Abstract
Background:
Dengue, chikungunya, and Zika viruses are emerging infectious disease threats wherever suitable vectors, hosts, and habitat are present. The aim of the present study was to use the bioagent transport and environmental modeling system (BioTEMS) to identify the potential for arbovirus-infected Aedes species to invade the Chabahar area in southeastern Iran.
Methods:
ArcGIS geospatial analysis software, Statistica software, and BioTEMS were used to analyze geographic information and conduct data analysis. BioTEMS utilizes up to several hundred abiotic and biotic factors to produce risk and vulnerability assessments for biological agents and infectious diseases. The output of BioTEMS was validated using published predictive models, and most importantly published collection data of Aedes species in Iran.
Results:
There appears to have been two separate invasion events by Ae. albopictus into the southern region of Iran, first preceding 2009 and then again in 2013. BioTEMS identified two probable areas of introduction during the 2009 time frame, either through one or both the Chabahar ports or the Iranshahr airport with subsequent spread through vehicular transport. BioTEMS identified the port as an introduction zone for ZIKAV with high-risk zones and identifies gap zones during the 2013 time frame. Recommended surveillance sites are provided.
Conclusion:
The air and maritime ports of Iran serve international customers, and are therefore vulnerable to import and invasion of mosquito vectors and arboviruses. Based on comparisons with other published low-resolution models, BioTEMS provides information for medical and public health professionals conducting integrated mosquito management, preventive medicine, and epidemiological surveillance.
Keywords: Integrated mosquito management , Vector control , Disease management , Iran
What’s Known
Previous studies and modeling of the suitability for the global distribution of Aedes aegypti, Aedes albopictus and the arbovirus species they transmit are often of low resolution (about 5 km2).
These published models sometimes produce contradictory results.
What’s New
A predictive output model for Aedes species and arboviruses at high resolution (up to 30 m2) is provided.
BioTEMS provides medical and public health officials a probable directional movement of invasive mosquito species, identifies zones where mosquito control should be prioritized, and identifies sites for human and vector epidemiologic surveillance.
Introduction
Mosquitoes and the pathogens they transmit can have a significant impact on the health and economy of a community. In the Islamic Republic of Iran, several arboviruses and malaria are endemic; non-human filariasis has been documented, but the status of human filariasis in Iran is unclear.1,2 Doosti et al. (2016) stated, “epidemics of mosquito-borne viral infections such as dengue, chikungunya, West Nile, and Rift Valley fevers in neighboring countries and the risk of introduction of exotic vectors into Iran have placed this country at a significant risk for these mosquito-borne diseases.”3 In the southeastern region of Iran, West Nile virus is present and the possibility for the introduction of Rift Valley fever (RVFV) remains a concern.4,5 Zika virus (ZIKAV) is invading globally where competent vectors, hosts, and habitats occur. ZIKAV is the most recent arbovirus put into status as a public health emergency by the World Health Organization; it has recently been removed as a public health emergency of international concern, but remains a significant and long-term public health problem.6
ZIKAV was first isolated in 1947 from a rhesus macaque monkey and in 1948 from Aedes africanus from cages placed on a tower in the Zika forest near Lake Victoria, Uganda.7 ZIKAV is no longer restricted to transmission by Ae. africanus in the tropical forest ecosystem in Africa. Several Aedes species, including Ae. albopictus, have been implicated in the transmission of ZIKAV to humans in urban habitats where the poor are particularly at risk.8 Sixty-one mosquito species have been recorded in Iran with no recent records of Ae. aegypti; Ae. albopictus was recently recorded in Iran in the Chabahar area in 2009.3 Aedes albopictus is one of the most medically important mosquito because of the number of pathogens it may transmit and its status as the most invasive mosquito species in the world. Twenty-six viruses have been associated with Ae. albopictus, and it is a viable vector of DENV, CHIKV, and ZIKAV.9-11 Assessing the risk of invasion and implementation of integrated mosquito management (IMM), at the local level is critical in protecting communities from medically important vectors. Where Ae. albopictus has already invaded, immigration of susceptible and infected haplotypes should be of concern. For example, risk modeling of invasive mosquito species in the United Arab Emirates indicates the port of Dubai is vulnerable to invasion by Ae. albopictus and Ae. vexans.12
