Significance
A majority of emerging infectious diseases in humans are transmitted from animals. It is generally agreed that our behavior can influence our exposure to such pathogens, but little is known regarding our role in shaping evolution in such pathogens. Such understanding would aid in their control, to the benefit of public health. Our results indicate that expansion of agriculture influenced not only the biogeography but also the virulence of Toxoplasma gondii. By linking landscape ecology to parasite virulence, our framework contributes a fundamentally unique perspective on the ecology and evolution of infectious disease.
Keywords: Toxoplasma gondii, population genetics, virulence, evolution, mathematical modeling
Abstract
A majority of emerging infectious diseases in humans are zoonoses. Understanding factors that influence the emergence and transmission of zoonoses is pivotal for their prevention and control. Toxoplasma gondii is one of the most widespread zoonotic pathogens known today. Whereas only a few genotypes of T. gondii dominate in the Northern Hemisphere, many genotypes coexist in South America. Furthermore, T. gondii strains from South America are more likely to be virulent than those from the Northern Hemisphere. However, it is not clear what factor(s) shaped modern-day genetic diversity and virulence of T. gondii. Here, our analysis suggests that the rise and expansion of farming in the past 11,000 years established the domestic cat/mouse transmission cycle for T. gondii, which has undoubtedly played a significant role in the selection of certain linages of T. gondii. Our mathematical simulations showed that within the domestic transmission cycle, intermediately mouse-virulent T. gondii genotypes have an adaptive advantage and eventually become dominant due to a balance between lower host mortality and the ability to superinfect mice previously infected with a less virulent T. gondii strain. Our analysis of the global type II lineage of T. gondii suggests its Old World origin but recent expansion in North America, which is likely the consequence of global human migration and trading. These results have significant implications concerning transmission and evolution of zoonotic pathogens in the rapidly expanding anthropized environment demanded by rapid growth of the human population and intensive international trading at present and in the future.
Most emerging infectious diseases in humans are zoonoses (1, 2). Among these pathogens, the zoonotic protozoan parasite Toxoplasma gondii is perhaps the most ubiquitous, having been identified in the tissues of a variety of animal hosts, including both mammalian and avian species. T. gondii is estimated to chronically infect one-third of the world’s human population, causing ocular toxoplasmosis in immunocompetent individuals and often-fatal encephalitis in the immunocompromised, as well as birth defects following vertical transmission to developing fetuses (3, 4). Globally, this parasite has distinct population structures for each major geographic region examined, with a striking contrast between the highly diverse, epidemic structure of the Central/South American region and the more clonal populations found in all other areas, wherein North America, Europe, North Africa, and East Asia are each dominated by particular clonal genotypes (5–8).
Do regional differences merely reflect subdivisions resulting from the founder effect and geographical separation, or has natural selection engendered and reinforced regional differences? The latter possibility seems plausible, given how markedly strains differ in their virulence to house mice, a phenotype for which the molecular basis has been subjected to careful study. Polymorphisms in several key effector rhoptry (ROP) proteins of the parasite secreted into host cells mediate different disease outcomes in the house mouse Mus musculus domesticus (9–13). This variability provides the primary systematic contrast between the three archetypal lineages, including the type I strains that are lethal to house mice, causing rapid death within 2 to 3 wk after infection with only one viable parasite; the type II strains that are intermediately virulent (LD50 = 102–104 parasites); and the type III strains that are predominantly nonvirulent to mice (LD50 > 105 parasites) (14). More virulent parasite strains are better able to superinfect those mice previously exposed to, and chronically infected with, a less virulent T. gondii strain (SI Appendix, Table S1). Chronic infection with a nonlethal strain of T. gondii likely provides sufficient immune protection for the mice to survive what would normally be a fatal infection by a highly virulent strain. Such an interaction would favor transmission of virulent parasites. In this way, more virulent strains may be more transmissible, especially in settings where infection is prevalent and strains must compete for access to previously infected hosts. However, this advantage might be offset by the high mortality rate (often 100%) associated with infection of naive mice, which could reduce the chances of subsequent transmission to new hosts. The observed natural variability in murine virulence might therefore have evolved as differing functional responses to outcomes that favor or disfavor transmission.
We hypothesize that the domestic life cycle of T. gondii, established by the advent of farming within the past 11,000 y, has allowed the parasite to adapt to the newfound transmission opportunities that disfavor virulence to mice and favor expansion of less virulent lineages. To test this hypothesis, we examined the impact of agricultural development on the T. gondii life cycle, and the patterns of mouse virulence among major clonal lineages worldwide, through the review and synthesis of relevant literature. In addition, we used an in silico modeling approach to simulate the domestic and sylvatic life cycles, monitoring infection and environmental contamination of lethal (highly virulent) and nonlethal (intermediately virulent and nonvirulent) strains of T. gondii. Finally, we analyzed the genetic diversity of globally distributed clonal type II samples using multilocus microsatellite markers to infer the lineage’s origin and evolution in recent history.
Results and Discussion
The Impact of Agricultural Development on the Life Cycle of T. gondii.
Before the rise of agrarian societies, transmission of T. gondii would have occurred through a variety of wild felids and intermediate host species in the natural environment, termed the sylvatic life cycle (Fig. 1). The small rodent house mouse (Mus musculus) was one among a large number of other intermediate hosts. M. musculus arose and diverged in the vicinity of the Indo-Pak subcontinent between 1.7 and 0.7 Mya, and its three major subspecies (M. m. musculus, M. m. domesticus, and M. m. castaneus) became adapted to life in woodland, shrubland, and grassland steppe, and radiated to different regions of the Eurasian continent and North Africa within the past 100,000 y (15) (Fig. 2A). However, house mice may only have played a minor role in transmission of T. gondii in the natural environment, given that they accounted for only a very minor proportion of small mammals before the development of agriculture (16).
