Significance
The domestication of horses and adoption of horse riding were critical processes that culminated in the emergence of mounted warriors and nomadic empires that shaped world history. The constraints of horse biology and riding equipment meant that equine veterinary care, particularly of teeth, was a core component of the success of the human–horse relationship. We report the earliest evidence of equine dentistry, from the Mongolian Steppe, at 1150 BCE. Key shifts in equine dentistry practice through time can be linked first to the emergence of horseback riding and later to the use of metal bits that enabled better control of horses. The maintenance of horse health through dentistry underwrote the key role of horses in cultures and economies around the world.
Keywords: horses, veterinary care, horseback riding, domestication, Mongolia
Abstract
From the American West to the steppes of Eurasia, the domestic horse transformed human societies, providing rapid transport, communication, and military power, and serving as an important subsistence animal. Because of the importance of oral equipment for horse riding, dentistry is an essential component of modern horse care. In the open grasslands of northeast Asia, horses remain the primary form of transport for many herders. Although free-range grazing on gritty forage mitigates many equine dental issues, contemporary Mongolian horsemen nonetheless practice some forms of dentistry, including the removal of problematic deciduous teeth and the vestigial first premolar (“wolf tooth”). Here, we present archaezoological data from equine skeletal remains spanning the past 3,200 y, indicating that nomadic dental practices have great antiquity. Anthropogenic modifications to malerupted deciduous central incisors in young horses from the Late Bronze Age demonstrate their attempted removal, coinciding with the local innovation or adoption of horseback riding and the florescence of Mongolian pastoral society. Horse specimens from this period show no evidence of first premolar removal, which we first identify in specimens dating to ca. 750 BCE. The onset of premolar extraction parallels the archaeological appearance of jointed bronze and iron bits, suggesting that this technological shift prompted innovations in dentistry that improved horse health and horse control. These discoveries provide the earliest directly dated evidence for veterinary dentistry, and suggest that innovations in equine care by nomadic peoples ca. 1150 BCE enabled the use of horses for increasingly sophisticated mounted riding and warfare.
Perhaps no single domestic animal has had a more recognizable effect on human history than the horse (Equus caballus). First domesticated in the steppes of northern Kazakhstan and southern Russia ca. 3500 BCE (1), by the 20th century, horses had prompted important changes to human patterns of interaction, exchange, and social organization on every continent except Antarctica (2). The history of Mongolia is intertwined especially closely with the history of horse transport. In contemporary Mongolia, domestic horses are essential to herding life, serving not only as the primary means of transportation but also as an important livestock animal, providing milk and dairy products in the summer and early fall and meat for the late fall and winter months (3). Although infamously associated with the conquests of Genghis Khan, horses and horse cavalry also underwrote the success of several northeast Asian nomadic empires over the past 2,500 y (4), including those of the Xiongnu (ca. late third century BCE through second century CE) and Turkic Khaganate (ca. sixth through eighth centuries CE). In fact, horses appear to have been used for mounted riding in the steppes of modern Mongolia since at least ca. 1200 BCE, as evidenced by ritual horse burials of the deer stone–khirigsuur (DSK) complex.
Deer stones are anthropomorphic standing stones, often found in association with khirigsuurs, large stone mounds that often contain human burials. Both kinds of monument date to the Late Bronze Age, ca. 1300–700 BCE, and are most distinctively characterized by large numbers of individual horse sacrifice mounds (SI Appendix, Fig. S1), sometimes numbering in the hundreds or thousands (5, 6). Precision chronology modeling demonstrates that horse sacrifice spread rapidly through DSK ritual across a wide region of eastern Eurasia, ca. 1200 BCE (7). Diagnostic changes to the equine skull indicate that DSK horses were heavily exerted and used for transport (8), while anthropogenic deformations to the nasal bones of the horse, caused by a bridle, are distinctively left-biased (SI Appendix, Fig. S2), a pattern that appears best explained by mounted horseback riding rather than chariots or cart use (9). This osteological evidence for horse riding coincides with the first clear evidence for mobile, horse-based pastoralism, suggesting a link between changes in horse transport and the intensification of nomadic herding in the Eastern Steppes (7, 10).
Equine Veterinary Care in Antiquity
The breeding, supply, and maintenance of healthy horses underwrote many of the biggest sociopolitical developments in ancient Eurasia over the past several millennia. Syrian texts from the Hittite Empire, dating to the 14th century BCE, describe the proper feeding of chariot horses and treatment of key ailments (11). In China, domestic horses first appeared during the end of the Shang Dynasty, ca. 1200 BCE (12). Following their introduction to the region, horses became the basis for long-distance communication and transport, as well as military power, within only a few hundred years (13). In later centuries, the total number of horses used in the Chinese postal relay system alone would number up to 200,000, but the supply of horses was controlled by the same steppe “barbarians” who used them to raid and destroy (14), and sold them to Chinese buyers at great expense (13). Without the ability to sustainably breed them in large numbers, the appropriate health care of these animals was paramount. As early as the first millennium BCE, Chinese states thus provided formal veterinary care for horses (15). One of the earliest equine veterinary texts is credited to a Chinese author from the Spring and Autumn Period, ca. 770–476 BCE, and over subsequent centuries, veterinary care of horses played an increasingly formalized role in Chinese government bureaucracy and state infrastructure (15). Greek and Roman texts also indicate the development of specialized horse care in the classical world by the mid- to late first millennium BCE, and many of the most important early veterinary texts focused heavily on the care of horses and other equids (16, 17).
Despite the apparent significance of equine veterinary care in China and other ancient societies, the origins of equine dental care are poorly understood. Dental health is of systemic importance to the health of almost any animal (18), and some scholars hypothesize that it must have been practiced by early charioteers in western Asia and the Near East (11); however, very little physical or textual evidence exists to support inferences of equine dentistry during the Eneolithic or Bronze Age. Some archaeologists hypothesize that a strange wear pattern found on a horse from the site of Buhen, Egypt, dated to ca. 1675 BCE was produced by dental filing of the lower second premolar (19), although the most likely explanation of this damage is “bit wear,” damage caused directly to a tooth by a bit during use (20). The first definitive record of horse dentistry also comes from early Chinese veterinary texts dating to ca. 600 BCE, which describe the method of aging a horse through changes in its dentition (21, 22). During the Roman Empire, Aristotle and others made detailed mention of equine periodontal disease in their writings (21). By the Middle Ages, numerous Islamic texts refer directly to the practice of filing of sharp points in the animal’s mouth (22, 23), clear evidence that intentional modification of the teeth was practiced by this time. Nonetheless, examples of major uncorrected occlusal issues in archaeological equids are known even from this period (18, 24), indicating that equine dental care was far from ubiquitous. Because of the ambiguity of both historical records and putative dental modifications found on early archaeofaunal remains, it is difficult to reliably assess when the first equine dental care arose, or its relationship to broader developments in the domestication, spread, and riding of horses across Eurasia.
