Abstract
Background:
Researchers suggest that benign breast disease (BBD) is a key risk factor for breast cancer. The present study aimed to determinate the risk level of breast cancer in terms of various BBD subgroups.
Methods:
A meta-analysis was performed to determinate the risk of breast cancer associated with BBD. Observational studies (traditional case-control studies, nested case-control studies, and cohort studies) published from January 2000 to June 2015 were assessed to evaluate the risk of developing breast cancer related to BBD. Various databases such as Medline (PubMed), Web of Science (ISI), Scopus, and Google Scholar were searched. The additional search included the Journal of Breast Cancer Research and Treatment and the Journal of Cancer Research.
Results:
Twenty studies out of 21 were used to estimate the risk of developing breast cancer related to proliferative disease without atypia versus non-proliferative disease and the reported risk ranged from 1.04 to 1.83. The reported risk of developing breast cancer related to proliferative disease with atypia versus non-proliferative disease in 21 studies ranged from 1.59 to 4.74. Based on 20 studies, the pooled risk estimates for developing breast cancer related to proliferative disease without atypia versus non-proliferative disease was 1.58 (95% CI: 1.51-1.66). Based on 21 studies, the pooled risk estimates for developing breast cancer related to proliferative disease with atypia versus non-proliferative disease was 3.49 (95% CI: 3.23-3.77).
Conclusion:
The overall result of this review showed an elevated risk for breast cancer related to BBD subtypes. We propose better strategies for screening recommendations for such women.
Keywords: Benign breast disease ; Dysplasia, mammary ; Breast neoplasms ; Risk factors ; Review
What’s Known
The association between benign breast diseases (BBD) and breast cancer has been the concern of researchers. BBD has been reported to increase the risk of breast cancer, but the risk level is still under discussion.
Few meta-analyses have been conducted to determine the risk level of breast cancer.
What’s New
It is shown that the risk of developing breast cancer related to proliferative disease with atypia versus non-proliferative disease has increased to 3.49, and the risk of developing breast cancer related to proliferative disease without atypia versus non-proliferative disease has increased to 1.58
Better strategies for screening recommendations are suggested
Introduction
Benign breast disease (BBD) includes a wide spectrum of histological changes,1,2 and it is a common disease in women worldwide.3-5 The incidence of BBD varies according to different diagnostic methods and pathological criteria. The incidence of confirmed BBD by biopsy is almost 10-20%, while it is approximately 50% by autopsy.6
There is insufficient information about the role of some breast cancer risk factors such as BBD, the age at time of BBD diagnosis, status of menopause, personal or family history of breast cancer; duration from BBD diagnosis,7 reproductive history, lifestyle, genetics; environmental factors,8 multiple BBD,9 radial scar,10 BBD biopsy,11 breast density,12 etc.
Researchers suggest that BBD is a key risk factor for breast cancer.13 The relationship between BBD and breast cancer has been a controversial topic for a long time. The first report on BBD progressing to breast cancer was published in the 1960’s.14 Over the last decades, the majority of research studies have demonstrated a strong relationship between BBD and breast cancer.14,15 It is shown that women with a diagnosis of BBD have a two-fold greater breast cancer risk than those without a BBD.2 The other topic that researchers would like to explore is whether the risk level of breast cancer differs with respect to various BBD subgroups.16 In previous studies, the risk level of proliferative lesions was estimated to be 3 to 5 times greater than that of non-proliferative lesions or no BBD.2,15,17 While recent studies have confirmed these results; however, the risk level of each BBD subtype remains unclear.18
Increasing the awareness of women with BBD regarding the disease’s main risk factors for breast cancer could be their strongest incentive to alter their health behavior and to prevent the consequences of breast cancer.19 In this regard, an accurate risk estimate would help decision-makers to design appropriate programs and interventions to improve the health of women at the risk of breast cancer.19,20 Hence, the present systematic review and meta-analysis aimed to assay the risk level of BBD subtypes leading to breast cancer.
Materials and Methods
Protocol and Registration
PROSPERO registration was utilized under the confirmation number 42016035243. The protocol of this meta-analysis has already been published in the Iranian Journal of Medical Sciences. The PRISMA checklist was used for reporting the present systematic review and meta-analysis.
Eligibility Criteria
Observational studies, including traditional case-control studies, nested case-control studies, and cohort studies were assessed to evaluate the risk of developing BBD related breast cancer. All of the included studies were published from January 2000 to June 2015. The inclusion criteria were articles in the English language, BBD classifications based on the pathologic criteria of Page et al. and Dupond et al., and containing the non-proliferative disease, proliferative disease without atypia, and proliferative disease with atypia.13 The extracted risk estimates from the studies were risk ratio, odds ratio, standardized incidence ratios; rate ratio, hazard ratio, and incidence rate ratio.
Information Sources and Search
In the present study, various databases such as Medline (PubMed), Web of Science (ISI), Scopus, and Google Scholar were searched. The additional search included the Journal of Breast Cancer Research and Treatment and the Journal of Cancer Research. The end-date for the search was June 2015.
The search of PubMed database was performed using the following query string syntax. (“2000/01/01”[Date-Publication]:”2015/06/21”[Date-Publication])”Breast cancer”[tiab] AND (“benign breast disease”[tiab] OR “non-proliferative breast disease” OR “Mammary Dysplasia”[tiab] OR “Mastopathy”[tiab] OR “Breast Fibrocystic Changes”[tiab] OR “Microglandular Adenos*”[tiab] OR “Chronic Cystic Mastitis”[tiab]). A total of 521 items was detected by the above-mentioned query from which 21 primary articles were identified. The search of the Web of Science (ISI) database was performed using the following query string syntax. “Breast cancer” AND “benign breast disease” OR “non-proliferative breast disease” OR “Mammary Dysplasia” OR “Mastopathy” OR “Breast Fibrocystic Changes” OR “Microglandular Adenos*” OR “Chronic Cystic Mastitis”. A total of 273 items was detected by the above-mentioned query from which 11 primary articles were identified. The search of the Scopus database was performed using the following query string syntax. “Breast cancer” AND “benign breast disease” OR “non-proliferative breast disease” OR “Mammary Dysplasia” OR “Mastopathy” OR “Breast Fibrocystic Changes” OR “Microglandular Adenos*” OR “Chronic Cystic Mastitis” (FROM2000-21 JUN 2015). A total of 868 items was detected by the above-mentioned query from which 15 primary articles were identified. The search of the Google Scholar database was performed using the query string syntax: “Breast cancer”+”benign breast disease” “Mastopathy”. A total of 362 items was detected by the above-mentioned query from which 6 primary articles were identified. The Journal of Breast Cancer Research and Treatment and the Journal of Cancer Research were also searched using the query string syntax: “Breast cancer” AND “benign breast disease” OR “non-proliferative breast disease”. The search yielded 262 and 97 search items, respectively, from which no items were considered suitable for inclusion in our meta-analysis.
