Abstract
BACKGROUND
Colorectal cancer (CRC) risk varies by race and sex. This study, 1 of 2 microsimulation analyses to inform the 2018 American Cancer Society CRC screening guideline, explored the influence of race and sex on optimal CRC screening strategies.
METHODS
Two Cancer Intervention and Surveillance Modeling Network microsimulation models, informed by US incidence data, were used to evaluate a variety of screening methods, ages to start and stop, and intervals for 4 demographic subgroups (black and white males and females) under 2 scenarios for the projected lifetime CRC risk for 40‐year‐olds: 1) assuming that risk had remained stable since the early screening era and 2) assuming that risk had increased proportionally to observed incidence trends under the age of 40 years. Model‐based screening recommendations were based on the predicted level of benefit (life‐years gained) and burden (required number of colonoscopies), the incremental burden‐to‐benefit ratio, and the relative efficiency in comparison with strategies with similar burdens.
RESULTS
When lifetime CRC risk was assumed to be stable over time, the models differed in the recommended age to start screening for whites (45 vs 50 years) but consistently recommended screening from the age of 45 years for blacks. When CRC risk was assumed to be increased, the models recommended starting at the age of 45 years, regardless of race and sex. Strategies recommended under both scenarios included colonoscopy every 10 or 15 years, annual fecal immunochemical testing, and computed tomographic colonography every 5 years through the age of 75 years.
CONCLUSIONS
Microsimulation modeling suggests that CRC screening should be considered from the age of 45 years for blacks and for whites if the lifetime risk has increased proportionally to the incidence for younger adults. Cancer 2018;124:2974‐85. © 2018 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: colorectal neoplasms, decision modeling, early detection of cancer, guidelines, personalized medicine
Short abstract
An established modeling method suggests that screening should be considered from the age of 45 years for African American men and women, and for white men and women if their lifetime risk of colorectal cancer increases similarly to trends observed at younger ages.
See also pages 2964‐73.
INTRODUCTION
Colorectal cancer (CRC) is the second leading cause of cancer deaths in the United States.1 Screening can prevent death from CRC and has been promoted by multiple organizations since 1980.2, 3 Recommendations for screening have evolved over the years, with the emergence of new screening technologies, new evidence on the performance of various screening methods, and evidence of differential risk across population subgroups.
It has been well documented that individuals with African ancestry have higher rates of CRC incidence and mortality than individuals of other races or ethnicities in the United States and that men are at higher risk than women.1 Similarly, longevity varies by race and sex.4 These differences in cause‐specific and other‐cause mortality could influence the optimal start age, duration, and intensity of screening.5 A higher risk of CRC may justify a more intensive screening approach, whereas a high risk of other‐cause morbidity and mortality may reduce the benefit from screening at older ages.
There are differences in the current US guidelines for the age to start CRC screening in African Americans. Currently, the American College of Gastroenterology and the US Multi‐Society Task Force recommend that African Americans begin screening at the age of 45 years, whereas those of other races should begin at the age of 50 years, regardless of sex.6, 7 The US Preventive Services Task Force (USPSTF) updated its screening recommendations for the US general population in 2016.8 Although it acknowledged that black and Alaska Native individuals have higher CRC incidence and mortality rates than the general population and that microsimulation analyses indicated that there may be some merit to starting screening at the age of 45 years rather than 50 years even for the general population, it concluded that the current evidence best supports a starting age of 50 years for all individuals at average risk.
To inform the update of its 2008 CRC screening guideline,9 the American Cancer Society requested a decision‐analytic modeling analysis to further explore the question of optimal CRC screening strategies by race and sex. An accompanying article by Peterse et al10 shows that based on increasing CRC rates in young birth cohorts, modeling supports earlier screening for the whole population. However, as explained in that article, there is controversy around the mechanism for that increase, with some arguing that it is a detection bias attributable to early uptake of screening rather than a true increase in risk.11 In this article, we explore the potential benefit and burden from earlier screening for black men and women versus whites, and we consider 2 scenarios for current background CRC risk: one based on original models informed by data from a period before screening was widely adopted that assumed stable risk and another based on increasing risk as described by Peterse et al.
