Significance
Acyl-homoserine lactone (AHL) quorum sensing (QS) has received interest as a possible target for controlling microbial activities and as a model for communication involved in coordinating activities of bacteria. Recent advances show there are two related families of AHL QS signal synthesis enzymes, which differ in whether they acquire the organic acid reaction substrate from biosynthetic or primarily catabolic pathways. Most known AHL synthases utilize fatty acids activated with an acyl carrier protein from fatty acid biosynthesis. Some AHL synthases use coenzyme-A–activated organic acids. We describe a coenzyme-A–dependent AHL synthase QS system. We also set forth an experimental approach for discovering new signals that will allow further expansion of our understanding of the diversity of AHL signaling.
Keywords: bacterial communication, Prosthecomicrobium, α-Proteobacteria, phenylacetate, sociomicrobiology
Abstract
Many species of Proteobacteria produce acyl-homoserine lactone (AHL) compounds as quorum-sensing (QS) signals for cell density-dependent gene regulation. Most known AHL synthases, LuxI-type enzymes, produce fatty AHLs, and the fatty acid moiety is derived from an acyl-acyl carrier protein (ACP) intermediate in fatty acid biosynthesis. Recently, a class of LuxI homologs has been shown to use CoA-linked aromatic or amino acid substrates for AHL synthesis. By using an informatics approach, we found the CoA class of LuxI homologs exists primarily in α-Proteobacteria. The genome of Prosthecomicrobium hirschii, a dimorphic prosthecate bacterium, possesses a luxI-like AHL synthase gene that we predicted to encode a CoA-utilizing enzyme. We show the P. hirschii LuxI homolog catalyzes synthesis of phenylacetyl-homoserine lactone (PA-HSL). Our experiments show P. hirschii obtains phenylacetate from its environment and uses a CoA ligase to produce the phenylacetyl-CoA substrate for the LuxI homolog. By using an AHL degrading enzyme, we showed that PA-HSL controls aggregation, biofilm formation, and pigment production in P. hirschii. These findings advance a limited understanding of the CoA-dependent AHL synthases. We describe how to identify putative members of the class, we describe a signal synthesized by using an environmental aromatic acid, and we identify phenotypes controlled by the aryl-HSL.
Many bacterial species use quorum-sensing (QS) signals as a proxy for cell density. Diffusible acyl-homoserine lactones (AHLs) are common QS signals in Proteobacteria. The AHL signals accumulate during growth and at sufficient concentrations bind cognate transcription factors, QS signal receptors, which affect transcription of genes in QS regulons (1–3).
Generally, AHL production is catalyzed by signal synthases, members of the LuxI protein family. Most known AHLs have straight-chain fatty acyl side groups of 4–18 carbons, with varying degrees of oxidation at the 3-C position (1). The substrates for characterized fatty acyl AHLs are S-adenosylmethionine (SAM) and an acylated-acyl carrier protein (acyl-ACP) (4, 5) from the fatty acid biosynthesis pathway.
Recently, LuxI homologs have been identified that produce AHLs signals with aromatic acid and branched amino acid side chains in three species of α-Proteobacteria: RpaI from Rhodopseudomonas palustris catalyzes production of p-coumaroyl-HSL (pC-HSL) (6); CinI from a photosynthetic Bradyrhizobium catalyzes production of cinnamoyl-HSL (cinn-HSL) (7); and BjaI from Bradyrhizobium japonicum catalyzes production of isovaleryl-HSL (IV-HSL) (8). These three enzymes use coenzyme-A (CoA)–activated acids rather than ACP-activated acids as substrates (8, 9). Presumably, the CoA substrates are derived from the activity of cellular acyl-CoA ligases involved in organic acid catabolism. In fact, R. palustris requires exogenous p-coumarate to produce pC-HSL (6). Phylogenomic analyses led to the conclusion that the CoA-utilizing family of AHL synthases resulted from an evolutionary exaptation event (10).
Relatively little is known about the acyl-CoA utilizing AHL synthases and the activities they control. Based on the sequences of the three known acyl-CoA utilizing LuxI homologs and phylogenomic analyses, there are now informatics tools, which can be used to predict whether a LuxI homolog is in the ACP- or CoA-dependent family (11–13). We became interested in the α-Proteobacterium Prosthecomicrobium hirschii when its genome sequence was published (14). This dimorphic prosthecate bacterium has genes coding for an AHL QS system, and we predicted the luxI homolog codes for a member of the CoA-utilizing family. P. hirschii is a saprophyte that can be isolated from freshwater lakes (15) and exhibits two different cell morphologies. Some cells have multiple long prostheca, and other cells have many short prostheca. Although there is very little known about the genus Prosthecomicrobium, it has been reported that the short prostheca cell type increases in relative abundance in the late logarithmic phase of growth (16). This further piqued our interest because it raises the possibility that cell type is influenced by QS.
We report here that P. hirschii produces phenylacetyl-homoserine lactone (PA-HSL) and uses exogenous phenylacetate for this purpose. We present evidence that the gene linked to the PA-HSL synthase gene codes for the PA-HSL receptor. This QS circuit not only positively autoregulates PA-HSL synthesis, it also controls aggregation and pigment production. We find that putative CoA-utilizing AHL synthases are common in α-Proteobacteria and rare in other Proteobacteria.
Results
The P. hirschii LuxI Homolog Clusters with Members of the CoA-Utilizing Family of AHL Synthases.
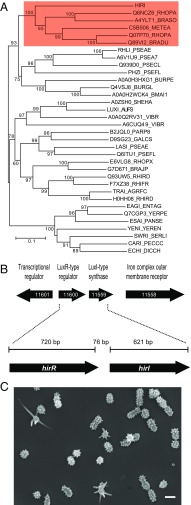
We developed a method involving the Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology (KO) database and an enzyme nomenclature (EC) populated database (13) to search for likely acyl-CoA–utilizing LuxI homologs. The KO associated with acyl-CoA–utilizing LuxI homologs (K18096) was encoded almost exclusively in genomes of α-Proteobacteria. We found 974 acyl-CoA-type luxI homologs (K018096) in genomes submitted to the Joint Genome Institute Integrated Microbial Genomes (JGI IMG) repository and 957 were in α-proteobacterial genomes. This represents about 30% of the 3,010 luxI homologs we found in sequenced α-proteobacterial genomes. The single luxI homolog in the recently sequenced genome of P. hirschii (14) was found within the family of genes encoding putative acyl-CoA–dependent AHL synthases by KO classification and in phylogenetic analyses (Fig. 1A and SI Appendix, Table S1). The P. hirschii luxI homolog is linked to a gene coding for a LuxR-like AHL-responsive transcription factor. We named this pair of genes hirI and hirR (Fig. 1B). P. hirschii is a dimorphic appendeged α-proteobacterial species, which exhibits two distinct cell morphologies (Fig. 1C). Because P. hirschii is a Rhizobiales as are the Rhodopseudomonas and Bradyrhizobium species, which produce aryl- or amino AHLs, we sought to identify the HirI-produced AHL.