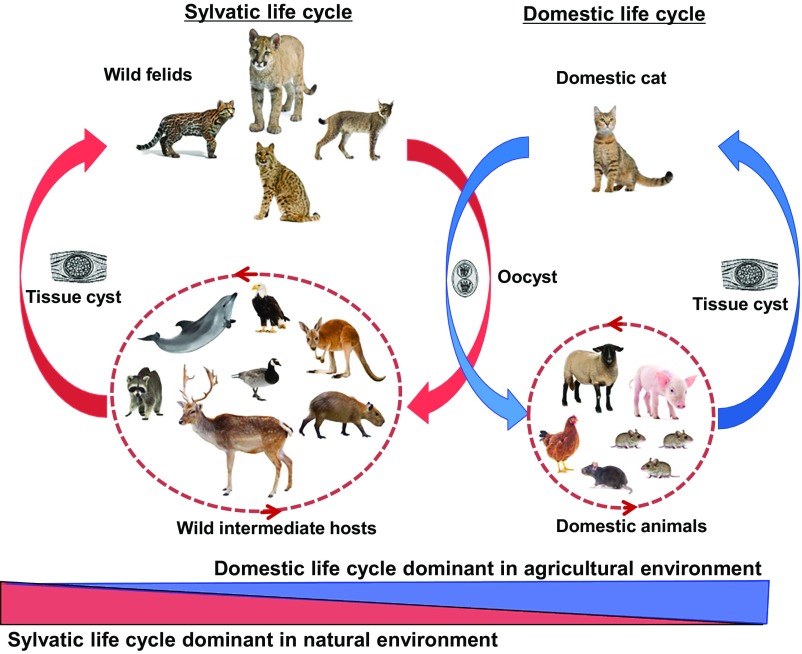
Fig. 1.
Life cycle of T. gondii. Parasites are shed by definitive feline hosts in the form of oocysts, which may then be ingested by intermediate hosts. Transmission back to definitive hosts may occur by ingestion of infected intermediate host tissue containing parasites in tissue cysts. The sylvatic cycle includes many definitive wild feline host species and varied mammalian and avian intermediate host species, whereas the domestic cycle includes the domestic cat as a definitive host and the house mouse as an important intermediate host. Transmission may also occur through scavenging among the intermediate hosts.
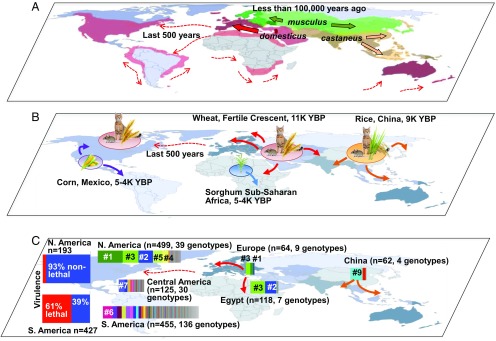
Fig. 2.
Association of global expansion and distribution of house mice, human agriculture, and population structure of T. gondii. (A) Global distribution of the three major house mouse subspecies, M. m. domesticus (red), M. m. musculus (green), and M. m. castaneus (brown). Colored arrows indicate directions of mouse subspecies expansion over the indicated time frames. (B) Origins and expansion of agriculture and association with domestic cats and house mice. Indicated are cultivation of wheat in the Middle East, rice in Southeast Asia (both associated with domestic cats and house mice), corn in South and Central America, and sorghum in Africa. Arrows indicate directions of outward agricultural expansion, including migration of European settlers to North America and concomitant exportation of domestic cats, house mice, and livestock. (C) Overall population structure and mouse virulence of T. gondii. Charts indicate proportions of color-coded T. gondii PCR restriction fragment length polymorphism (RFLP) genotypes present on corresponding continents. Populations in the Northern Hemisphere are largely clonal in structure, with small numbers of highly dominant lineages, whereas the South American population is much more diverse, without notably dominant individual genotypes. Most isolates from North (N.) America were nonvirulent to mice, whereas the opposite was true for South (S.) America. The numbers following the number sign (#) are PCR-RFLP genotype numbers.
Human agricultural societies independently arose from several locations worldwide between about 11,000 and 4,000 y ago (Fig. 2B) (17). Among these locations, southwestern Asia (the Fertile Crescent), central China, Mexico, and western Africa were the cradles for the domestication and cultivation of grain crops. Wheat was first cultivated about 11,000 y ago in the Fertile Crescent, rice 9,000 y ago in central China, corn 5,000 y ago in Mexico, and legumes 5,000 y ago possibly in western Africa (17). Farming in the Fertile Crescent and central China originated much earlier than in Mexico and western Africa; it spread to large areas surrounding the centers of origin and likely had a broader impact on human societies in these regions. Within a few millennia, the farming system developed in the Fertile Crescent had spread over vast areas of the Old World: to Britain in the west, to central Asia in the east, to Egypt and North Africa in the south, and to Pakistan and India in the southeast. Similarly, agriculture developed in central China also dispersed to a wide area, reaching southeastern Asia and eastern India rapidly. Following the initial domestication of plants, sedentary agricultural societies began to develop, enabling the production of reliable and more abundant food sources, and subsequently leading to rapid expansion of the human population (17). The house mouse is remarkable in its propensity for commensalism with humans in the context of a settled agricultural environment, a relationship that began developing with the very beginnings of agriculture and the creation of the first grain stores in the Fertile Crescent about 11,000 y ago (18). The house mouse now occupies an exceptionally widespread geographical range and is arguably the most successful and ubiquitous invasive mammal next to human beings (16). Easy access to food, such as stored grains in human settlements, may have allowed the mouse population to expand rapidly. Zooarchaeological studies of Neolithic sites in Syria and Turkey revealed that the occurrence of house mice among small mammals was less than 5% in the vicinities of hunter-gatherer communities about 12,000 y ago, but this proportion jumped to an overwhelming 80% in association with the earliest human agricultural settlements of 11,000 y ago (16). Genetic assessment of domestic cats and their wild progenitors has revealed that cats were domesticated in the Near East, coincident with agricultural settlement in the Fertile Crescent, where they probably began their commensal association with humans, feeding on the rodent pests, such as house mice, that infested the grain stores of the first farmers (19, 20).