MATERIALS AND METHODS
Four US demographic subgroups were distinguished: white females, black females, white males, and black males. There were insufficient data to include other races or to distinguish Hispanic ethnicity. Two independently developed microsimulation models for CRC were used to evaluate a large number of possible screening strategies with various screening modalities, ages to begin, ages to end, and screening intervals for each subgroup. Models were developed within the National Cancer Institute–funded Cancer Intervention and Surveillance Modeling Network. Apart from the distinction of race‐ and sex‐specific population subgroups and scenarios considered for current CRC risk, the analyses were similar to those performed to inform USPSTF guideline recommendations (see Supporting Table 1 for a summary of all differences).12, 13
Model Description
The Microsimulation Screening Analysis–Colon (MISCAN‐Colon) and the Simulation Model of Colorectal Cancer (SimCRC) have been described extensively in other studies14 and in the Cancer Intervention and Surveillance Modeling Network model registry.15 Each model consists of 3 components, which are used to simulate individual life histories from birth to death under alternative CRC screening strategies. First, the demography component determines each simulated person's date of birth and death in the absence of CRC. Second, the natural history component is used to simulate the potential development of CRC and reductions in the overall years of life. The natural history of CRC is assumed to follow the adenoma‐carcinoma sequence (Supporting Fig. 1). Simulated individuals may develop 1 or more adenomas. An adenoma may grow in size and develop into CRC, which then may transition through stages I to IV without symptoms or be clinically diagnosed at any stage. Depending on the varying rates of CRC progression and survival, simulated individuals may die of either other causes or clinically diagnosed CRC. The third component, the model's screening component, allows a simulated person's life trajectory to be altered because of the detection of preclinical CRC or the detection and removal of an adenoma.
The demography component was informed by all‐cause mortality rates from the 2013 US life tables by race and sex.4 For the natural history component, the age‐specific adenoma onset was based on the prevalence and multiplicity of adenomas as observed in autopsy studies.16, 17, 18, 19, 20, 21, 22, 23, 24, 25 Race‐ and sex‐specific CRC incidence by age, stage, and localization was calibrated to data from the Surveillance, Epidemiology, and End Results Program (SEER) from the period before screening was widely adopted26: SimCRC was calibrated to 1975‐1979 data, a period devoid of screening, and MISCAN‐Colon was calibrated to 1990‐1994 data, a period with limited screening but more pronounced racial disparities in CRC risk (see Supporting Fig. 2 for a comparison of incidence by period). Race‐ and sex‐specific CRC survival in both models was based on recent SEER data.27 The screening component was informed by data on the sensitivity and specificity of the test performed and, for endoscopic tests, the proportion visualizing the complete colon or rectum (Table 1).
Table 1.
Screening Test Characteristics Used in the Analysisa
| Test Characteristic | Colonoscopy (per Lesion Within Reach)b | FIT (per Person) | HSgFOBT (per Person) | FIT‐DNA (per Person) | SIG (per Lesion Within Reach) | CTC (per Lesion) |
|---|---|---|---|---|---|---|
| Sensitivity for adenomas ≤ 5 mm, % | 75 (70‐79) | 7.6 (6.7‐8.6)c | 7.5 (7.5‐7.5)d | 17.2 (15.9‐18.6)c | 75 (70‐79) | — |
| Sensitivity for adenomas of 6‐9 mm, % | 85 (80‐92) | 12.4 (10‐26.2) | 85 (80‐92) | 57 (48.9‐71.6) | ||
| Sensitivity for adenomas ≥ 10 mm, % | 95 (93.1‐99.5) | 23.8 (20.8‐27)e | 23.9 (17.7‐49.4) | 42.4 (38.7‐46.2)e | 95 (93.1‐99.5) | 84 (75.6‐92.4) |
| Sensitivity for CRC, % | 95 (93.1‐99.5) | 73.8 (62.3‐83.3) | 70 (61.5‐79.4) | 92.3 (84‐97) | 95 (93.1‐99.5) | 84 (75.6‐92.4) |
| Specificity, % | 86f | 96.4 | 92.5 | 89.8 | 87f | 88g |
| Proportion completed, % | 95h | 100 | 100 | 100 | 76h | 100 |
| Risk of fatal complications, % | 0.01i | 0 | 0 | 0 | 0i | 0 |
Abbreviations: CRC, colorectal cancer; CTC, computed tomographic colonography; FIT, fecal immunochemical testing with a positivity cutoff of ≥100 ng of hemoglobin/mL of buffer (≥20 μg of hemoglobin/g of feces); FIT‐DNA, fecal immunochemical testing with a DNA stool test (multitarget stool DNA testing); HSgFOBT, high‐sensitivity guaiac‐based fecal occult blood testing; SIG, flexible sigmoidoscopy
The ranges evaluated in the sensitivity analysis are presented in parentheses after the base‐case characteristics.