Fig. 1.
The P. hirschii AHL synthase phylogeny, gene organization, and images of P. hirschii cells. (A) Protein phylogeny of AHL synthases. All sequences except HirI are labeled with the uniprot identifier. A description of each LuxI homolog is provided in SI Appendix, Table S1. The clade containing the CoA-utilizing AHL synthases is shaded in red. RpaI is Q6NCZ6_RHOPA, CinI is A4YLT1_BRASO, and BjaI is Q89VI1. The percentage that each branch was observed during bootstrap resampling (n = 1,000) is shown next to the branch. (B) The hirR-hirI genomic region. Locus designations are within the gene arrow (prefix for all is Ga0100838_ as annotated in the JGI IMG resource). (C) An SEM of P. hirschii, short-stalked cells predominate. (Scale bar: 1 μm.)
P. hirschii Produces Phenylacetyl-HSL.
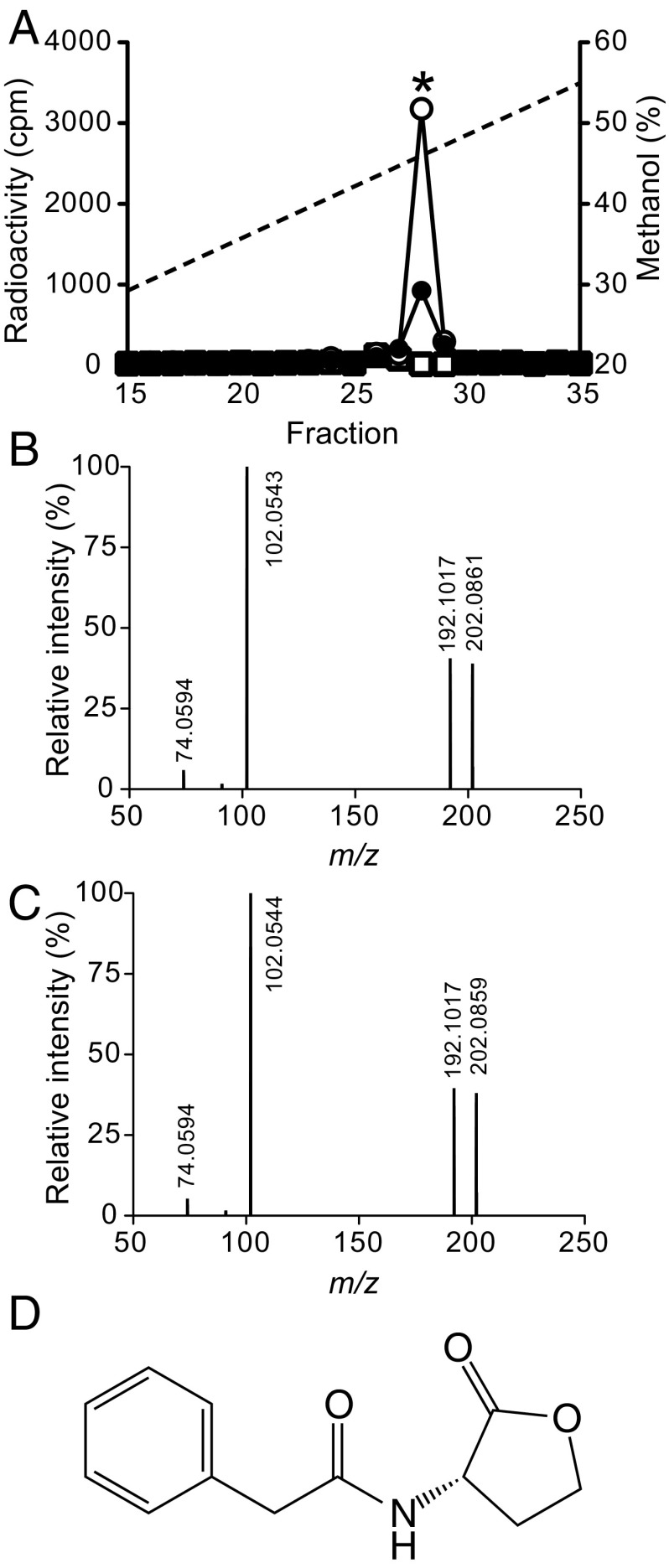
As a first step toward identification of the AHL produced by HirI, we grew this bacterium in the presence of l-[1-14C]-methionine. To synthesize AHLs, bacteria use the common metabolic intermediate SAM derived from methionine, and when grown in the presence of l-[1-14C]-methionine, the AHL ring is labeled with a 14C atom (17, 18). Because AHLs are diffusible and solvent extractable, we extracted the culture supernatant fluid with ethyl acetate and fractionated the extract by C18-reverse phase HPLC. Nearly all of the radiolabel was found in a single HPLC fraction (Fig. 2A). As expected if the radiolabeled compound was an AHL, the radiolabeled HPLC fraction was eliminated by pretreatment with purified AiiA AHL lactonase (Fig. 2A). None of the synthetic AHLs we tested, including pC-HSL, cinn-HSL, and IV-HSL, coeluted with the radiolabeled P. hirschii compound. This indicated that P. hirschii produces an AHL, which has not been described previously.
Fig. 2.
Identification of the P. hirschii AHL. (A) HPLC profiles of 14C-AHLs synthesized by P. hirschii grown in MMB with no addition (filled circles), 0.1 mM phenylacetic acid added (open circles), or AiiA lactonase added (open squares). The asterisk indicates the fraction where synthetic PA-HSL is eluted. The dashed line shows the methanol gradient. (B) MS/MS daughter scan of the m/z 220 (M + H) ion from the natural product. (C) MS/MS daughter scan of the m/z 220 (M + H) ion from chemically synthesized PA-HSL. (D) The proposed P. hirschii QS signal, PA-HSL.
To further characterize the putative P. hirschii AHL, we ethyl acetate extracted material from the supernatant fluid of a 1-L P. hirschii culture and collected the fraction at which the radiolabel was eluted. The material in this fraction was analyzed by high-resolution liquid chromatography tandem mass spectrometry (LC-MS/MS) and scanned for the presence of an aminobutyrolactone fragment ion (M + H) of 102, common to most AHLs (6). There was one peak with the diagnostic 102-fragment ion (Fig. 2B), and the mass of this peak was 220.0966, which is in agreement with the molecular formula C12H13NO3. This formula does not correspond to any known acyl-, amino, or aryl-HSL, but does correspond to PA-HSL. Chemically synthesized PA-HSL (19) coeluted with the 14C product from P. hirschii (Fig. 2A), and its LC-MS/MS profile was indistinguishable from the P. hirschii compound (Fig. 2C). This is strong evidence that P. hirschii catalyzes the synthesis of PA-HSL (Fig. 2D).
