Significance
Reduction in clinical cases of vector-borne diseases is strongly dependent on the ability to reduce the number of infectious insect bites. Here we describe a treatment concept based on single-dose administration of an insecticidal isoxazoline drug to a human population, which leads to killing of blood-fed insect vectors and a predicted sharp decline in disease transmission. Based on the long half-life observed in preclinical species, a single human dose of <500 mg is predicted to provide plasma exposure above the insecticidal threshold for longer than 2 months. Importantly, we show that isoxazolines are active against a range of vector species, which holds promise for expanding the concept of drug-based vector control from malaria to leishmaniasis and arboviral diseases.
Keywords: vector control, insecticide, malaria, zika fever, isoxazoline
Abstract
Isoxazolines are oral insecticidal drugs currently licensed for ectoparasite control in companion animals. Here we propose their use in humans for the reduction of vector-borne disease incidence. Fluralaner and afoxolaner rapidly killed Anopheles, Aedes, and Culex mosquitoes and Phlebotomus sand flies after feeding on a drug-supplemented blood meal, with IC50 values ranging from 33 to 575 nM, and were fully active against strains with preexisting resistance to common insecticides. Based on allometric scaling of preclinical pharmacokinetics data, we predict that a single human median dose of 260 mg (IQR, 177–407 mg) for afoxolaner, or 410 mg (IQR, 278–648 mg) for fluralaner, could provide an insecticidal effect lasting 50–90 days against mosquitoes and Phlebotomus sand flies. Computational modeling showed that seasonal mass drug administration of such a single dose to a fraction of a regional population would dramatically reduce clinical cases of Zika and malaria in endemic settings. Isoxazolines therefore represent a promising new component of drug-based vector control.
Vector-borne diseases, including malaria, Zika fever, and leishmaniasis, remain major causes of mortality and morbidity in (sub)tropical regions (1, 2). Temperate areas are also at risk for such diseases, for instance, due to the reintroduction of West Nile Virus in Europe (3). Elimination of these diseases will require not only clinical development of new drugs and vaccines, but also effective control of vector populations (4, 5). Drug-based vector control is a new strategy that involves administration of an oral insecticidal drug to a human population at risk to kill the insect vector on blood feeding, thereby reducing the vector population and preventing disease transmission (6). This approach has the advantage of being effective against mosquito populations feeding outdoors, which are increasingly important for malaria transmission (7) and escape the killing effects of traditional vector control methods such as insecticide-treated bed nets and indoor residual spraying. In contrast to approaches targeting livestock, administering an insecticidal drug to a human population would directly prevent onward transmission of human vector-borne pathogens and reduce the population size of fully anthropophilic mosquitoes and sand flies, which play major roles in the transmission of malaria in Africa and of visceral leishmaniasis in India, respectively (8). An oral insecticidal drug should preferentially have a long-lasting effect at a single dose to minimize logistical challenges and cost of mass drug administration (9), have a very broad safety window, and show activity against a wide range of disease vector species.
Isoxazolines are a class of compounds recently licensed as veterinary drugs for protection of companion animals against fleas and ticks (10, 11), with very long in vivo half-lives that provide weeks to months of protection after a single oral administration (12, 13). Even though these compounds have neuronal targets (Fig. 1A), they have been generally shown to be safe in mammals, as they show limited brain penetration (14) and significant selectivity for insect over mammalian receptors (12, 15). Herein we evaluate two representatives of this compound class in multiple disease-carrying vector species and support the case for their potential use as human oral vector-control drugs.
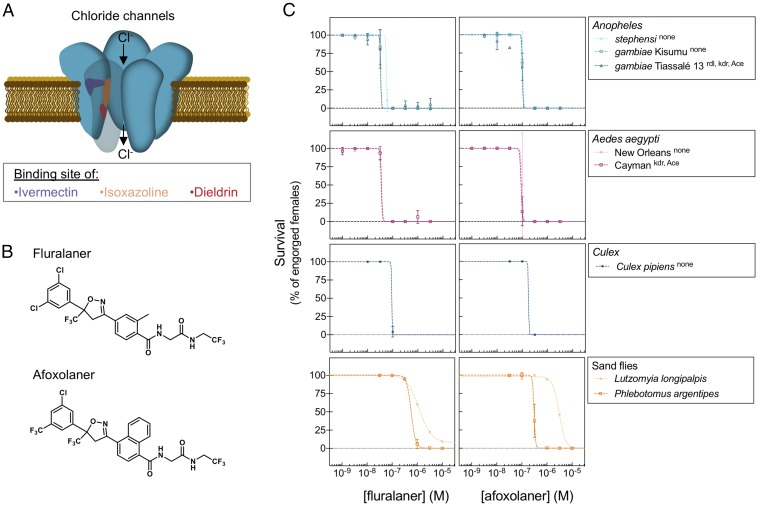
Fig. 1.
Insecticidal activity of fluralaner and afoxolaner on vectors. (A) Cartoon of the binding pockets of insecticides on the GABA or the glutamate-gated chloride channel (16, 17). The putative binding sites of ivermectin, isoxazolines, and dieldrin are depicted on a subunit of the pentameric structure of the channel, in purple, orange, and red, respectively. (B) Chemical structures of fluralaner and afoxolaner. (C) Survival (expressed as percentage of engorged individuals) of Anopheles, A. aegypti, and C. pipiens mosquitoes and P. argentipes and L. longipalpis sand flies at 24 h after feeding on fluralaner- (Left) and afoxolaner- (Right) supplemented blood meals. The species or strain is indicated in the legend of each graph with the known resistance mutation(s) in superscript. Error bars for the Anopheles and Aedes data indicate SEs based on duplicate experiments with approximately 30 mosquitoes each; on the Culex data, they indicate SEs based on four replicate experiments with approximately 15 mosquitoes each; on the Phlebotomus data, they indicate SEs based on duplicate experiments with 100 flies each. Data points of the Lutzomyia data indicate results of a single experiment with 100 flies per concentration and per compound.