Test characteristics were similar to those used in an analysis for the US Preventive Services Task Force13.
It was assumed that the same test characteristics for screening colonoscopies applied to colonoscopies for diagnostic follow‐up or for surveillance.
For individuals with 1‐ to 5‐mm adenomas, it was assumed that the sensitivity was equal to the positivity rate in individuals without adenomas. The sensitivity for individuals with 6‐ to 9‐mm adenomas was such that the weighted average sensitivity for individuals with 1‐ to 9‐mm adenomas equaled that for nonadvanced adenomas.
It was assumed that 1‐ to 5‐mm adenomas did not bleed and, therefore, could not cause a positive stool test. It was also assumed that HSgFOBT could be positive because of bleeding from other causes, the probability of which was equal to the positivity rate in individuals without adenomas.
Sensitivity for individuals with advanced adenomas (ie, adenomas ≥ 10 mm or adenomas with advanced histology). Sensitivity was not reported for the subset of individuals with ≥10‐mm adenomas.
The lack of specificity with endoscopy reflects the detection of nonadenomatous polyps, which, in the case of sigmoidoscopy, may lead to unnecessary diagnostic colonoscopy and, in the case of colonoscopy, leads to unnecessary polypectomy, which is associated with an increased risk of colonoscopy complications.
The lack of specificity with CTC reflects the detection of ≥6‐mm nonadenomatous lesions, artifacts, stool, and adenomas smaller than the 6‐mm threshold for referral to colonoscopy that are measured as ≥6 mm.
With colonoscopy, 95% reached the end of the colorectum (cecum); for the remaining 5%, the endpoint was distributed between the cecum and the rectum. With SIG, 76% reached the end of the sigmoid colon; 14% had an endpoint between the beginning and the end of the sigmoid colon; and 12% had an endpoint between the beginning and end of the descending colon.
The risk of complications is conditional on polypectomy. Case fatality was derived as the combination of the overall perforation rate from Warren et al28 and the mortality given perforation (0.0519) from Gatto et al.29, 30 Sigmoidoscopy was modeled without biopsy or polypectomy of detected lesions and was, therefore, assumed to have a 0 mortality risk.
The models have been validated against the mortality reductions of the UK Flexible Sigmoidoscopy Screening (UKFSS) trial of once only sigmoidoscopy.31 The MISCAN‐Colon model has also been validated in the Norwegian Colorectal Cancer Prevention (NORCCAP) trial32 and the Screening for Colon and Rectum (SCORE) trial.33
Study Population
For each of the 4 population subgroups described previously, the models simulated outcomes for 40‐year‐old individuals without a prior CRC diagnosis.