Like the UAE, the ports of Chabahar and surrounding areas in southeastern Iran may also be susceptible to invasion by Ae. albopictus. Medical and public health officials at the local level in Chabahar and elsewhere in Iran would benefit from high-resolution models, maps, and information concerning sites of possible invasion of mosquitoes infected with arboviruses. Once introduced into an area, the invasive mosquito species can spread rapidly across regions through ground transport.13,14 In addition to the import of infected mosquitoes, introduction of arboviruses into a new geographic area can occur when local mosquitoes bite infected travelers and become infected, or when people become infected through sex or contaminated blood.15,16
The air and maritime ports of Iran serve international customers and are therefore at risk for the introduction of mosquito vectors and arboviruses, as well as other infectious diseases. The Bioagent transport and environmental modeling system (BioTEMS) was used to model the potential for Ae. albopictus and arboviruses to enter the region through the Chabahar port area spread into Chabahar County and Sistan-Baluchistan Province. Identifying probable invasive mosquitoes and developing an IMM plan for communities is essential. BioTEMS output identifying recommended IMM zones, control methods, and surveillance sites is discussed in order to provide practical information to local medical and public health professionals.
Materials and Methods
The maritime port of Chabahar was evaluated for invasion by Ae. albopictus. Areas at risk of arboviruses and IMM zones were developed based on the BioTEMS TIGER model should an arbovirus be introduced through the port. ArcGIS geospatial analysis software, Statistica software, and BioTEMS were used to analyze geographic information and conduct data analysis. BioTEMS has previously been used for modeling biological weapons defense and infectious diseases in several countries.17 BioTEMS utilizes up to several hundred abiotic and biotic factors to produce risk and vulnerability assessments for biological agents and infectious diseases. Examples of biotic and biotic factors include pathogen strain, vector/host relationship, vectorial capacity, host/vector physiology, colonization ability, population dynamics of hosts and vectors, soil, shade, and weather conditions, such as wind, temperature, precipitation, shade. Analytical methods within BioTEMS include artificial intelligence, fuzzy logic, niche analysis, and general additive regression.
Ecological niche and dynamic change modeling are often used to predict the potential for invasive species.18,19 Ecological niche and dynamic change modeling are used within BioTEMS to identify areas at risk for invasion by arboviruses and provide information for integrated mosquito management. The BioTEMS TIGER model has been used in several countries, e.g. Bangladesh, Brazil, Honduras, United Arab Emirates, and United States to assist in the identification of areas at high risk for invasive mosquito species and mosquito-borne disease, optimize surveillance, and treatment zones.12,20-22 The acronym TIGER represents the six stages in the invasion of a mosquito species or haplotype:12
Transport: Identifies the point of origin, method, and rate of transport to a locality.
Introduction: The point or area of initial introduction/immigration of species or haplotypes and preliminary spread into a locality.
Gap: Determines the area where vector/pathogen infiltrates and initially spreads once it has gained a foothold.
Escalade: Incorporates abiotic and biotic factors as possible resistance to invasion.
Residence and recruitment: Incorporates factors and area where vector/pathogen adds to genetic diversity or becomes endemic and recruits con-specifics/haplotypes.
The output from BioTEMS was compared to collection information of Ae. aegypti and Ae. albopictus in Iran and to global predictive maps.3,23-8 BioTEMS and ArcGIS were used to produce output into Google® Earth.