Results and Discussion
Insecticidal Activity of Fluralaner and Afoxolaner.
The isoxazolines afoxolaner and fluralaner (Fig. 1B) were tested on various strains of Anopheles, Aedes aegypti, and Culex pipiens, which are important vectors for malaria, Zika/dengue, and West Nile virus, respectively. Mosquitoes were fed on drug-supplemented human blood by membrane feeding. At 24 h after the blood meal, fluralaner showed IC50 values in the range of 33–92 nM against all mosquito strains tested, whereas afoxolaner was slightly less active, with IC50 values ranging from 90 to 177 nM (Fig. 1C and Table 1). Isoxazolines occupy a binding site that is distinct from the targets of known modulators of ionotropic GABA receptors (Fig. 1A) (16, 17). In line with this notion, fluralaner and afoxolaner were fully active against the Anopheles gambiae Tiassalé 13 strain that carries the resistance-to-dieldrin (rdl) mutation in the GABA receptor (Fig. 1C and SI Appendix, Table S1). In addition, they were equipotent against pyrethroid- and carbamate-resistant strains carrying mutations in the kdr sodium channel and acetylcholine esterase (ace-1) genes (Fig. 1C and SI Appendix, Table S1).
Table 1.
Insecticidal activity of fluralaner and afoxolaner against disease vectors
| Fluralaner | Afoxolaner | |||
| Vector | IC50, nM | 95% CI | IC50, nM | 95% CI |
| Anopheles stephensi | 56.0 | [55–57] | 106.8 | [102–118] |
| Anopheles gambiae Kisumu | 33.3 | [33–34] | 101.4 | [100–103] |
| Anopheles gambiae Tiassalé | 33.2 | [32–34] | 100.7 | [99–102] |
| Aedes aegypti New Orleans | 34.2 | [33–35] | 100.0 | [93–107] |
| Aedes aegypti Cayman | 35.8 | [34–37] | 90.2 | [87–94] |
| Culex pipiens | 92.5 | [89–96] | 177.5 | [175–180] |
| Phlebotomus argentipes | 575.4 | [503–658] | 305.5 | ND |
| Lutzomyia longipalpis | 1,183.0 | [971–1,439] | 3,051.0 | [2,455–3,792] |
Listed are IC50 values (with 95% CIs in brackets) determined from the curves shown in Fig. 1C that represent 24-h survival of mosquito and sand fly strains in the presence of increasing concentrations of fluralaner or afoxolaner. ND, not determined.
The compounds were further tested by membrane feeding of sand flies, which are important vectors of Leishmania. Afoxolaner and fluralaner showed IC50 values of 305 and 575 nM, respectively, against Phlebotomus argentipes (Fig. 1C and Table 1), a vector of Leishmania donovani on the Indian subcontinent (2). Both compounds were less active against the South American vector, Lutzomyia longipalpis, with IC50 values of 1–3 µM (Fig. 1C and Table 1). The difference in isoxazoline activity seen between sand flies and mosquitoes suggests that the targeted pocket in the GABA receptor is not conserved among insects.
Human Dose Prediction.
Neither fluralaner nor afoxolaner was measurably metabolized when incubated with dog or human hepatocytes, suggesting that the low in vivo intrinsic clearance observed in dogs (12, 13) could be similar in humans (Table 2). Both compounds were highly bound to plasma proteins (Table 2), which could further contribute to a long in vivo half-life. Using published dog pharmacokinetic parameters (SI Appendix, Table S2) (12, 18), we performed allometric scaling to predict human plasma exposure following oral dosing. Obviously, plasma concentrations after dosing of an eventual drug product will vary among human subjects due to variations in absorption rates, genotypes and expression of CYP450 drug metabolizing enzymes, etc. The exact distribution of human pharmacokinetic parameters will not be known until human population pharmacokinetics data become available. However, to estimate variability, a stochastic simulation approach was adopted using a fixed coefficient of variation (CV) of 20% with a log-normal distribution for each parameter used in the single compartment model, i.e., clearance (Cl), volume of distribution (V), absorption rate (Ka) and bioavailability (F). This variation is in line with data reported for the dog studies, where CV values ranged from 14 to 24% (12, 18). Using this approach, we predicted the human dose resulting in circulating drug concentrations above the mosquitocidal IC99 of Anopheles and Aedes for 90 d (SI Appendix, Fig. S1). The results indicate an estimated single total human median dose level of 260 mg (IQR, 177–407 mg) of afoxolaner or 410 mg (IQR, 278–648 mg) of fluralaner. For Culex, this dose would lead to circulating drug levels above IC99 for 74 d. As sand flies appeared less sensitive to the compounds (Table 1), a 410-mg dose of fluralaner would not yield sufficient plasma exposure for a sand fly killing effect, whereas a 260-mg dose of afoxolaner would yield plasma concentrations above the Phlebotomus IC99 for 50 d.
Table 2.