Scenarios for Background Risk
Two scenarios were considered for the projected lifetime risk of CRC in 40‐year‐olds in the absence of screening. In the first scenario, the conventional scenario in microsimulation models for CRC screening,12, 13 age‐specific risks of CRC were assumed to have remained at the level observed before screening was widely adopted in the United States. In the second scenario, age‐specific CRC risks for all ages older than 40 years were assumed to have increased proportionally to observed trends in incidence for individuals younger than 40 years old.34 Hence, the assumed relative increase in lifetime risk across models was 1.80 to 1.90 for white females, 1.24 to 1.27 for black females, 2.07 to 2.13 for white males, and 1.41 to 1.56 for black males. The increase was assumed to have arisen from an increased rate of adenoma onset, primarily in the rectum and distal colon. More details on the background and methodology for these assumptions are in the article by Peterse et al.10
Screening Strategies
Six screening modalities were evaluated: colonoscopy, fecal immunochemical testing (FIT) with a positivity cutoff at hemoglobin levels ≥ 20 μg/g of stool, high‐sensitivity guaiac‐based fecal occult blood testing (HSgFOBT), multitarget stool DNA testing (fecal immunochemical testing with a DNA stool test [FIT‐DNA]), flexible sigmoidoscopy (SIG), and computed tomographic colonography (CTC). For each modality, multiple ages to begin and end screening and multiple screening intervals were evaluated for a total of 132 unique strategies for each population subgroup or 528 across all race and sex combinations (Table 2). In all evaluated strategies, individuals in whom adenomas were detected and removed received colonoscopy surveillance through the age of 85 years. It was assumed that there was 100% adherence to all procedures to avoid compensation of lower adherence rates by shorter recommended screening intervals. As a result, predicted outcomes from the model reflect the potential lifetime benefits and burden of screening with the assumption of full adherence to the entire screening process.
Table 2.
Screening Strategies Evaluated by the Model for Each Race and Sex Subgroupa
| Screening Modality | Age to Begin Screening, y | Age to End Screening, y | Screening Interval, y | No. of Strategies (Unique)b |
|---|---|---|---|---|
| No screening | 1 | |||
| Stool‐based screening | ||||
| FIT | 45, 50, 55 | 75, 80, 85 | 1, 2, 3 | 27 (27) |
| HSgFOBT | 45, 50, 55 | 75, 80, 85 | 1, 2, 3 | 27 (27) |
| FIT‐DNA | 45, 50, 55 | 75, 80, 85 | 1, 3, 5 | 27 (27) |
| SIG screening | 45, 50, 55 | 75, 80, 85 | 5, 10 | 18 (15) |
| CTC screening | 45, 50, 55 | 75, 80, 85 | 5, 10 | 18 (15) |
| Colonoscopy screening | 45, 50, 55 | 75, 80, 85 | 5, 10, 15 | 27 (20) |
| Total No. of (unique) screening strategies evaluated in the model | 145 (132) |
Abbreviations: CTC, computed tomographic colonography; FIT, fecal immunochemical testing; FIT‐DNA, fecal immunochemical testing with a DNA stool test (multitarget stool DNA testing); HSgFOBT, high‐sensitivity guaiac‐based fecal occult blood testing; SIG, flexible sigmoidoscopy.
Strategies were similar to those evaluated in an analysis for the US Preventive Services Task Force.13 Combinations of SIG with stool‐based screening were not considered here.
The number of unique strategies excludes the strategies that result in the same screening regimen (eg, colonoscopy every 10 years from the ages of 50‐80 years and colonoscopy every 10 years from the ages of 50‐85 years both include colonoscopies at the ages of 50, 60, 70, and 80 years and thus are not unique strategies).
Main Outcomes
The benefit or effectiveness of screening was measured by the number of life‐years gained (LYG) from the screening strategy; this accounted for life‐years lost because of fatal screening complications. The primary fatal complication is perforation of the colon, which occurs at a rate less than 1 per 1000 colonoscopies; approximately 5% of these cases result in death.35, 36 The number of required colonoscopies was used as a measure of the aggregate burden of screening, and it included colonoscopies for screening, follow‐up, surveillance, and the diagnosis of symptomatic cancer. We controlled for the burden of tests other than colonoscopy by grouping and comparing tests with similar noncolonoscopy burdens. This resulted in 4 classes of screening modalities: colonoscopy, stool‐based modalities, SIG, and CTC.
