromolecules and inorganic crystals.
Keywords: ice-binding protein, ice crystallization, growth rates, Fragilariopsis cylindrus, DUF3494
Abstract
Ice-binding proteins (IBPs) affect ice crystal growth by attaching to crystal faces. We present the effects on the growth of an ice single crystal caused by an ice-binding protein from the sea ice microalga Fragilariopsis cylindrus (fcIBP) that is characterized by the widespread domain of unknown function 3494 (DUF3494) and known to cause a moderate freezing point depression (below 1 °C). By the application of interferometry, bright-field microscopy, and fluorescence microscopy, we observed that the fcIBP attaches to the basal faces of ice crystals, thereby inhibiting their growth in the c direction and resulting in an increase in the effective supercooling with increasing fcIBP concentration. In addition, we observed that the fcIBP attaches to prism faces and inhibits their growth. In the event that the effective supercooling is small and crystals are faceted, this process causes an emergence of prism faces and suppresses crystal growth in the a direction. When the effective supercooling is large and ice crystals have developed into a dendritic shape, the suppression of prism face growth results in thinner dendrite branches, and growth in the a direction is accelerated due to enhanced latent heat dissipation. Our observations clearly indicate that the fcIBP occupies a separate position in the classification of IBPs due to the fact that it suppresses the growth of basal faces, despite its moderate freezing point depression.
Ice-binding proteins (IBPs) have a prominent position among molecules and particles interacting with ice due to the fact that they are particularly successful in affecting ice crystal growth. Also referred to as antifreeze proteins (AFPs), IBPs have been found in several polar or cold-tolerant organisms (reviewed in ref. 1). These proteins cause, among other effects, a thermal hysteresis (TH; i.e., a shift of the freezing point below the melting point). Based on this activity, two main IBP groups have been identified. At identical protein concentrations, moderate IBPs cause a TH of up to 1 °C, while hyperactive IBPs induce a much stronger freezing point depression. Moreover, the variance of effectiveness becomes evident from morphological changes of the ice crystal (2). For example, at temperatures slightly below the freezing point, ice crystals in the presence of moderate fish IBPs have a bipyramidal shape and grow preferentially along the c axis. However, the preferential growth occurs along the a axis in the presence of hyperactive IBPs, resulting in planar ice crystals.
The mechanisms by which IBPs inhibit crystal growth and underlying causes of TH activity differences among various protein groups remain unclear. In this regard, several studies have pointed to a match between certain molecules on the ice-binding site (IBS) of the proteins and defined crystallographic faces of the ice crystals, which allow the formation of stable attachment of IBPs to the ice. In contrast, the high effectiveness of hyperactive proteins has been often attributed to their additional match to the basal face and therefore, their ability to inhibit growth of the crystals along the c axis (3, 4).
The ice-binding proteins from the sea ice diatom Fragilariopsis cylindrus (fcIBPs) belong to the protein family characterized by the “domain of unknown function” 3494 (DUF3494) as the domain is referred to in the Pfam database. This IBP family was described at first with proteins from the snow mold fungus Typhula ishikariensis (5) and from a few diatom species (6). It was later identified in several polar organisms, having likely been transported by horizontal gene transfer (7, 8). The DUF3494 IBPs represent today the most widespread of the known IBP families and can be found in bacteria (9–11), diatoms (12, 13), yeast, and other fungi (14–17) among others.
The classification of fcIBPs as moderate or hyperactive from properties described for these proteins is challenging. If we apply the classical scheme described above, which considers the link between TH activity, face affinity, and crystal morphology, fcIBPs and other DUF3494 IBPs cannot be described as moderate or hyperactive. For example, fcIBP11 is known to have a moderate TH activity, but the crystal morphology shows a planar dendritic pattern (18) as is usually attributed to hyperactive IBPs. The peculiarity of this protein family has been mentioned in the description of other DUF3494 IBPs with moderate TH activity (11, 19, 20) and can also be observed in the IBP from the grass Lolium perenne (21).
However, any data on ice growth kinetics in the presence of fcIBPs or any DUF3494 IBP for that matter are entirely missing. Previous publications regarding crystal morphology and growth direction in the presence of DUF3494 IBPs or ice-binding proteins from L. perenne (LpIBPs) refer to image analyses using only the smallest crystal sizes, around 10–50 μm in diameter (16, 21–23). Although this approach is very helpful for the assessment of protein activity, the usage of small microcrystals does not provide a clear distinction of morphological details and may, therefore, introduce ambiguities in the determination of the orientation of crystallographic axes. Furthermore, experimental factors, like cooling rate and initial crystal size, have been shown to affect the results (24).
In the following, we present a characterization of the growth process of ice single crystals in the presence of fcIBP11. We show the morphology and growth rates of ice crystals and the localization of fluorescently labeled fcIBP11 on the different faces of ice crystals. We interpret our results in the context of growth kinetics and protein activity and question whether fcIBP11 occupies a separate position in the actual classification of IBPs as moderate or hyperactive proteins.
Materials and Methods
The Protein.
We used the IBP isoform 11 from the Antarctic sea-ice diatom F. cylindrus, fcIBP11 (GenBank accession no. DR026070). Previous works on this isoform stressed its relevance as IBP in vivo, showing that fcIBP11 is actively expressed by F. cylindrus (6) and suggesting that it plays a role in the diatom as a response to freezing environmental conditions (12), that it binds to ice, and that it has TH activity (18). In this study, we focus on the effect of only one single isoform among the different fcIBPs to better understand its interaction with ice from the viewpoint of ice growth physics. Although cooperative effects with other isoforms are important, the elucidation of these effects will be our next challenge in the future after the clarification of the nature of fcIBP11. The protein is characterized by the domain classified in the Pfam database as DUF3494 and has a molecular mass of 26 kDa. fcIBP11 was recombinantly expressed (EMBL Heidelberg) as described in a previous publication (18), whereby the protein solution was diluted and dialyzed in ultrapure water with a resistivity of 18 MΩ·cm. The use of pure water was chosen to focus on the effect of fcIBP11 on ice, excluding the possible influence of salts on ice crystal growth. Previous experiments had indicated that, at the protein concentrations considered here, the activity of fcIBP11 is not significantly affected by the absence of a buffer (SI Appendix, SI Text). The concentration of the fcIBP11 solution was limited by the low solubility of the protein in pure water and did not exceed 3 μM (80 μg/mL). For fluorescence observations, we used a fusion protein created by recombinantly joining the gene of a fluorescent protein [monomeric Kusabira Orange (mKO)] to the fcIBP11 gene. The mKO gene was placed at the 5′ end of the IBP sequence (GenScript and EMBL Heidelberg). The fusion protein mKO-fcIBP11 (molecular mass, 50 kDa) was expressed following the protocol for fcIBP11 (EMBL Heidelberg), and the protein solution was diluted and dialyzed in ultrapure water. Working solutions had a concentration of 1.6 μM (80 μg/mL). The fluorescent mKO protein has an excitation maximum at 548 nm (optimal for excitation with a green laser light) while emitting bright orange light at 559 nm.