Eventually, after reviewing all primary articles and eliminating duplicated studies, 21 articles were used in the meta-analysis. Among them, 20 articles reported the risk estimate (95% CI) for proliferative disease without atypia versus non-proliferative disease.1,2,8,9,12-15,21-36 All articles reported the risk estimate (95% CI) for proliferative disease with atypia versus non-proliferative disease.34
Study Selection
Eligibility assessment was conducted by two independent reviewers that evaluated the titles, abstracts, inclusion and exclusion criteria, and full-text. Possible disagreements were resolved in a panel discussion; otherwise, the issue was referred to a third reviewer. For quality assessment, a modified version of the quality assessment checklist for observational studies (STROBE) was used (appendix I).
appendix I.
Data extraction form
| Review title | |
|---|---|
| 1. General information | |
| Article code | |
| Reference number | |
| Reviewer initials | |
| Publication details: | |
| First author | |
| Journal title and year | |
| Volume and first page | |
| 2. Study eligibility | |
| Name of the country | |
| Total study period | |
| Participants: | |
| Study setting (e.g. urban, rural, hospital-based, population-based) | |
| Inclusion criteria (in the study) | |
| Exclusion criteria (in the study) | |
| Total population at start of study | |
| Age of study population | |
| Type of outcome measures | Odds ratio Risk ratio Rate ratio Hazard ratio |
| Should this study be included in the review? | Yes No Maybe Reasons for No or Maybe: ........ |
| 3. Method | |
| Aims of the study | |
| Study design | Traditional case-control Nested case-control Case-cohort Cohort |
| Ethical approval obtained for the study | |
| 4. Risk of bias assessment | |
| Quality scale | Yes=1 No=0 Unclear=0 |
| Items | |
| Abstract | 1. Clearly defined study design and main results of the study |
| Objectives | 2. State specific objectives, including any prespecified hypotheses |
| Study design | 3. Present key elements of study design early in the paper |
| Setting | 4. Describe the setting |
| 5. Locations | |
| 6. Including periods of recruitment | |
| 7. Case definition/exposure | |
| Participants | 8. Give the eligibility criteria |
| 9. The sources and methods for the selection of participants | |
| 10. Describe methods of follow-up/Give the rationale for the choice of cases and controls | |
| 11. For matched studies, give matching criteria and the number of exposed/unexposed controls per case | |
| Variables | 12. Clearly define all outcomes |
| 13. Clearly define potential confounders and effect modifiers | |
| Data sources/measurement | 14. Give sources of data and details of methods of assessment |
| Bias | 15. Address potential sources of bias |
| Study size | 16. Explain how the study size was derived |
| Statistical methods | 17. Describe all statistical methods |
| 18. Including those used to control for confounding | |
| 19. If applicable, explain how lost to follow-up/matching of cases and controls was addressed | |
| 20. Describe any sensitivity analyses | |
| Total | |
| 5. Results: | |
| Outcome | |
| Number of case | |
| Number of control | |
| Number of exposed | |
| Number of unexposed | |
| Crude results (95% CI) | |
| Adjusted results (95% CI) | |
| Adjusted for which confounders? | |
Data Collection Process
A dedicated data extraction form was developed that included the general information of the studies (e.g. article code, article title, reference number, reviewer initials, publication details, first author, journal title and year, volume, first page), study eligibility (e.g. country name, total study period, participants, study setting, inclusion criteria, exclusion criteria, total population at the start of the study, age of the study population, type of outcome measures), methods (e.g. aim of the study, study design (traditional case-control, nested case-control, cohort), ethical approval), risk of bias assessment, and results (risk estimates) including relative risks (e.g. odds ratio, risk ratio, rate ratio, hazard ratio) (appendix II).
appendix II.
Quality assessment form (modified form of STROBE)
| Items | Yes=1 | No=0 | Unclear=0 | |
|---|---|---|---|---|
| Abstract | 1. Clearly defined study design and main results of the study | |||
| Objectives | 2. State specific objectives, including any prespecified hypotheses | |||
| Study design | 3. Present key elements of study design early in the paper | |||
| Setting | 4. Describe the setting | |||
| 5. Locations | ||||
| 6. Including periods of recruitment | ||||
| 7. Case definition/ exposure | ||||
| Participants | 8. Give the eligibility criteria | |||
| 9. The sources and methods for the selection of participants | ||||
| 10. Describe methods of follow-up/ Give the rationale for the choice of cases and controls | ||||
| 11. For matched studies, give matching criteria and the number of exposed and unexposed/ controls per case | ||||
| Variables | 12. Clearly define all outcomes | |||
| 13. Clearly define potential confounders and effect modifiers | ||||
| Data sources/measurement | 14. Give sources of data and details of methods of assessment | |||
| Bias | 15. Address potential sources of bias | |||
| Study size | 16. Explain how the study size was derived | |||
| Statistical methods | 17. Describe all statistical methods | |||
| 18. Including those used to control for confounding | ||||
| 19. If applicable, explain how lost to follow-up/matching of cases and controls was addressed | ||||
| 20. Describe any sensitivity analyses | ||||
| Total | ||||
Synthesis of Results
The data were processed using the STATA software version 12.0. A random effect model was performed to check the heterogeneity of the studies and the analyses were performed by the “metan” command. After checking for crude and adjusted risk estimates in the primary studies, less than 10% difference was found between them which indicated no confounding effect. Therefore, it was decided to use the crude risk estimates of BBD for developing breast cancer since all primary studies had reported exposure and outcome in 2×2 tables. Crude OR was used to synchronize the result of the studies. To examine heterogeneity across studies, Q statistic test (I-square) was used. The publication bias was examined using funnel plot, Begg’s test, and Egger’s test. The “trim and fill” method was used to confirm the Egger’s test result.