In vitro metabolism of fluralaner and afoxolaner
| Human hepatocytes | Dog hepatocytes | Rat hepatocytes | Human plasma, unbound fraction (%) | ||||
| Compound | t1/2, min | CLint, μL/min/ 106 cells | t1/2, min | CLint, μL/min/ 106 cells | t1/2, min | CLint, μL/min/ 106 cells | |
| Fluralaner | >216.8 | <6.4 | >216.8 | <6.4 | >216.8 | <6.4 | 1.6 |
| Afoxolaner | >216.8 | <6.4 | >216.8 | <6.4 | >216.8 | <6.4 | ND |
| 7-Ethoxy-coumarin | 20.1 | 69.1 | 14.0 | 99.3 | 61.8 | 22.4 | — |
The table shows in vitro degradation of fluralaner and afoxolaner and, as a control, ethoxycoumarin, by human, dog and rat primary hepatocytes. The results are shown as the half-life (t1/2) and intrinsic in vitro clearance (CLint) of each compound. The data are based on 5-point time courses. The last column shows the percentage of afoxolaner and fluralaner unbound to human plasma. The results are based on 3 replicate experiments. ND, not detected; no peak was observed in the buffer sample, indicating that the molecule may be highly bound to plasma proteins.
Since the dose levels described above are reasonable amounts to be formulated and delivered in a single-dose mass drug administration, we used the associated 90-d period of efficacy in Aedes and Anopheles mosquitoes to model the potential effect on two mosquito-borne diseases.
Modeled Impact on Zika Fever Incidence.
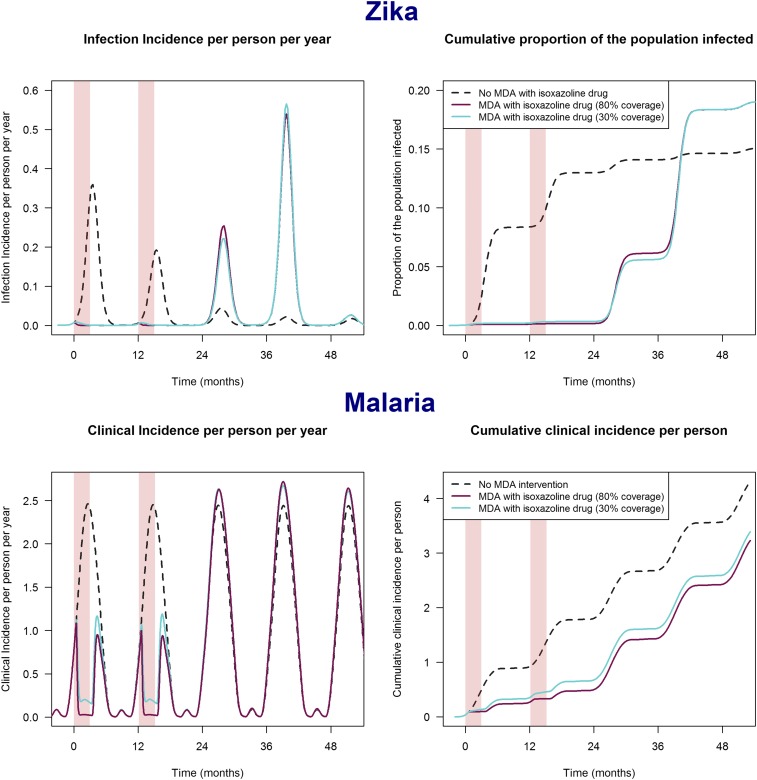
Zika is an immunizing infection, meaning that once an individual has been infected, he or she is no longer susceptible to new infections. We modeled the effect of an intervention in a population with historical exposure to Zika at a moment in time when herd immunity had declined to a degree that permits a new epidemic (19). Two scenarios in which different proportions of the human population over age 5 years would be treated were explored. A 30% coverage scenario assumes that women of childbearing potential are excluded from treatment, because of the absence of human teratogenicity data on initial entry of an isoxazoline drug on the market. A second 80% coverage scenario implies that sufficient data have been gathered to also treat women who are potentially pregnant. The results show little difference between the two scenarios and indicate that administration of an isoxazoline drug once yearly is predicted to prevent >97% of all clinical cases during the years of administration, even if only 30% of the population is treated (Fig. 2A). As soon as the intervention ceases, transmission restarts, then it peaks in the second year after cessation of the intervention and wanes again thereafter. The rebound in transmission may result in a higher cumulative number of cases in the years postintervention compared with a scenario without treatment. The data presented in Fig. 2A show that in the absence of an intervention, 15% of the population would be affected by the epidemic, whereas an epidemic delayed by a 2-y isoxazoline intervention would affect 18% of the population. The overshoot in the number of cases after intervention is explained by a reduction in herd immunity (due to deaths of immune individuals) and an increase in the susceptible population (due to births) during the years of intervention.
Fig. 2.
Modeled impact of mass drug administration of an isoxazoline drug. Reduction in the incidence of both symptomatic (clinical) and asymptomatic infections in Zika (Top) and clinical incidence and cumulative clinical incidence in malaria (Bottom) after 2 y of fluralaner/afoxolaner mass drug administration (MDA) during the transmission season (indicated by the pink shaded areas), with either 30% or 80% of the population age >5 y receiving the drug each year. The model assumes a mosquitocidal drug dose resulting in blood levels >IC99 for 90 d. The two initial years of treatment are followed by three transmission seasons without further intervention.