For synthesis of pC-HSL, R. palustris requires p-coumarate in the growth medium (6). We found that P. hirschii produced PA-HSL without addition of phenylacetic acid to the growth medium, but P. hirschii requires small amounts (0.015%) of peptone and tryptone for growth and there is likely some phenylacetic acid present in these components. Thus, it is possible that, like R. palustris, P. hirschii uses an exogenous organic acid for AHL production. It is also possible that P. hirschii can produce a different AHL if provided the proper organic acid exogenously. To gain insights about these two possibilities, we grew P. hirschii in the presence of l-[1-14C]-methionine plus one of 14 different aromatic acids (Materials and Methods) and analyzed culture fluid extracts by HPLC. Added phenylacetic acid resulted in about three times more 14C-label in the HPLC fraction that coelutes with PA-HSL, than the no addition control (Fig. 2A). None of the other aromatic acids affected the level of 14C incorporation into the PA-HSL–coeluting peak nor did growth with these aromatic acids give rise to any other peak of radioactivity. These data indicate that P. hirschii requires, or at least utilizes, exogenous phenylacetic acid for PA-HSL synthesis and that the P. hirschii AHL is in fact PA-HSL.
The hirR and hirI Gene Products Constitute a PA-HSL QS Circuit.
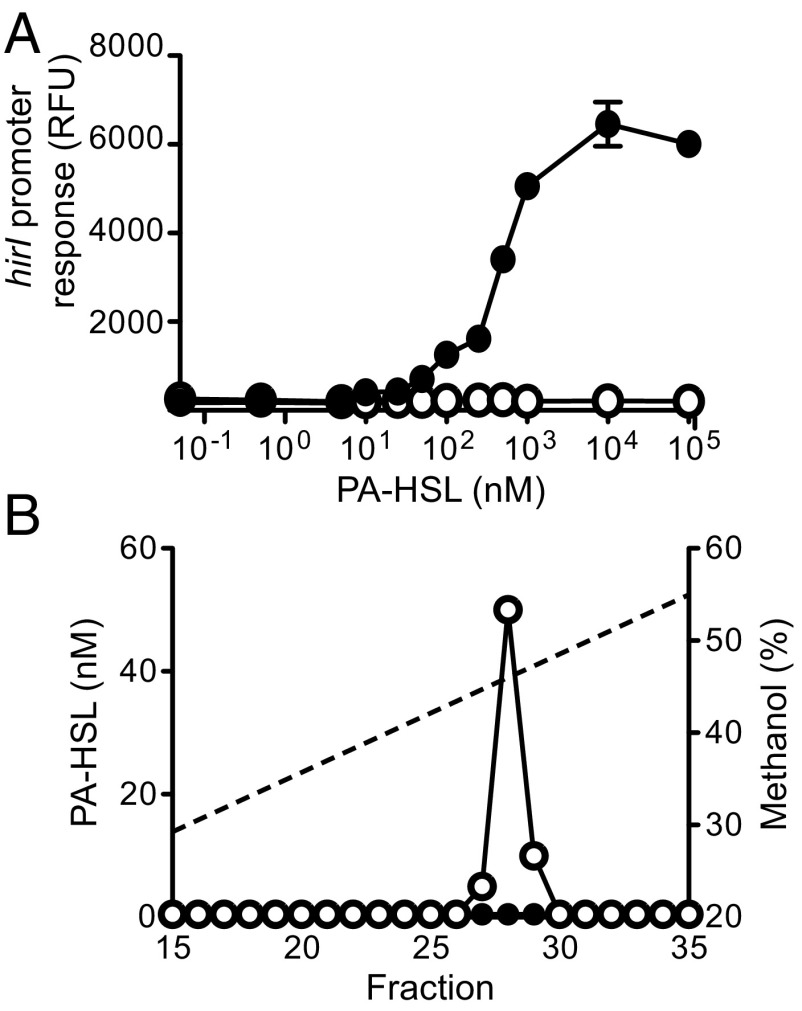
In most LuxR-LuxI–type QS circuits, the luxI-like gene is positively autoregulated by its own AHL product together with the luxR-like gene product (2, 20). Is this the case for hirI-hirR? Because a genetic system for P. hirschii has not been established, we addressed the question by using recombinant Pseudomonas putida F1. The genome of this bacterium has a high GC content similar to that of P. hirschii, it can use phenylacetic acid as a carbon and energy source and the first step in phenylacetate catabolism involves a phenylacetyl-CoA ligase (21, 22). Finally, P. putida F1 does not have its own luxI homolog. We constructed a plasmid (pLL1) with hirR and a hirI promoter-controlled mCherry gene. P. putida (pLL1) fluorescence showed a saturable dose-dependent response to PA-HSL (Fig. 3A). As a control we constructed pLL2, which contained the hirI promoter-controlled mCherry but lacked hirR. Expression of mCherry was not influenced by PA-HSL in P. putida (pLL2) (Fig. 3A). This shows the response to PA-HSL is HirR dependent. Fluorescence of P. putida (pLL1) was unaffected by pC-HSL, cinn-HSL, IV-HSL, C4-HSL, C8-HSL, or 3-oxo-C12-HSL even at high (1 μM) concentrations. These findings indicate HirR responds specifically to PA-HSL with an EC50 of about 250 nM.
Fig. 3.
HirR-HirI constitute a quorum-sensing circuit, and P. putida can use exogenous phenylacetate for PA-HSL synthesis. (A) PA-HSL–dependent hirI promoter activity requires HirR. Expression of hirI::mCherry P. putida containing pLL1 (filled circles), or pLL2 (open circles). There is a hirR gene in pLL1 but not in pLL2. Data are the mean, and the errors bars indicate the range of three biological replicates. (B) HPLC analysis of PA-HSL produced by P. putida (pHirI) grown in MSB broth with (open circles) or without (filled circles) 0.1 mM phenylacetate. The dashed line shows the methanol gradient.
To determine whether HirI is itself a PA-HSL synthase, we ethyl acetate extracted material from P. putida with the hirI plasmid pHirI grown with or without added phenylacetate. We assayed PA-HSL concentrations in the extracted material by using P. putida with the hirR-hirI promoter-mCherry fusion plasmid pLL1 in a PA-HSL bioassay (Materials and Methods). P. putida (pHirI) grown in a defined medium without added phenylacetate did not produce PA-HSL, but it did when grown with added phenylacetate (Fig. 3B). When P. putida (pHirI) was grown to late-logarithimic phase in rich Luria–Bertani (LB) broth, PA-HSL levels were about 1 μM. We hypothesized that the phenylacetate acquired from the growth medium was activated by a P. putida phenylacetyl-CoA ligase. To test this hypothesis, we grew a strain with a paaF phenylacetate deletion mutation containing pHirI in LB broth as above and found no detectable PA-HSL.
PA-HSL Is a QS Signal That Controls Specific P. hirschii Functions in a Cell Density-Dependent Manner.