Additional Analyses
Subgroup analysis was used to control the effect of potential risk modifiers. The quality (low/high) and population-based/hospital-based categories were considered as the main subgroups for additional analysis.
Results
Study Selection
An initial search of the databases yielded 2,383 potential literature, among which 96 related articles were identified in the systematic search. Based on the exclusion criteria, 21 studies were included in the quantitative synthesis of the meta-analysis (figure 1).
Figure1.
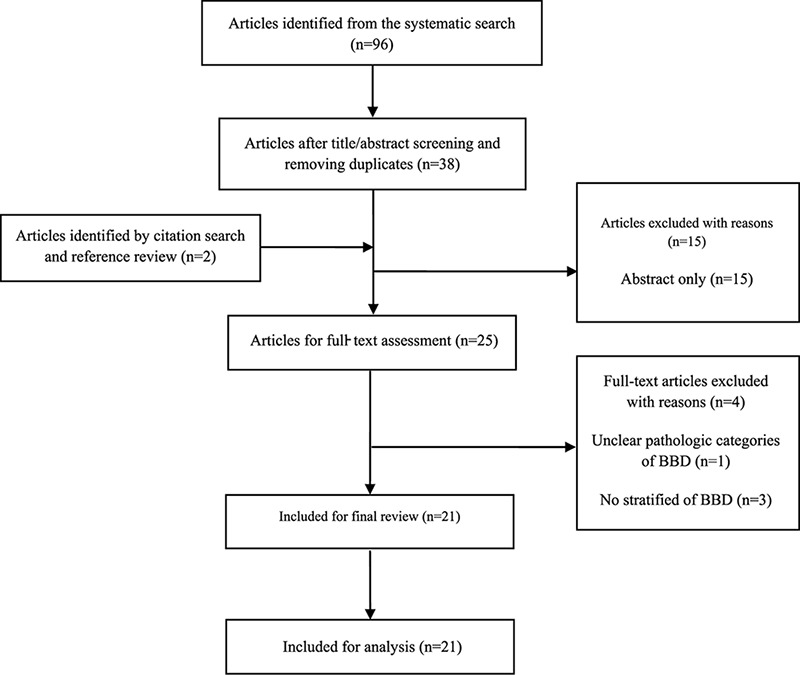
Flow diagram of the systematic search and selection process of articles for BBD as a risk factor of breast cancer.
Study Characteristics
All observational studies with a cohort design (n=10), nested case-control design (n=10), and traditional case-control design (n=1) which were published from 2000 to 2015 and evaluated breast cancer risk for proliferative disease without atypia (PDWA) and proliferative disease with atypia (PDA) versus non-proliferative disease (NPD) were included. All data usages were reported from 1946 to 2011.
Risk of Bias Within Studies
For the quality assessment, a modified version of the quality assessment checklist for observational studies (STROBE) was used (appendix II). The checklist of each study was entered by two independent reviewers. To determine the eligibility of the articles, the sum score of quality items was used. The score ranged from 13 to 19. The mean±SD of the quality score for the primary studies was 15.66±1.85. Of the 21 studies, 11 (52.38%) were of a low-quality (≤15) and 10 (47.62%) were of a high-quality (>15).
Results of Individual Studies
Twenty studies out of 21 were used to estimate the risk of developing breast cancer related to PDWA versus NPD. The reported risk was from 1.04 to 1.83 (figure 2). Risk estimation for PDWA versus NPD disease in high-quality studies ranged from 1.04 to 1.75 and in low-quality studies ranged from 1.21 to 1.83 (figure 3). Risk estimation for PDWA versus NPD disease in population-based studies ranged from 1.35 to 1.81 and in hospital-based studies ranged from 1.04 to 1.83 (figure 4). Risk estimation for PDWA versus NPD disease in cohort studies ranged from 1.21 to 1.83 and in case-control studies ranged from 1.04 to 1.75 (figure 5). The reported risks of developing breast cancer related to PDA versus NPD in 21 studies ranged from 1.59 to 4.74 (figure 6). Risk estimation for PDA versus NPD disease in high-quality studies ranged from 2.14 to 4.56 and in low-quality studies ranged from 1.59 to 4.74 (figure 7). Risk estimation for PDA versus NPD disease in population-based studies ranged from 1.59 to 4.51 and in hospital-based studies ranged from 3.18 to 4.74 (figure 8). Risk estimation for PDA versus NPD disease in cohort studies ranged from 1.59 to 4.74 and in case-control studies ranged from 2.14 to 4.56. (figure 9).
Figure2.
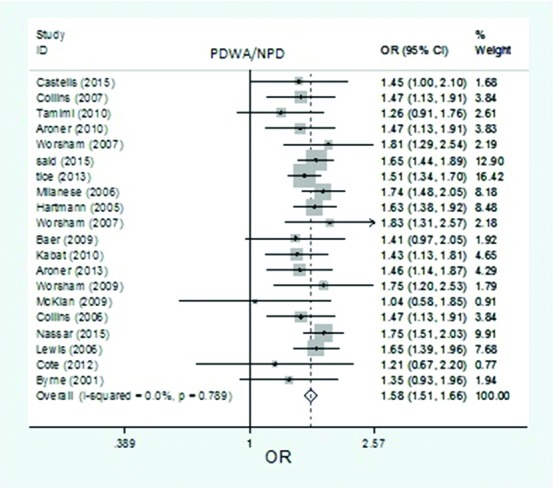
The forest plot of pooled risk estimates of developing breast cancer in patients with proliferative disease without atypia versus non-proliferative disease. Based on 20 studies, the pooled risk estimates of developing breast cancer related to PDWA versus NPD was 1.58 (95% CI: 1.51-1.66).