The time scale and magnitude of the modeled epidemic are consistent with past incidence data for Zika, most notably from multiple Latin American countries in 2015–2017 (20, 21). Thus, mass drug administration of a mosquitocidal drug is very efficient in delaying Zika transmission in populations but needs to be maintained to sustainably prevent outbreaks. This risk of disease rebound is well known from mass drug administration programs (22, 23), and the duration and coverage of any such campaigns must be carefully chosen to optimize the risk-benefit ratio.
Modeled Impact on Malaria Incidence.
Naturally acquired immunity to malaria is mainly nonsterilizing but reduces the severity of infections, and any infectious mosquito bite could potentially cause a new infection. Therefore, a transient intervention, such as afoxolaner/fluralaner administration, would result in a temporary reduction in malaria incidence and a large reduction in cumulative incidence over a specified period. For example, in a transmission setting with 17% malaria parasite prevalence by microscopy and a short transmission season (approximately 5–6 mo), 80% coverage of the population with the drug would result in a 75% reduction in malaria cases in the intervention year (Fig. 2B). Under the same conditions, 66% reduction in cases is achieved with a population coverage of only 30% (Fig. 2B). Despite a slight rebound in clinical incidence after cessation of treatment, the total relative reduction in numbers of cases over a period of 4 y (with intervention in the first 2 y) is 28% and 33% for scenarios with 30% and 80% population coverage, respectively, compared with a scenario with 0% coverage (no intervention). The potential impact of an isooxazoline-based intervention seems much greater than the predicted impact of mosquitocidal doses of ivermectin, which is in line with the notion that the duration of the mosquitocidal effect is one of the main drivers of clinical efficacy (24).
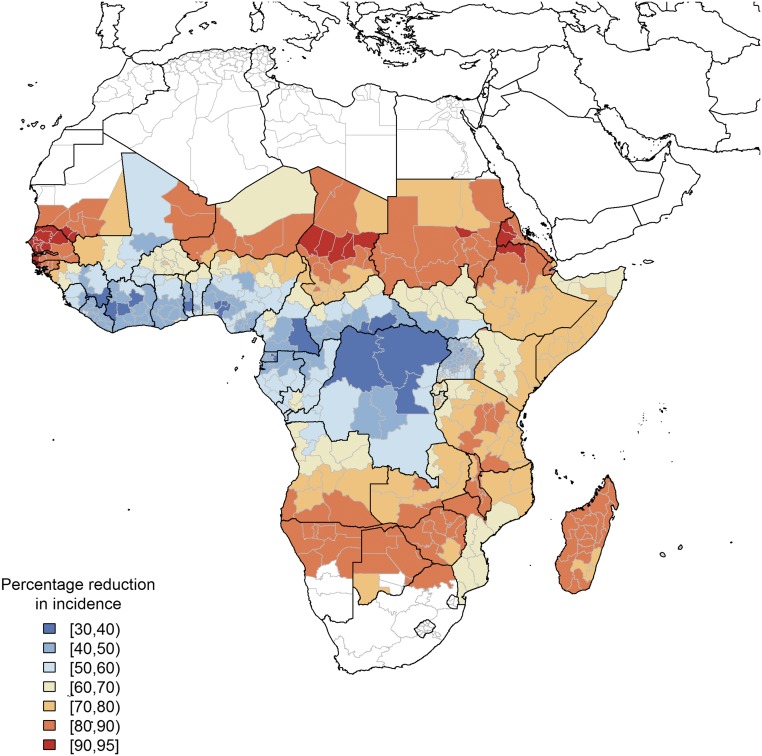
To further study the impact of prevalence and seasonality, we estimated the reduction in clinical malaria cases for the malaria endemic areas of sub-Saharan Africa based on previously used parameterization of malaria transmission heterogeneity (25) (SI Appendix, Fig. S2). This model is based on data from 2015 and does not take into account the results of continuing efforts in malaria control and prevention that may have changed the malaria landscape at the time when an isoxazoline drug will reach the market (26). However, it is in line with current assessments of mass “screening and treatment” and drug administration campaigns as provided by the Malaria Modeling Consortium and does provide a comparison with the current state of the art (27). Assuming a population coverage of 30%, the modeling results show that the intervention has the greatest impact (>70% reduction in clinical cases) in areas with low and very seasonal transmission, such as Senegal, Sudan, Madagascar, Namibia, Botswana, and Zimbabwe (Fig. 3). In these countries, a large proportion of annual transmission occurs in a short period. Therefore, administration of a single dose of an isoxazoline drug at the beginning of this season would dramatically decrease the number of cases over the full year. In the rest of the continent, isoxazoline administration is predicted to have less impact but still to result in a minimum 30% reduction in clinical cases. In terms of absolute impact, a 30% reduction in cases in countries like the Democratic Republic of the Congo, where the World Health Organization estimated 16–26 million cases in 2015, may be more significant than a 70% reduction in, for example, Senegal, where the estimated number of cases was 1.1–2.8 million (28).
Fig. 3.
Predicted impact of mass administration of an isoxazoline drug on malaria incidence in Africa. The figure shows cumulative reduction in incidence during 2 y of fluralaner/afoxolaner mass drug administration covering 30% of the population age >5 y, dosed once per year optimally timed in relation to the start of the transmission season. The model uses available data on regional disease prevalence in 2015 and a seasonality profile as illustrated in SI Appendix, Fig. S2.