Ice Growth Cell.
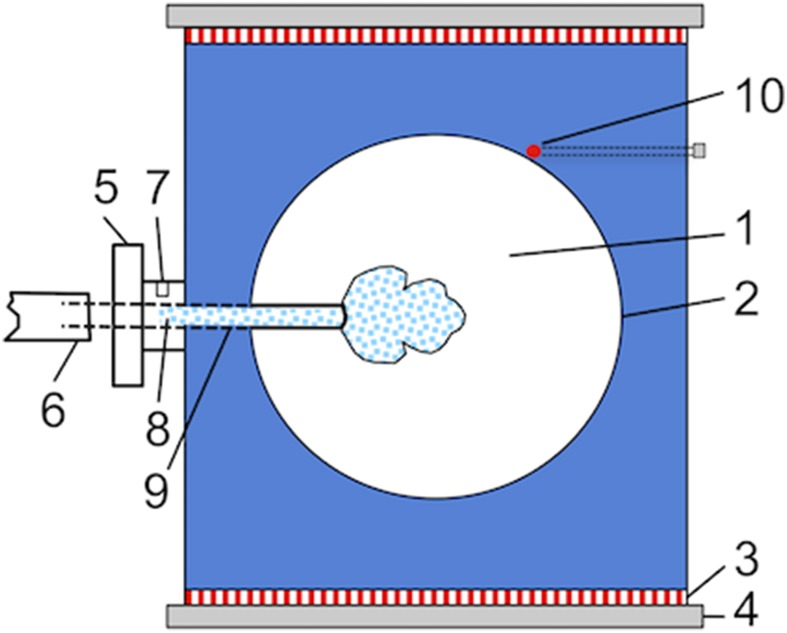
We used the same ice growth cell in all measurements in the case of morphology and growth rates determination as well as for fluorescence microscopy. Ice single crystals were grown in a homemade free growth cell filled with the protein solution (Fig. 1). The details of the cell have been explained in detail elsewhere (25). The cell’s inner chamber (Fig. 1, 1) was filled with 1 mL of fcIBP11 solution set to a constant temperature with an accuracy of ±0.02 °C using a thermistor (Fig. 1, 10) and two Peltier elements (Fig. 1, 3), one at the top and the other at the bottom of the cell. A thin glass capillary, also filled with the solution (Fig. 1, 9), protruded from outside of the cell into its inner chamber. Rapid freezing was initiated by the injection of a cold spray at the inlet (Fig. 1, 7) to induce the nucleation of ice crystals inside the capillary (Fig. 1, 8). After the formation of many microcrystals, usually only one single crystal reached the tip of the capillary due to geometrical selection and thereafter, was able to expand freely within the supercooled solution inside the chamber. The crystal orientation was adjusted for optimal visualization by rotating the capillary holder (Fig. 1, 5); an example is shown in SI Appendix, SI Text. The experiment was halted when growing ice reached the walls of the inner chamber. This moment was determined by monitoring for a temperature increase due to the release of latent heat. The temperature was then set to 5 °C until all ice was melted. At least three runs were made for each experimental condition.
Fig. 1.
Schematic drawing of the free growth cell with an ice single crystal (highlighted by blue dots) growing from the solution. Details of the setup are given by the inner growth chamber (1) filled with the protein solution, triple-glass windows (2), Peltier elements (3), heat sink (4) with circulating cooling water, capillary holder (5), tube filled with solution (6), inlet for cold shock application (7), the seed crystal formation area (8) located in the glass capillary (9), and the thermistor (10).
Morphology and Growth Rates.
To determine the morphology of ice single crystals as well as ice growth rates along the a and c axes, the free growth cell was set in a microscopic device that combined ordinary bright-field microscopy with Mach–Zehnder interferometry.
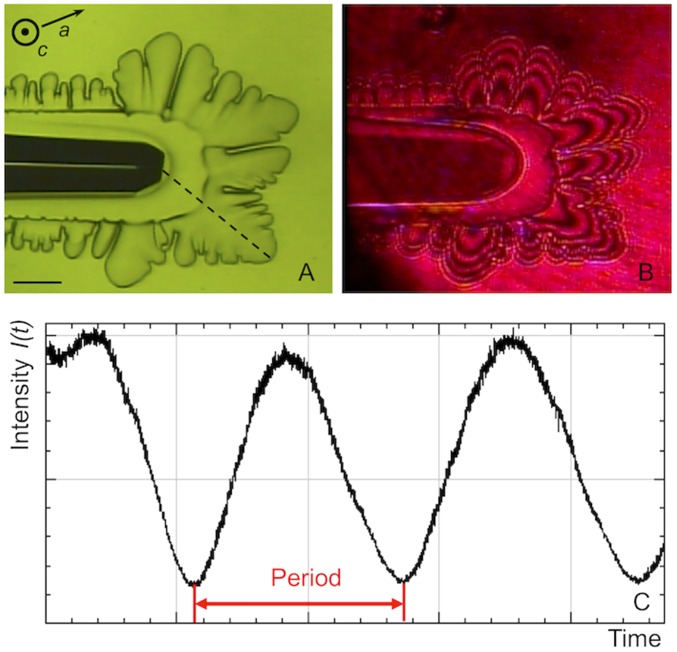
Bright-field microscopy was applied to determine the growth rates along the a axis by measuring the displacement of the dendrite’s tips over time (Fig. 2A) as described previously by Vorontsov et al. (26). The growth rates of the basal faces growing in the c direction were measured by Mach–Zehnder interferometry. We used a red laser beam of 670-nm wavelength and 5-mW power. The laser beam, which passed through the sample mostly perpendicular to the basal face, created interference patterns recorded by a CCD camera (Fig. 2B). The changes of the interference pattern with time were directly related to changes in ice thickness ∆h along the c axis. The time-dependent changes in signal intensity I(t) (Fig. 2C), measured at selected points on the basal face of the crystal, were analyzed to determine ∆h as follows:
Fig. 2.