Contemporary Equine Dental Care
Horse teeth are subject to continuous wear that is replaced by tooth eruption throughout the animal’s life, which means that minor issues with posture and occlusion can cause chronic dental problems. Much of contemporary equine dental work in the United States and other Western countries is focused on correcting these occlusal issues, which can cause issues with feeding and behavior. A second major goal of horse dentistry centers on the extraction of deciduous teeth, which may erupt incorrectly as they are pushed out by permanent teeth, particularly the central incisors (X01 in the modified Triadan nomenclature system), which are susceptible to trauma, causing issues with behavior, feeding, or willingness to accept a bridle and bit (25). Of particular concern are horses that develop a “wolf tooth,” or first premolar. This vestigial tooth, which could have been part of either the permanent or deciduous ancestral dentition, may develop on the upper jaw, lower jaw, or both. It is situated anterior to the normal cheek row and serves no important function in mastication. It typically erupts during the horse’s first year of life, and it often falls out on its own by the time an animal reaches around 3 y of age. Because of its forward position in the mouth, it may interfere with the normal activity of modern bits and cause pain or tissue damage (26). As a consequence, it is standard practice for all horses to have wolf teeth removed at a young age, typically between 1 and 2 y of age (27). Beyond this, many horse dentists also recommend “flotation” of the upper and lower second premolars, a sometimes controversial practice alternatively referred to as a “bit seat.” Flotation is used commonly on race and competition horses, and involves filing off the anterior tooth margin to prevent pinching or damage of tissues caused by interaction with the bit.
Because hypsodont dentition evolved to handle gritty, low-quality steppe forage, many dental issues related to occlusion can be traced to stabling and a diet of nonabrasive plants (24). These issues are mitigated among freely grazed horses, particularly in the steppes of northeast Asia, where natural forage wears teeth in a manner similar to the ancestral caballine diet. Nonetheless, contemporary Mongolian herders still practice deciduous tooth extraction. During our ethnographic interviews with individuals from Khuvsgul province in northern Mongolia and Uvurkhangai province in central Mongolia, herders reported conducting extractions of problematic deciduous teeth that were interfering with animal behavior using pliers. Contemporary herders also extract wolf teeth during the spring of the animal’s first year using simple tools, such as a screwdriver (Fig. 1), in conjunction with the spring roundup and hair-cutting event (28). Although some informants describe this removal in more abstract terms (stating, for example, that extraction increases the “power” of the horse), we observed that contemporary Mongol bits cause regular damage to the anterior margin of the second premolar (SI Appendix, Fig. S3), meaning that modern bits also interact with wolf teeth when present. Consequently, wolf tooth extraction plays a practical role in preventing pain and tissue damage during riding.
Fig. 1.
Mongolian herder removing first premolar, or “wolf tooth,” from a young horse during the spring roundup using a screwdriver. Photo courtesy of D.S.
The practice of horse dentistry by contemporary nomadic peoples in Mongolia, coupled with the centrality of horse transport to Mongolian life, both now and in antiquity, raises the possibility that dental care played an important role in the development of nomadic life and domestic horse use in the past. To investigate, we conducted a detailed archaeozoological study of horse remains from tombs and ritual horse inhumations across the Mongolian Steppe, assessing evidence for anthropogenic dental modifications and comparing our findings with broader patterns in horse use and nomadic material culture.
Results
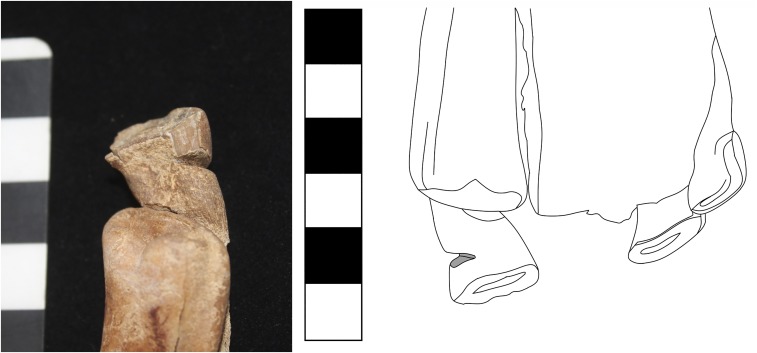
Although many of the specimens analyzed (SI Appendix, Table S1) were taphonomically damaged or otherwise incomplete, two of 10 juvenile Bronze Age DSK horses with complete dental preservation displayed unequivocal evidence of anthropogenic modification to the deciduous teeth. These specimens are deciduous incisors: one complete tooth (upper left central incisor/601) and one retained tooth fragment (501/502 or 601/602). They originate from the sites of Uguumur in Zavkhan province, west-central Mongolia (Fig. 2 and Table 1, specimen 014), and the Egiin Gol Valley in east-central Mongolia (Fig. 2 and Table 1, specimen 059). Both teeth display exterior damage to the enamel consistent with attempted removal (Figs. 3 and 4 and SI Appendix, Figs. S4–S6). Although the first specimen, a young (2–2.5 y old) horse from the site of Uguumur in central Mongolia, is fragmented and missing much of the maxillary bone structure, all anterior dentition was present at the time of analysis, with the exception of the upper right deciduous central incisor (Fig. 3, Right). We interpret this as evidence that this tooth had fallen out before the animal’s death, causing the upper left deciduous central incisor (601) to begin to grow medially and orienting the occlusal surface of the tooth at an uncomfortable angle (Fig. 3, Right). This would have caused the animal difficulty with mastication.
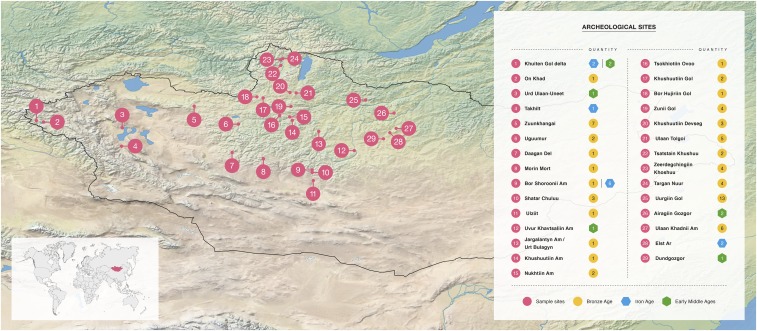
Fig. 2.
Map of Mongolia and archaeological sites mentioned in the text, along with time periods and numbers of samples.
Table 1.