Because up-to-date information on population size at the administrative unit scale is lacking (29), data on the absolute reduction in cases cannot be provided here. Nevertheless, from this simplified but illustrative approach (see Materials and Methods for limitations), the key message is that this intervention is predicted to have a significant impact on malaria transmission, with the greatest efficacy in areas with low transmission and a short transmission season.
Preliminary Assessment of Human Safety.
Nonclinical safety studies of fluralaner (30, 31) and afoxolaner (32–34) have been conducted for veterinary indications and can be leveraged to offer a preliminary assessment of the human safety of a single dose of these isoxazoline molecules. The reported oral toxicity profile of afoxolaner consists of a diuretic effect (rats only), effects secondary to a reduction in food consumption (rats and rabbits only), and occasional vomiting and/or diarrhea in dogs following high oral doses. No treatment-related effects on vomiting or diarrhea were noted in dogs following oral afoxolaner doses of up to 31.5 mg/kg once monthly for 3 mo (33). Fluralaner showed similar mild gastrointestinal events (diarrhea, vomiting, inappetence, and drooling) in dogs, no adverse events in a single-dose toxicity study in rats, and some histopathological abnormalities in lung, thymus, and liver in a repeat dose study in rats in the highest dose groups (400–600 mg/kg) with liver as the main target, showing hepatocellular fatty changes (30). Importantly, there was no significant neurotoxicity findings in rats or beagle dogs. This is consistent with both in vitro and in vivo data showing that these compounds have no significant interaction with the mammalian GABA receptor (12, 15). Overall, isoxazolines were well tolerated and showed mild and clinically monitorable adverse events. Moreover, both fluralaner and afoxolaner were negative in mutagenicity/genotoxicity studies, and no effects on embryo-fetal development in rats were observed below maternal toxic levels.
To assess a maximum human tolerated dose, we used the guidelines provided by the US Food and Drug Administration to translate the reported rat and dog no-adverse effect levels (NOAELs) to cognate human dose levels. These allometry guidelines are based on data showing that toxic endpoints scale well between species when doses are normalized to body surface area (35). The results show that anticipated median single doses of fluralaner (410 mg) and afoxolaner (260 mg) in humans are comparable to or lower than equivalent doses considered to be NOAELs based on acute and repeat-dose toxicity studies in rats and dogs (SI Appendix, Table S3). Interestingly, veterinary formulations of afoxolaner and fluralaner are based on racemic mixtures, whereas the S-enantiomer has been reported to be the active component against ectoparasites (15, 36). Thus, future development of a human application of these molecules will benefit from further characterization of the activity and toxicology profile of the active enantiomer, which may lead to a 50% reduction in the required dose. The veterinary application of the racemic form of these drugs provides a first assessment of their safety. Given the indirect clinical benefit of an isoxazoline-based intervention, an extremely favorable human safety margin would be essential, and detailed additional nonclinical studies (toxicology, pharmacokinetics, and metabolism) will be required before a first-in-human isoxazoline drug application can be filed with a regulatory authority. Alternatively, administration to livestock could be considered as a means of controlling the vector population. This could be effective in areas where a large proportion of blood meals is of livestock origin but would be less effective in areas where insects are mainly anthropophilic (37). Furthermore, the clinical impact would be significantly less compared with a scenario of human administration, as the direct effect on pathogen transmission would be lacking.
Conclusion
Drug-based vector control holds great promise for reducing disease burden. Pioneering work with ivermectin, a broadly used antihelminthic that shows adulticidal activity against Anopheles mosquitoes, has delivered an important proof of concept by demonstrating reduced survival of blood-fed mosquitoes and likelihood of malaria transmission (38, 39). However, ivermectin’s half-life (18 h in human) necessitates the use of multiple doses, a limitation that poses challenges in terms of logistics and drug compliance (6). Long-lasting formulations and higher doses are being pursued to solve this issue (9, 38), but these will require de novo safety studies, delaying entry into clinical studies and ultimate market approval. Effectively, development times of novel ivermectin formulations may be comparable to those of an isoxazoline drug, although the latter cannot benefit from the vast amount of pharmacovigilance data available for ivermectin. The doses of ivermectin used today to impact anopheline mosquitoes are insufficient for control of Aedes or Culex mosquitoes that transmit arboviral diseases (40, 41). In contrast, our data show potent effects of isoxazolines against a range of disease vectors.The impact of mass isoxazoline drug administration may be greatest in diseases where human is the single vertebrate host, as opposed to diseases that cycle between a human and an animal reservoir (e.g., West Nile Virus, leishmaniasis) (3, 42). As shown in our modeling data, even a 30% population coverage could lead to a substantial reduction in the clinical incidence of malaria or Zika fever. In conclusion, repurposing of isoxazoline compounds offers substantial promise for the development of a single-dose vector-control drug based on a novel mode of action compared with commonly used insecticides, with activity against a broad range of relevant disease vectors.
Materials and Methods
Chemical Extraction and Purification.
Fluralaner and afoxolaner were obtained by extraction and purification from Bravecto (Merck Animal Health) and Nexgard (Merial) pills, respectively. The pills were smashed into fine powder by mortar and pestle. A solvent of dichloromethane and methanol (1:1) was then added to the powder. The mixture was stirred at room temperature for 1 h and then filtered. The filtrate was concentrated under a rotary evaporator, and the product was purified by silica gel chromatography. LCMS and NMR matched values reported in the literature (43, 44).
Mosquito Colonies.