An ice single crystal visualized simultaneously by bright-field microscopy (A) and Mach–Zehnder interferometry (B). The typical interference fringes are visible in B. The direction of observation is perpendicular to the basal face. The movement of the fringes over time is detected with the variation of signal intensity I(t) measured at selected points on the basal face (C). One period equals the time between the passage of two subsequent fringes and corresponds to a change in ice crystal thickness of 26.9 μm. (Scale bar: 200 μm.)
where λ is the laser wavelength and Nw = 1.3327 and Ni = 1.3078 are the refractive indices of water and ice, respectively, at temperature T = 0 °C. The change of the refractive indices in the temperature range analyzed here is negligible. Thus, the change in intensity I(t) for one period between the passage of the fringes corresponds to a change in crystal thickness ∆h = 26.9 μm. The growth rate in c direction Rc could be determined as Rc = ∆h/(2∆t) under the assumption that ∆h was due in equal parts to changes on the two opposite basal faces of the crystal.
We performed experiments in a range of supercooling ∆T between 0 °C and 0.5 °C, where ∆T = Tm − T with ice melting temperature Tm = 0 °C. We used fcIBP11 concentrations of 0.30, 1.5, and 3.0 μM, corresponding to 8.0, 40, and 80 μg/mL, respectively.
Fluorescence Microscopy.
The protein localization at the ice–liquid interfaces in 1.5 μM mKO-fcIBP11 solutions was visualized by laser confocal fluorescence microscopy. The same free growth chamber as described above was used, applying supercooling between 0.1 °C and 0.2 °C. At first, the growth of an ice single crystal was monitored in the absence of laser light to avoid early photobleaching of mKO-fcIBP11 molecules. As soon as the crystal inside the capillary reached the tip, the green laser light with wavelength of 532 nm was turned on, and the fluorescence images were recorded. The signal intensity of the fluorescence images, which was directly proportional to the local concentration of fluorescent molecules, was analyzed along selected transects using tools of image analysis.
Results
Morphology of Ice Crystals.
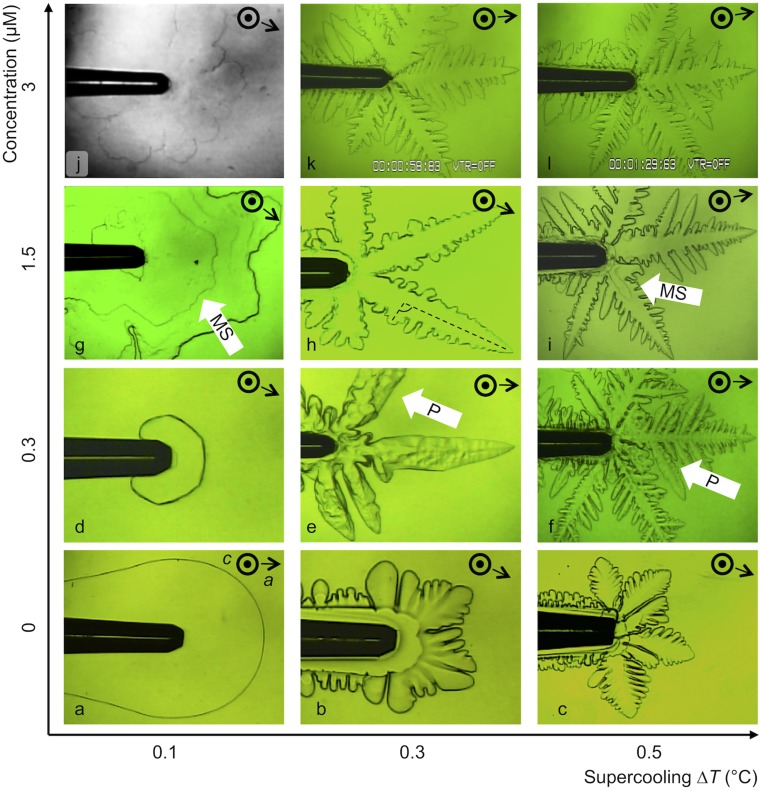
Ice single crystals in pure water at low supercooling (Fig. 3A) grew as circular plates. The two basal faces of the ice crystal (i.e., those parallel to the observation plane) appeared flat and even. At higher supercoolings (0.3 °C to 0.5 °C), rounded-tip dendritic structures grew in direction of the a axis.
Fig. 3.
Different morphologies of ice single crystals observed by bright-field microscopy. Crystals were growing at fcIBP11 concentrations of 0 μM (A–C), 0.3 μM (D–F), 1.5 μM (G–I) and 3 μM (J–L). Pictures were chosen as representative of the morphology at each experimental condition. White arrows point exemplarily to special features, like macrosteps (MSs) and pits (Ps). The outer diameter of the capillary tips corresponds to 200 μm. The captions in K and L show standard settings of the microscopic device and are of no relevance for these measurements.
The morphology of the crystals was clearly affected by fcIBP11 (Fig. 3 D–L). At low supercooling (∆T = 0.1 °C), the crystals became faceted and appeared as hexagonal plates at 0.3 μM (Fig. 3D). Increases in fcIBP11 concentration up to 1.5 and 3 μM led to the formation of faceted stellar (Fig. 3G) and daisy-like (Fig. 3J) crystal shapes, respectively. At higher supercooling, the faceted shapes developed into dendritic morphologies. These dendrite branches were finer and sometimes skeletal, and their tips appeared less rounded than those residing in pure water. At 1.5 μM and 0.3 °C supercooling, the angles between primary and secondary dendrites seemed to randomly deviate from the regular 60° (Fig. 3H, highlighted by dashed lines). The thickness of the crystals along the c direction became smaller with increasing protein concentration, such that, in some cases, it could barely be detected (Fig. 3J).