Age, dental modifications, taphonomic data, and radiocarbon age/cultural affiliation for key specimens cited in main text
| Specimen | Estimated age, y (basis) | Dental modifications | Alveolar healing? | Archaeological period/site type | Postdepositional alveolar damage? | Laboratory no. | 14C YBP | Radiocarbon error range (+/−) | Calibrated age range (2-sigma) |
| Uguumur DS2 F1 (014) | 2–2.5 y (eruption) | Sawed deciduous I1 | — | DSK | — | GrM11927 | 2,936 | 14 | 1211–1056 cal. BCE |
| Darkhan Uul Khirigsuur 75 F2 (059) | 3–4 y (eruption) | Sawed deciduous I1 | — | DSK | — | GrM11925 | 2,802 | 14 | 1002–912 cal. BCE |
| Bor Shoroonii Am SB 2.1.1 (070) | 1–2 (eruption) | Extracted P1 (upper) | Yes (UR, UL) | Slab Burial | No | AA110195 | 2,545 | 28 | 800–551 cal. BCE |
| Bor Shoroonii Am SB 2.1.2 (068) | 1–2 (eruption) | Extracted P1 (lower) | Yes (LL) | Slab Burial | No | AA110195 | 2,545 | 28 | 800–551 cal. BCE |
| Bor Shoroonii Am SB 2.1.3 (073) | 2–2.5 y (eruption) | Extracted P1 (upper) | Yes (UL) | Slab Burial | Yes (lower jaw only) | AA110195 | 2,545 | 28 | 800–551 cal. BCE |
| Khuiten Gol Delta (Biluut 2-2) (012) | 2–2.5 y (eruption) | Extracted P1 (upper) | Yes (UL, UR) | Pazyryk | No | BETA-308477 | 2,070 | 30 | 174–1 cal. BCE |
| Elst-Ar Burial 14 (079) | ∼1 y (eruption) | Extracted P1 (upper) | Yes (UL) | Xiongnu | No | GrM11928 | 2,002 | 14 | 38 cal. BCE–50 cal. CE |
| Airagiin Gozgor Burial 2 (086) | 2–2.5 y (eruption) | Chipped lower P2 and extracted lower P1 | Yes (LR) | Late Xiongnu | No (maxilla missing) | GrM11864 | 1,919 | 13 | 56–126 cal. CE |
| Urd Ulaan-Uneet (087) | 4–4.5 (eruption) | P1 worn through bit damage | — | Post-Xiongnu | No | IAAA-170205 | 1,737 | 20 | 243–380 cal. CE |
Radiocarbon dates were calibrated using IntCAL13 via OxCal. I1, first/central incisor; LL, lower left; LR, lower right; P1, first premolar (wolf tooth); P2, second premolar; UL, upper left; UR, upper right.
Fig. 3.
Anthropogenically modified deciduous, upper left first incisor (201) from the site of Uguumur, Zavkhan, central Mongolia, recovered from a ritual horse burial belonging to the Late Bronze Age DSK complex. (Scale bar: 1 square, 1 cm.)
Fig. 4.
(Left) Photomicrograph showing a modified I1 margin from Uguumur under 20× magnification, revealing a triangular-shaped and exfoliated tooth margin. (Center) SEM photomicrograph showing the modified I1 margin. (Right) SEM close-up view showing anthropogenic striation, indicated by white arrows, on the exposed surface. (Scale bar: 500 μm.)
The maleruption of this deciduous tooth appears to have prompted an attempted removal or occlusal correction by Late Bronze Age herders. The specimen exhibits a triangular notch parallel to the normal occlusal surface of the incisor row (Fig. 3, Right). The modified surface was examined by scanning electron microscopy (SEM) directly under a JEOL JSM-IT100 InTouchScope scanning electron microscope. This analysis revealed that the Uguumur tooth was cut through both the enamel and dentine, fraying enamel at the point of first contact and exposing the tooth interior (Fig. 4). SEM also revealed a transverse cut mark on the notch surface in the incisal direction, indicative that the notch was produced through human modification (Fig. 4B). We examined several particles that looked embedded within the exposed dentine using energy dispersive spectroscopy (EDS) (SI Appendix, Fig. S4). The major elements seen in all of the examined particles were indicative of bone or silicate composition. On the basis of current information, it is impossible to say whether these silicate inclusions were introduced naturally through diet or taphonomic processes (e.g., embedded sand). However, the lack of metal residues, which have been identified in Iron Age horse teeth modified by bit use (29), would seem to point toward the use of a stone cutting tool, which may have been efficient at cutting through enamel and dentine. On the basis of the available evidence, it is difficult to say whether the remover intended to saw completely through the crown or whether the removal process might have had additional steps (e.g., breaking the crown off from the root).
A second tooth specimen from the Egiin Gol Valley (a fragment of a deciduous central incisor 501/502 or 601/602, SI Appendix, Fig. S5) also shows apparent modification related to extraction. The specimen appears to have broken during natural tooth eruption, resulting in the retention of a deciduous tooth fragment alongside the permanent dentition that might have similarly caused behavioral or dietary issues in the young (3–4 y old) horse (25). A grooved divot in the tooth’s buccal surface, oriented parallel to the natural occlusal plane and concentrated on a single side of the tooth fragment (SI Appendix, Figs. S5A and S6), may also reflect a cutting motion from the exterior of the horse’s mouth. This perpendicular cutting activity appears to have caused a “slab” or uncomplicated crown fracture (wherein a perpendicular force causes a flat slice of enamel material to separate from the rest of the tooth, but not the pulp cavity). This fracture exposed the underlying dentine (shown by SEM in SI Appendix, Fig. S5B). Unlike the Uguumur specimen, the Egiin Gol horse tooth does not appear to have been sawed by a sharp object. Instead, it exhibits a rounded surface morphology suggestive of a blunt instrument (SI Appendix, Fig. S5C). While some form of developmental enamel defect is also a plausible explanation for this feature, the co-occurrence of an uncomplicated crown (slab) fracture makes this less likely. As both teeth were recovered in situ, it appears that both removal attempts were unsuccessful or aborted, leaving the damaged incisor fragment in the animal’s mouth until its death and burial. Direct radiocarbon dates on other teeth from these same horses place these early dental procedures at ca. 1150 BCE [mean calibrated (cal.) BCE], or between 1211 and 1056 (2-sigma range) cal. BCE (Table 1). Later specimens from Iron and Middle Age contexts revealed no evidence of similar modifications.