The colony of Anopheles stephensi (Sind-Kasur Nijmegen strain) (45) was maintained at the Radboud University Medical Center at 30 °C and 70–80% humidity and on a 12/12-h day/night cycle. The A. gambiae strains Kisumu and Tiassalé 13 and the A. aegypti strains New Orleans and Cayman were reared at the Liverpool Insect Testing Establishment. Kisumu and New Orleans are insecticide-susceptible laboratory strains (46). The New Orleans strain was originally colonized by the Centers for Disease Control and Prevention (47). The Tiassalé 13 strain was colonized from southern Côte D’Ivoire, where resistance to all classes of insecticide is found (46), and the Cayman strain was colonized from Grand Cayman, where A. aegypti are highly resistant to DDT and pyrethroid insecticides (47). Both resistant strains are routinely selected with insecticides to ensure the maintenance of resistance (0.75% permethrin for Cayman and 0.05% deltamethrin for Tiassalé), and profiled for resistance to a range of insecticides, including 4% dieldrin, to which Tiassalé is resistant but Cayman is susceptible (SI Appendix, Table S1). The colony of C. pipiens biotype pipiens originated from egg rafts collected from aboveground habitats in 2015 in Best, The Netherlands, and was maintained at Wageningen University, The Netherlands, on a 16/8-h day/night cycle at 23 °C and 60% humidity (48).
Systemic Test of Insecticidal Activity on Mosquitoes.
Blood meals containing 50% human red blood cells and 50% human serum were supplemented with different doses of either isoxazoline (3.16 µM, 1 µM, 316 nM, 100 nM, 31.6 nM, 10 nM, 3.16 nM, or 1 nM) diluted first in DMSO and then in serum to reach a final DMSO concentration of 0.1%. A vehicle control (0.1% DMSO) was included in all experiments. The supplemented blood meals were then fed to 3-to 10-d old female mosquitoes in a membrane feeder system previously described for standard membrane feeding assays (49). At 24 h after feeding, the numbers of dead and live mosquitoes among the fed population were recorded. Survival was calculated as the percentage of live mosquitoes at 24 h postfeeding out of the total number of fed mosquitoes per treatment.
Sand Fly Colonies and Feeding.
P. argentipes (originating from India, 2008) and L. longipalpis (originating from Brazil, 1991) have been reared at Laboratory of Vector Biology, Charles University, Prague, for many generations. Sand flies were maintained under standard conditions as described previously (50). For evaluation of insecticidal activity of isoxazolines on sand flies, we tested different doses of fluralaner and afoxolaner (31.6 nM, 100 nM, 316 nM, 1 µM, 3.16 µM, and 10 µM) diluted in DMSO and mixed with sterile defibrinated rabbit blood. One hundred females (3–5 d old) of both species were fed through a chick-skin membrane on rabbit blood with different doses of insecticides. Fully blood fed females were separated, and mortality was recorded at 24 h after a blood meal. The experiments with both isoxazolines were done twice in P. argentipes and once in L. longipalpis. A negative control (0.1% DMSO diluted in rabbit blood) was used in all experiments. The displayed results are normalized to control mortality (<3% in all experiments).
Data Analysis for Viability Assays.
IC50 values were calculated by applying a four-parameter logistic regression model using a least squares method to find the best fit using the GraphPad Prism 5.0 software package.
WHO Insecticide Susceptibility Test.
The 2- to 5-d-old female mosquitoes were routinely profiled for resistance to insecticides and colonies selected following the methodology recommended by the World Health Organization (51) and using test kits and insecticide impregnated papers supplied by Universiti Sains Malaysia.
Hepatocyte Metabolism Assays.
Isoxazolines and control compounds (7-ethoxycoumarin and 7-hydroxycoumarin; Sigma-Aldrich) were dissolved in DMSO at 10 and 30 mM, respectively, then diluted first 20-fold with 45% methanol in water and another 10-fold in prewarmed Williams’ Medium E. Cryopreserved human, dog, and rat hepatocytes (In Vitro Technologies) were thawed, isolated by Percoll gradient, and suspended in Williams’ Medium E. They were then dispensed into the wells of 96-well plates containing 10 µL of diluted compounds, to reach a final concentration of 0.5 × 106 cells/mL and either 1 µM isoxazolines or 3 µM control. After an incubation at 37 °C of 0, 15, 30, 60 or 90 min, the reaction was stopped with acetonitrile. The samples were then shaken for 10 min at 500 rpm and then centrifuged at 3,220 × g for 20 min. Supernatants were transferred and stored at 4 °C until LC-MS-MS analysis.
Human Dose Prediction.
Dog pharmacokinetic parameters reported previously (12, 18) were used to estimate corresponding human parameters and derive drug doses necessary to reach human plasma concentrations with insecticidal activity. Details of the allometry methods are provided in SI Appendix.
Modeling of Mosquito-Borne Disease Incidence.
The models of malaria and Zika fever incidence were adapted from published transmission models (19, 24) to include yearly administration of an isoxazoline drug efficacious against Anopheles and Aedes mosquitoes for 90 d. Details of the model parameters are provided in SI Appendix.
Estimation of Human No Adverse Effect Levels.
Mouse, rat, and dog NOAELs obtained from publicly available safety studies on fluralaner (30, 31) and afoxolaner (32–34) have been scaled to human values using allometry factors of 0.08, 0.16, and 0.54 respectively, assuming a body weight of 60 kg for humans (35).