The fcIBP11 molecules caused the formation of macrosteps, which appeared at irregular time intervals and were located randomly on the basal face. The lateral surface of the crystal, perpendicular to the basal face, was therefore characterized by a visible alternation of flat terraces and macrosteps. Irregularities in the form of pits on the basal face were overgrown by new layers of ice.
Growth Rates of Ice Crystals.
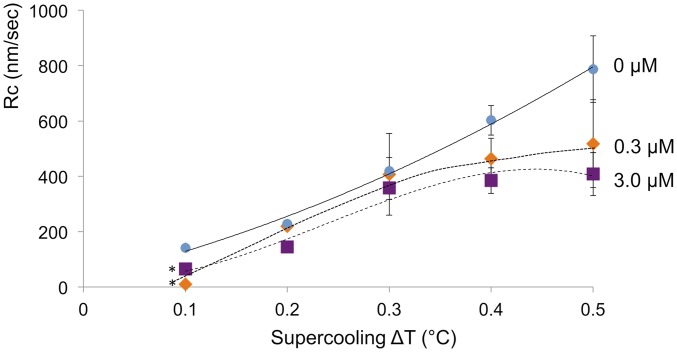
The growth rates Rc and Ra of ice crystals in the c and a directions changed depending on supercooling and fcIBP11 concentration (Figs. 4 and 5).
Fig. 4.
Growth rates Rc of ice crystals in the c direction as a function of supercooling at fcIBP11 concentrations of 0, 0.3, and 3.0 μM. The data represent the average of sample size n = 3, except where indicated. Error bars represent the SD. Trend lines were drawn by hand; *, n = 2.
Fig. 5.
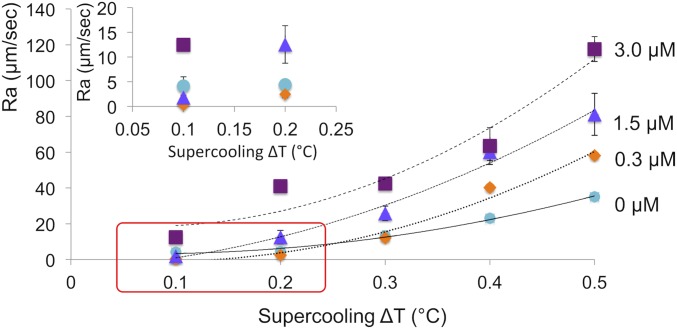
Growth rates Ra of ice crystals in the a direction as a function of supercooling at fcIBP11 concentrations of 0, 0.3, 1.5, and 3.0 μM. The data represent the average of sample size n = 3. The error bars represent the SD. Trend lines were drawn by hand. Inset shows a magnification of the area marked by the red rectangle.
fcIBP11 inhibited the growth of the basal face along the c axis (Fig. 4). The suppression effect was more evident at ∆T > 0.3 °C, when the slope of the growth rate curve decreased dramatically with increasing fcIBP11 concentration. Inhibition increased with increasing fcIBP11 concentration and is, therefore, consistent with the morphological observations of growth suppression along the c axis described above (Fig. 3 D, G, and J). The growth of ice in the a direction was suppressed at low supercoolings (∆T = 0.1 °C to 0.3 °C and ∆T = 0.1 °C in 0.3 and 1.5 μM fcIBP11 solutions, respectively) (Fig. 5). When supercooling exceeded these values, crystal growth along the a axis became faster than in pure water. In contrast, the proteins enhanced ice growth in the a direction irrespective of the supercooling at fcIBP11 concentration of 3 μM.
Fluorescence Analysis.
Laser confocal fluorescence microscopy showed the localization of the mKO-fcIBP11 molecules on different faces of an ice crystal. Before observation by fluorescence microscopy, we tested mKO-fcIBP11 using bright-field microscopy and confirmed that it caused the formation of similar ice crystal shapes like fcIBP11, indicating that fcIBP11 and mKO-fcIBP11 have comparable affinities for ice crystal faces.
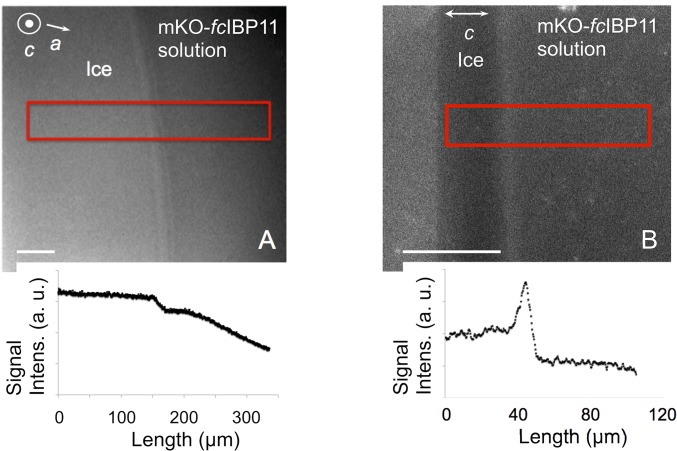
Depicted in Fig. 6 are the laser confocal fluorescence microscopy images of growing ice crystals. The basal face was located in the image plane and in the focal plane (Fig. 6A), and as one crystal was tilted by 90°, the basal face was oriented perpendicular to the image plane, with the focal plane set inside the crystal (Fig. 6B). Fig. 6, Lower represents the fluorescence signal intensities measured in the area marked by red rectangles in Fig. 6, Upper. Fluorescence intensity on the basal face was significantly higher than that in the mKO-fcIBP11 solution surrounding the ice crystal, which suggests that the mKO-fcIBP11 molecules accumulated on the basal face. The fluorescence inside the crystal was lower than at the ice–liquid interfaces or in the bulk solution (Fig. 6B).
Fig. 6.
Fluorescence confocal microscopy images of a growing ice crystal in an mKO-fcIBP11 solution (1.5 μM). The basal face is oriented parallel (A) and perpendicular (B) to the observation plane. Lower shows the fluorescence intensities measured in the area marked by the red rectangles. (Scale bar: 50 μm.)