Figure3.
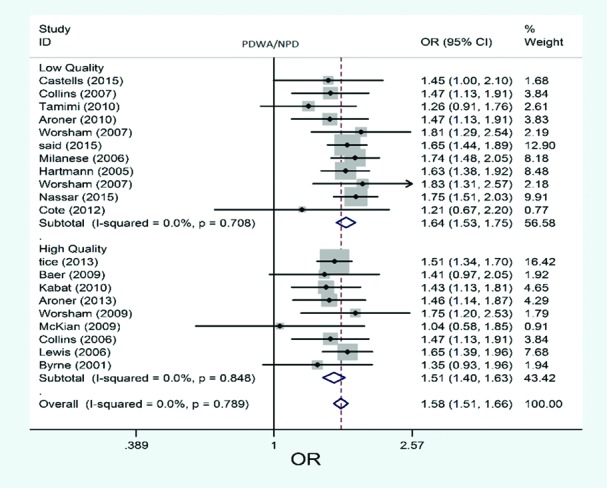
The forest plot of pooled risk estimates of developing breast cancer in patients with proliferative disease without atypia versus proliferative disease by quality. Risk estimation for PDWA versus NPD disease in high-quality studies ranged from 1.04 to 1.75, and in low-quality studies ranged from 1.21 to 1.83.
Figure4.
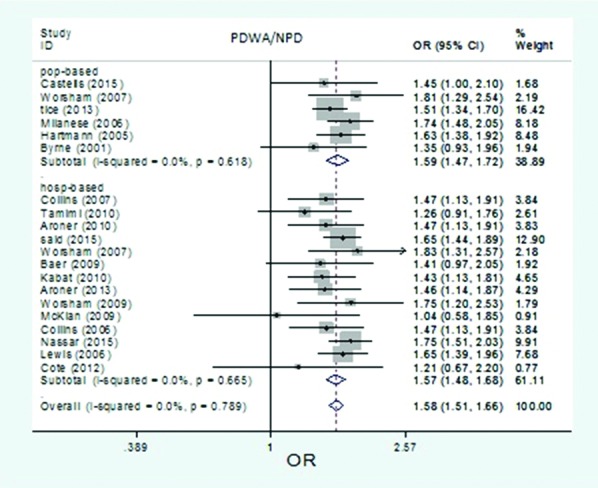
The forest plot of pooled risk estimates of developing breast cancer in patients with proliferative disease without atypia versus non-proliferative disease by study setting of studies. Risk estimation for PDWA versus NPD disease in population-based studies ranged from 1.35 to 1.81, and in hospital-based studies ranged from 1.04 to 1.83.
Figure5.
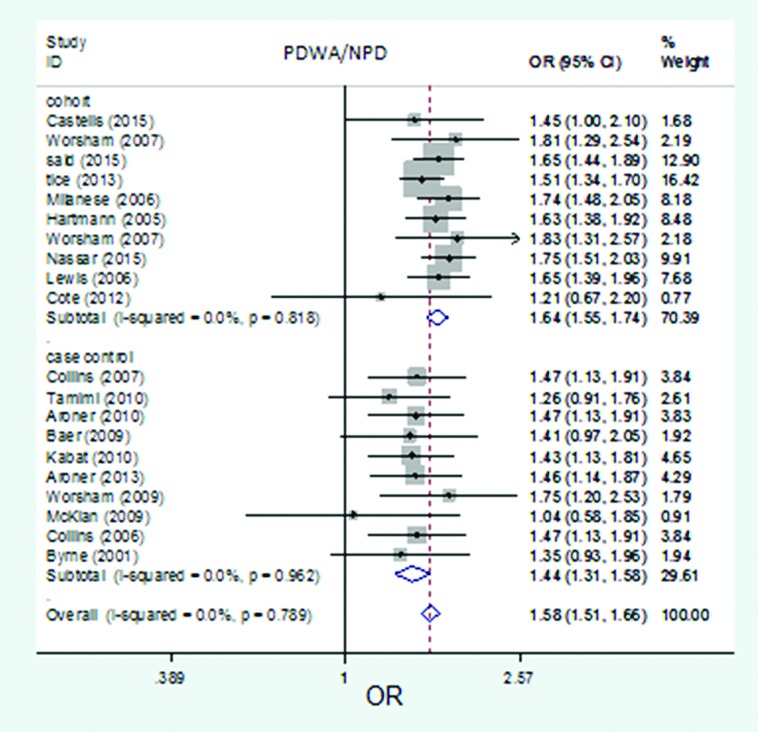
The forest plot of pooled risk estimates of developing breast cancer in patients with proliferative disease without atypia versus non-proliferative disease by study design. Risk estimation for PDWA versus NPD disease in cohort studies ranged from 1.21 to 1.83, and in case-control studies ranged from 1.04 to 1.75.
Figure6.
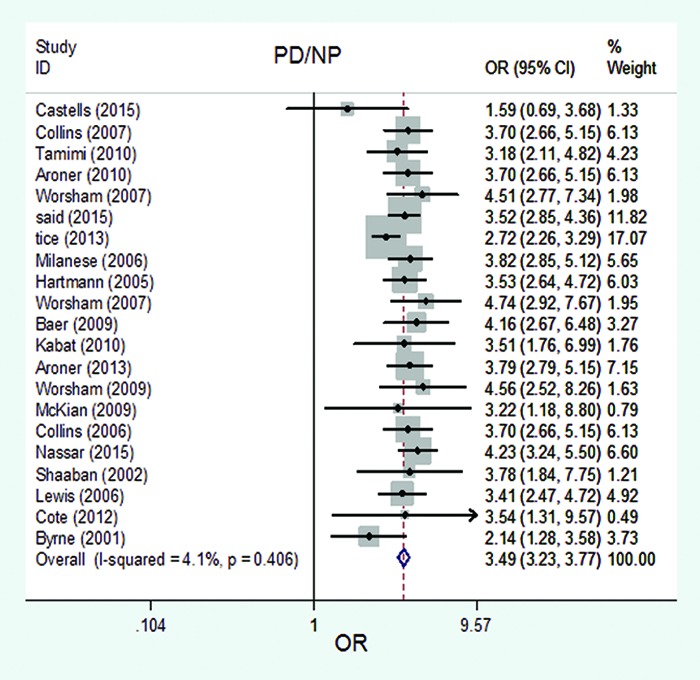
The forest plot of pooled risk estimates of developing breast cancer in patients with the proliferative disease with atypia versus non-proliferative disease. The pooled risk estimates of developing breast cancer related to PDA versus NPD was 3.49 (95% CI: 3.23-3.77), based on 21 studies.