Results
The BioTEMS TIGER model predicted the suitability for the invasion of arbovirus-infected Ae. albopictus into southern Iran (figure 1). There appears to have been two separate invasion events by Ae. albopictus into the southern region of Iran, first in 2009 and then 2013. BioTEMS identified two probable areas of introduction into the region during 2009, through either or both the Chabahar ports or the Iranshahr airport with subsequent spread through vehicular transport (figure 1). BioTEMS identified the port as an introduction zone for ZIKAV with high-risk zones and identifies gap zones during the 2013 time frame (figure 2). High-risk zones are defined as an area likely to be invaded or have already been invaded by infected mosquitoes or to have localized transmission. The gap zone includes areas where ZIKAV will spread through infected mosquitoes. Recommended surveillance sites in the Chabahar and Ramin ports, Chabahar and along Highway 95 are provided (figure 2).
Figure1.
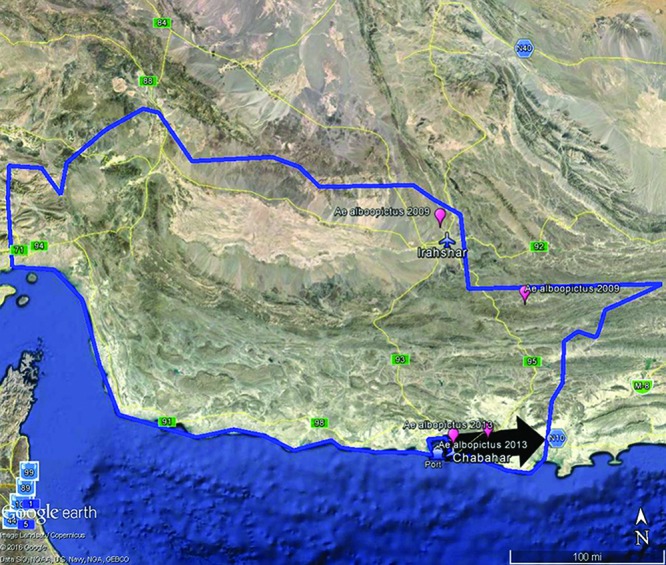
Sites where Aedes albopictus has been reported in southeastern Iran in pink balloon (Doosti et al., 2016) with maritime ports and airports shown. Blue outline is the predicted area of ZIKAV excerpted from Carlson et al. (2016). Ship and plane symbols represent BioTEMS predicted areas of invasion by Aedes albopictus into southeastern Iran. Black arrow indicates BioTEMS primary direction of spread of Aedes albopictus if introduced through the maritime ports or airports in Chabahar.
Figure2.
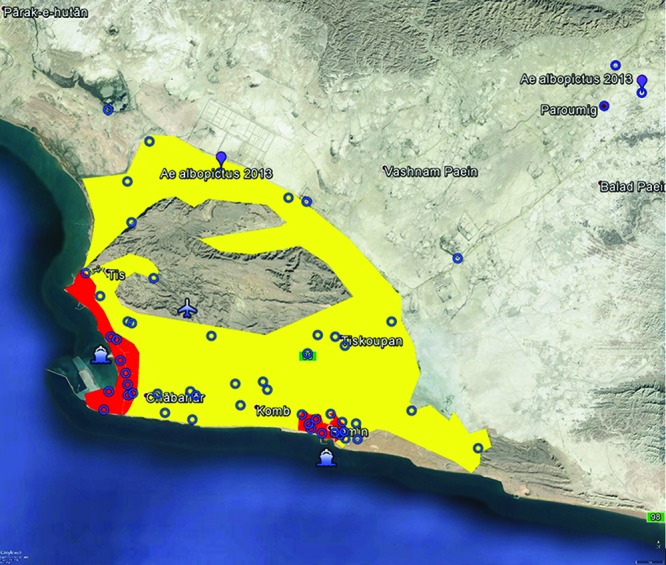
Introduction zones (red) and gap zone (yellow) surrounding Chabahar City where surveillance and control efforts should be prioritized. Blue circles represent recommended surveillance sites. Sites where Aedes albopictus were collected in 2013 are shown in purple (Doosti et al. 2016).