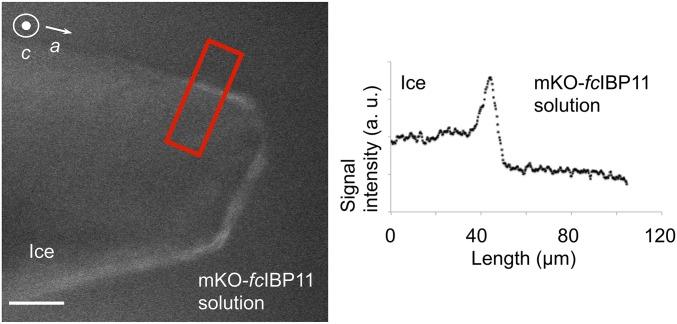
Finally, we noted that, when the ice crystal growth was stopped and the crystal was neither growing nor melting (Fig. 7), mKO-fcIBP11 also became visible on the prism faces, thereby indicating protein adsorption over these faces.
Fig. 7.
Fluorescence confocal microscopy image of an ice single crystal in an mKO-fcIBP11 solution (1.5 μM) when ice crystal growth was halted. Right shows the fluorescence signal intensity measured in the area marked by the red rectangle. (Scale bar: 50 μm.)
Discussion
Growth Kinetics of Ice Crystals.
We show that the morphology of ice crystals strongly depends on supercooling and fcIBP11 concentration.
The shapes of the ice crystals observed in pure water are typical for crystals growing from their melt (27). At low supercooling values (∆T = 0.1 °C), the ice crystal has a plate-like shape defined by two flat basal faces and a rounded lateral surface (Fig. 3A). With increasing supercooling, a dendritic structure develops on the prism faces due to the morphological instability driven by the diffusion of latent heat. Under these conditions, the basal face is known to be molecularly smooth and to grow layer by layer, exhibiting a much slower growth rate than the rough lateral faces. The growth rates in the a and c directions determined in this study agree well with the data previously reported (28).
fcIBP11 significantly inhibits the growth rate Rc of the basal faces in the whole ranges of supercooling and fcIBP11 concentrations examined in this study as shown in Fig. 4. This result strongly suggests that fcIBP11 molecules adsorb on the basal faces. The appearance of macrosteps (Fig. 3 G and I) and pits (Fig. 3 E and F) observed on the basal faces can be explained by the retardation of the lateral movement of elementary steps by adsorbed fcIBP11 molecules. An example of the process of the formation of macrosteps in a solution with impurities can be found in Land et al. (29). Note that the decrease in the growth rate of the basal faces with increasing fcIBP11 concentration decreases the amount of latent heat generation, having the effect of increasing the supercooling over the lateral faces.
The effects of fcIBP11 on the growth of the prism faces exhibit more complicated results than those on the basal faces (Figs. 3 and 5). When the fcIBP11 concentration is low (0.3 µM), the crystal has a hexagonal shape (Fig. 3D) at a supercooling of 0.1 °C. The growth rate Ra is lower than in pure water. This result clearly shows that fcIBP11 molecules adsorb on the prism faces and suppress their growth. In contrast, at higher supercooling (ΔT ≥ 0.3 °C), the ice crystals exhibit a dendritic shape induced by morphological instability (Fig. 3 E and F), and Ra is accelerated beyond that of pure water. When the fcIBP11 concentration is higher (1.5 and 3.0 µM), the ice crystals exhibit a dendritic shape bounded by facets (prism faces), as evident in Fig. 3 G and J, and accelerated growth rates Ra, even at ΔT < 0.3 °C. This phenomenon can be explained by taking into account the following two processes: morphological changes of the ice crystal by fcIBP11 and the release of latent heat by the growing ice crystal. The mass growth rate of an ice crystal is determined by the balance between the production of latent heat and its dissipation. The tips of the dendritic crystal branches become sharper with increasing protein concentration, as shown in Fig. 3, due to the fact that the growth of the lateral faces of the dendrite branches, which correspond to the prism faces adsorbed by fcIBP11, is suppressed. Consequently, the linear growth rate at the dendrite tip may be increased to compensate for the depression of latent heat release that occurs by the reduced growth of the lateral faces. Since the growth rate Rc of the basal faces was also reduced by attachment of fcIBP11, the growth rate Ra at the dendrites tips may be further increased. We conclude that the formation of facetted dendrites observed in the supercooled solution of fcIBP11 is caused by the anisotropic adsorption of this protein on the crystal surface.
The attachment of fcIBP11 to the basal and prism faces was further confirmed by laser confocal fluorescence microscopy. Fig. 6 shows an accumulation of fluorescent protein on the basal face of the ice crystal, which can be caused by different processes such as strong and permanent protein attachment to the basal plane, loose and reversible attachment, or rejection of the protein from the growing ice surface. However, we still detect a fluorescence signal on the basal face in Fig. 7, which shows a crystal that is not growing. Therefore, we can conclude that the signal is not caused by rejected mKO-fcIBP11, since the molecules would have diffused away in the absence of crystal growth, not resulting in any specific signal over the basal face. Instead, our results indicate attachment of mKO-fcIBP11 or at least of a part of it to the basal face. With regard to the prism faces, when the ice crystal is growing (Fig. 6), exposure time of the ice surface to the protein solution is relatively short, and mKO-fcIBP11 molecules cannot accumulate on the surface of the prism faces in an amount significant enough to produce a detectable fluorescence signal. However, when crystal growth is halted (and hence, exposure time increased) (Fig. 7), we see mKO-fcIBP11 accumulation on the prism faces. We, therefore, conclude that the fluorescence signal is a result of mKO-fcIBP11 molecules attaching to the ice surface.
The absence of a fluorescence signal from inside the ice crystal (Fig. 6B) can be explained by a low concentration of mKO-fcIBP11 or by a conformation change of the mKO protein caused by its contact with ice. Both cases are conceivable. IBPs have been shown to be attached to ice strongly and irreversibly (30–32), resulting in inclusion within the ice, while other IBPs display a reversible binding mode with detachment of the protein from the crystal surface on ice growth (33, 34). Also, fluorescence can react in different ways to inclusion inside a crystal and can be either maintained (35) or lost (36). After attachment to ice by fcIBP11, a destabilization of the mKO protein engulfed in the crystal can easily result in a reversible or irreversible change of its fluorescent properties. Further studies are needed to elucidate whether mKO-fcIBP11 is included in the ice crystal.