Therefore, the rise of agriculture established the domestic life cycle for T. gondii by bringing together mice and cats into human settlements (Fig. 1). Abundance of food inevitably led to a high density of house mice in this anthropic environment, and the control of such rodent species was likely a major incentive for the initial domestication of cats. As a consequence, mice would likely have become a more important prey animal for domestic cats early in the history of farming, in turn, sustaining a high density of cats. Such an intimate association of mice and cats in a relatively compact space would have led to a high contamination of oocysts in the environment, causing increased infection rates of T. gondii in farm animals (21).
The Patterns of Mouse Virulence Among Major Clonal Lineages Worldwide.
T. gondii has a distinct global population structure, with a highly diverse, endemic genetic structure in the Central/South American region and essentially clonal populations in all other regions (5–8) (Fig. 2C). By synthesizing published genotypic and virulence data, summarized in a recent report (8), we found that 61% of 427 isolates characterized from South/Central America were highly virulent to house mice, whereas only 7% of 193 isolates from North America were virulent. Among these samples, about 80% were from domestic animals and 20% were from wildlife, and there is no evidence of an association of virulence with host species. These observations suggest that virulence is far more characteristic of T. gondii strains in Central/South America than in North America.
In this study, we examined parasite virulence in laboratory mice (derived from house mice) for the genotypes that predominate in the Northern Hemisphere, and for a frequently occurring genotype in South America and Africa (SI Appendix, Table S2). We found that all predominant genotypes from the Northern Hemisphere are intermediately virulent or nonvirulent to laboratory mice. However, the most frequently isolated genotype in Brazil (ToxoDB genotype 6) is highly virulent to these mice. The virulence of T. gondii may have placed a strong selective pressure on the evolution of defense mechanisms in mice. This is evidenced by the murine IFN-γ-inducible, 47-kDa, immunity-related GTPase (IRG) protein family. Both laboratory and wild mice in Eurasia have two highly polymorphic clusters of IRGs (22). The IRG proteins are essential for mouse immunity to defend against T. gondii infection. However, laboratory mice are highly sensitive to virulent T. gondii strains, whereas the wild mice are more resistant. This is due to expression by virulent strains of specific ROP protein alleles that confer resistance to laboratory mouse IRG proteins (9–13, 23, 24). The existence of polymorphisms in both mouse IRG proteins and parasite ROP proteins suggests that the mice and T. gondii have been engaged in a long-term coevolutionary arms race (13, 25). The difference in resistance to T. gondii infection between house mice and wild mice will inevitably exert different evolutionary pressures to shape the population structure of the parasite in human vs. wild environments.
Simulation of Transmission Dynamics Among T. gondii Strains with Different Levels of Virulence.
To assess the consequences of virulence levels on long-term infection dynamics of T. gondii, we performed simulations of parasite infection/environmental contamination for both the domestic and sylvatic transmission cycles. There are three major components of the transmission cycle: the definitive host, the intermediate host, and the environment (Fig. 1 and SI Appendix, Supplementary Text and Fig. S1). Three virulence levels of T. gondii were used, including the highly virulent (HV), intermediately virulent (IV), and nonvirulent (NV) types, each causing different mortality rates in infected house mice during domestic cycle simulations. Transmission was simulated for two modes: (i) a mode in which superinfection is permitted in chronically infected mice challenged with a more virulent genotype and (ii) a mode in which only single infection is permitted, without the possibility of superinfection. An example of simulation dynamics is presented in Fig. 3 A and B. For the domestic cycle, the results showed that when superinfection was modeled, the HV type could not be maintained in transmission and was eliminated quickly (Fig. 3 A and C). However, the IV and NV types were able to become fixed in the population, with the IV type being more likely to become fixed than the NV type. In contrast, when infection was simulated without superinfection, although the HV type was still consistently eliminated, the IV and NV types were able to become fixed with equal probability (Fig. 3D).
Fig. 3.
T. gondii transmission dynamics in the domestic and sylvatic cycles. (A) Simulation of domestic life cycles with superinfection for 25 y. The IV type becomes fixed, while the HV and NV types disappear. (B) Simulation of sylvatic life cycles with superinfection for 25 y. The HV type becomes fixed, while the NV and IV types disappear. (C) Domestic cycle with superinfection. There were significant differences in the frequencies of fixation among the three populations, with IV > NV > HV types (P < 0.001). (D) Domestic cycle without superinfection. The IV and NV types were significantly higher than the HV type (P < 0.001). There was no difference between the IV and NV types (P > 0.01). (E) Sylvatic cycle with superinfection. The HV type was significantly higher than the IV and NV types (P < 0.001). The IV type was also significantly higher than the NV type (P < 0.01). (F) Sylvatic cycle without superinfection. There was no difference among the three populations (P > 0.01). ANOVA was performed using SAS (9.4) proc glimmix. *P < 0.01; **P < 0.001; #P > 0.01.
To model the sylvatic cycle, in which most intermediate hosts are resistant to T. gondii, we set the mortality of infected animal hosts to a low level, indistinguishable among the genotypes. When superinfection was introduced in this scenario, the HV type was more likely to become fixed than either the IV or NV type, and the IV type was more likely to become fixed than the NV type (Fig. 3 B and E), indicating a general fitness advantage for strains with greater virulence and superinfection probability. However, when each animal was modeled to encounter at most one infection without the possibility of superinfection, each of the three genotypes had the same probability of becoming fixed (Fig. 3F).
We also examined the dynamics of simulated populations over a range of values for both superinfection and virulence levels for the domestic cycle. Increasing the probability of superinfection increased the prevalence of the IV type while decreasing that of the NV type in cats, mice, and the environment (SI Appendix, Fig. S2), whereas increasing the virulence of the IV type decreased its prevalence (SI Appendix, Fig. S3), indicating that superinfection and virulence could be two counterbalanced aspects of a process that has influenced the prevalence of different T. gondii genotypes.