Discussion
The principal factor responsible for the invasion of disease vectors is through air and ship transport.29,30 Various models have been developed in order to identify and better understand the bionomics, treatment, epidemiology, and potential for geographic spread of ZIKAV. For example, A129 mice may provide an urgently required small animal model for testing of antivirals and vaccines because they are highly susceptible to infection by ZIKAV.31 Modeling sexual transmission and migration of humans demonstrated that sexual transmission influences the magnitude of an outbreak and migration influences the magnitude over time.32 When projecting the number of infections among childbearing women, Perkins et al. (2016) cautioned that a number of conditions would affect whether a local epidemic will take place such as; dispersal limitation, stochastic fadeout, and mismatches in geographic seasonality.33 Several Aedes species have been implicated in the transmission of ZIKAV, e.g. Ae. aegypti, Ae. albopictus, Ae. africanus, Ae. luteocephalus, Ae. vitattus, Ae. furcifer, Ae. Hensilii, Ae. apicoargenteus, , and Ae. taylori.34-40 However, ZIKAV models of mosquito vectors have focused on the two primary and globally distributed vectors; Ae. aegypti and Ae. albopictus.13,41
Most geographic models of low-resolution are valuable for ascertaining the current and potential geographic range of vector species and the pathogens they transmit, e.g. the 5 km2 resolution maps for Ae. aegypti and Ae. albopictus.25,27,28,33 Low-resolution models (>1 km2) have limited utility for IMM and control efforts at the community level. There are various and contradictory conclusions drawn from published predictive global models of Aedes and arboviruses. Messina et al. (2016) categorized the entire country of Iran as having limited suitability for ZIKAV; however, Carlson et al. (2016) identified geographic variation in suitability for ZIKAV in Iran.25,27 BioTEMS supports the geographic variation in susceptibility proposed by Carlson et al. (2016) and the suitability of the environment and vector availability for ZIKAV in southeastern Iran proposed by Samy et al. (2016).25,28 The validity of the BioTEMS model for predicting the presence and spread of Ae. albopictus in southeastern Iran was also confirmed by the collection records of both adult and larvae stages of Ae. albopictus.3 In the Chabahar area, Kraemer et al. (2015) identifies the area as suitable for Ae. aegypti, which is a vector of ZIKAV; however, they did not predict the presence of Ae. albopictus.26 It is possible that Ae. aegypti was indeed previously found in the Chabahar area, but when faced with invasion and competition by Ae. albopictus, Ae. aegypti is often eradicated or numbers significantly reduced.42 The BioTEMS model also fell within the combined global model of Ae. aegypti, Ae. africanus, and Ae. albopictus of Carlson et al. (2106) for the Chabahar region (figure 1).
When validated against several models, and more importantly local capture of Aedes vectors of ZIKAV, the high resolution of BioTEMS (often less than 30 m2) provides several features; e.g. 1) Point/area of invasion, 2) Identifying risk zones for prioritizing control and epidemiologic surveys, 3) Sites for surveillance efforts, and 4) Identifying where the infected invasive mosquitoes will most likely spread (figure 2). These features are not available in other lower resolution models. Using high-resolution models in IMM can greatly reduce the cost of pesticides and labor as well as reduce the risk to community health and environmental impact resulting from pesticide application.