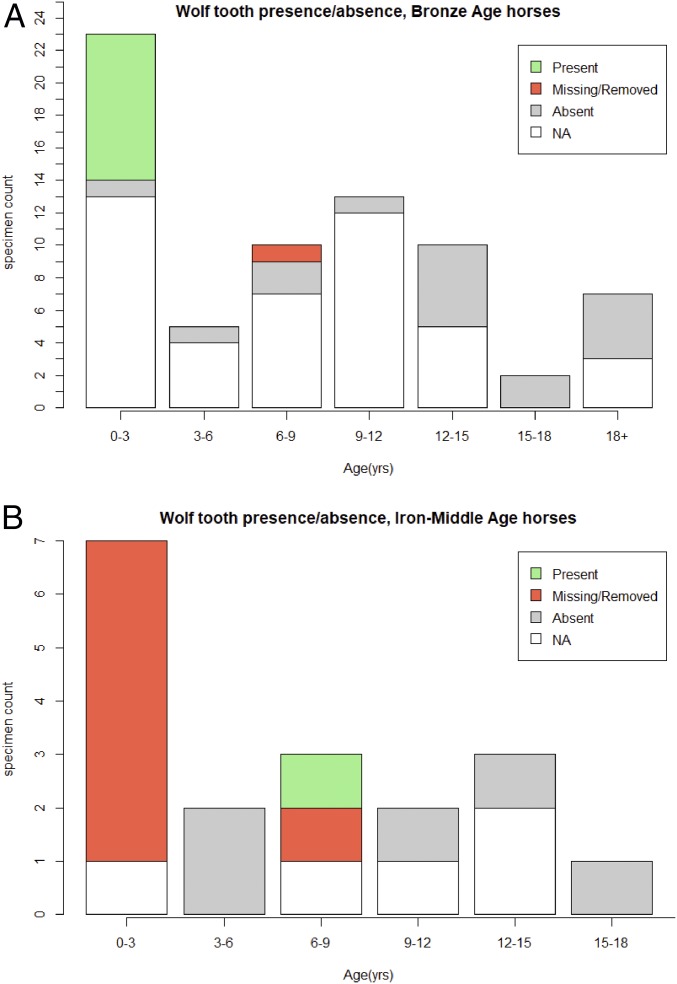
Despite this evidence for incisor modification, Bronze Age horses analyzed here exhibited no changes to the first premolar/wolf tooth (X05 in the modified Triadan system). Although many Bronze Age horses were poorly preserved to the point that the alveolar matrix could not be assessed, nearly all of those juvenile specimens with sufficient preservation to assess tooth presence/absence retained a complete wolf tooth (Figs. 5A and 6A). Without human interference, as many as 90% of contemporary yearlings will develop at least one wolf tooth, with between 13% and 32% of animals retaining these teeth into adulthood (25, 26). In the Bronze Age sample, nearly all (n = 9 of 10 total) of the observed specimens younger than 3 y of age with sufficient preservation for assessment displayed an intact wolf tooth of some kind. A single adult specimen, a male horse with an estimated age of 8 y, did exhibit an empty alveolus for a large upper wolf tooth. However, this specimen had experienced obvious postdepositional breakage that resulted in postmortem (and likely postrecovery) tooth loss. These observed patterns in first premolar persistence suggest that during the Late Bronze Age, wolf teeth were likely present in their natural frequency and that natural processes of wolf tooth loss left a minimal archaeological signature (i.e., few or no empty alveoli with evidence of premortem loss). One explanation for this pattern may be that unextracted wolf teeth undergo natural root resorption (26).
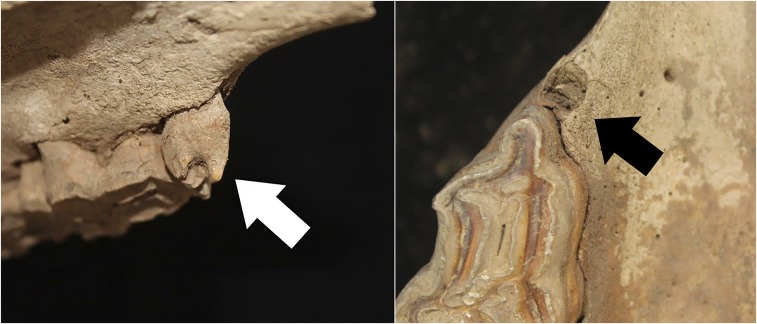
Fig. 5.
Intact P1 or wolf tooth from a juvenile Bronze Age horse (Left, arrow) and from a horse of similar age from an Early Iron Age (EIA) context with an empty alveolus that has been infilled with sediment (Right, arrow).
Fig. 6.
Wolf tooth presence or absence in observed archaeological samples from the Late Bronze Age (1200–700 BCE) (Top) and those dating to later periods (Bottom). Specimens with insufficient preservation to identify the presence or absence of a wolf tooth are labeled NA (not available).
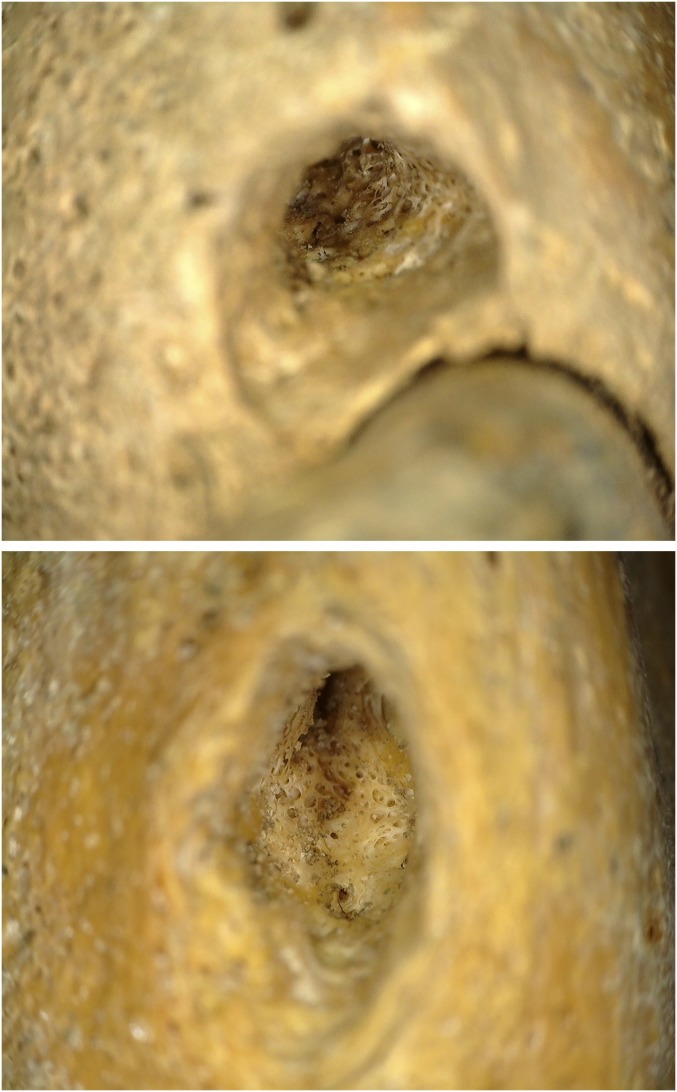
Bronze Age patterns of wolf tooth retention contrast greatly with the sample of juvenile specimens from the Iron and Middle Ages, in which nearly all of the analyzed juvenile archaeological horse specimens exhibited an empty alveolus (Figs. 5B and 6B). A few of these specimens displayed fracturing or evidence of postdepositional damage, but most had no indication of taphonomic damage to the alveolar bone matrix and many alveoli were still filled with original sediment from excavation at the time of analysis (Fig. 5B and Table 1). Cleaning and alveolar inspection under 20× to 50× magnification with a DinoLITE digital microscope indicated new bone formation in at least one empty alveolus for six of seven specimens from the Early Iron Age (Slab Burial, Pazyryk), Late Iron Age (Xiongnu), and Early Middle Ages (post-Xiongnu) (Fig. 7). New cancellous bone formation replacing the previously smooth alveolar margin is an indicator that the teeth were extracted or otherwise lost before the animal’s death. This is in contrast to evidence of advanced periodontal disease, where teeth become mobile (loose) due to the loss of bony support.
Fig. 7.
Photomicrograph showing new bone formation at the lower margin of the P1 alveolus in horses from first millennium BCE sites in Khuiten Gol Delta, western Mongolia (Top) and Elst-Ar, central Mongolia (Bottom), indicating that healing had begun before the animal’s death. Images taken at 20× to 50× magnification.