Overall, the outcome of simulations employing the superinfection virulence model most closely mirrors actual real-world observations, wherein the majority of highly prevalent T. gondii genotypes in Europe, North Africa, North America, and China are intermediately virulent, with none being lethal and few being nonvirulent. In contrast, the majority of T. gondii isolates from Central/South America (where sylvatic transmission is still widespread) are highly virulent to mice, with coexistence of less virulent genotypes (SI Appendix, Table S2). This pattern is correlated with the distribution of agriculture, house mice, and domestic cats (Fig. 2). These results imply a balance between factors that favor or disfavor the transmission of parasites when traits that imperil naive hosts also augment superinfection potential. Where house mice are important hosts, dominance of IV and NV T. gondii genotypes would be expected to result. In addition, the low diversity of primary and intermediate hosts in agricultural environments may further reduce the heterogeneity of T. gondii, whereas the highly diverse felids and intermediate hosts in tropical Central/South America may differentially select heterologous T. gondii genotypes with HV phenotypes, thereby maintaining a genetically diverse parasite population. However, this hypothesis remains to be tested. It would seem profitable to examine, in the future, what adaptations this parasite may have made to other intermediate hosts prevalent in anthropic environments (e.g., livestock, other rodent pests, cats); at present, these hosts are generally considered resistant to clinical disease, but we lack much information on their specific responses to particular parasite genotypes.
The Most Recent Common Ancestor of the Dominant Type II T. gondii Lineage.
To reveal the most recent common ancestor (MRCA) and transmission pattern of the widespread type II lineage of T. gondii, we genotyped 296 type II and five non-type II samples (Dataset S1) using 15 microsatellite markers reported previously (26). Within-population analysis showed that samples from Europe have the highest diversity, followed by those from Africa, Central/South America, and North America (Table 1). Between-population analysis indicated that European and African populations are more similar to each other than they are to North American or Central/South American populations. In addition, both North American and Central/South American populations are more similar to European populations than to African populations (Table 2). The rooted neighbor-joining tree showed that the samples from Europe and Africa are placed near the root of the tree and samples from North America are clustered at the tip (SI Appendix, Fig. S4). Together, these results suggest that the MRCAs of type II T. gondii have originated from the Old World (likely Europe), been transmitted globally, and expanded in North America only recently.
Table 1.
Within-population diversity of type II samples
| Data category | Africa | Europe | South/Central America | North America |
| No. of isolates | 59 | 116 | 18 | 99 |
| No. of microsatellite genotypes | 43 | 90 | 11 | 59 |
| Average gene diversity | 0.3504 ± 0.1903 | 0.3686 ± 0.1975 | 0.2641 ± 0.1544 | 0.2193 ± 0.1259 |
Geographical regions that have less than 10 samples are excluded from analysis due to small sample size. Supportive data for this table are provided in Dataset S1.
Table 2.
Between-population pairwise differences of type II samples
| Regions | Africa | Europe | Central/South America | North America |
| Africa | 0.00000 | |||
| Europe | 0.08446 | 0.00000 | ||
| Central/South America | 0.24601 | 0.13984 | 0.00000 | |
| North America | 0.32890 | 0.17325 | 0.38623 | 0.00000 |
There are significant differences between all pairwise comparisons (F-statistic, P < 0.001). Supportive data for this table are provided in Dataset S1.
Conclusion and Perspective
In this study, our findings suggest that virulence to house mice and the capability of overcoming existing chronic infection through superinfection may be important factors that affect the genetic diversity and population structure of T. gondii. The domestic and sylvatic transmission cycles of T. gondii likely represent distinct regimes of selection. The domestic life cycle associated with human agricultural settlements may have favored the development of clonal populations dominated by genotypes that are less virulent to house mice. In contrast, within natural environments, such as the tropical Amazon rainforest, the sylvatic cycle seems to favor development of a diverse population supporting highly virulent T. gondii genotypes. The extant conditions in tropical South America, where much of the Amazon rainforest region remains relatively unaffected by the agricultural landscapes that predominate in the Northern Hemisphere, likely resemble the primordial state of the parasite, whereas the genetic uniformity observed elsewhere represents a relatively recent anthropogenic state. At present, very little is known about T. gondii population genetics in tropical regions in Africa and Asia; future studies in these areas will provide us with important information regarding evolution of T. gondii in tropical regions in general.
Habitat structure may slow or even prevent competitive exclusion (27), and “virulence” has sometimes been conceived as the ability of a pathogen to infect those animals already harboring chronic infection with other parasite strains (28, 29). The rates of superinfection have been shown to determine whether virulent or avirulent parasites should prevail and whether coexistence or competitive exclusion should result (28, 30). Our model affirms the strong influence that superinfection rates and host population dynamics have on competing parasites, here modeled in a spatial array, instantiating tradeoffs between strategies that maintain host longevity (in the case of T. gondii, prolonging opportunities for female mice to transmit infection to their offspring and for all mice to fall prey to cats) against strategies that displace prior infections, favored only in scenarios entailing frequent superinfection.
Besides virulence and superinfection, other factors may contribute to the contemporary genetic diversity and population structure of T. gondii, characterized by the dominance of a few strains that are not highly virulent to mice in the Northern Hemisphere but by diverse strains with high virulence in South America. First, the dramatic expansion of human civilization, most notably in the short time span of the past several hundred years, has brought striking changes in the global landscape, altering faunal populations throughout the world (31, 32). The spread of domesticated animals with human settlements has generally resulted in the reduction or, often, eradication of many diverse wild animal species and their replacement with large, uniform populations of domesticated animals. This shift in host population structure would be expected to produce correspondingly significant changes in the selective fitness of parasitic microbes adapted to survival in a particular region. Indeed, analysis of genetic diversity among numerous animal parasites appears to implicate human activity as an important influence on population structure (33). In contrast, high diversity of fauna in tropical rainforests, such as the Amazon River Basin, will likely facilitate and maintain high diversity among parasite populations (34).