After invasion, one of the principal routes of spread of Aedes species across a region is through vehicular transport.43 The Kraemer model identifies the Chahabar area as suitable for Ae. aegypti but not Ae. albopictus.26 However, recent mosquito surveys have only detected Ae. albopictus and not Ae. aegypti in the Chabahar area.44 In previous studies, BioTEMS was accurate in predicting the presence of ZIKAV cases in Brazil and the USA through the import of infected Aedes species and infection of local mosquitoes.21,22 ZIKAV is primarily introduced into a new geographic area through an infected human traveler or by invasion of an infected mosquito. There is sometimes the failure of public health officials to recognize these two possibilities. For example, in a recent CBS 60 Minutes broadcast, Dr. Anthony Fauci (the head of infectious diseases at the U.S. National Institutes of Health) stated, “The mosquito did not fly from Rio de Janeiro to Florida. The mosquito flies 500 feet in a lifetime. It is the people who travel.”45 Ignoring the possibility of invasive mosquitoes in the contribution to the spread of mosquito-borne diseases ignores hundreds of years of evidence for vector mosquitoes being introduced into new geographic areas. Aedes albopictus is probably the most successful invasive mosquito species and it has been rapidly spreading globally, primarily through the trade in tires and lucky bamboo, arriving by ship and then spreading along highways.43 Seaports play a critical role in the invasion of Aedes species, this includes recruitment of new haplotypes.46 Both maritime ports and airports are important routes of invasion of mosquitoes infected with arboviruses.47 Focusing control and surveillance efforts primarily on travelers and not including ports of entry do a disservice to the population to whom public health officials are charged to protect. For example, if Miami and Rio de Janeiro had an active ZIKAV surveillance system in place for mosquitoes in the port areas, the chance of finding an infected mosquito would have been increased and IMM could have been initiated sooner.
As part of IMM, education of the local population on reducing breeding sites by emptying containers and the use of personal protective measures can greatly reduce the risk of mosquito-borne infections. These include; personal protection by using repellents, daytime avoidance of mosquito bites for pregnant mothers and ZIKAV infected patients, and community-level surveillance and control measures.48 Previously published models provide little information with which local medical and public health officials can incorporate in their IMM strategy and planning beyond only recognizing that there is a possibility of invasion by mosquitoes infected with arboviruses. The output from the BioTEMS model to aid in IMM can be improved in the Chabahar area through the input of additional data. It is recommended that increased monthly surveillance for three or more years be implemented in the area of Chabahar to aid in mitigation and vector control efforts.
Vaux and Medlock (2015) implemented the following surveillance procedures in port areas in the United Kingdom: 1) Establish a baseline of mosquito breeding habitats, 2) Conduct active surveillance for invasive mosquitoes at the ports, 3) Identify appropriate surveillance method suited to port environments, and 4) Develop the capability and capacity of port health officers to conduct invasive mosquito surveillance.49 In addition to surveillance, prevention of establishment of invasive species into the port area is critical. Application of pesticides on ships, cargo, and port areas can reduce the risk of invasion by mosquitoes; however, the continuous spraying of pesticides is expensive and may damage the environment. Low cost and environmentally friendly methods using new pesticide technologies can be used to lower the risk of the establishment of invasive species while reducing the local mosquito population. Pesticides with mosquito bait can be delivered using devices, such as the ProVector, hung in structures to reduce the mosquito population without the need for spraying for up to several months.20,50
Conclusion
In summary, local transmission of DNV, CHIKV, and ZIKAV have not been documented in Chabahar. The risk is increased with the recent invasion of Ae. albopictus, most likely through ports in southeastern Iran. The BioTEMS model provides high-resolution information that medical and public health officials can use to assess the risk of invasive mosquito species, arboviruses, and integrated mosquito management planning. Active mosquito control and epidemiologic surveillance of mosquitoes and humans, particularly surrounding air and marine ports and in vehicle maintenance facilities are critical in reducing the risk of the introduction and establishment of arboviruses in Chabahar.
Acknowledgement
The views expressed in this publication are those of the author’s and do not reflect the official policy of the United States Army or United States Government.
Conflict of Interest:None declared.