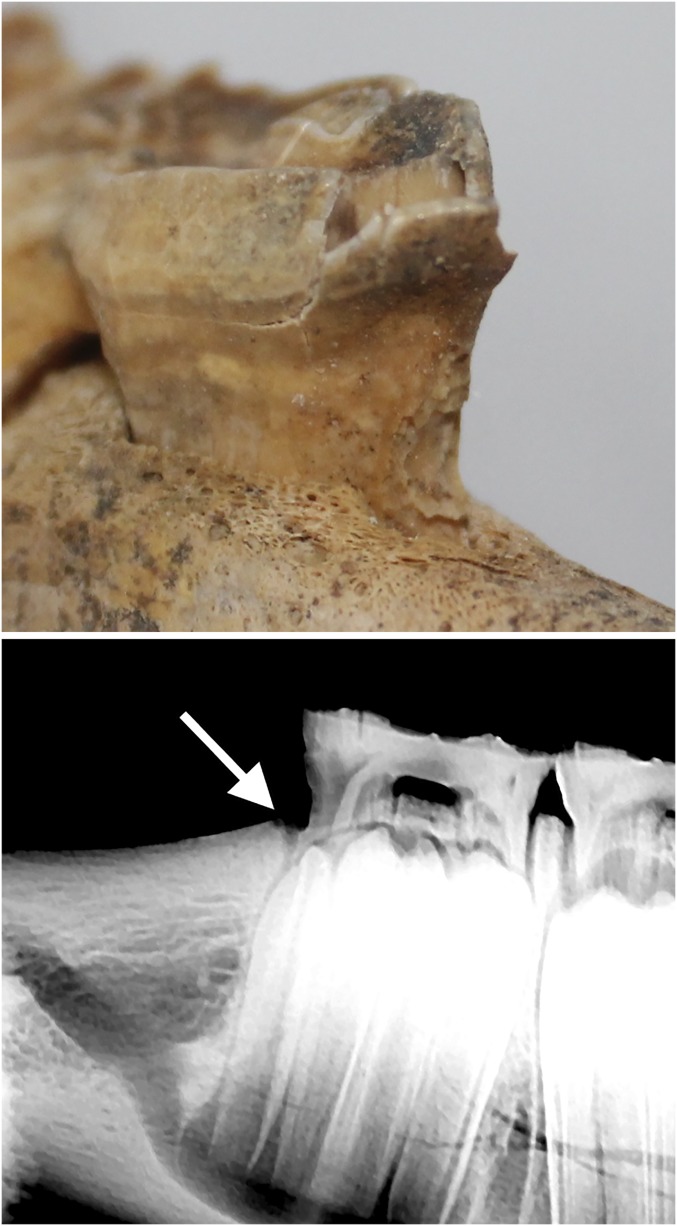
One specimen exhibited a severely damaged deciduous lower second premolar (Fig. 8A, tooth 806) that may provide direct evidence of dental practices. The horse, a juvenile (2–2.5 y old) specimen from the late Xiongnu site of Airagiin Gozgor in central Mongolia, has a large area of exposed dentine with jagged margins on the tooth’s lower anterior surface, near the alveolar margin. Despite postdepositional taphonomic damage to the upper portion of the tooth root, the lower portion of the tooth is undamaged by postdepositional processes (Fig. 8A). The damaged area shows patination and bone remodeling indicative of a predepositional and premortem occurrence. Minor remodeling along the anterior alveolar margin (Fig. 8A) indicates that the tooth was destabilized, and surrounding bone subsequently healed, following trauma to the anterior tooth margin. A radiograph reveals that while no alveolus is visible at the surface, a wolf tooth was originally present in this location (Fig. 8B). The event that detached this tooth fragment was invasive enough to remove or damage a portion of the tooth root. Because of this, we suggest that it was most likely caused by a leveraging action against the anterior 706 margin during wolf tooth extraction. Although this damage could have been caused by a different anthropogenic process besides veterinary dental extraction (e.g., traumatic contact with a bit), mandibular wolf teeth are a rare occurrence (26), making the presence of a healed mandibular alveolus in this case a striking coincidence. Moreover, the lower left deciduous premolar, or 706 (opposite the damaged tooth), is intact and undamaged, and the specimen lacks other kinds of toothwear often associated with metal bit use (20, 30). Consequently, we consider anthropogenic damage during wolf tooth extraction to be the most likely cause of this pathology.
Fig. 8.
(Top) Damaged anterior margin of the deciduous lower right second premolar (406) in a horse dated to the early centuries CE, which appears to have been caused by a botched dental procedure (removal of the P1). A large spall of enamel has begun to peel and fragment from the tooth postdepositionally. (Bottom) Radiograph showing the former (healed) alveolus (arrow).
The ubiquity of premortem wolf tooth loss among juvenile specimens of varying ages provides a dramatic contrast to the Late Bronze Age sample, and is difficult to explain without invoking human activity. We argue that these patterns are best explained by more frequent tooth extraction during the Early Iron Age and onward, increasing the frequency of empty and partially remodeled wolf tooth alveoli in the archaeological record. Considered alongside evidence for damage directly caused by extraction efforts, our data strongly suggest that the absence of wolf teeth in juvenile horses from Iron and Middle Age contexts was caused by premortem extraction by pastoral herders.
Discussion
Detailed analysis of archaeological horse remains shows that the practice of equine dentistry by nomadic peoples in the Mongolian Steppe can be traced back over 3,000 y, to the period linked with the first evidence for both horseback riding and specialized horse pastoralism in northeastern Eurasia (31–33). Although other explanations for the observed equine dental changes may be possible, indentations to exterior margins of two deciduous incisors from subadult horses appear to indicate the attempted removal of incorrectly erupted milk teeth. In contemporary Western equid dental procedures, deciduous teeth are removed when they begin to erupt incorrectly and interfere with other teeth or affect proper occlusion or feeding behavior (34). However, deciduous tooth extraction is typically performed by using forceps and elevators, the latter of which is a sharp, curved instrument that can be used to separate the tooth from alveolar bone and cut the periodontal ligament and underlying connective tissues (35). In both Late Bronze Age archaeological specimens, the removal procedure appears to have consisted of sawing the crown of the tooth directly from the mouth exterior, presumably using a stone instrument (at least in the case of Uguumur). Removing the upper part of the tooth in this fashion, which leaves the roots intact, would have been a slow, laborious process throughout which the animal would be likely to bite or strike. This technique suggests a kind of experimentation with dental procedures rather than sophisticated knowledge of equine dentition. This pattern is consistent with other evidence for experimentation with key elements of pastoral subsistence during the Late Bronze Age (36), and suggests that genuine innovation in equine dental care was closely linked to broader pastoral social developments.
The apparently delayed emergence of wolf tooth extraction in the archaeofaunal record shares striking parallels with technological developments in northeast Asian horse bits. Although no horse tack has been found from DSK horse burials or other second millennium BCE contexts, the earliest dated finds of bridle equipment from the early first millennium BCE consist of cheekpieces without a connecting mouthpiece (37, 38). In one case, at the site of Jargalantyn Am, four horse skulls were recovered with bronze cheekpieces and remnant leather mouthpieces (39). Although the original remains from this site were apparently destroyed or lost, we later dated a horse tooth recovered from site backfill to ca. 2,520 ± 30 14C years before present (YBP) (ca. 790–540 cal. BCE, 2-sigma range). Given the absence of other bitting damage associated with horses used for transport during this period, it appears that these soft organic bits caused little direct damage to the dentition (38), despite the observation that such bits can occasionally wear the anterior margin of the second premolar (1). Unextracted wolf teeth in a horse controlled with an organic bit may have led to noticeable issues with equine behavior or health. Such organic bits apparently remained in use at least occasionally throughout the Xiongnu Period, or ca. 200 BCE–200 CE (40).