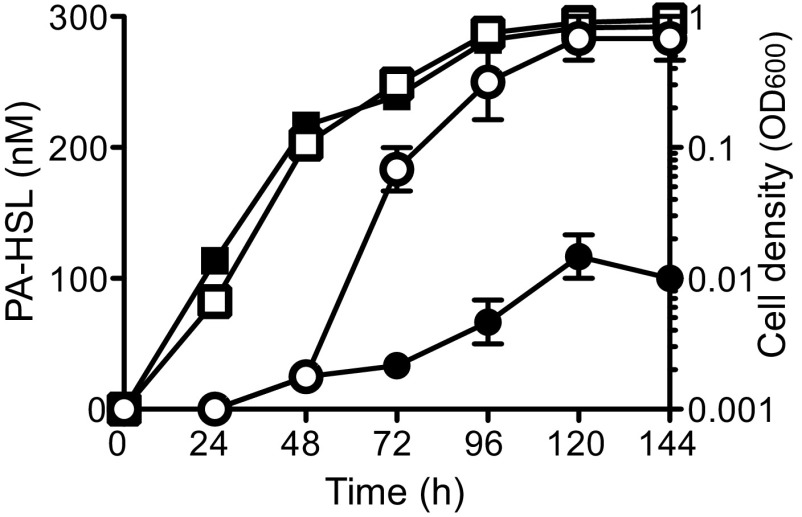
As discussed above, most luxI-like AHL synthase genes are positively autoregulated by the AHL they produce together with the cognate LuxR homolog. Total PA-HSL levels were higher in P. hirschii cultures when phenylacetate was added to the medium (300 nM vs. 100 nM), but in both conditions, PA-HSL levels remained low during early and midlogarithmic growth and then increased rapidly during late logarithmic and early stationary phase (Fig. 4). This is typical of AHL systems (2, 20).
Fig. 4.
PA-HSL is produced in a cell density-dependent manner. PA-HSL production (circles) and growth (squares) of P. hirschii grown with (open symbols) or without (filled symbols) 0.1 mM phenylacetate. Data are means, and the errors bars indicate the range of three biological replicates.
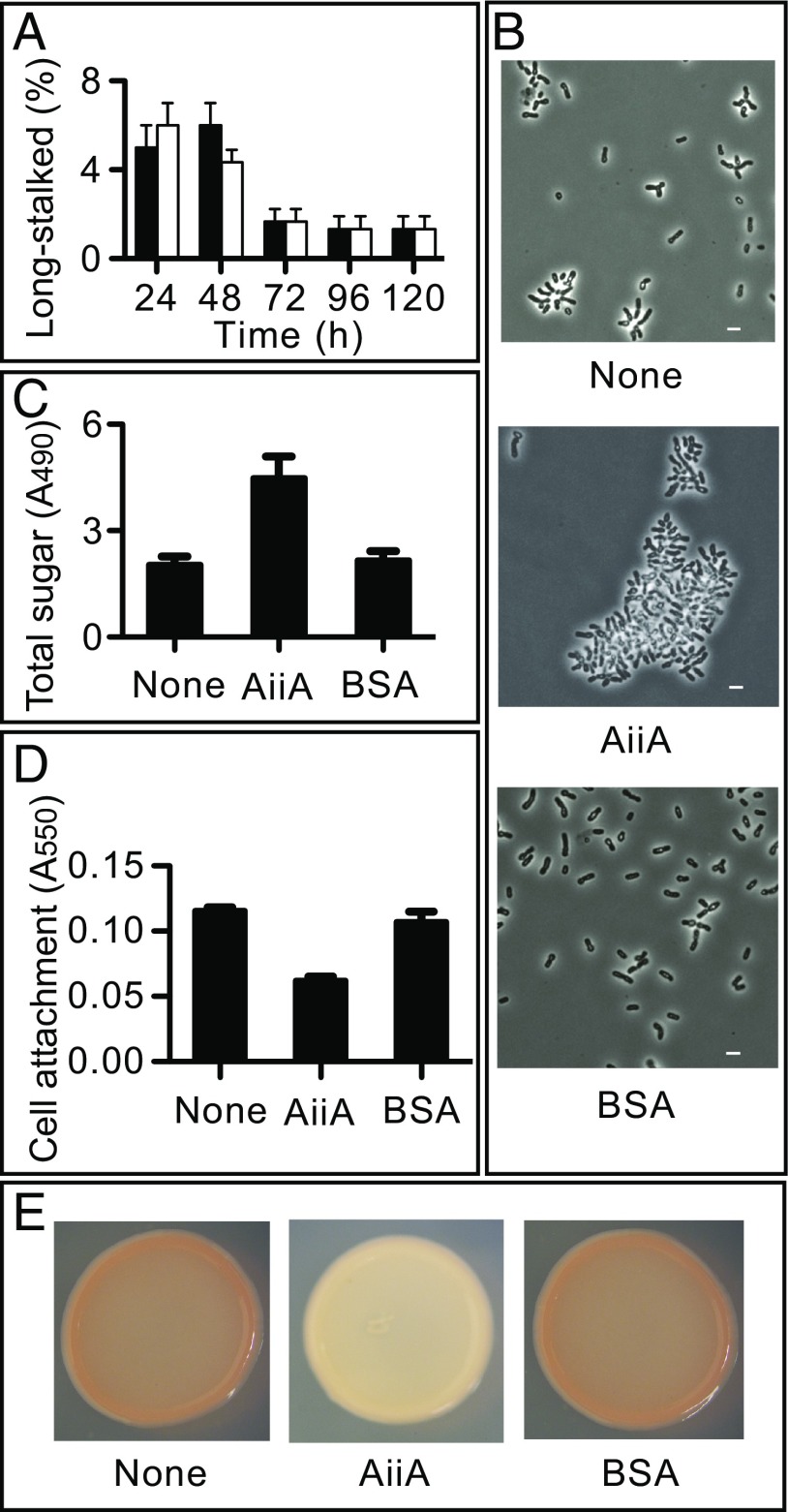
Does PA-HSL control other P. hirschii phenotypes? We were particularly interested in this question for several reasons. First, there is a correlation between the relative abundances of the two P. hirschii cell morphotypes and cell density during growth (16). Could morphological development be QS dependent? Second, we are not aware of evidence describing pC-HSL, cinn-HSL, or IV-HSL regulation of functions other than their own synthesis in the α-Proteobacteria, which produce these compounds. In fact, in an extensive transcriptome analysis of pC-HSL dependent gene transcription in R. palustris, we identified only a single gene activated directly by pC-HSL and that was the pC-HSL synthase gene rpaI itself (23). Because we were unable to manipulate P. hirschii genetically and delete either hirI or hirR, we approached this issue by growing P. hirschii in the presence of an AHL lactonase we showed degrades PA-HSL (Fig. 2A). Our examination of cell density-dependent cell morphology confirmed the previous report that the ratio of long to short stalked cells decreases in the late logarithmic phase of growth (16), but the PA-HSL–degrading lactonase did not influence this result (Fig. 5A).
Fig. 5.