Second, coevolution of the host and parasite may favor transmission of certain parasite strains. A recent study on T. gondii strains isolated from anthropized regions of French Guiana showed that the parasites are more transmissible through domestic cats than those isolated from jungle ecosystems (35), suggesting that the capacity for transmission in domestic cats may be an important source of selective pressure in human-associated environments. Such anthropic environments established the domestic transmission cycle for T. gondii (Fig. 1), which may impose a different selective pressure on the evolution of this parasite than the sylvatic cycle. Consequently, expansion of agriculture from the centers of their origins may facilitate the spread of T. gondii genotypes that are adapted to the agricultural environments and replace the native T. gondii genotypes in the path of agricultural expansion. Third, domestic cats may heavily contaminate areas surrounding the households and farms with T. gondii oocysts (36), leading to increased infection rates in farm animals and resulting in clonal expansion of a subset of T. gondii genotypes. Expansion of agriculture would further distribute this subset of parasite strains, consequently reducing regional diversity of the parasite (21).
One question is why different clonal genotypes dominate in the Northern Hemisphere. This may be explained by the founder effect in addition to selective pressure. It is reasonable to assume that T. gondii as a species had already radiated worldwide well before the time of human agricultural development. It is also likely that the T. gondii strains in different geographical regions had already diverged by that point, providing the genetic background of founder strains for selection at different geographic origins of agriculture. In addition, T. gondii genotypes that were selected and adapted to agricultural environments will likely have spread with the expansion of agriculture, and possibly replaced the native parasite genotypes in the path of such expansion. A case in point is our analysis of the global type II lineage of T. gondii, the results of which suggest an Old World origin but recent expansion in North America, which is likely the consequence of recent global human migration and trading. This may also explain the wide distribution of the Chinese 1 strain of T. gondii (ToxoDB 9) in East Asia.
This study provides insight into the probable mechanisms governing parasite population dynamics since the expansion of human civilization and may also aid in predicting the future course of evolution in rapidly developing areas of the world, such as the tropical regions of South America. It is reasonable to predict that, in the long term, as human influence continues to expand and more of Earth’s ecosystems are altered, the global T. gondii population structure may continue to evolve toward uniformity and reduced mouse virulence. In the short term, however, encroachment into unexplored natural environments, such as the tropical Amazon, will potentially expose us to more virulent T. gondii strains, increasing the risk of contracting more severe forms of toxoplasmosis. Although a causal association has not yet been definitively demonstrated between parasite genotype and human disease outcome, mounting evidence supports a correlation between the mouse virulence of T. gondii strains and the severity of symptoms in human infections. This could have important implications regarding the epidemiological trajectory of human toxoplasmosis in developing regions, where highly virulent strains currently predominate. In Brazil, where, as noted, the T. gondii population is highly diverse and comprises many unique genotypes with much higher average virulence in mice compared with populations in the Northern Hemisphere, severe symptoms have been found to be associated with ocular toxoplasmosis at a rate fivefold that of European cases (37). In French Guiana, a severe form of systemic acquired toxoplasmosis has emerged among immunocompetent adults. This form of the disease, termed “Amazonian toxoplasmosis,” generally requires intensive medical treatment and has resulted in deaths from multiple organ failure (38, 39). In cases where parasites have been isolated from patient tissues, isolates were found to possess unique genotypes that were highly virulent in mice (38, 40–42). Even in North America, where the type II and III clonal lineages are highly dominant, clinical symptoms have been reported to be disproportionately associated with infections caused by nonclonal strains (43). Thus, evolutionary trends driven by selective pressures in mice may also play a role in governing future developments of human disease. Although the long-term effect of human agricultural expansion is expected to reduce the genetic diversity and virulence of T. gondii throughout much of the world, human-associated changes to ecosystems, such as dramatic increases in the densities of house mouse and domestic cat populations (including feral cats) on farms, may lead to heavier environmental contamination of T. gondii in animals (29), resulting in increased infection rates of T. gondii within human populations.
Aside from house mice, other intermediate hosts with variable sensitivity to particular strains of T. gondii could similarly exert selective pressure on the parasite. Wild rabbits (lagomorphs), which are highly sensitive to T. gondii infection (3), have been reported to be the major diet for domestic cats and bobcats in certain regions of Great Britain (44, 45). Among the small rodents in North America, the deer mouse (Peromyscus maniculatus) is the most abundant species (46). Deer mice can be easily infected with T. gondii, and infections may be transmitted vertically to offspring (47). Therefore, they may play an important role in T. gondii transmission in North America, where the dominant genotype (type 12) in wildlife is considered intermediately virulent to house mice (48–51). The impact of genetic variation in other intermediate hosts, such as rats, cats, sheep, chickens, and pigs, is not clear. Given that these hosts are more resistant to T. gondii infection than house mice, regardless of parasite genotype, these animals may favor more virulent parasite strains if doing so maximizes their basic reproductive rate (52), akin to what we have observed in sylvatic isolates and in model conditions intended to represent the sylvatic cycle (Fig. 3E). Similarly, it would be interesting in future efforts to examine any differences among strains in their capacity to manipulate intermediate host behavior in ways that make it more likely for such hosts to fall prey to cats (53), the varying effects of distinct genotypes on the reproductive fitness of mice (54), and the consequence of strain variation on sexual transmission of T. gondii (55).
While our current study provides a general view regarding the consequences of domestic versus sylvatic transmission cycles on the population genetics in T. gondii, many details are still missing. Future studies are needed to better understand the role of different animal hosts in the transmission of T. gondii and their sensitivity to infection by different parasite strains, as well as the influence of different anthropic settings, such as urban versus rural environments, on the population genetics of this ubiquitous protozoan parasite. Elucidation of the selective pressures shaping the evolutionary trajectory of T. gondii will also aid in understanding the transmission patterns of zoonotic pathogens in general, thereby laying the foundation for the development of effective parasite control strategies.
Materials and Methods
Determination of T. gondii Virulence in Mice Based on Published Reports.
Data regarding mouse virulence of South, Central, and North American T. gondii isolates were obtained from references in a recent review paper (8). Specific information regarding methods for virulence determination may be found in the original publications. The majority of data were obtained via bioassay, in which mice were inoculated with tissue from infected host animals and observed for disease presentation and mortality. Some studies also included inoculation via i.p. injection of tachyzoites from cell-cultured strains, or via oral inoculation with oocysts. Given the variation of methodologies used, parasite virulence was roughly categorized as virulent (lethal), killing all infected mice, and nonvirulent (nonlethal), from which surviving mice are either seropositive or produce tissue cysts of the parasites in their brain tissues.