Although organic mouthpieces remained in occasional use for several centuries more, bits made of metal (bronze and, later, iron) first became widespread across Central Asia during the late second or early first millennium BCE (31, 32). Bronze jointed “snaffle” bits, which function by applying pressure to the corners of the mouth and provide improved control, made their first appearance in territories immediately adjoining Mongolia during the ninth century BCE. This is demonstrated by archaeological discoveries in Xinjiang, China dated to ca. 850 BCE (33, 41) and Arzhan, Russia dating to ca. 795–815 cal. BCE (42), coeval with the end of the DSK period (7). In Mongolia itself, metal bits first enter the archaeological record in the Early Iron Age, via burials of the Slab Burial or Duruvljin Bulsh culture (33). This period witnessed widespread social transformations and perhaps upheaval: Deer stones across Mongolia were uprooted and recycled to build slab burials (43). Three juvenile specimens in our analyzed sample from a single Slab Burial in Bayankhongor, central Mongolia, displayed empty wolf tooth alveoli with evidence of postextraction healing (Table 1). A direct radiocarbon date on one of these specimens dates this feature to ca. 800–551 cal. BCE (2 sigma), with a median age of 753 cal. BCE. The estimated age of these and later Iron and Early Middle Age animals with evidence for dental extraction falls between a tight age range of 1–2.5 y (Table 1), strikingly similar to the average age of 1.4 y reported for modern wolf tooth extraction in some veterinary reports (27). The coincident timing between the regional appearance of metal bits and the initiation of wolf tooth extraction suggests that the adoption of metal bit technology prompted innovations in equine dental practice, which had been initiated, perhaps for the first time, by nomadic herders living in Mongolia several centuries before.
Like the controversial Buten horse (44), many of our analyzed Iron and Middle Age specimens display a kind of damage or modification to the occlusal surface of the second premolar (X06 in the modified Triadan nomenclature system) referred to as bit wear (20), which has been described elsewhere (9). Although this kind of wear could conceivably be caused by intentional dental filing similar to modern flotation (23, 45), the occlusal wear observed in our Iron Age and Early Middle Age samples was accompanied by other kinds of damage that are more definitively indicative of contact between the bit, teeth, and mandibular bone (9), including damage to the anterior margin of the second premolar (30) and new bone formation at the corners of the mouth where a jointed bit would rest. Bit wear was also entirely absent from the DSK sample, corroborating evidence from early horse equipment for Late Bronze Age organic bit use (9, 38). Critically, then, the paired emergence of both metal bits and metal bit wear in the archaeological record of the Early Iron Age supports the inference of a link between metal bit use and the initiation of wolf tooth extraction.
One early Middle Age horse specimen, a mummy from Urd Ulaan-Uneet (SI Appendix, Fig. S7), provides some additional insights into the relationship between first premolar removal and bit use. This animal has a single, large retained upper wolf tooth that displays occlusal beveling caused by a metal bit. The occlusal damage to this specimen is indicative that, when unextracted, wolf teeth would interact directly with the jointed metal snaffle bits used in antiquity. Considered together, the simplest interpretation of these patterns is that the use of metal bits caused new challenges related to interaction with and damage to the first premolar, prompting the development of wolf tooth removal as a cultural practice in northeast Asia.
This Late Bronze Age dental modification counts among the earliest documented instances of equine veterinary care, and the oldest known evidence for horse dentistry. At first glance, the detailed historical record of early equine veterinary care in places such as China, Greece, Rome, and Syria, which spans the late second millennium BCE through the early centuries CE (11, 15, 16), might imply that equine dentistry emerged in the sedentary civilizations of the Old World. However, the earliest textual references describe only nonsurgical medicinal treatments and make few mentions of oral health (11). Recent archaeological discoveries suggest that human care of domestic animals was practiced by hunter-gatherers as far back as the Paleolithic (46), and that pastoralists may have occasionally practiced surgical procedures on domestic animals as early as the Neolithic in Europe (47). The evidence presented here indicates that horse dentistry was developed by nomadic pastoralists living on the steppes of Mongolia and northeast Asia during the Late Bronze Age, concurrent with the local adoption of the metal bit and many centuries before the first mention of dental practices in historical accounts from sedentary Old World civilizations.
Our results reveal a fundamental link between equine dentistry and the emergence of horsemanship in the steppes of Eurasia. At the turn of the first millennium BCE, militarized, horse-mounted peoples reshaped the social and economic landscape of many areas of the Eurasian continent. Conflagrations with equestrian peoples, such as those between the Persian Empire and the Pontic “Scythians,” plagued alluvial civilizations from the Near East to India and China, while large-scale movements of people linked East and West in never-before-seen ways (48). The archaeological and historical records indicate that the earliest horseback riding was accomplished without stirrups or saddles, and probably using only bitless or organic-mouthpiece bridles (49, 50). The bronze snaffle bit, and the improved control it provided, was a key technological development that enabled the use of horseback riding for more stressful and difficult activities, such as long-distance transportation and warfare (32). We argue that these technological improvements in horse control were preceded and sustained by innovations in veterinary dentistry by nomadic peoples living in the continental interior. By increasing herd survival and mitigating behavioral and health issues caused by horse equipment, innovations in equine dentistry improved the reliability of horseback riding for ancient nomads, enabling horses to be used for nonpastoral activities like warfare, high-speed riding, and distance travel.
Conclusion
Archaeozoological data from Mongolian horses indicate that the nomadic practice of equine dentistry dates back more than 3,000 y to the DSK complex, a Late Bronze Age culture associated with the first mounted horseback riding and mobile pastoralism in eastern Eurasia. Attempted removal of deciduous incisors through sawing of the exterior suggests experimentation with dental extraction, but not the removal of wolf teeth. The appearance of extracted first premolars in the first millennium BCE coincides with the arrival of metal bits in the archaeological record and oral trauma linked with metal bit use, suggesting that innovations in dental practice were an adaptation to the mechanical changes in horse equipment. These bronze and metal bits provided greater control over the horse, facilitating the development of military uses for the horse, but also introduced new dental problems with the first premolar. Our results indicate that, coincident with the earliest evidence for metal bit use, wolf tooth extraction was practiced in Mongolia by ca. 750 BCE and continued through the early Middle Ages (Table 1). These results push back the earliest dates for equine dentistry by more than a millennium and suggest that nomadic peoples developed key innovations in veterinary care that enabled more sophisticated horse control, ultimately changing the structure of communication, exchange, and military power in ancient Eurasia.