Figure7.
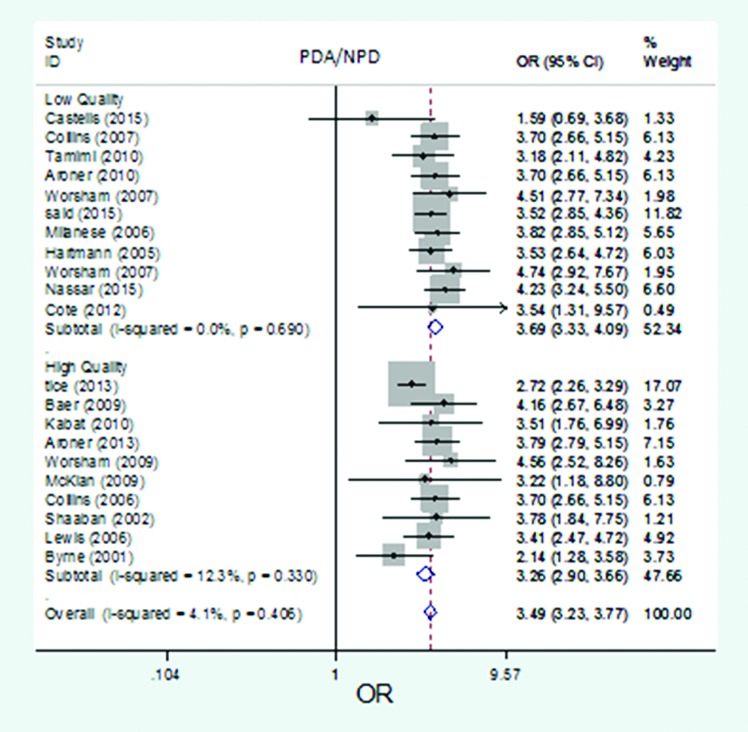
The forest plot of pooled risk estimates of developing breast cancer in patients with the proliferative disease with atypia versus proliferative disease by quality. Risk estimation for PDA versus NPD disease in high-quality studies ranged from 2.14 to 4.56, and in low-quality studies ranged from 1.59 to 4.74.
Figure8.
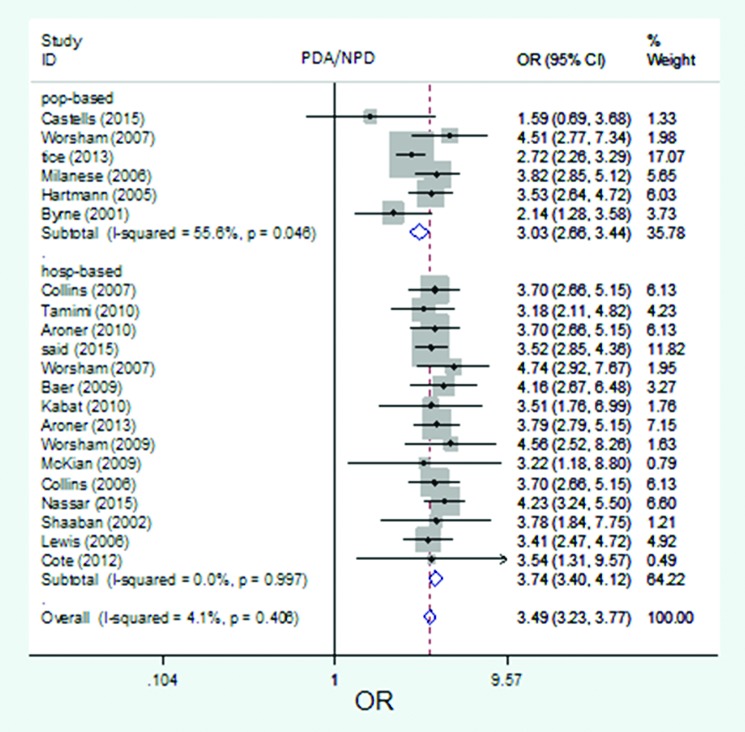
The forest plot of pooled risk estimates of developing breast cancer in patients with the proliferative disease with atypia versus proliferative disease by setting. Risk estimation for PDA versus NPD disease in population-based studies ranged from 1.59 to 4.51, and in hospital-based studies ranged from 3.18 to 4.74.
Figure9.