Determining Cumulative Mortality of Mice Infected with the Dominant Genotype in East Asia.
To determine cumulative mortality in mice infected with genotype 9 (dominant in East Asia), we performed mortality assays in outbred Kuming mice. Use of animals was approved by the Institutional Animal Care and Use Committee of Lanzhou Veterinary Research Institute. We assayed three isolates of genotype 9 from different animal hosts (cat, pig, and sheep) in different regions in China, together with reference genotype 10 (type I, RH) and genotype 1 (type II, PRU). Low-passage parasite strains were used to minimize change of virulence phenotype during intensive passages in cell culture. Tachyzoites of T. gondii strains were prepared as described previously (56). Six groups of 10 mice each were inoculated with 10−1, 100, 101, 102, 103 and 104 tachyzoites by i.p. injection, respectively. Mortality and time to death were recorded for 30 d postinfection. Blood samples were collected on day 30 postinfection from the retroorbital sinus with sodium citrate. Antibodies against T. gondii were determined using the modified agglutination test (MAT). Sera with MAT titers of 1:25 or higher were considered positive for T. gondii antibodies. Cumulative mortality was determined by the number of killed mice divided by the total number of infected mice in three dosage groups in which the lowest one only infected a proportion of mice (survivors excluded if MAT-negative).
Simulation of T. gondii Life Cycles.
A model was developed to simulate transmission dynamics of T. gondii in domestic and sylvatic life cycles to envision the implications of a tradeoff when increased virulence also increases the ability of a strain to superinfect a chronically infected mouse. The model was adapted from previously published reports (57, 58). This model takes into account the complete life cycle of T. gondii, which includes the transitions of the parasite from cats to the environment through feces, from the contaminated environment to mice through oocysts, from mice to cats through tissue cysts, and from the environment to cats through oocysts, as well as the vertical transmission among mice. In this study, we included superinfection in the model. A flow chart for the model is presented in SI Appendix, Fig. S1. The simulation was run using the software NetLogo, version 5.1.0 (https://ccl.northwestern.edu/netlogo/). The simulation code is shown in Dataset S2. Because many of the parameters involved in this model are not clearly defined by experimental data, we carried out simulations under a variety of conditions relating to mouse mortality rate and probability of reinfection for parasite virulence type, as well as host and parasite starting population sizes and duration of the simulation (more information about the modeling is provided in SI Appendix, Supplementary Text). Simulated environments consisted of variables that included cats, mice, the environment, and parasites. The parasites comprised three categories: NV, IV, and HV. The cats and mice were programmed to move randomly within the area of a spatial patch, interacting when a cat and a mouse occupied the same point on the patch. Co-occurrence resulted in the cat consuming the mouse. If the mouse were a carrier of T. gondii, the cat would become infected. If the consumed mouse were infected with two different parasite strains, the cat would randomly become infected by one or the other strain. Following infection, cats shed oocysts into the environment, which persisted for a specified period of time. Encounter of oocysts by mice or other cats resulted in those animals becoming infected. If mice were to become infected by T. gondii, they would die between 7 and 21 d later with a probability specified for each parasite virulence category. Survivors remained chronic carriers of the parasite for the duration of their life span. If a chronically infected mouse were to encounter an oocyst of a different virulence category, the strain would reinfect that mouse with a probability value specified for each virulence category. Vertical transmission was allowed with a specific probability for all T. gondii strains. Birth and natural death rates of cats and mice were also separately specified. Simulations were run for specified numbers of days, with one event occurring for each organism per day; that is, each organism would move to one new location each day and process any developments that the new location and/or progression of time might dictate, including birth, death, predation, infection, or oocyst shedding.
Four conditions were simulated: (i) domestic cycle with superinfection, (ii) domestic cycle without superinfection, (iii) sylvatic cycle with superinfection, and (iv) sylvatic cycle without superinfection. Each condition was simulated 10 times, and each simulation had 50 runs. Mouse mortality rates of NV, IV, and HV types were set to 0.1%, 1%, and 90%, respectively. Superinfection rates were set at 70% for HV to NV, 40% for IV to NV, and 0% for NV to NV. Transmission was simulated for 25 y. The frequencies of fixation of a given genotype were determined and analyzed to determine statistical significance. To analyze simulation data, we performed a two-way ANOVA using SAS (9.4) proc glimmix. As the dependent variable (proportion/percentage of total runs) was not distributed normally, the data were rank-transformed, and statistical analysis (nonparametric, two-way ANOVA rank test) was performed by the Friedman’s test.
Multilocus Microsatellite Typing.
DNA samples were collected from previous studies in our laboratories (Dataset S1). Information about the location and host for these isolates was obtained from previously published papers. Genotyping of T. gondii isolates was performed using 15 microsatellite markers located on 11 different chromosomes in a single multiplex PCR assay (26). The multilocus microsatellite typing data were coded for all genetic loci. For a given locus, the DNA-banding pattern was coded with a string of 1’s and 0’s. A phylogenetic network and neighbor-joining tree were generated by SplitsTree 4.8 (59). A rooted neighbor-joining tree for 301 samples (296 type II samples and five non-type II samples as an outgroup) was constructed using FigTree v1.4.2 (tree.bio.ed.ac.uk/software/figtree/). Basic statistics for quantitative gene diversity within populations were calculated using Arlequin v 3.5 (60).
Supplementary Material
Acknowledgments
We thank Xiaojuan Zhu for statistical analysis of data. This study was supported, in part, by the University of Tennessee Bridge Fund (C.S.), National Science Foundation Grant CMMI-0845753 (to X.Z.), a National Institute for Mathematical and Biological Synthesis grant (to X.Z. and C.S.), National Natural Science Foundation of China Grants 31228022 (to C.S. and X.-Q.Z.) and 31230073 (to X.-Q.Z.), and South African Medical Research Council and National Research Foundation grants (to K.H.-A. and P.v.H.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722202115/-/DCSupplemental.