Materials and Methods
We conducted a detailed study of archaeological horse collections spanning the past 3,200 y, including those from the Late Bronze Age DSK complex (ca. 1200–700 BCE, n = 70), Early Iron Age Slab Burial culture (ca. 700–300 BCE, n = 4), Pazyryk culture (ca. 600–200 BCE, n = 2), Late Iron Age Xiongnu Empire (ca. 200 BCE–200 CE, n = 3), Early Middle Ages post-Xiongnu period (ca. 100–550 CE, n = 3), and Turkic Khaganate (ca. 600–800 CE, n = 3). This assemblage comprises all archaeological horse remains in collections at the National Museum of Mongolia. The greater number of individual horse burials from the Bronze Age sample arises from the fact that these animals were inhumed in small, relatively shallow burials that are found in large numbers around monuments of this era (5, 6), while animals from other periods are primarily recovered from within human burials. For each specimen, we estimated age and sex using dental eruption schedules (51), incisor wear patterns (52), and crown height wear curves (53). We analyzed all teeth and alveolar margins from each specimen under low-power (20×) and high-power (200×) magnification for evidence of human modification, including mechanical changes to the tooth surface and evidence of tooth extraction (missing dentition, damage to the tooth margin or alveolar bone, and alveolar remodeling). For those juvenile specimens lacking a first premolar but with an intact alveolus present, we carefully brushed the alveolar surface using brushes and a small air bladder, and examined the alveolar cavity under 20× to 50× magnification, using a DinoLITE Premier digital microscope for evidence of bone remodeling and healing, to identify evidence for healing indicative of premortem extraction or loss. Specimens with apparent modifications were sampled for radiocarbon dating (using an unmodified intact tooth from the same specimen). Direct radiocarbon dates were calibrated using OXCAL and the IntCal13 calibration curve, and are reported in Table 1.
Two specimens displaying apparent human modifications were examined directly under a JEOL JSM-IT100 InTouchScope scanning electron microscope at the Max Planck Institute for the Science of Human History. The specimens were viewed uncoated in backscattered electron mode under low vacuum at 10 kV. Specimens were also tilted up to 18° to view different surfaces of the modified teeth (Figs. 4 and 5). Particles that looked embedded within the modifications were examined with EDS using the built-in JEOL Dry Extra EDS detector. EDS analysis, in this case, was qualitative (i.e., nonquantitative), and the major elements seen in all of examined particles were indicative of bone or silicate particles (SI Appendix, Fig. S4).
Finally, we considered these data alongside taphonomic evidence (including the completeness of each specimen, state of curation, and presence of postdepositional damage) to assess implications for ancient human activity (reported in SI Appendix, Table S1). We compared our inferences with the archaeological record for horse bridling and tack (38) to evaluate the contribution of changes in horse equipment and use to observed dental patterns. All data collected in the analysis are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank Dr. Rodney Flint Taylor and Dr. Dave Fly for their valuable input on equine dentistry, Dr. Gantuya and Dr. Justin Woolsey (Enerekh Animal Hospital, Ulaanbaatar, Mongolia) for their assistance with radiographs, and the National Museum of Mongolia and the American Center for Mongolian Studies in Ulaanbaatar for facilitating this research. This research was funded, in part, by National Geographic Society Young Explorer’s Grant 9713-15, Fulbright US Student Research Program Grant 34154234, and the US Embassy in Mongolia’s Ambassador’s Fund for Cultural Heritage Preservation. Additional funding was provided by the Max Planck Institute for the Science of Human History.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721189115/-/DCSupplemental.
References
- 1.Outram AK, et al. The earliest horse harnessing and milking. Science. 2009;323:1332–1335. doi: 10.1126/science.1168594. [DOI] [PubMed] [Google Scholar]
- 2.Olsen S. 2017. The role of humans in horse distribution through time. Wild Equids: Ecology, Management, and Conservation, eds Ransom J and Kaczenzsky P (Johns Hopkins Univ Press, Baltimore), pp 105–120.
- 3.Bold B-O. Eques Mongolica: Introduction to Mongolian Horsemanship. Bold & Boi; Reykjavik, Iceland: 2012. [Google Scholar]
- 4.Rogers JD. Inner Asian states and empires: Theories and synthesis. J Archaeol Res. 2012;20:205–256. [Google Scholar]
- 5.Allard F, Erdenebaatar D. Khirigsuurs, ritual and mobility in the Bronze Age of Mongolia. Antiquity. 2005;79:547–563. [Google Scholar]
- 6.Fitzhugh WW. The Mongolian deer stone–khirigsuur complex: Dating and organization of a Late Bronze Age menagerie. In: Bemmann J, Parzinger H, Pohl E, Tseveendorj D, editors. Current Archaeological Research in Mongolia. Bonn University Press; Bonn, Germany: 2009. pp. 183–199. [Google Scholar]
- 7.Taylor WTT, et al. A Bayesian chronology for early domestic horse use in the Eastern Steppe. J Archaeol Sci. 2017;81:49–58. [Google Scholar]
- 8.Taylor WTT, Bayarsaikhan J, Tuvshinjargal T. Equine cranial morphology and the identification of riding and chariotry in Late Bronze Age Mongolia. Antiquity. 2015;89:854–871. [Google Scholar]
- 9.Taylor WTT, Tuvshinjargal T. Horseback riding, asymmetry, and changes to the equine skull: Evidence for mounted riding in Mongolia’s Late Bronze Age. In: Bartosiewicz L, Gál E, editors. Care or Neglect?: Evidence of Animal Disease in Archaeology. Oxbow Books; Oxford: 2018. pp. 134–154. [Google Scholar]
- 10.Beardsley RK. Hypotheses on inner Asian pastoral nomadism and its culture area. Mem Soc Am Archaeol. 1953:24–28. [Google Scholar]
- 11.Donaghy T. The origins of equine medicine. J Vet Hist Soc. 2010;15:227–248. [Google Scholar]
- 12.Linduff KM. A walk on the wild side: Late Shang appropriation of horses in China. In: Levine M, Renfrew C, Boyle K, editors. Prehistoric Steppe Adaptation and the Horse. McDonald Institute for Archaeological Research; Cambridge, UK: 2003. pp. 139–162. [Google Scholar]
- 13.Gazagnadou D. The Diffusion of a Postal Relay System in Premodern Eurasia. Editions Kimé; Paris: 2016. [Google Scholar]
- 14.Cooke B. Imperial China: The Art of the Horse in Chinese History: Exhibition Catalog. Art Media Resources Limited; Kentucky Horse Park, Lexington, KY: 2000. [Google Scholar]
- 15.Meserve RI. Chinese hippology and hippiatry: Government bureaucracy and inner Asian influence. Z Dtsch Morgenl Ges. 1998;148:277–314. [Google Scholar]
- 16.Bartosiewicz L. 2018. Taphonomy and disease prevalence in animal palaeopathology: The proverbial “veterinary horse.”Care or Neglect?: Evidence of Animal Disease in Archaeology, eds Bartosiewicz L, Gál E (Oxbow Books, Oxford), pp 185–207.