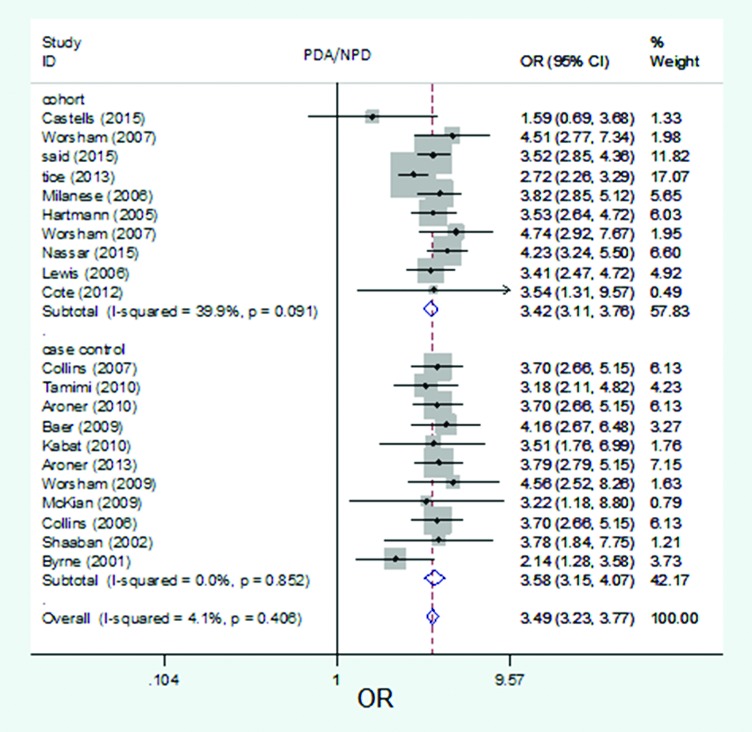
The forest plot of pooled risk estimates of developing breast cancer in patients with the proliferative disease with atypia versus proliferative disease by study design. Risk estimation for PDA versus NPD disease in cohort studies ranged from 1.59 to 4.74, and in case-control studies ranged from 2.14 to 4.56.
Synthesis of Results
Based on the 20 studies, the pooled risk estimates of developing breast cancer related to PDWA versus NPD was 1.58 (95% CI: 1.51-1.66) (figure 2). No significant heterogeneity was seen in the studies that used risk estimates related to PDWA versus NPD (I2=0.0%; df=19, P=0.789). Based on the 20 studies, the pooled risk estimates of developing breast cancer related to PDA versus NPD was 3.49 (95% CI: 3.23-3.77) (figure 6). No significant heterogeneity was seen in the studies that used risk estimates related to PDA versus NPD (I2=4.1%; df=20, P=0.406).
Risk of Bias Across Studies
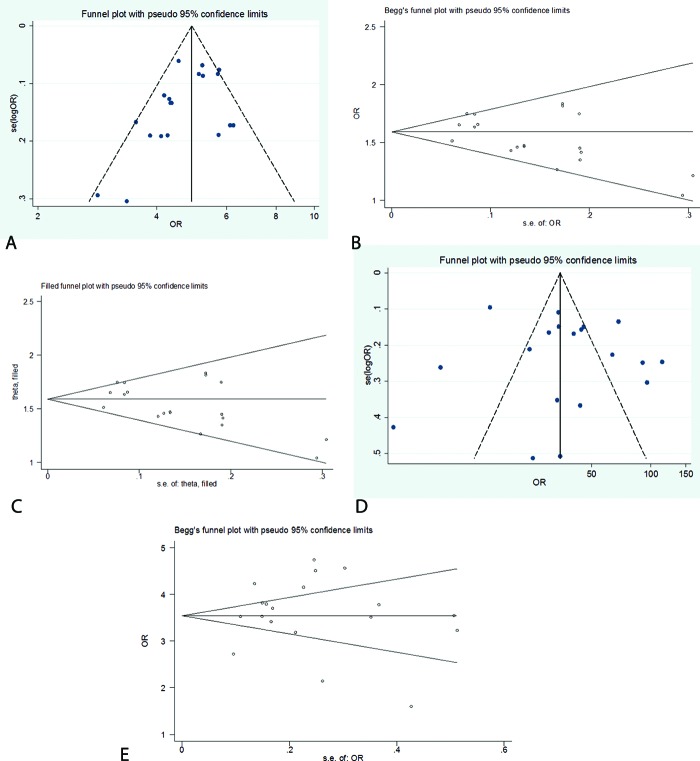
To estimate the risk of developing breast cancer related to proliferative disease without atypia versus non-proliferative disease, the publication bias was assessed by the funnel plot (figure 10a), Egger’s test, and Begg’s test (figure 10b) plots. Egger’s test showed a low publication bias (P=0.049). Therefore, the “trim and fill” method was used to confirm the Egger’s test result. Based on the result of the trim test, the publication bias from all studies was omitted in the present analysis (figure 10c).
Figure10.
a) The funnel plot for publication bias of the risk estimate of developing breast cancer in patients with proliferative disease without atypia versus non-proliferative disease. No significant publication bias was seen according to the funnel plot. b) The Begg plot for the publication bias of risk estimate of developing breast cancer in patients with proliferative disease without atypia versus non-proliferative disease. The Egger’s test showed a low publication bias (P=0.049). c) The “trim and fill” plot for the publication bias of risk estimate of developing breast cancer in patients with proliferative disease without atypia versus non-proliferative disease. According to the trim test result, publication bias was rejected and none of the studies were added to the present study. d) The funnel for publication bias of the risk estimate of developing breast cancer in patients with the proliferative disease with atypia versus non-proliferative disease. No significant publication bias was seen according to the funnel plot. e) The Begg plot for the publication bias of risk estimate of developing breast cancer in patients with the proliferative disease with atypia versus non-proliferative disease. No significant publication bias was seen according to the Begg plot.
To estimate the risk of developing breast cancer related to proliferative disease with atypia versus non-proliferative disease, the publication bias was assessed by the funnel plot (figure 10d), Egger’s test (P=0.445), and Begg’s test (figure 10e) plots. Overall, no significant publication bias was observed.
Additional Analysis
The present meta-analysis involved two types of subgroup analysis in BBD subtypes risk estimation. The pooled risk estimate of developing breast cancer related to proliferative disease without atypia versus non-proliferative disease in high-quality studies (n=9) was 1.51 (95% CI: 1.40-1.63), in low-quality studies (n=11): 1.64 (95% CI: 1.53-1.75), in population-based studies (n=6): 1.59 (95% CI: 1.47-1.72), and in hospital-based studies (n=14): 1.57 (95% CI: 1.48-1.68).
The pooled risk estimate of developing breast cancer related to proliferative disease with atypia versus non-proliferative disease in high-quality studies (n=10) was 3.26 (95% CI: 2.90-3.66), in low-quality studies (n=11): 3.69 (95% CI: 3.33-4.09), in population-based studies (n=6): 3.03 (95% CI: 2.66-3.44), and in hospital-based studies (n=15): 3.74 (95% CI 3.40-4.12).