References
- 1.Azari-Hamidian S, Yaghoobi-Ershadi MR, Javadian E, Abai MR, Mobedi I, Linton YM, et al. Distribution and ecology of mosquitoes in a focus of dirofilariasis in northwestern Iran, with the first finding of filarial larvae in naturally infected local mosquitoes. Med Vet Entomol. 2009;23:111–21. doi: 10.1111/j.1365-2915.2009.00802.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health, Organization [Internet] Filariasis. Regional Office Eastern Region. c2017- [cited 2014 May 2] Available from: [http://www.emro.who.int/health-topics/filariasis/index.html. ]
- 3.Doosti S, Yaghoobi-Ershadi MR, Schaffner F, Moosa-Kazemi SH, Akbarzadeh K, Gooya MM, et al. Mosquito Surveillance and the First Record of the Invasive Mosquito Species Aedes (Stegomyia) albopictus (Skuse) (Diptera: Culicidae) in Southern Iran. Iran J Public Health. 2016;45:1064–73. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghaie A, Aaskov J, Chinikar S, Niedrig M, Banazadeh S, Mohammadpour HK. Frequency of West Nile Virus Infection in Iranian Blood Donors. Indian J Hematol Blood Transfus. 2016;32:343–6. doi: 10.1007/s12288-015-0567-5. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinikar S, Nariman SH, Mostafavi E, Moradi M, Khakifirouz S, Jalali T, et al. Surveillance of rift valley fever in Iran between 2001 and 2011. The All Results Journals: Biol. 2013;4:16–8. [Google Scholar]
- 6.World Health, Organization [Internet] Fifth meeting of the Emergency Committee under the International Health Regulations (2005) regarding microcephaly, other neurological disorders and Zika virus. [cited 18 November 2016] Available from: [ ]
- 7.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 8.Atif M, Azeem M, Sarwar MR, Bashir A. Zika virus disease: a current review of the literature. Infection. 2016;44:695–705. doi: 10.1007/s15010-016-0935-6. [DOI] [PubMed] [Google Scholar]
- 9.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui*Ondo S, Jiolle D, et al. Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:e2681. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–77. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 11.Kampango A, Abilio AP. The Asian tiger hunts in Maputo city--the first confirmed report of Aedes (Stegomyia) albopictus (Skuse, 1895) in Mozambique. Parasit Vectors. 2016;9:76. doi: 10.1186/s13071-016-1361-4. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollars T, Kollars PG, Hulsey B. Reducing the risk to marine ports from invasive mosquito species, zika, dengue, chikungunya viruses and filariasis. Int J Med. 2016;4:70–3. doi: 10.14419/ijm.v4i2.5922. [DOI] [Google Scholar]
- 13.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, Zeller H, et al. A review of the invasive mosquitoes in Europe: ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–47. doi: 10.1089/vbz.2011.0814. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindsay MD, Jardine A, Giele C, Armstrong P, McCarthy S, Whittle A, et al. Investigation of the First Case of Dengue Virus Infection Acquired in Western Australia in Seven Decades: Evidence of Importation of Infected Mosquitoes? . PLoS Negl Trop Dis. 2015;9:e0004114. doi: 10.1371/journal.pntd.0004114. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, et al. Transmission of Zika Virus Through Sexual Contact with Travelers to Areas of Ongoing Transmission - Continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215–6. doi: 10.15585/mmwr.mm6508e2. [DOI] [PubMed] [Google Scholar]
- 16.Musso D, Stramer SL, Committee AT-TD, Busch MP. International Society of Blood Transfusion Working Party on Transfusion-Transmitted Infectious D. Zika virus: a new challenge for blood transfusion. Lancet. 2016;387:1993–4. doi: 10.1016/S0140-6736(16)30428-7. [DOI] [PubMed] [Google Scholar]