The inhibition influence of fcIBP11 can be described by the classical Cabrera–Vermilyea model (37) or similar pinning models (38, 39). The proteins adsorbed on the crystal become obstacles for step and surfaces, and the curvature of the ice surface locally increases where the flow percolates through pinned fcIBP11 molecules. Step flow and surface growth are reduced and eventually stop when the curvature reaches a critical radius as described by the Gibbs–Thomson equation. The adsorbed fcIBP11 molecules act as inhibitors to the growth of the basal and prism surfaces at low supercoolings (between 0.1° °C and 0.5 °C) (Figs. 4 and 5). In the case of the prism faces, this results in the formation of a ragged surface (instead of rounded interfaces typically observed in pure water) and in polygonized and sharp tips of the dendrites.
Note that some previous studies (28, 40) reported the possibility that IBPs may decrease the free energies of ice–water interfaces, resulting in faster growth kinetics and more pronounced morphological instability. In this study, the effect of fcIBP11 on the free energy of ice–water interfaces remains uncertain.
A Moderate IBP with Affinity to the Basal Face.
IBPs have different effects on the growth of the basal face (an overview is presented in SI Appendix, Table S2). Detailed crystal growth analyses were carried out with fish AFGP7-8 (41) and fish AFP III (28). Although attachment of AFGPs to the basal face has been reported (42), results of growth analyses indicate that AFGPs and AFP III enhance crystal growth along the c axis, whereas fcIBP11 presented here has the opposite effect. The promotion of basal face growth is also displayed by other moderate fish IBPs as shown by morphological observations carried out with the smallest ice crystals observed by light microscopy (43, 44). However, hyperactive IBPs suppress the growth along the c axis, and growing crystals display a planar dendritic pattern (2). The DUF3494 IBP family is composed of several proteins: some moderate and others hyperactive. Different activities are sometimes displayed by the various isoforms of the same organism, despite high structural similarity (23). However, irrespective of the activity of the protein, ice crystals in the presence of the wide majority of DUF3494 IBPs grow preferentially along the a axis (11, 15, 17, 45, 46), and a similar behavior has been described for the LpIBP (21). In the field of IBPs, it has been previously assumed that moderate IBPs adsorb only on the lateral surface of the crystals and that hyperactive IBPs adsorb also on the basal faces. The ability to suppress or restrict crystal growth of the basal face has been repeatedly mentioned as the basis for hyperactivity (1, 3, 45, 47–49). Our study clearly shows that fcIBP11 adsorbs on both basal and prism faces of ice crystals and thereby, inhibits growth along the c axis, similar to hyperactive IBPs. However, fcIBP11 is characterized by a moderate TH activity, similar to that of several DUF3494 IBPs (5, 11, 17, 18, 50). Hence, we can conclude that fcIBP11 (and presumably, several other DUF3494 IBPs) should occupy a separate position in the wide spectrum of IBPs. We show that the suppression of growth of the basal face is necessary, but not sufficient, for hyperactivity. A few structural studies have highlighted the role of hydrophobicity of the IBS of DUF3494 IBPs and the relevance of its geometrical match to the ice surface for conferring hyperactivity to the proteins (10, 23, 45). We suggest that the binding of fcIBP11 on ice crystal surfaces is much weaker than that of hyperactive IBPs (i.e., the binding energy of IBPs on ice crystal surfaces plays an important role in exhibiting hyperactive TH activity).
The natural environment of fcIBP11 is within sea ice brine inclusions, and the icy surface of these inclusions is dominated by the basal face (51). It seems, therefore, of crucial relevance that fcIBP11 can attach to these planes (SI Appendix, SI Text). It is conceivable that fcIBP11s, secreted by the diatom cells, bind to the surface of the ice crystals enclosing the brine and affect their growth and morphology, increasing the habitability of the ice and possibly altering the physical properties and biogeochemical imprint (52, 53).
Conclusions
We have observed that fcIBP11 induces significant modifications to the shapes of both bulk ice crystals and dendrites. Furthermore, fcIBP11 also affects the crystal growth rates Ra and Rc. This is manifested as reduced growth rates Rc with increasing protein concentration and supercooling and as faceted ice crystals with reduced ice growth rates Ra in the a direction or dendritic forms with increased Ra, depending on the protein concentration and on supercooling. We conclude that fcIBP11 molecules attach to both the basal and prism faces as supported by microscopy observations with fluorescent mKO-fcIBP11 molecules. The protein’s effect on crystal growth rates Ra can be explained by the morphological changes induced by fcIBP11 and their resulting effects on the balance between the production and the dissipation of latent heat. We speculate that the affinity of fcIBP11 for multiple crystal faces, including the basal face, is of crucial importance for survival within sea ice brine (where the basal face is dominant at the interface between ice and brine) and that the proteins actively shape the structure of their icy habitat. Finally, our observations clearly indicate that the basal plane affinity and reduction of growth of the basal face are not sufficient to explain the hyperactivity of IBPs.
Supplementary Material
Acknowledgments
We acknowledge support from Deutsche Forschungsgemeinschaft SPP1158 (German Research Association Special Program 1158) Grant BA 3694/2-1, Japan Society for the Promotion of Science Invitational Fellowships PE16746 and L17515 and Kakenhi Grant 16K13672, and Hokkaido University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807461115/-/DCSupplemental.