Supplementary Material
Acknowledgments
We thank Geert-Jan van Gemert and Laura Pelser-Posthumus (Radboud University Medical Center) for their help with the A. stephensi experiments. We also thank David Malone from the Innovative Vector Control Consortium and Helen S. Williams and the other members of the Liverpool Insect Testing Establishment for help with mosquito experiments. Finally, we thank the DuPont Company for supporting the initiation of our research on isoxazolines. K.P., M.J., and P.V. were supported by the UNCE (204072) and Infravec2 (H2020 and 731060) Projects.
Footnotes
Conflict of interest statement: K.J.D. and R.W.S. hold stock in TropIQ Health Sciences.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801338115/-/DCSupplemental.
References
- 1.Benelli G, Mehlhorn H. Declining malaria, rising of dengue and Zika virus: Insights for mosquito vector control. Parasitol Res. 2016;115:1747–1754. doi: 10.1007/s00436-016-4971-z. [DOI] [PubMed] [Google Scholar]
- 2.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27:123–147. doi: 10.1111/j.1365-2915.2012.01034.x. [DOI] [PubMed] [Google Scholar]
- 3.Rizzoli A, et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Euro Surveill. 2015;20:21135. doi: 10.2807/1560-7917.es2015.20.20.21135. [DOI] [PubMed] [Google Scholar]
- 4.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika virus. N Engl J Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 6.Foy BD, Kobylinski KC, da Silva IM, Rasgon JL, Sylla M. Endectocides for malaria control. Trends Parasitol. 2011;27:423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govella NJ, Ferguson H. Why use of interventions targeting outdoor biting mosquitoes will be necessary to achieve malaria elimination. Front Physiol. 2012;3:199. doi: 10.3389/fphys.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco AO, Gomes MG, Rowland M, Coleman PG, Davies CR. Controlling malaria using livestock-based interventions: A one health approach. PLoS One. 2014;9:e101699. doi: 10.1371/journal.pone.0101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellinger AM, et al. Oral, ultra-long-lasting drug delivery: Application toward malaria elimination goals. Sci Transl Med. 2016;8:365ra157. doi: 10.1126/scitranslmed.aag2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoop WL, et al. Discovery and mode of action of afoxolaner, a new isoxazoline parasiticide for dogs. Vet Parasitol. 2014;201:179–189. doi: 10.1016/j.vetpar.2014.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Gassel M, Wolf C, Noack S, Williams H, Ilg T. The novel isoxazoline ectoparasiticide fluralaner: Selective inhibition of arthropod γ-aminobutyric acid- and L-glutamate-gated chloride channels and insecticidal/acaricidal activity. Insect Biochem Mol Biol. 2014;45:111–124. doi: 10.1016/j.ibmb.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Letendre L, et al. The intravenous and oral pharmacokinetics of afoxolaner used as a monthly chewable antiparasitic for dogs. Vet Parasitol. 2014;201:190–197. doi: 10.1016/j.vetpar.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Walther FM, Allan MJ, Roepke RK. Plasma pharmacokinetic profile of fluralaner (Bravecto) and ivermectin following concurrent administration to dogs. Parasit Vectors. 2015;8:508. doi: 10.1186/s13071-015-1123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walther FM, Paul AJ, Allan MJ, Roepke RK, Nuernberger MC. Safety of fluralaner, a novel systemic antiparasitic drug, in MDR1(−/−) collies after oral administration. Parasit Vectors. 2014;7:86. doi: 10.1186/1756-3305-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozoe Y, Asahi M, Ozoe F, Nakahira K, Mita T. The antiparasitic isoxazoline A1443 is a potent blocker of insect ligand-gated chloride channels. Biochem Biophys Res Commun. 2010;391:744–749. doi: 10.1016/j.bbrc.2009.11.131. [DOI] [PubMed] [Google Scholar]
- 16.Casida JE, Durkin KA. Novel GABA receptor pesticide targets. Pestic Biochem Physiol. 2015;121:22–30. doi: 10.1016/j.pestbp.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Weber T, Selzer PM. Isoxazolines: A novel chemotype highly effective on ectoparasites. ChemMedChem. 2016;11:270–276. doi: 10.1002/cmdc.201500516. [DOI] [PubMed] [Google Scholar]
- 18.Kilp S, Ramirez D, Allan MJ, Roepke RK, Nuernberger MC. Pharmacokinetics of fluralaner in dogs following a single oral or intravenous administration. Parasit Vectors. 2014;7:85. doi: 10.1186/1756-3305-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson NM, et al. Countering the Zika epidemic in Latin America. Science. 2016;353:353–354. doi: 10.1126/science.aag0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netto EM, et al. High Zika virus seroprevalence in Salvador, northeastern Brazil limits the potential for further outbreaks. MBio. 2017;8:e01390-17. doi: 10.1128/mBio.01390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saba Villarroel PM, et al. Zika virus epidemiology in Bolivia: A seroprevalence study in volunteer blood donors. PLoS Negl Trop Dis. 2018;12:e0006239. doi: 10.1371/journal.pntd.0006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkot T, Ichimori K. The PacELF programme: Will mass drug administration be enough? Trends Parasitol. 2002;18:109–115. doi: 10.1016/s1471-4922(01)02221-8. [DOI] [PubMed] [Google Scholar]
- 23.Cheah PY, White NJ. Antimalarial mass drug administration: Ethical considerations. Int Health. 2016;8:235–238. doi: 10.1093/inthealth/ihw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slater HC, Walker PG, Bousema T, Okell LC, Ghani AC. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: A modelling study. J Infect Dis. 2014;210:1972–1980. doi: 10.1093/infdis/jiu351. [DOI] [PubMed] [Google Scholar]
- 25.Walker PG, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: A modelling study. Lancet Glob Health. 2016;4:e474–e484. doi: 10.1016/S2214-109X(16)30073-0. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M, et al. Spectrum-malaria: A user-friendly projection tool for health impact assessment and strategic planning by malaria control programmes in sub-Saharan Africa. Malar J. 2017;16:68. doi: 10.1186/s12936-017-1705-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brady OJ, et al. Role of mass drug administration in elimination of Plasmodium falciparum malaria: A consensus modelling study. Lancet Glob Health. 2017;5:e680–e687. doi: 10.1016/S2214-109X(17)30220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Global Malaria Programme . World Malaria Report. World Health Organization; Geneva, Switzerland: 2015. [Google Scholar]
- 29.Cairns M, et al. Estimating the potential public health impact of seasonal malaria chemoprevention in African children. Nat Commun. 2012;3:881. doi: 10.1038/ncomms1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency, Committee for Medicinal Products for Veterinary Use 2016 CVMP assessment report for Bravecto for spot-on solution for dogs and cats. Available at www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/veterinary/002526/WC500163860.pdf. Accessed June 11, 2018.