References
- 1.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gebreyes WA, et al. The global one health paradigm: Challenges and opportunities for tackling infectious diseases at the human, animal, and environment interface in low-resource settings. PLoS Negl Trop Dis. 2014;8:e3257. doi: 10.1371/journal.pntd.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dubey JP. Toxoplasmosis of Animals and Humans. 2nd Ed CRC Press; Boca Raton, FL: 2010. [Google Scholar]
- 4.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 5.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J Infect Dis. 1995;172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 6.Ajzenberg D, Bañuls AL, Tibayrenc M, Dardé ML. Microsatellite analysis of Toxoplasma gondii shows considerable polymorphism structured into two main clonal groups. Int J Parasitol. 2002;32:27–38. doi: 10.1016/s0020-7519(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 7.Dardé ML, Ajzenberg D, Su C. Molecular epidemiology and population structure of Toxoplasma gondii. In: Weiss LM, Kim K, editors. Toxoplasma gondii–The Model Apicomplexan: Perspectives and Methods. 2nd Ed Elsevier; San Diego: 2014. [Google Scholar]
- 8.Shwab EK, et al. Geographical patterns of Toxoplasma gondii genetic diversity revealed by multilocus PCR-RFLP genotyping. Parasitology. 2014;141:453–461. doi: 10.1017/S0031182013001844. [DOI] [PubMed] [Google Scholar]
- 9.Taylor S, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 10.Saeij JPJ, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reese ML, Zeiner GM, Saeij JPJ, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci USA. 2011;108:9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etheridge RD, et al. The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe. 2014;15:537–550. doi: 10.1016/j.chom.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reese ML, Shah N, Boothroyd JC. The Toxoplasma pseudokinase ROP5 is an allosteric inhibitor of the immunity-related GTPases. J Biol Chem. 2014;289:27849–27858. doi: 10.1074/jbc.M114.567057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki H, Aplin KP, Pialek J. Phylogeny and biogeography of the genus Mus in Eurasia. In: Macholan M, Baird SJE, Munclinger P, Pialek J, editors. Evolution of the House Mouse. Cambridge Univ Press; Cambridge, UK: 2012. pp. 35–64. [Google Scholar]
- 16.Cucchi T, Auffray JC, Vigne JD. On the origin of the house mouse synanthropy and dispersal in the Near East and Europe: Zooarchaeological review and perspectives. In: Macholan M, Baird SJE, Munclinger P, Pialek J, editors. Evolution of the House Mouse. Cambridge Univ Press; Cambridge, UK: 2012. pp. 65–93. [Google Scholar]
- 17.Bellwood P. First Farmers: Origins of Agricultural Societies. Blackwell Publishing; Malden, MA: 2005. [Google Scholar]
- 18.Bonhomme F, Searle JB. House mouse phylogeography. In: Macholan M, Baird SJE, Munclinger P, Pialek J, editors. Evolution of the House Mouse. Cambridge Univ Press; Cambridge, UK: 2012. pp. 278–296. [Google Scholar]
- 19.Vigne JD, Guilaine J, Debue K, Haye L, Gérard P. Early taming of the cat in Cyprus. Science. 2004;304:259. doi: 10.1126/science.1095335. [DOI] [PubMed] [Google Scholar]
- 20.Driscoll CA, et al. The Near Eastern origin of cat domestication. Science. 2007;317:519–523. doi: 10.1126/science.1139518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann T, et al. Transmission dynamics of Toxoplasma gondii on a pig farm. Infect Genet Evol. 2003;3:135–141. doi: 10.1016/s1567-1348(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 22.Lilue J, Müller UB, Steinfeldt T, Howard JC. Reciprocal virulence and resistance polymorphism in the relationship between Toxoplasma gondii and the house mouse. eLife. 2013;2:e01298. doi: 10.7554/eLife.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behnke MS, et al. The polymorphic pseudokinase ROP5 controls virulence in Toxoplasma gondii by regulating the active kinase ROP18. PLoS Pathog. 2012;8:e1002992. doi: 10.1371/journal.ppat.1002992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard JC, Hunn JP, Steinfeldt T. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr Opin Microbiol. 2011;14:414–421. doi: 10.1016/j.mib.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Gazzinelli RT, Mendonça-Neto R, Lilue J, Howard J, Sher A. Innate resistance against Toxoplasma gondii: An evolutionary tale of mice, cats, and men. Cell Host Microbe. 2014;15:132–138. doi: 10.1016/j.chom.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ajzenberg D, Collinet F, Mercier A, Vignoles P, Dardé M-L. Genotyping of Toxoplasma gondii isolates with 15 microsatellite markers in a single multiplex PCR assay. J Clin Microbiol. 2010;48:4641–4645. doi: 10.1128/JCM.01152-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilman D. Competition and biodiversity in spatially structured habitats. Ecology. 1994;75:2–16. [Google Scholar]
- 28.Nowak MA, May RM. Superinfection and the evolution of parasite virulence. Proc Biol Sci. 1994;255:81–89. doi: 10.1098/rspb.1994.0012. [DOI] [PubMed] [Google Scholar]
- 29.Levin S, Pimentel D. Selection of intermediate rates of increase in parasite-host systems. Am Nat. 1981;117:308–315. [Google Scholar]
- 30.Castillo-Chavez C, Velasco-Hernández JX. On the relationship between evolution of virulence and host demography. J Theor Biol. 1998;192:437–444. doi: 10.1006/jtbi.1998.0661. [DOI] [PubMed] [Google Scholar]
- 31.Crosby AW. Ecological Imperialism: The Biological Expansion of Europe, 900-1900. 2nd Ed Cambridge Univ Press; New York: 2004. [Google Scholar]
- 32.Goudie AS. The Human Impact on the Natural Environment: Past, Present, and Future. 7th Ed Wiley; Oxford: 2013. [Google Scholar]