Analysis
Efficient and near‐efficient screening strategies
Efficient strategies were identified via the plotting of LYG with respect to the number of required colonoscopies. A strategy was considered efficient when it provided the largest incremental increase in LYG per additional colonoscopy performed in comparison with the next less colonoscopy‐intensive screening strategy within the same class of screening modalities. The line connecting all efficient strategies is the efficient frontier. Near‐efficient strategies were defined as strategies just below the efficient frontier that provided at least 98% of the maximum incremental benefit per additional colonoscopy performed in comparison with the next less effective strategy on the efficient frontier. For efficient and near‐efficient screening strategies, the incremental number of colonoscopies (ΔCOL), the incremental number of life‐years gained (ΔLYG), and the burden‐to‐benefit ratio (or efficiency ratio [ER]: ΔCOL/ΔLYG) in comparison with the next less effective strategy on the efficient frontier were calculated.
Model recommendations
Model‐recommendable strategies fulfilled 3 main criteria: efficiency within their class of screening modality, comparable overall benefit as measured by LYG, and an acceptable balance of burden and benefit (see Fig. 1).13 First, an optimal colonoscopy screening strategy was selected. This was an efficient or near‐efficient colonoscopy screening strategy required to provide at least as many LYG as the current general population recommendation of screening colonoscopy (every 10 years between 50 and 75 years) and to have a burden‐to‐benefit ratio of no more than 50 additional colonoscopies per LYG. This threshold was similar to the accepted balance in USPSTF model recommendations across models13 and was judged to be an acceptable balance for this analysis by the American Cancer Society. The most effective colonoscopy strategy (defined by most LYG) meeting these requirements was recommended. Second, for each alternative class of screening modalities, all strategies with the same ages to begin and end screening as the optimal colonoscopy strategy (benchmark strategy) were identified, and within‐class efficiency was re‐assessed. Model‐recommendable strategies were efficient or near‐efficient strategies with at least 90% of the LYG of the benchmark colonoscopy strategy and with a burden‐to‐benefit ratio lower than the benchmark. Again, the most effective strategies within each class meeting the requirements were considered model‐recommendable. It was possible to have no recommendable strategy within a class of screening modalities.
Figure 1.
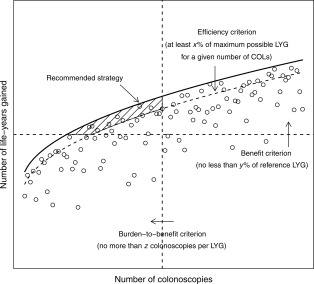
Illustration of the selection algorithm for model‐recommendable strategies. Each dot represents the hypothetical outcome for a single screening strategy. The bold line is the efficient frontier connecting efficient strategies (not plotted as separate dots). Dashed lines represent thresholds imposed by the decision algorithm: the efficiency criterion ensures that recommended strategies are efficient in terms of the yield in LYG for any level of COL requirement, the benefit criterion ensures that LYG do not lag far behind a selected reference strategy, and the burden‐to‐benefit criterion ensures that the incremental number of required COLs per LYG does not exceed a predefined number. The shaded area encompasses strategies fulfilling all 3 decision criteria. The model‐recommended strategy is the strategy within this area with the highest predicted number of LYG. COL indicates colonoscopy; LYG, life‐years gained.
Sensitivity analysis
In sensitivity analyses, 3 alternative scenarios were evaluated. First, we evaluated best‐case and worst‐case scenarios for the sensitivity of each evaluated screening modality, including potential follow‐up or surveillance colonoscopy, to reflect uncertainty in the estimates of the diagnostic performance of each modality (Table 1). Second, we varied the minimum acceptance threshold for LYG to 75% instead of 90% for alternative screening strategies in comparison with colonoscopy screening. Third, we lowered the acceptance threshold for burden to benefit from 50 additional colonoscopies per LYG to 40. For each alternative scenario, model recommendations were re‐assessed.