PA-HSL is a QS signal that controls specific P. hirschii functions. (A) Percentage of long-stalked P. hirschii cells grown with (black) or without (white) AiiA lactonase over time. One hundred cells were scored for each sample. The bars show the range of three biological replicates. (B) Phase contrast micrographs of P. hirschii cells from late logarithmic phase cultures. (Scale bars: 1 μm.) (C) Total sugar in EPS extracted from P. hirschii late logarithmic phase cultures (72 h). Data are means and ranges for three biological replicates. (D) Biofilm biomass of P. hirschii (72 h). Data are the means and ranges of four biological replicates. (E) Pigmentation of P. hirschii cells grown on agar for 10 d (inoculum was a 10-μL spot of a late-logarithmic phase culture). The colonies measured ∼5 mm in diameter. In B–E, None indicates no addition to the culture broth, AiiA indicates addition of lactonase, and BSA indicates addition of BSA as a control.
Although cell morphology appeared to be independent of PA-HSL, some phenotypic differences between cells grown with and without the PA-HSL–degrading lactonase were obvious. Notably, when cells were grown in the presence of the lactonase, we found visible cell aggregates in the late logarithmic phase. Some of these aggregates, or flocs, were of sufficient size to settle to the bottom of unshaken culture tubes. As shown by phase contrast microscopy, cells from flocculant, lactonase-grown cultures were found primarily in large clusters compared with cells grown with no addition or grown in the presence of BSA (Fig. 5B). Often bacterial cell aggregation is in part due production of extracellular polymeric substances (EPS), which can be complex mixtures of polysaccharides, proteins, and sometimes DNA. EPS is also important in biofilm formation. To measure P. hirschii EPS, we extracted cell-free supernatant fluid by using cold ethanol and measured total sugar content in the extract by using a phenol sulfuric acid protocol (Materials and Methods). We found that with growth in the presence of the lactonase there was about twice as much total sugar as with growth in the absence of the lactonase (Fig. 5C). This lactonase-dependent increase in EPS correlated with a 50% reduction in biofilm formation as measured in a microtiter dish assay (Fig. 5D), suggesting that when cells aggregate, they may be less likely to attach to plastic surfaces. EPS-overproducing bacterial colonies often appear rugose or rough when grown on agar plates (24); however, when agar plates contained lactonase, we did not observe rough or wrinkly colonies (Fig. 5E). Rather, we observed a striking but different phenotype. Without lactonase colonies were pink, but colonies on plates with lactonase-containing agar did not develop the pink pigmentation (Fig. 5E). Apparently, PA-HSL QS influences cellular aggregation or flocculation, biofilm formation, and pigment production.
Discussion
We describe a LuxR-LuxI–type QS system in P. hirschii that synthesizes and responds to PA-HSL. It is interesting that a variety of substituted derivatives of PA-HSL have been synthesized, and several are potent antagonists and agonists of other AHL-type QS systems (19, 25–27). PA-HSL has been used as a synthetic scaffold for the design of nonnative chemical probes to study LuxR-type receptor activity (28). PA-HSL itself was not very active as an agonist or antagonist of other QS systems (26). As far as we are aware, HirI is only the second AHL synthase reported to use an exogenously supplied organic acid. In this way it is similar to R. palustris, which requires exogenous p-coumarate for pC-HSL production (6). That phenylacetate is incorporated directly into a bacterial signal is interesting, as this plant metabolite is known to function as a growth hormone (auxin) (29). Furthermore, phenylacetate is abundant in decaying plant matter and phenylacetate degradation pathways are present in about 16% of sequenced bacterial genomes (30).
We describe the use of an AHL-degrading lactonase during culture growth to identify PA-HSL–dependent cellular activities. Our approach is similar to those reported recently to identify QS-controlled activities in other bacteria (31, 32). Using this approach, we showed PA-HSL influences P. hirschii pigment production, flocculation, EPS production, and biofilm formation (Fig. 5). Control of aggregation and biofilm development is a common feature of AHL QS systems (1). In many bacteria QS enhances biofilm development, but in some bacteria, it serves as a dispersal signal as it does in P. hirschii. Because the P. hirschii QS-dependent phenotypes likely depend on availability of environmental phenylacetate, the HirI-HirR-PA-HSL system must integrate information about both bacterial population density and the availability of a specific plant metabolite. This is similar to the situation with the pC-HSL system in R. palustris, but other than control of its own production, we do not know what the R. palustris pC-HSL regulates (6, 23). The P. hirschii system represents a model to begin to understand the biological relevance of systems, which integrate QS with other environmental factors, directly.
We hypothesize the substrate for PA-HSL synthesis is phenylacetyl-CoA for several reasons: (i) HirI is in the family of CoA-utilizing LuxI homologs (K18096) and groups with the CoA-utilizing enzymes (Fig. 1A). (ii) The first step in phenylacetate degradation pathways is ligation with CoA via a PA-CoA ligase enzyme (EC 6.2.1.30) (21, 22). The P. hirschii genome encodes three putative PA-CoA ligases, including one (Ga0100838_112492) that has 65% amino acid sequence identity with the sole PA-CoA-ligase (PaaF) in P. putida F1 (21). (iii) When P. putida with a hirI expression vector is grown with exogenous phenylacetate it produces PA-HSL, but under the same growth conditions a P. putida PaaF mutant does not produce PA-HSL. There remains a formal possibility that in P. hirschii and P. putida there is conversion of phenylacetyl-CoA to phenyacetyl-ACP. This conversion has been demonstrated in vitro with an enzyme from Escherichia coli, phenylacetyl-CoA, and Streptomyces ACP (33).
The ability to utilize the EC and KEGG classification systems to identify potential CoA-utilizing LuxI homologs is a valuable tool for those interested in exploring AHL signal diversity. A previous analysis of LuxI diversity using the Enzyme Function Initiative-Enzyme Similarity toolkit (34) with BjaI as a seed sequence is another useful method for predicting CoA-type LuxI homologs (9). However, such a protocol requires additional analysis, while the EC and KEGG ortholog resources are already integrated in some genome annotation pipelines (e.g., JGI IMG; https://img.jgi.doe.gov/). Our finding that CoA-type luxI homologs are prevalent and quite restricted to genomes in α-Proteobacteria indicates that the CoA-dependent signal synthases are common among this group of Proteobacteria. Because bacterial degradation of plant metabolites often includes a CoA-activation step (35) and α-Proteobacteria have been shown to be enriched in plant tissues (36, 37) and environments rich in plant material (38), perhaps it is logical that when the exaptation event resulting in a CoA-utilizing LuxI enzyme occurred in the lineage, this provided ample opportunity to diversify the CoA-derived AHL substrate range.