References
- 1.Bar Dolev M, Braslavsky I, Davies PL. Ice-binding proteins and their function. Annu Rev Biochem. 2016;85:515–542. doi: 10.1146/annurev-biochem-060815-014546. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Dolev M, Celik Y, Wettlaufer JS, Davies PL, Braslavsky I. New insights into ice growth and melting modifications by antifreeze proteins. J R Soc Interface. 2012;9:3249–3259. doi: 10.1098/rsif.2012.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scotter AJ, et al. The basis for hyperactivity of antifreeze proteins. Cryobiology. 2006;53:229–239. doi: 10.1016/j.cryobiol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Pertaya N, Marshall CB, Celik Y, Davies PL, Braslavsky I. Direct visualization of spruce budworm antifreeze protein interacting with ice crystals: Basal plane affinity confers hyperactivity. Biophys J. 2008;95:333–341. doi: 10.1529/biophysj.107.125328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoshino T, et al. Antifreeze proteins from snow mold fungi. Can J Bot. 2003;81:1175–1181. [Google Scholar]
- 6.Janech MG, Krell A, Mock T, Kang J-S, Raymond JA. Ice-binding proteins from sea ice diatoms (Bacillariophyceae) J Phycol. 2006;42:410–416. [Google Scholar]
- 7.Sorhannus U. Evolution of antifreeze protein genes in the diatom genus fragilariopsis: Evidence for horizontal gene transfer, gene duplication and episodic diversifying selection. Evol Bioinform Online. 2011;7:279–289. doi: 10.4137/EBO.S8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond JA, Kim HJ. Possible role of horizontal gene transfer in the colonization of sea ice by algae. PLoS One. 2012;7:e35968. doi: 10.1371/journal.pone.0035968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raymond JA, Fritsen C, Shen K. An ice-binding protein from an Antarctic sea ice bacterium. FEMS Microbiol Ecol. 2007;61:214–221. doi: 10.1111/j.1574-6941.2007.00345.x. [DOI] [PubMed] [Google Scholar]
- 10.Do H, Kim S-J, Kim HJ, Lee JH. Structure-based characterization and antifreeze properties of a hyperactive ice-binding protein from the Antarctic bacterium Flavobacterium frigoris PS1. Acta Crystallogr D Biol Crystallogr. 2014;70:1061–1073. doi: 10.1107/S1399004714000996. [DOI] [PubMed] [Google Scholar]
- 11.Mangiagalli M, et al. Cryo-protective effect of an ice-binding protein derived from Antarctic bacteria. FEBS J. 2017;284:163–177, and erratum (2017) 284:831. doi: 10.1111/febs.13965. [DOI] [PubMed] [Google Scholar]
- 12.Bayer-Giraldi M, Uhlig C, John U, Mock T, Valentin K. Antifreeze proteins in polar sea ice diatoms: Diversity and gene expression in the genus Fragilariopsis. Environ Microbiol. 2010;12:1041–1052. doi: 10.1111/j.1462-2920.2009.02149.x. [DOI] [PubMed] [Google Scholar]
- 13.Gwak IG, Jung WS, Kim HJ, Kang SH, Jin E. Antifreeze protein in Antarctic marine diatom, Chaetoceros neogracile. Mar Biotechnol (NY) 2010;12:630–639. doi: 10.1007/s10126-009-9250-x. [DOI] [PubMed] [Google Scholar]
- 14.Raymond JA, Janech MG. Ice-binding proteins from enoki and shiitake mushrooms. Cryobiology. 2009;58:151–156. doi: 10.1016/j.cryobiol.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Xiao N, et al. Comparison of functional properties of two fungal antifreeze proteins from Antarctomyces psychrotrophicus and Typhula ishikariensis. FEBS J. 2010;277:394–403. doi: 10.1111/j.1742-4658.2009.07490.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee JK, et al. An extracellular ice-binding glycoprotein from an Arctic psychrophilic yeast. Cryobiology. 2010;60:222–228. doi: 10.1016/j.cryobiol.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Hashim NHF, et al. Characterization of Afp1, an antifreeze protein from the psychrophilic yeast Glaciozyma antarctica PI12. Extremophiles. 2013;17:63–73. doi: 10.1007/s00792-012-0494-4. [DOI] [PubMed] [Google Scholar]
- 18.Bayer-Giraldi M, Weikusat I, Besir H, Dieckmann G. Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryobiology. 2011;63:210–219. doi: 10.1016/j.cryobiol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Kondo H, et al. Ice-binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc Natl Acad Sci USA. 2012;109:9360–9365. doi: 10.1073/pnas.1121607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KS, et al. Characterization of the ice-binding protein from Arctic yeast Leucosporidium sp. AY30. Cryobiology. 2012;64:286–296. doi: 10.1016/j.cryobiol.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Middleton AJ, et al. Antifreeze protein from freeze-tolerant grass has a beta-roll fold with an irregularly structured ice-binding site. J Mol Biol. 2012;416:713–724. doi: 10.1016/j.jmb.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 22.Xiao N, et al. Annealing condition influences thermal hysteresis of fungal type ice-binding proteins. Cryobiology. 2014;68:159–161. doi: 10.1016/j.cryobiol.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 23.Cheng J, Hanada Y, Miura A, Tsuda S, Kondo H. Hydrophobic ice-binding sites confer hyperactivity of an antifreeze protein from a snow mold fungus. Biochem J. 2016;473:4011–4026. doi: 10.1042/BCJ20160543. [DOI] [PubMed] [Google Scholar]
- 24.Takamichi M, Nishimiya Y, Miura A, Tsuda S. Effect of annealing time of an ice crystal on the activity of type III antifreeze protein. FEBS J. 2007;274:6469–6476. doi: 10.1111/j.1742-4658.2007.06164.x. [DOI] [PubMed] [Google Scholar]
- 25.Zepeda S, Nakatsubo S, Furukawa Y. Apparatus for single ice crystal growth from the melt. Rev Sci Instrum. 2009;80:115102. doi: 10.1063/1.3222739. [DOI] [PubMed] [Google Scholar]
- 26.Vorontsov DA, Sazaki G, Hyon S-H, Matsumura K, Furukawa Y. Antifreeze effect of carboxylated ε-poly-L-lysine on the growth kinetics of ice crystals. J Phys Chem B. 2014;118:10240–10249. doi: 10.1021/jp507697q. [DOI] [PubMed] [Google Scholar]