Discussion
The present meta-analysis was performed on a large number of primary studies from different countries across the world. Only two meta-analyses that evaluated the risk of developing breast cancer in women with BBD were found.8,35 The date for data collection in these reports was 2011. Only one systematic review on proliferative epithelial disease and the risk of developing breast cancer was found, where the data were collected in October 2013.36
From the primary articles, all data that evaluated the risk of developing breast cancer associated with BBD subtypes and published during 2000-2015 were collected. In line with the previous two meta-analyses, it was found that the risk level for breast cancer in women with a history of biopsy-proven proliferative disease with atypia was higher than those without atypia. The highest increase in the relative risk was 4.74 and the final result showed that having a history of proliferative disease with atypia increases the risk of developing breast cancer by up to 3.5 times. Additionally, women with proliferative disease without atypia had an increased risk than those with the non-proliferative disease. There was no heterogeneity among the studies, thus it was not used in the present meta-analysis.
Considering the inclusion of many studies and the fact that breast cancer involves multi-risk factors, various multivariate statistical models can be used to adjust these factors. In regard to uniformity, the difference between crude risk measures and every adjusted measure in primary studies was checked. Less than 10% difference was found between them. Therefore, 2×2 tables from the primary studies were used to calculate the crude risks. Then, the meta-analysis was performed based on the crude odds ratios. This uniformity will improve the knowledge of clinicians, educate patients, and guide screening recommendations.8
The main strength of the present study is the risk level estimation of progressing breast cancer associated with BBD subtypes based on the quality of primary studies. In this review, the results of subgroup analysis showed that high-quality studies reported lower risk estimates. Experience shows that large studies with exact methodologies and high-quality provide a better estimation of the true risk estimate.
The main limitation of the present study is related to various pathological classifications in previous studies (i.e. three main histological categories were used). Other meta-analyses also noted such limitation.8 Another limitation was related to the primary studies that did not report the risk level of breast cancer associated with non-proliferative disease versus no BBD. Thus, we could not estimate the risk level of breast cancer related to this type of BBD.
Conclusion
The result of the present meta-analysis showed that benign proliferative breast disease raises the risk of breast cancer and the risk level is different with respect to the type of BBD. It is recommended to provide better screening and management strategies for women who are potentially susceptible to develop breast cancer based on the histological classification of BBD.
Acknowledgement
The authors are deeply grateful to the Vice Chancellor for Research of Guilan University of Medical Sciences for supporting this project and approving the protocol of this meta-analysis.
Conflict of Interest:None declared.
References
- 1.Kabat GC, Jones JG, Olson N, Negassa A, Duggan C, Ginsberg M, et al. A multi-center prospective cohort study of benign breast disease and risk of subsequent breast cancer. Cancer Causes Control. 2010;21:821–8. doi: 10.1007/s10552-010-9508-7. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tamimi RM, Rosner B, Colditz GA. Evaluation of a breast cancer risk prediction model expanded to include category of prior benign breast disease lesion. Cancer. 2010;116:4944–53. doi: 10.1002/cncr.25386. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldacre MJ, Abisgold JD, Yeates DG, Vessey MP. Benign breast disease and subsequent breast cancer: English record linkage studies. J Public Health (Oxf) 2010;32:565–71. doi: 10.1093/pubmed/fdq001. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh SJ, Oh H, Peterson MA, Almendro V, Hu R, Bowden M, et al. The Proliferative Activity of Mammary Epithelial Cells in Normal Tissue Predicts Breast Cancer Risk in Premenopausal Women. Cancer Res. 2016;76:1926–34. doi: 10.1158/0008-5472.CAN-15-1927. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Visscher DW, Frost MH, Hartmann LC, Frank RD, Vierkant RA, McCullough AE, et al. Clinicopathologic features of breast cancers that develop in women with previous benign breast disease. Cancer. 2016;122:378–85. doi: 10.1002/cncr.29766. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onstad M, Stuckey A. Benign breast disorders. Obstet Gynecol Clin North Am. 2013;40:459–73. doi: 10.1016/j.ogc.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Schnitt SJ. Benign breast disease and breast cancer risk: morphology and beyond. Am J Surg Pathol. 2003;27:836–41. doi: 10.1097/00000478-200306000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Dyrstad SW, Yan Y, Fowler AM, Colditz GA. Breast cancer risk associated with benign breast disease: systematic review and meta-analysis. Breast Cancer Res Treat. 2015;149:569–75. doi: 10.1007/s10549-014-3254-6. [DOI] [PubMed] [Google Scholar]
- 9.Worsham MJ, Raju U, Lu M, Kapke A, Cheng J, Wolman SR. Multiplicity of benign breast lesions is a risk factor for progression to breast cancer. Clin Cancer Res. 2007;13:5474–9. doi: 10.1158/1078-0432.CCR-07-0928. [DOI] [PubMed] [Google Scholar]
- 10.Berg JC, Visscher DW, Vierkant RA, Pankratz VS, Maloney SD, Lewis JT, et al. Breast cancer risk in women with radial scars in benign breast biopsies. Breast Cancer Res Treat. 2008;108:167–74. doi: 10.1007/s10549-007-9605-9. [DOI] [PubMed] [Google Scholar]