Our genomic analysis indicates the potential for discovery of novel CoA-derived AHL signals is high, but how might signal diversity be studied more systematically? One key is to utilize the radiolabel methionine protocol (Materials and Methods and Fig. 2A) for AHL screening. In principal this protocol should allow detection of AHL biosynthesis regardless of the nature of the acyl group as long as there is cellular uptake of methionine (17). The AiiA lactonase treatment provides a confirmation that a radiolabeled product is an AHL (Fig. 2A). We believe an additional informatics step will enhance discovery. It is clear that for some LuxI homologs exogenous acyl substrate is required or at the very least enhances AHL production, as is the case for P. hirschii. There are 55 categories of CoA ligases. A useful strategy may involve determining the CoA ligase inventory encoded in the genome of a bacterial species of interest, and growing cells with radiolabeled methionine and a mix of putative CoA-ligase substrates for analysis.
Here, we not only describe an AHL QS system and some functions it controls, but we describe an approach to discovery of other AHL QS systems. We imagine that there is a large amount of untapped AHL-type signal diversity among the α-Proteobacteria with virtually any organic acid for which there is a CoA-ligase serving as the acyl-group for an acyl-CoA–dependent LuxI homolog. As long as the AHL product is reasonably hydrophobic and reasonably water-soluble it can serve as a QS signal.
Materials and Methods
Acyl-HSL Synthase Phylogeny.
For phylogenetic tree construction, the analysis was similar to that described elsewhere (10). Sequences were aligned with MUSCLE (39), trimmed with the alignment editor of MEGA7 (40), and the evolutionary history was inferred by using the neighbor-joining method (40) with evolutionary distances computed using the p-distance method in MEGA7.
Bacterial Strains and Growth Conditions.
Strains and plasmids are described in SI Appendix, Table S2. With the exception of the cells prepared for scanning electron microscopy (SEM), P. hirschii was grown in minimal medium broth, which consisted of 0.15 g of peptone, 0.15 g of yeast extract, 1.0 g of glucose, 0.25 g of ammonium sulfate, 20 mL of Hutner’s modified salts solution, 10 mL of vitamin solution per liter (41) at 26 °C with shaking. For SEM, P. hirschii was grown in peptone yeast extract (PYE) broth (42), which consisted of 2 g of peptone, 1 g of yeast extract, 0.3 g of MgSO4·7H2O, 70 mg of CaCl2·2H2O per liter of tap water. E. coli was grown in LB or Terrific Broth (TB) (43). P. putida was grown in LB broth or mineral salts base (MSB) broth (44) plus 10 mM succinate at 30 °C with shaking. Antibiotics were used as needed at the following concentrations: 100 μg/mL ampicillin and 15 μg/mL gentamicin. For plating, media contained 1.5% agar.
Chemicals.
AHLs tested for HirR activity (1 μM concentrations unless otherwise indicated): PA-HSL (45), pC-, and cinn-HSL (Syntech Solution LLC); IV-HSL (8), C4-, C8-, and 3-oxo-C12-HSL (Cayman Chemicals). Where indicated, the following aromatic salts (pH 7) were added to media at a final concentration of 0.1 mM: phenylacetic acid, benzoate, p-coumarate, m-coumarate, o-coumarate, caffeate, cinnamate, ferulate, p-hydroxybenzoate, methoxycinnamate, phenylalanine, sinapate, tryptophan, or vanillate.
Radiotracer Analysis of AHLs.
A 14C-radiotracer assay (17, 18) was used to detect AHLs produced in P. hirschii as follows: The inoculum for these experiments was 1 mL of a midlogarithmic phase culture (OD600 of 0.5) in 5 mL of fresh medium in 15-mL plastic tubes (including any aromatic acid or AiiA lactonase additions), to which 5 μCi of l-[1-14C] methionine (American Radiolabeled Chemicals) was added. After 24 h at 30 °C with shaking, AHLs were extracted from total cultures with two equal volumes of acidified ethyl acetate. Ethyl acetate extracts were separated by C18 reverse-phase HPLC in a 10–100% methanol gradient as described previously (17), except that the flow rate was 0.75 mL per min. One-minute fractions were collected, a portion of which were mixed with 4 mL of scintillation mixture (3a70b; Research Products International), and radioactivity determined by liquid scintillation counting. To develop a protocol for further purification (see below) the radiolabel-containing fraction (fraction 28, Fig. 2A) was concentrated and subjected to isocratic HPLC (20% methanol-in-water). One-minute fractions were collected, radioactivity counted, and the 14C-AHL made by P. hirschii was eluted in fractions 33 and 34.
AiiA-Lactonase Treatment.
AiiA lactonase was purified as a maltose-binding protein (MalE) fusion protein from E. coli cells grown in TB plus 0.2% glucose by using amylose resin affinity purification as described previously (46). Purified AiiA lactonase was added every 24 h (100 μg/mL) to P. hirschii cultures. Control cultures included P. hirschii grown without added lactonase and P. hirschii with BSA (100 μg/mL) added every 24 h in place of lactonase. For agar plates AiiA or BSA (100 μg/mL) was added to agar cooled to 55 °C before pouring plates.
Purification and Identification of PA-HSL.
We purified the P. hirschii AHL from 1 L of culture fluid from which cells had been removed by centrifugation. Purification was guided by results of our radiotracer experiments, and the HPLC fractionation was similar to that described elsewhere (8) except flow rate was 0.75 mL/min, we used the 30% and 50% methanol cuts from the C18 Sep-pak cartridge (Waters) step, and the second, isocratic HPLC was with 20% methanol. Purified material and synthetic PA-HSL were analyzed by LC-MS/MS in a Waters Acquity UPLC C18 RP column (1.7 μM, 2.1 mm × 30 mm) with a Thermo Linear Trap Quadrupole Orbitrap MS System (collision energy = 40 eV, cone = 35 V).
Plasmid and Strain Construction.
Primers are described in SI Appendix, Table S3. To construct the pLL1 hirR-PhirI-mCherry plasmid, we combined the following three PCR fragments by using E. coli DH5α-mediated assembly (47): (i) the hirR ORF plus the downstream 86-bp intergenic region containing the hirI promoter (PCR amplified with the hirR-F and hirR-R primers and P. hirschii genomic DNA as template), (ii) the mCherry ORF and ribosomal binding site (RBS) (PCR amplified with the primers mCherry-F and mCherry-R, and pUT18-mini Tn7 as template DNA), and (iii) PCR-linearized pBBRgdh (PCR amplified by using the primers pBBR-hirR-F and pBBR-mCherry-R). All primers contained 25 bp of homologous overlap, which is required for E. coli DH5α-mediated assembly. To create pLL2 we used the pBBR-mcherry-R and pBBR-mcherry-F as primers for PCR amplification a PCR fragment lacking the hirR gene from pLL1 and assembled it in E. coli DH5α. To create pHirI, we combined a 633-bp hirI DNA fragment including the hirI RBS) that was PCR amplified from genomic DNA by using the hirI-F and hirI-R primers with a 4,207-bp fragment of pBBRgdh PCR amplified by using the primers pBBR-hirI-F and pBBR-hirI-R). To construct pHirI we used E. coli-mediated assembly as above. Transformants were selected on LB-gentamicin agar plates and plasmid DNA sequences were confirmed by DNA sequencing. The sequence-confirmed plasmids were then used to electrotransform P. putida.