- 27.Jackson KA. Kinetic Processes: Crystal Growth, Diffusion, and Phase Transitions in Materials. Wiley-VCH Verlag GmbH & Co KGaA; Weinheim, Germany: 2010. [Google Scholar]
- 28.Vorontsov DA, et al. Growth of ice crystals in the presence of type III antifreeze protein. Cryst Growth Des. 2018;18:2563–2571. [Google Scholar]
- 29.Land TA, Martin TL, Potapenko S, Palmore GT, De Yoreo JJ. Recovery of surfaces from impurity poisoning during crystal growth. Nature. 1999;399:442–445. [Google Scholar]
- 30.Celik Y, et al. Microfluidic experiments reveal that antifreeze proteins bound to ice crystals suffice to prevent their growth. Proc Natl Acad Sci USA. 2013;110:1309–1314. doi: 10.1073/pnas.1213603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drori R, Davies PL, Braslavsky I. When are antifreeze proteins in solution essential for ice growth inhibition? Langmuir. 2015;31:5805–5811. doi: 10.1021/acs.langmuir.5b00345. [DOI] [PubMed] [Google Scholar]
- 32.Hudait A, Odendahl N, Qiu Y, Paesani F, Molinero V. Ice-nucleating and antifreeze proteins recognize ice through a diversity of anchored clathrate and ice-like motifs. J Am Chem Soc. 2018;140:4905–4912. doi: 10.1021/jacs.8b01246. [DOI] [PubMed] [Google Scholar]
- 33.Zepeda S, Yokoyama E, Uda Y, Katagiri C, Furukawa Y. In situ observation of antifreeze glycoprotein kinetics at the ice interface reveals a two-step reversible adsorption mechanism. Cryst Growth Des. 2008;8:3666–3672. [Google Scholar]
- 34.Mochizuki K, Molinero V. Antifreeze glycoproteins bind reversibly to ice via hydrophobic groups. J Am Chem Soc. 2018;140:4803–4811. doi: 10.1021/jacs.7b13630. [DOI] [PubMed] [Google Scholar]
- 35.Kurimoto M, et al. 2000. Intrasectoral zoning of proteins and nucleotides in simple crystalline hosts. Morphology and Dynamics of Crystal Surfaces in Complex Molecular Systems, MRS Proceedings, eds De Yoreo J, Casey W, Malkin A, Vlieg E, Ward M, Vol 620, p M9.8.1 Cambridge Univ Press, Cambridge, UK.
- 36.Bhatnagar BS, Bogner RH, Pikal MJ. Protein stability during freezing: Separation of stresses and mechanisms of protein stabilization. Pharm Dev Technol. 2007;12:505–523. doi: 10.1080/10837450701481157. [DOI] [PubMed] [Google Scholar]
- 37.Cabrera N, Vermilyea DA. The growth of crystals from solution. In: Doremus RH, Roberts BW, Turnbull D, editors. Growth and Perfection of Crystals. Wiley; New York: 1958. pp. 393–410. [Google Scholar]
- 38.Sander LM, Tkachenko AV. Kinetic pinning and biological antifreezes. Phys Rev Lett. 2004;93:128102. doi: 10.1103/PhysRevLett.93.128102. [DOI] [PubMed] [Google Scholar]
- 39.Kubota N. Effect of impurities on the growth kinetics of crystals. Cryst Res Technol. 2001;36:749–769. [Google Scholar]
- 40.Kutschan B, Morawetz K, Thoms S. Dynamical mechanism of antifreeze proteins to prevent ice growth. Phys Rev E Stat Nonlin Soft Matter Phys. 2014;90:022711. doi: 10.1103/PhysRevE.90.022711. [DOI] [PubMed] [Google Scholar]
- 41.Furukawa Y, et al. Oscillations and accelerations of ice crystal growth rates in microgravity in presence of antifreeze glycoprotein impurity in supercooled water. Sci Rep. 2017;7:43157. doi: 10.1038/srep43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson PW, Beaglehole D, Devries AL. Antifreeze glycopeptide adsorption on single crystal ice surfaces using ellipsometry. Biophys J. 1993;64:1878–1884. doi: 10.1016/S0006-3495(93)81559-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao H, DeLuca CI, Davies PL. Mixing antifreeze protein types changes ice crystal morphology without affecting antifreeze activity. FEBS Lett. 1995;357:183–186. doi: 10.1016/0014-5793(94)01357-7. [DOI] [PubMed] [Google Scholar]
- 44.Gauthier SY, et al. A re-evaluation of the role of type IV antifreeze protein. Cryobiology. 2008;57:292–296. doi: 10.1016/j.cryobiol.2008.10.122. [DOI] [PubMed] [Google Scholar]
- 45.Hanada Y, Nishimiya Y, Miura A, Tsuda S, Kondo H. Hyperactive antifreeze protein from an Antarctic sea ice bacterium Colwellia sp. has a compound ice-binding site without repetitive sequences. FEBS J. 2014;281:3576–3590. doi: 10.1111/febs.12878. [DOI] [PubMed] [Google Scholar]
- 46.Kim M, Gwak Y, Jung W, Jin E. Identification and characterization of an isoform antifreeze protein from the Antarctic marine diatom, Chaetoceros neogracile and suggestion of the core region. Mar Drugs. 2017;15:E318. doi: 10.3390/md15100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Graether SP, et al. Beta-helix structure and ice-binding properties of a hyperactive antifreeze protein from an insect. Nature. 2000;406:325–328. doi: 10.1038/35018610. [DOI] [PubMed] [Google Scholar]
- 48.Jia Z, Davies PL. Antifreeze proteins: An unusual receptor-ligand interaction. Trends Biochem Sci. 2002;27:101–106. doi: 10.1016/s0968-0004(01)02028-x. [DOI] [PubMed] [Google Scholar]
- 49.Davies PL. Ice-binding proteins: A remarkable diversity of structures for stopping and starting ice growth. Trends Biochem Sci. 2014;39:548–555. doi: 10.1016/j.tibs.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, et al. Structural basis for antifreeze activity of ice-binding protein from arctic yeast. J Biol Chem. 2012;287:11460–11468. doi: 10.1074/jbc.M111.331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas DN, Dieckmann GS. Sea Ice. 2nd Ed Wiley-Blackwell; Hoboken, NJ: 2010. [Google Scholar]
- 52.Raymond JA. Algal ice-binding proteins change the structure of sea ice. Proc Natl Acad Sci USA. 2011;108:E198. doi: 10.1073/pnas.1106288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krembs C, Eicken H, Deming JW. Exopolymer alteration of physical properties of sea ice and implications for ice habitability and biogeochemistry in a warmer Arctic. Proc Natl Acad Sci USA. 2011;108:3653–3658. doi: 10.1073/pnas.1100701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.