- 17.Kollars TM. BioTEMS-Biology based modeling to determine bioagent fate. Chemical Biological Weapons Delivery Methods and Consequence Assessment Modeling Conference. Boston: National Geospatial Intelligence Center, Weapons Intelligence, Nonproliferation and Arms Control; 2008. [Google Scholar]
- 18.Thuiller W, Midgley GF, Hughes GO, Bomhard B, Drew G, Rutherford MC, et al. Endemic species and ecosystem sensitivity to climate change in Namibia. Glob Chang Biol. 2006;12:759–76. [Google Scholar]
- 19.Peterson AT. Uses and requirements of ecological niche models and related distributional models. Biodiv Inf. 2006; 3:59–72. [Google Scholar]
- 20.Kollars*Jr Risk assessment of zika virus and optimization of mosquito surveillance in the port and city of Chittagong, Bangladesh. Asian Journal of Science and Technology. 2016;7:3833–6. [Google Scholar]
- 21.Kollars TM, Jr Kollars, JW The Invasion of Zika Virus into Rio De Janeiro and Fortaleza, Brazil, Inside Out or Outside In? Ann Community Med Pract. 2016;2:1015. [Google Scholar]
- 22.Kollars TM. Assessing likely invasion sites of Zika virus-infected mosquitoes in civilian and naval maritime ports in Florida. Res Rep Trop Med. 2017;8:1–6. doi: 10.2147/RRTM.S123456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaim M. The distribution and larval habitat characteristics of Iranian Culicinae. J Am Mosq Control Assoc. 1987;3:568–73. [PubMed] [Google Scholar]
- 24.Azari-Hamidian S. Checklist of Iranian mosquitoes (Diptera: Culicidae) J Vector Ecol. 2007;32:235–42. doi: 10.3376/1081-1710(2007)32[235:coimdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Carlson CJ, Dougherty ER, Getz W. An Ecological Assessment of the Pandemic Threat of Zika Virus. PLoS Negl Trop Dis. 2016;10:e0004968. doi: 10.1371/journal.pntd.0004968. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015;4:e08347. doi: 10.7554/eLife.08347. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messina JP, Kraemer MU, Brady OJ, Pigott DM, Shearer FM, Weiss DJ, et al. Mapping global environmental suitability for Zika virus. Elife. 2016:5. doi: 10.7554/eLife.15272. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samy AM, Thomas SM, Wahed AA, Cohoon KP, Peterson AT. Mapping the global geographic potential of Zika virus spread. Mem Inst Oswaldo Cruz. 2016;111:559–60. doi: 10.1590/0074-02760160149. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci U S A. 2006;103:6242–7. doi: 10.1073/pnas.0508391103. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyerson LA, Mooney HA. Invasive alien species in an era of globalization. Frontiers in Ecology and the Environment. 2007;5:199–208. [Google Scholar]
- 31.Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, et al. A Susceptible Mouse Model for Zika Virus Infection. PLoS Negl Trop Dis. 2016;10:e0004658. doi: 10.1371/journal.pntd.0004658. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baca-Carrasco D, Velasco-Hernandez JX. Sex, Mosquitoes and Epidemics: An Evaluation of Zika Disease Dynamics. Bull Math Biol. 2016;78:2228–42. doi: 10.1007/s11538-016-0219-4. [DOI] [PubMed] [Google Scholar]
- 33.Alex*Perkins T, Siraj AS, Ruktanonchai CW, Kraemer MU, Tatem AJ. Model-based projections of Zika virus infections in childbearing women in the Americas. Nat Microbiol. 2016;1:16126. doi: 10.1038/nmicrobiol.2016.126. [DOI] [PubMed] [Google Scholar]
- 34.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 35. European Centre for Disease Prevention and Control Rapid Risk Assessment [Internet] Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barr syndrome. ECDC, Stockholm. [cited 2015 December 10] . Available from: [http://ecdc.europa.eu/en/publications/Publications/zika-virus-americas-association-with-microcephaly-rapid-risk-assessment.pdf. ]