PA-HSL Bioassays.
AHLs were extracted from broth cultures with two equal volumes of acidified ethyl acetate (0.1 mL of glacial acetic acid per liter of solvent). Levels of PA-HSL from crude extracts or HPLC fractions were measured by using P. putida (pLL1) as follows: Cell culture extracts, HPLC fractions, or synthetic AHLs (used to generate a standard curve) were added to 16-mm glass tubes and the solvent removed by evaporation under N2 gas. One milliliter of P. putida pLL1 (subcultured from an overnight culture to an OD600 of 0.01 in fresh LB broth) was added to each tube and incubated for 16 h at 30 °C with shaking. To measure fluorescence, we transferred 150-μL samples to wells of 96-well black microtiter plates. mCherry fluorescence (587 nm excitation, 610 nm emission) was measured by using a Biotek H1 plate reader. Standard curves were generated by measuring the fluorescence response to different concentrations of synthetic PA-HSL.
Exopolysaccharide and Biofilm Assays.
Exopolysaccharide was extracted from P. hirschii cell culture fluid as described elsewhere (48). Briefly, bacteria were grown to midlogarithmic phase in MMB broth with or without added AiiA lactonase or BSA (100 μg/mL each) as indicated. Two milliliters of cell-free culture fluid were extracted with three volumes of cold ethanol, and the extract was incubated at −20 °C overnight. The ethanol-precipitated EPS was collected by centrifugation (16,800 × g for 30 min at 4 °C), and the pellet was suspended in 100 μL of Milli-Q water. We measured total sugar by using phenol-sulfuric acid; briefly, 50 µL of suspended EPS pellet, 150 µL of concentrated sulfuric acid, and 30 µL of 5% phenol (wt/vol) were mixed in 96-well microtiter plate wells and sugar content was measured as A490 in a Biotek H1 plate reader. To assess the role of PA-HSL in biofilm development we used a crystal violet assay (49). Briefly logarithmic phase P. hirschii cells were used to inoculate MMB broth (starting OD600 of 0.05) with or without added AiiA lactonase or BSA (100 μg/mL). The inoculated broth was dispensed (100 μL) into wells of a 96-well polyvinyl microtiter plate. After 72 h, 30 °C wells were washed twice with Milli-Q water and then stained with crystal violet (125 μL of a 0.1% wt/vol solution) for 15 min, rinsed four times with water, and dried. Crystal violet bound to the wells was measured by dissolving it in 30% acetic acid and measuring A550 with a Biotek H1 plate reader).
Microscopy.
For SEM imaging, P. hirschii was grown in PYE, cells were fixed with 3% glutaraldehyde and 1% osmium tetroxide, coated with palladium-gold (80:20), and imaged as with a JEOL-JSM 5800 electron microscope operating at 15 kV (16). Phase contrast microscopy employed an inverted Nikon Eclipse 80i Light microscope with a 100× oil immersion lens, a Digital Sight-series camera, and Nikon NIS-Elements imaging software. Photographs of P. hirschii cells growing on agar plates (Fig. 5E) were taken with a digital camera mounted on an Olympus SZX12 dissecting microscope.
Supplementary Material
Acknowledgments
We thank Drs. James Staley and Rebecca Parales for helpful discussions and Dr. Carolyn Brotherton for assistance with phylogenetic tree analysis. This research was sponsored by Innovative R&D Team Program of Guangdong Province, China Grant 2013S0340 (to E.P.G.); National Key Project for Basic Research of China Grant 2015CB150605; China Scholarship Council fellowship program Grant 20608440308 (to L.L.); US Public Health Service Grant GM59026 (to E.P.G.); National Science Foundation Grant IO1557806 (to P.J.B.B.); and the Genomic Science Program, US Department of Energy, Office of Science, Biological and Environmental Research, as part of the Plant Microbe Interfaces Scientific Focus Area (pmi.ornl.gov). Oak Ridge National Laboratory is managed by UT-Battelle LLC for the US Department of Energy under Contract DE-AC05-00OR22725.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808351115/-/DCSupplemental.
References
- 1.Whiteley M, Diggle SP, Greenberg EP. Progress in and promise of bacterial quorum sensing research. Nature. 2017;551:313–320. doi: 10.1038/nature24624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in gram-negative bacteria. FEMS Microbiol Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 3.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 4.Schaefer AL, Val DL, Hanzelka BL, Cronan JEJ, Jr, Greenberg EP. Generation of cell-to-cell signals in quorum sensing: Acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA. 1996;93:9505–9509. doi: 10.1073/pnas.93.18.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moré MI, et al. Enzymatic synthesis of a quorum-sensing autoinducer through use of defined substrates. Science. 1996;272:1655–1658. doi: 10.1126/science.272.5268.1655. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer AL, et al. A new class of homoserine lactone quorum-sensing signals. Nature. 2008;454:595–599. doi: 10.1038/nature07088. [DOI] [PubMed] [Google Scholar]
- 7.Ahlgren NA, Harwood CS, Schaefer AL, Giraud E, Greenberg EP. Aryl-homoserine lactone quorum sensing in stem-nodulating photosynthetic bradyrhizobia. Proc Natl Acad Sci USA. 2011;108:7183–7188. doi: 10.1073/pnas.1103821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindemann A, et al. Isovaleryl-homoserine lactone, an unusual branched-chain quorum-sensing signal from the soybean symbiont Bradyrhizobium japonicum. Proc Natl Acad Sci USA. 2011;108:16765–16770. doi: 10.1073/pnas.1114125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong SH, et al. Molecular basis for the substrate specificity of quorum signal synthases. Proc Natl Acad Sci USA. 2017;114:9092–9097. doi: 10.1073/pnas.1705400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen QH, Brecht RM, Dudekula D, Greenberg EP, Nagarajan R. Evolution of acyl-substrate recognition by a family of acyl-homoserine lactone synthases. PLoS One. 2014;9:e112464. doi: 10.1371/journal.pone.0112464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald AG, Boyce S, Tipton KF. ExplorEnz: The primary source of the IUBMB enzyme list. Nucleic Acids Res. 2009;37:D593–D597. doi: 10.1093/nar/gkn582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDonald AG, Tipton KF. Fifty-five years of enzyme classification: Advances and difficulties. FEBS J. 2014;281:583–592. doi: 10.1111/febs.12530. [DOI] [PubMed] [Google Scholar]
- 14.Daniel JJ, Givan SA, Brun YV, Brown PJ. Draft genome sequence of Prosthecomicrobium hirschii ATCC 27832T. Genome Announc. 2015;3:e01355-15. doi: 10.1128/genomeA.01355-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staley JT. Prosthecomicrobium hirschii, a new species in a redefined genus. Int J Syst Bacteriol. 1984;34:304–308. [Google Scholar]