- 31.U.S. Food and Drug Administration 2014 Corrected Freedom of Information Summary, NADA 141-426. Available at https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/1502. Accessed June 11, 2018.
- 32.U.S. Food and Drug Administration 2014 Freedom of Information Summary, NADA 141-406. Available at https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/912. Accessed June 11, 2018.
- 33.Committee for Medicinal Products for Veterinary Use 2013. CVMP assessment report for NexGard (European Medicines Agency, London), EMEA/V/C/002729/0000.
- 34.Merial Australia Pty Ltd 2014 Safety data sheet: NexGard. Available at www.frontlineplus.com.au/resource_centre//files/pdf/SDS_Afoxolaner_2%2027_Chewable%20Tablets_Masked_Formulation_061313_Australia_241014.pdf. Accessed June 11, 2018.
- 35.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research 2005 Guide for industry: estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Available at https://www.fda.gov/downloads/Drugs/Guidance/UCM078932.pdf. Accessed June 11, 2018.
- 36.McTier TL, et al. Discovery of sarolaner: A novel, orally administered, broad-spectrum, isoxazoline ectoparasiticide for dogs. Vet Parasitol. 2016;222:3–11. doi: 10.1016/j.vetpar.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Chaccour C, Rabinovich NR. Ivermectin to reduce malaria transmission II: Considerations regarding clinical development pathway. Malar J. 2017;16:166. doi: 10.1186/s12936-017-1802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alout H, Foy B. Ivermectin: A complementary weapon against the spread of malaria? Expert Rev Anti Infect Ther. 2016;15:231–240. doi: 10.1080/14787210.2017.1271713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouédraogo AL, et al. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: A double-blind, randomized, clinical trial. Clin Infect Dis. 2015;60:357–365. doi: 10.1093/cid/ciu797. [DOI] [PubMed] [Google Scholar]
- 40.Kobylinski KC, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derua YA, Kisinza WN, Simonsen PE. Differential effect of human ivermectin treatment on blood feeding Anopheles gambiae and Culex quinquefasciatus. Parasit Vectors. 2015;8:130. doi: 10.1186/s13071-015-0735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akhoundi M, et al. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl Trop Dis. 2016;10:e0004349. doi: 10.1371/journal.pntd.0004349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Annis GD. 2009. E.I. Du Pont De Nemours and Company Patent Application WO 2009126668.
- 44.García-Reynaga P, Zhao C, Sarpong R, Casida JE. New GABA/glutamate receptor target for [3H]isoxazoline insecticide. Chem Res Toxicol. 2013;26:514–516. doi: 10.1021/tx400055p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldmann AM, Ponnudurai T. Selection of Anopheles stephensi for refractoriness and susceptibility to Plasmodium falciparum. Med Vet Entomol. 1989;3:41–52. doi: 10.1111/j.1365-2915.1989.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 46.Edi CV, Koudou BG, Jones CM, Weetman D, Ranson H. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, southern Côte d’Ivoire. Emerg Infect Dis. 2012;18:1508–1511. doi: 10.3201/eid1809.120262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris AF, Rajatileka S, Ranson H. Pyrethroid resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg. 2010;83:277–284. doi: 10.4269/ajtmh.2010.09-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogels CB, Fros JJ, Göertz GP, Pijlman GP, Koenraadt CJ. Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasit Vectors. 2016;9:393. doi: 10.1186/s13071-016-1677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponnudurai T, et al. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology. 1989;98:165–173. doi: 10.1017/s0031182000062065. [DOI] [PubMed] [Google Scholar]
- 50.Volf P, Volfova V. Establishment and maintenance of sand fly colonies. J Vector Ecol. 2011;36(Suppl 1):S1–S9. doi: 10.1111/j.1948-7134.2011.00106.x. [DOI] [PubMed] [Google Scholar]
- 51.WHO Pesticide Evaluation Scheme . Test Procedures for Insecticide Resistance Monitoring in Malaria Vectors, Bio-Efficacy and Persistence of Insecticides on Treated Surfaces. World Health Organization; Geneva, Switzerland: 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.