- 11.Kabat GC, Jones JG, Olson N, Negassa A, Duggan C, Ginsberg M, et al. Risk factors for breast cancer in women biopsied for benign breast disease: a nested case-control study. Cancer Epidemiol. 2010;34:34–9. doi: 10.1016/j.canep.2009.12.005. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tice JA, O’Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density, and the risk of breast cancer. J Natl Cancer Inst. 2013;105:1043–9. doi: 10.1093/jnci/djt124. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castells X, Domingo L, Corominas JM, Tora-Rocamora I, Quintana MJ, Bare M, et al. Breast cancer risk after diagnosis by screening mammography of nonproliferative or proliferative benign breast disease: a study from a population-based screening program. Breast Cancer Res Treat. 2015;149:237–44. doi: 10.1007/s10549-014-3208-z. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worsham MJ, Abrams J, Raju U, Kapke A, Lu M, Cheng J, et al. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J. 2007;13:115–21. doi: 10.1111/j.1524-4741.2007.00388.x. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. Magnitude and laterality of breast cancer risk according to histologic type of atypical hyperplasia: results from the Nurses’ Health Study. Cancer. 2007;109:180–7. doi: 10.1002/cncr.22408. [DOI] [PubMed] [Google Scholar]
- 16.Kotsopoulos J, Chen WY, Gates MA, Tworoger SS, Hankinson SE, Rosner BA. Risk factors for ductal and lobular breast cancer: results from the nurses’ health study. Breast Cancer Res. 2010;12:R106. doi: 10.1186/bcr2790. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Degnim AC, Dupont WD, Radisky DC, Vierkant RA, Frank RD, Frost MH, et al. Extent of atypical hyperplasia stratifies breast cancer risk in 2 independent cohorts of women. Cancer. 2016;122:2971–8. doi: 10.1002/cncr.30153. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posso M, Corominas JM, Serrano L, Roman M, Tora-Rocamora I, Domingo L, et al. Biomarkers expression in benign breast diseases and risk of subsequent breast cancer: a case-control study. Cancer Med. 2017;6:1482–9. doi: 10.1002/cam4.1080. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park K, Chang SJ, Kim HC, Park EC, Lee ES, Nam CM. Big gap between risk perception for breast cancer and risk factors: nationwide survey in Korea. Patient Educ Couns. 2009;76:113–9. doi: 10.1016/j.pec.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Dumitrescu RG, Cotarla I. Understanding breast cancer risk -- where do we stand in 2005? J Cell Mol Med. 2005;9:208–21. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Costantino JP, Tan-Chiu E, Wickerham DL, Paik S, Wolmark N. Lower-category benign breast disease and the risk of invasive breast cancer. J Natl Cancer Inst. 2004;96:616–20. doi: 10.1093/jnci/djhs105. [DOI] [PubMed] [Google Scholar]
- 22.Aroner SA, Collins LC, Schnitt SJ, Connolly JL, Colditz GA, Tamimi RM. Columnar cell lesions and subsequent breast cancer risk: a nested case-control study. Breast Cancer Res. 2010;12:R61. doi: 10.1186/bcr2624. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Said SM, Visscher DW, Nassar A, Frank RD, Vierkant RA, Frost MH, et al. Flat epithelial atypia and risk of breast cancer: A Mayo cohort study. Cancer. 2015;121:1548–55. doi: 10.1002/cncr.29243. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milanese TR, Hartmann LC, Sellers TA, Frost MH, Vierkant RA, Maloney SD, et al. Age-related lobular involution and risk of breast cancer. J Natl Cancer Inst. 2006;98:1600–7. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann LC, Sellers TA, Frost MH, Lingle WL, Degnim AC, Ghosh K, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–37. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 26.Baer HJ, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Tamimi RM. Lobule type and subsequent breast cancer risk: results from the Nurses’ Health Studies. Cancer. 2009;115:1404–11. doi: 10.1002/cncr.24167. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aroner SA, Collins LC, Connolly JL, Colditz GA, Schnitt SJ, Rosner BA, et al. Radial scars and subsequent breast cancer risk: results from the Nurses’ Health Studies. Breast Cancer Res Treat. 2013;139:277–85. doi: 10.1007/s10549-013-2535-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Worsham MJ, Raju U, Lu M, Kapke A, Botttrell A, Cheng J, et al. Risk factors for breast cancer from benign breast disease in a diverse population. Breast Cancer Res Treat. 2009;118:1–7. doi: 10.1007/s10549-008-0198-8. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKian KP, Reynolds CA, Visscher DW, Nassar A, Radisky DC, Vierkant RA, et al. Novel breast tissue feature strongly associated with risk of breast cancer. J Clin Oncol. 2009;27:5893–8. doi: 10.1200/JCO.2008.21.5079. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins LC, Baer HJ, Tamimi RM, Connolly JL, Colditz GA, Schnitt SJ. The influence of family history on breast cancer risk in women with biopsy-confirmed benign breast disease: results from the Nurses’ Health Study. Cancer. 2006;107:1240–7. doi: 10.1002/cncr.22136. [DOI] [PubMed] [Google Scholar]
- 31.Nassar A, Visscher DW, Degnim AC, Frank RD, Vierkant RA, Frost M, et al. Complex fibroadenoma and breast cancer risk: a Mayo Clinic Benign Breast Disease Cohort Study. Breast Cancer Res Treat. 2015;153:397–405. doi: 10.1007/s10549-015-3535-8. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaaban AM, Sloane JP, West CR, Moore FR, Jarvis C, Williams EM, et al. Histopathologic types of benign breast lesions and the risk of breast cancer: case-control study. Am J Surg Pathol. 2002;26:421–30. doi: 10.1097/00000478-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Lewis JT, Hartmann LC, Vierkant RA, Maloney SD, Shane Pankratz V, Allers TM, et al. An analysis of breast cancer risk in women with single, multiple, and atypical papilloma. Am J Surg Pathol. 2006;30:665–72. doi: 10.1097/00000478-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Cote ML, Ruterbusch JJ, Alosh B, Bandyopadhyay S, Kim E, Albashiti B, et al. Benign breast disease and the risk of subsequent breast cancer in African American women. Cancer Prev Res (Phila) 2012;5:1375–80. doi: 10.1158/1940-6207.CAPR-12-0175. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou WB, Xue DQ, Liu XA, Ding Q, Wang S. The influence of family history and histological stratification on breast cancer risk in women with benign breast disease: a meta-analysis. J Cancer Res Clin Oncol. 2011;137:1053–60. doi: 10.1007/s00432-011-0979-z. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hornberger J, Chen SC, Li Q, Kakad P, Quay SC. Proliferative epithelial disease identified in nipple aspirate fluid and risk of developing breast cancer: a systematic review. Curr Med Res Opin. 2015;31:253–62. doi: 10.1185/03007995.2014.988209. [DOI] [PubMed] [Google Scholar]