- 16.Williams M, et al. Short-stalked Prosthecomicrobium hirschii cells have a Caulobacter-like cell cycle. J Bacteriol. 2016;198:1149–1159. doi: 10.1128/JB.00896-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer AL, Greenberg EP, Parsek MR. Acylated homoserine lactone detection in Pseudomonas aeruginosa biofilms by radiolabel assay. Methods Enzymol. 2001;336:41–47. doi: 10.1016/s0076-6879(01)36576-x. [DOI] [PubMed] [Google Scholar]
- 18.Schaefer AL, Harwood CS, Greenberg EP. “Hot stuff”: The many uses of a radiolabel assay in detecting acyl-homoserine lactone quorum-sensing signals. Methods Mol Biol. 2018;1673:35–47. doi: 10.1007/978-1-4939-7309-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geske GD, et al. Comparative analyses of N-acylated homoserine lactones reveal unique structural features that dictate their ability to activate or inhibit quorum sensing. Chembiochem. 2008;9:389–400. doi: 10.1002/cbic.200700551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz RL, Greenberg EP. Positive autoregulation of an acyl-homoserine lactone quorum-sensing circuit synchronizes the population response. MBio. 2017;8:e01079-17. doi: 10.1128/mBio.01079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson DT, Hensley M, Yoshioka H, Mabry TJ. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 22.Luu RA, et al. Taxis of Pseudomonas putida F1 toward phenylacetic acid is mediated by the energy taxis receptor Aer2. Appl Environ Microbiol. 2013;79:2416–2423. doi: 10.1128/AEM.03895-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirakawa H, et al. Activity of the Rhodopseudomonas palustris p-coumaroyl-homoserine lactone-responsive transcription factor RpaR. J Bacteriol. 2011;193:2598–2607. doi: 10.1128/JB.01479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yildiz FH, Schoolnik GK. Vibrio cholerae O1 El Tor: Identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geske GD, O’Neill JC, Miller DM, Mattmann ME, Blackwell HE. Modulation of bacterial quorum sensing with synthetic ligands: Systematic evaluation of N-acylated homoserine lactones in multiple species and new insights into their mechanisms of action. J Am Chem Soc. 2007;129:13613–13625. doi: 10.1021/ja074135h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geske GD, O’Neill JC, Blackwell HE. N-phenylacetanoyl-L-homoserine lactones can strongly antagonize or superagonize quorum sensing in Vibrio fischeri. ACS Chem Biol. 2007;2:315–319. doi: 10.1021/cb700036x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geske GD, Mattmann ME, Blackwell HE. Evaluation of a focused library of N-aryl L-homoserine lactones reveals a new set of potent quorum sensing modulators. Bioorg Med Chem Lett. 2008;18:5978–5981. doi: 10.1016/j.bmcl.2008.07.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh MA, Blackwell HE. Chemical probes of quorum sensing: From compound development to biological discovery. FEMS Microbiol Rev. 2016;40:774–794. doi: 10.1093/femsre/fuw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugawara S, et al. Distinct characteristics of indole-3-acetic acid and phenylacetic acid, two common auxins in plants. Plant Cell Physiol. 2015;56:1641–1654. doi: 10.1093/pcp/pcv088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teufel R, et al. Bacterial phenylalanine and phenylacetate catabolic pathway revealed. Proc Natl Acad Sci USA. 2010;107:14390–14395. doi: 10.1073/pnas.1005399107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feltner JB, et al. LasR varient cystic fibrosis isolates reveal an adaptable quorum-sensing hierachy in Pseudomonas aeruginosa. MBio. 2016;7:e01513-16. doi: 10.1128/mBio.01513-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellbye BL, Giguere AT, Bottomley PJ, Sayavedra-Soto LA. Quorum quenching of Nitrobacter winogradskyi suggests that quorum sensing regulates fluxes of nitrogen oxide(s) during nitrification. MBio. 2016;7:e01753-16. doi: 10.1128/mBio.01753-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carreras CW, Gehring AM, Walsh CT, Khosla C. Utilization of enzymatically phosphopantetheinylated acyl carrier proteins and acetyl-acyl carrier proteins by the actinorhodin polyketide synthase. Biochemistry. 1997;36:11757–11761. doi: 10.1021/bi971350+. [DOI] [PubMed] [Google Scholar]
- 34.Gerlt JA, et al. Enzyme function initiative-enzyme similarity tool (EFI-EST): A web tool for generating protein sequence similarity networks. Biochim Biophys Acta. 2015;1854:1019–1037. doi: 10.1016/j.bbapap.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harwood CS, Burchhardt G, Herrmann H, Fuchs G. Anaerobic metabolism of aromatic compounds via the benzoyl-CoA pathway. FEMS Microbiol Rev. 1998;22:439–458. [Google Scholar]
- 36.Cregger MA, et al. The Populus holobiont: Dissecting the effects of plant niches and genotype on the microbiome. Microbiome. 2018;6:31. doi: 10.1186/s40168-018-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottel NR, et al. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. Appl Environ Microbiol. 2011;77:5934–5944. doi: 10.1128/AEM.05255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oda Y, et al. Multiple genome sequences reveal adaptations of a phototrophic bacterium to sediment microenvironments. Proc Natl Acad Sci USA. 2008;105:18543–18548. doi: 10.1073/pnas.0809160105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S, Stecher G, Tamura K. Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staley JT. The genera Prosthecomicrobium and Ancalomicrobium. In: Starr MP, Stolp H, Truper HG, editors. The Prokaryotes. Springer; Berlin: 1981. pp. 456–460. [Google Scholar]
- 42.Poindexter JS. Biological properties and classification of the Caulobacter group. Bacteriol Rev. 1964;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- 44.Stanier RY, Palleroni NJ, Doudoroff M. The aerobic pseudomonads: A taxonomic study. J Gen Microbiol. 1966;43:159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- 45.Oliver CM, Schaefer AL, Greenberg EP, Sufrin JR. Microwave synthesis and evaluation of phenacylhomoserine lactones as anticancer compounds that minimally activate quorum sensing pathways in Pseudomonas aeruginosa. J Med Chem. 2009;52:1569–1575. doi: 10.1021/jm8015377. [DOI] [PubMed] [Google Scholar]
- 46.Thomas PW, Fast W. Heterologous overexpression, purification, and in vitro characterization of AHL lactonases. Methods Mol Biol. 2011;692:275–290. doi: 10.1007/978-1-60761-971-0_20. [DOI] [PubMed] [Google Scholar]
- 47.Kostylev M, Otwell AE, Richardson RE, Suzuki Y. Cloning should be simple: Escherichia coli DH5α-mediated assembly of multiple DNA fragments with short end homologies. PLoS One. 2015;10:e0137466. doi: 10.1371/journal.pone.0137466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rühmann B, Schmid J, Sieber V. Methods to identify the unexplored diversity of microbial exopolysaccharides. Front Microbiol. 2015;6:565. doi: 10.3389/fmicb.2015.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.