- 17.Mitchell P. The Donkey in Human History. Oxford Univ Press; Oxford: 2018. [Google Scholar]
- 18.Bartosiewicz L, Gal E. Shuffling Nags, Lame Ducks: The Archaeology of Animal Disease. Oxbow Books; Oxford: 2013. [Google Scholar]
- 19.Raulwing P, Clutton-Brock J. The Buhen horse: Fifty years after its discovery (1958–2008) J Egypt Hist. 2009;2:1–106. [Google Scholar]
- 20.Anthony DW, Brown DR. Eneolithic horse rituals and riding in the steppes: New evidence. In: Levine M, Renfrew C, Boyle K, editors. Prehistoric Steppe Adaptation and the Horse. McDonald Institute for Archaeological Research; Cambridge, UK: 2003. pp. 55–68. [Google Scholar]
- 21.Easley K. Veterinary dentistry: Its origin and recent history. J Hist Dent. 1999;47:83–85. [PubMed] [Google Scholar]
- 22.Fahrenkrug P. Proceedings of the North American Veterinary Conference. Eastern States Veterinary Association; Gainesville, FL: 2005. The history and future of equine dental care; pp. 151–154. [Google Scholar]
- 23.Cross PJ. Where have all the mares gone? Sex and “gender” related pathology in archaeological horses: Clues to horse husbandry and use practices. In: Bartosiewicz L, Gál E, editors. Care or Neglect?: Evidence of Animal Disease in Archaeology. Oxbow Books; Oxford: 2018. pp. 155–175. [Google Scholar]
- 24.Viranta S, Mannermaa K. A tall rostral hook in a medieval horse premolar tooth. Int J Paleopathol. 2017;17:79–81. doi: 10.1016/j.ijpp.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Griffin C. Routine dentistry in juvenile performance horses. Comp Equine. 2009;4:402–415. [Google Scholar]
- 26.Griffin C. An extraction of the first premolar teeth. Comp Equine. 2009;4:68–76. [Google Scholar]
- 27.Hole SL. Wolf teeth and their extraction. Equine Vet Educ. 2016;28:344–351. [Google Scholar]
- 28.Stazewski D. June 23, 2016 A haircut for Mongolia’s half-wild horses. The Diplomat. Available at https://thediplomat.com/2016/06/a-hair-cut-for-mongolias-half-wild-horses/. Accessed June 13, 2018.
- 29.Bendrey R. Identification of metal residues associated with bit-use on prehistoric horse teeth by scanning electron microscopy with energy dispersive X-ray microanalysis. J Archaeol Sci. 2011;38:2989–2994. [Google Scholar]
- 30.Bendrey R. New methods for the identification of evidence for bitting on horse remains from archaeological sites. J Archaeol Sci. 2007;34:1036–1050. [Google Scholar]
- 31.Bokovenko NA. The origins of horse riding and the development of ancient Central Asian nomadic riding harnesses. In: Davis-Kimball J, Murphy E, Koryakova L, Yablonsky L, editors. Kurgans, Ritual Sites, and Settlements: Eurasian Bronze and Iron Age. Archaeopress; Oxford: 2000. pp. 304–310. [Google Scholar]
- 32.Drews R. Early Riders: The Beginnings of Mounted Warfare in Asia and Europe. Routledge; London: 2004. [Google Scholar]
- 33.Honeychurch W. Inner Asia and the Spatial Politics of Empire: Archaeology, Mobility, and Culture Contact. Springer; New York: 2015. [Google Scholar]
- 34.Pence P. Equine Dentistry: A Practical Guide. Wiley; Hoboken, NJ: 2008. [Google Scholar]
- 35.Rawlinson J, Carmalt JL. Extraction techniques for equine incisor and canine teeth. Equine Vet Educ. 2014;26:657–671. [Google Scholar]
- 36.Wright J. The honest labour of stone mounds: Monuments of Bronze and Iron Age Mongolia as costly signals. World Archaeol. 2017;49:547–567. [Google Scholar]
- 37.Turbat T, Amartuvshin C, Erdenebat U. 2003. [Egiin Golyn Sav Nutag Dakh’ Arkheologiin Dursgaluud] (Institute of Archaeology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia). Mongolian.
- 38.Taylor WTT, Tuvshinjargal T, Bayarsaikhan J. Reconstructing equine bridles in the Mongolian Bronze Age. J Ethnobiol. 2016;36:554–570. [Google Scholar]
- 39.Sanjmyatav T. 1993. [Arkhangai Aimag Nutag Dakhi Ertnii Tuukh Soyoliin Dursgal] (Arkhangai Aimagiin Zasag Dargiin Tamgiin Gazar, Ulaanbaatar, Mongolia). Mongolian.
- 40.Batsaikhan Ts, Bataarsuren Ch. 2015. [Morin Soyoliin Tonog Heregsel] (Munkhii Useg Group, Ulaanbaatar, Mongolia). Mongolian.
- 41.Wagner M, et al. Radiocarbon-dated archaeological record of early first millennium B.C. mounted pastoralists in the Kunlun Mountains, China. Proc Natl Acad Sci USA. 2011;108:15733–15738. doi: 10.1073/pnas.1105273108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zaitseva GI, et al. Chronology of key barrows belonging to different stages of the Scythian period in Tuva (Arzhan-1 and Arzhan-2 barrows) Radiocarbon. 2007;49:645–658. [Google Scholar]
- 43.Fitzhugh WW. Stone Shamans and flying deer of northern Mongolia: Deer goddess of Siberia or chimera of the steppe? Arctic Anthropol. 2009;46:72–88. [Google Scholar]
- 44.Clutton-Brock J. The Buhen horse. J Archaeol Sci. 1974;1:89–100. [Google Scholar]
- 45.Sasada Y. An alternative theory for ‘bit wear’ found on the lower second premolar of the Buhen horse. In: Veldmeijer AJ, Ikram S, editors. Chasing Chariots: Proceedings of the First International Chariot Conference. Sidestone Press; Leiden, The Netherlands: 2013. pp. 229–236. [Google Scholar]
- 46.Janssens L, et al. A new look at an old dog: Bonn-Oberkassel reconsidered. J Archaeol Sci. 2018;92:126–138. [Google Scholar]
- 47.Ramirez Rozzi F, Froment A. Earliest animal cranial surgery: From cow to man in the Neolithic. Sci Rep. 2018;8:5536. doi: 10.1038/s41598-018-23914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelekna P. The Horse in Human History. Cambridge Univ Press; Cambridge, UK: 2009. [Google Scholar]
- 49.Dietz UL. Horseback riding: Man’s access to speed. In: Levine M, Renfrew C, Boyle K, editors. Prehistoric Steppe Adaptation and the Horse. McDonald Institute for Archaeological Research; Cambridge, UK: 2003. pp. 189–199. [Google Scholar]
- 50.Littauer MA. Bits and pieces. Antiquity. 1969;43:289–300. [Google Scholar]
- 51.Evans P, Jack N, King D, Jones S. Aging Horses by Their Teeth. University of Arkansas Division of Agriculture; Fayetteville, AR: 2007. [Google Scholar]
- 52.Academy of Equine Dentistry . Teeth of the Horse-Chart for Accurately Telling the Age from Six Months to Twenty-Nine Years. Academy of Equine Dentistry; Glens Ferry, ID: 2013. [Google Scholar]
- 53.Levine MA. The use of crown height measurements and eruption-wear sequences to age horse teeth. In: Wilson B, Grigson C, Payne S, editors. Ageing and Sexing Animal Bones from Archaeological Sites. Vol 109. Archaeopress; Oxford, UK: 1982. pp. 223–249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.