- 36.Hayes EB. Zika virus outside Africa. Emerg Infect Dis. 2009;15:1347–50. doi: 10.3201/eid1509.090442. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tognarelli J, Ulloa S, Villagra E, Lagos J, Aguayo C, Fasce R, et al. A report on the outbreak of Zika virus on Easter Island, South Pacific, 2014. Arch Virol. 2016;161:665–8. doi: 10.1007/s00705-015-2695-5. [DOI] [PubMed] [Google Scholar]
- 38.Petersen LR, Jamieson DJ, Powers AM, Honein MA. Zika Virus. N Engl J Med . 2016;347:1552–63. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 39.Wikan N, Suputtamongkol Y, Yoksan S, Smith DR, Auewarakul P. Immunological evidence of Zika virus transmission in Thailand. Asian Pac J Trop Med. 2016;9:141–4. doi: 10.1016/j.apjtm.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Yakob L, Walker T. Zika virus outbreak in the Americas: the need for novel mosquito control methods. Lancet Glob Health. 2016;4:e148–9. doi: 10.1016/S2214-109X(16)00048-6. [DOI] [PubMed] [Google Scholar]
- 41.Monaghan AJ, Morin CW, Steinhoff DF, Wilhelmi O, Hayden M, Quattrochi DA, et al. On the Seasonal Occurrence and Abundance of the Zika Virus Vector Mosquito Aedes Aegypti in the Contiguous United States. PLoS Curr. 2016;8 doi: 10.1371/currents.outbreaks*.50dfc7f46798675fc63e7d7da563da76. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heusel JL, Moulis RA, Peaty LFAW, [Internet] Chikungunya, Zika, and how do you stop a flying Tiger? Available from: [http://www.mamca.org/2016Meeting/0331_1330_Heusel.pdf. ]
- 43.Akiner MM, Demirci B, Babuadze G, Robert V, Schaffner F. Spread of the Invasive Mosquitoes Aedes aegypti and Aedes albopictus in the Black Sea Region Increases Risk of Chikungunya, Dengue, and Zika Outbreaks in Europe. PLoS Negl Trop Dis. 2016;10:e0004664. doi: 10.1371/journal.pntd.0004664. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moosa-Kazemi S, Vatandoost H, Nikookar H, Fathian M. Culicinae (Diptera: culicidae) mosquitoes in chabahar county, sistan and baluchistan province, southeastern iran. Iran J Arthropod Borne Dis. 2009;3:29–35. [ PMC Free Article] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPook J, [Internet] What’s being done to fight the Zika virus? c2016- [cited 2015 December 15] Available from: [http://www.cbsnews.com/news/60-minutes-zika-in-the-united-states-mosquito-disease. ]
- 46.Futami K, Valderrama A, Baldi M, Minakawa N, Marin*Rodriguez R, Chaves LF. New and Common Haplotypes Shape Genetic Diversity in Asian Tiger Mosquito Populations from Costa Rica and Panama. J Econ Entomol. 2015;108:761–8. doi: 10.1093/jee/tou028. [DOI] [PubMed] [Google Scholar]
- 47.Gardner L, Sarkar S. A global airport-based risk model for the spread of dengue infection via the air transport network. PLoS One. 2013;8:e72129. doi: 10.1371/journal.pone.0072129. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogoch II, Brady OJ, Kraemer MUG, German M, Creatore MI, Kulkarni MA, et al. Anticipating the international spread of Zika virus from Brazil. Lancet. 2016;387:335–6. doi: 10.1016/S0140-6736(16)00080-5. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaux AG, Medlock JM. Current status of invasive mosquito surveillance in the UK. Parasit Vectors. 2015;8:351. doi: 10.1186/s13071-015-0936-9. [ PMC Free Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yalwala S, Kollars JW, Kasembeli G, Barasa C, Senessie C, Kollars PG, et al. Preliminary Report on the Reduction of Adult Mosquitoes in Housing Compounds in Western Kenya Using the ProVector Flower and Entobac Bait Pads Containing Bacillus thuringiensis israelensis With Honey Bait. J Med Entomol. doi: 10.1093/jme/tjw075. [DOI] [PubMed] [Google Scholar]