- 33.Rosenthal BM. How has agriculture influenced the geography and genetics of animal parasites? Trends Parasitol. 2009;25:67–70. doi: 10.1016/j.pt.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Ajzenberg D, et al. Genetic diversity, clonality and sexuality in Toxoplasma gondii. Int J Parasitol. 2004;34:1185–1196. doi: 10.1016/j.ijpara.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Khan A, et al. Geographic separation of domestic and wild strains of Toxoplasma gondii in French Guiana correlates with a monomorphic version of chromosome1a. PLoS Negl Trop Dis. 2014;8:e3182. doi: 10.1371/journal.pntd.0003182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotteland C, et al. Spatial distribution of Toxoplasma gondii oocysts in soil in a rural area: Influence of cats and land use. Vet Parasitol. 2014;205:629–637. doi: 10.1016/j.vetpar.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Gilbert RE, et al. European Multicentre Study on Congenital Toxoplasmosis (EMSCOT) Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis. 2008;2:e277. doi: 10.1371/journal.pntd.0000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demar M, et al. Fatal outbreak of human toxoplasmosis along the Maroni river: Epidemiological, clinical, and parasitological aspects. Clin Infect Dis. 2007;45:e88–e95. doi: 10.1086/521246. [DOI] [PubMed] [Google Scholar]
- 39.Demar M, et al. Acute toxoplasmoses in immunocompetent patients hospitalized in an intensive care unit in French Guiana. Clin Microbiol Infect. 2012;18:E221–E231. doi: 10.1111/j.1469-0691.2011.03648.x. [DOI] [PubMed] [Google Scholar]
- 40.Dardé ML, Villena I, Pinon JM, Beguinot I. Severe toxoplasmosis caused by a Toxoplasma gondii strain with a new isoenzyme type acquired in French Guyana. J Clin Microbiol. 1998;36:324. doi: 10.1128/jcm.36.1.324-324.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carme B, Demar M, Ajzenberg D, Dardé ML. Severe acquired toxoplasmosis caused by wild cycle of Toxoplasma gondii, French Guiana. Emerg Infect Dis. 2009;15:656–658. doi: 10.3201/eid1504.081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delhaes L, et al. Severe congenital toxoplasmosis due to a Toxoplasma gondii strain with an atypical genotype: Case report and review. Prenat Diagn. 2010;30:902–905. doi: 10.1002/pd.2563. [DOI] [PubMed] [Google Scholar]
- 43.Grigg ME, Ganatra J, Boothroyd JC, Margolis TP. Unusual abundance of atypical strains associated with human ocular toxoplasmosis. J Infect Dis. 2001;184:633–639. doi: 10.1086/322800. [DOI] [PubMed] [Google Scholar]
- 44.Liberg O. Food habits and prey impact by feral and house-based domestic cats in a rural area in southern Sweden. J Mammal. 1984;65:424–432. [Google Scholar]
- 45.Delibes M, Zapata SC, Blazques MC, Rodriguez-Estrella R. Seasonal food habits of bobcats (Lynx rufus) in subtropical Baja California Sur, Mexico. Can J Zool. 1997;75:478–483. [Google Scholar]
- 46.Witmer GW, Moulton RS. Deer mice (Peromyscus spp.) biology, damage and management: A review. In: Timm RM, editor. Proceedings of the 25th Vertebrate Pest Conference. University of California; Davis, CA: 2012. pp. 213–219. [Google Scholar]
- 47.Rejmanek D, et al. Congenital transmission of Toxoplasma gondii in deer mice (Peromyscus maniculatus) after oral oocyst infection. J Parasitol. 2010;96:516–520. doi: 10.1645/GE-2372.1. [DOI] [PubMed] [Google Scholar]
- 48.Miller MA, et al. An unusual genotype of Toxoplasma gondii is common in California sea otters (Enhydra lutris nereis) and is a cause of mortality. Int J Parasitol. 2004;34:275–284. doi: 10.1016/j.ijpara.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Wendte JM, Gibson AK, Grigg ME. Population genetics of Toxoplasma gondii: New perspectives from parasite genotypes in wildlife. Vet Parasitol. 2011;182:96–111. doi: 10.1016/j.vetpar.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan A, et al. Genetic analyses of atypical Toxoplasma gondii strains reveal a fourth clonal lineage in North America. Int J Parasitol. 2011;41:645–655. doi: 10.1016/j.ijpara.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dubey JP, et al. Genetic characterisation of Toxoplasma gondii in wildlife from North America revealed widespread and high prevalence of the fourth clonal type. Int J Parasitol. 2011;41:1139–1147. doi: 10.1016/j.ijpara.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 52.Anderson RM, May RM. Coevolution of hosts and parasites. Parasitol. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 53.Lélu M, Langlais M, Poulle M-L, Gilot-Fromont E, Gandon S. When should a trophically and vertically transmitted parasite manipulate its intermediate host? The case of Toxoplasma gondii. Proc Biol Sci. 2013;280:20131143. doi: 10.1098/rspb.2013.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dvorakova-Hortova K, et al. Toxoplasma gondii decreases the reproductive fitness in mice. PLoS One. 2014;9:e96770. doi: 10.1371/journal.pone.0096770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asgari Q, Keshavarz Valian H, Rezaeian M, Shojaee S, Mehrabani D. Toxoplasma gondii: Sexual transmission in mice. J Parasit Dis. 2015;39:253–257. doi: 10.1007/s12639-013-0330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou DH, et al. Comparative proteomic analysis of different Toxoplasma gondii genotypes by two-dimensional fluorescence difference gel electrophoresis combined with mass spectrometry. Electrophoresis. 2014;35:533–545. doi: 10.1002/elps.201300044. [DOI] [PubMed] [Google Scholar]
- 57.Jiang W, Sullivan AM, Su C, Zhao X. An agent-based model for the transmission dynamics of Toxoplasma gondii. J Theor Biol. 2012;293:15–26. doi: 10.1016/j.jtbi.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 58.Gotteland C, McFerrin BM, Zhao X, Gilot-Fromont E, Lélu M. Agricultural landscape and spatial distribution of Toxoplasma gondii in rural environment: An agent-based model. Int J Health Geogr. 2014;13:45. doi: 10.1186/1476-072X-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- 60.Excoffier L, Lischer HEL. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564–567. doi: 10.1111/j.1755-0998.2010.02847.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.