RESULTS
In the absence of screening, the model‐predicted life expectancy and CRC risk among 40‐year‐olds varied by race and sex. Life expectancy from the age of 40 years ranged from 35.3 to 42.3 years in the scenario of stable CRC risk and was lowest for black males and highest for white females (Supporting Table 2). In the conservative scenario of stable background CRC risk, the predicted lifetime CRC risk ranged from 59.3 to 70.7 per 1000 adults across population subgroups in MISCAN‐Colon and from 58.7 to 78.6 per 1000 adults in SimCRC. In the scenario of increased CRC risk, predicted lifetime risk across population subgroups increased to 76.7 to 149.0 in MISCAN‐Colon and to 79.9 to 162.5 in SimCRC. The predicted risk was highest for white males, but the rank order for other demographic subgroups differed across models and scenarios and in comparison with life‐years lost to CRC; this reflected differences in incidence by age in the data used to inform each model (Supporting Fig. 2).
Screening Benefit and Burden
Screening was predicted to result in clinically significant LYG in comparison with no screening by both models, regardless of the population subgroup and scenario for CRC risk.
In the scenario of stable age‐specific CRC risk, predicted LYG across models, strategies, and population subgroups ranged from 117 to 348 per 1000 adults (Supporting Tables 3‐6). The burden of screening, as measured by the lifetime number of required colonoscopies, varied from fewer than 800 per 1000 adults for triennial FIT during the ages of 55 to 75 years to almost 8000 per 1000 adults for colonoscopy every 5 years during the ages of 45 to 85 years. The predicted benefit of screening varied across population subgroups and was higher in SimCRC than MISCAN‐Colon. In MISCAN‐Colon, black females had the highest benefit from any of the screening strategies (range, 159‐306 LYG per 1000 adults), white females had the lowest benefit (117‐223 LYG), and white males and black males had similar intermediate benefits (141‐258 and 149‐284 LYG, respectively). In SimCRC, in contrast, black females and white males had the highest benefit from screening (175‐348 and 194‐334 LYG per 1000 40‐year‐olds, respectively), white females had somewhat fewer LYG (168‐307 LYG), and black males had the lowest benefit from screening (142‐272 LYG). In general, the lifetime number of colonoscopies required for screening was somewhat lower for black males and females because of their lower life expectancy in comparison with their white counterparts.
In the scenario with increased age‐specific CRC risk, the predicted benefit from screening was substantially higher than that in the scenario of stable risk, with LYG ranging from 203 to 556 per 1000 adults in MISCAN‐Colon and from 211 to 673 per 1000 adults in SimCRC; the maximum benefit from screening thus exceeded 0.5 LYG per individual in both models (Supporting Tables 3‐6). The required number of colonoscopies was only moderately higher for most colonoscopy‐based strategies in comparison with the scenario of stable risk and ranged from 684 to 8024 per 1000 adults across models, strategies, and population subgroups.
Efficient and Near‐Efficient Strategies
The set of strategies constituting the efficient frontier was similar across demographic subgroups and scenarios for CRC risk but differed between models (Fig. 2, Supporting Figs. 3‐5, and Supporting Tables 3‐6). In general, the predicted LYG range across evaluated strategies was smaller in MISCAN‐Colon than SimCRC, and this resulted in a wider set of strategies considered near‐efficient. The incremental burden‐to‐benefit ratio across models and population subgroups ranged from 6.0 to 2032.9 from the least to most resource‐intensive colonoscopy‐based screening strategy in the scenario of stable CRC risk. The biggest increase in LYG per additional screening colonoscopy was derived from lowering the starting age for screening, with lower resulting ERs for blacks versus whites in MISCAN‐Colon. The previously recommended strategy of colonoscopy every 10 years between the ages of 50 and 75 years was among the efficient strategies for all population subgroups in MISCAN‐Colon (range of ERs, 37.7‐43.2) but was less efficient in SimCRC than colonoscopy every 15 years between the ages of 45 and 75 years (range of ERs, 24.4‐31.6). For strategies other than colonoscopy‐based screening, the range of ERs across models, strategies, and population subgroups was smaller (2.0‐149.8). Among the stool‐based testing strategies, FIT screening was more efficient than most HSgFOBT and FIT‐DNA strategies, regardless of race and sex.
Figure 2.
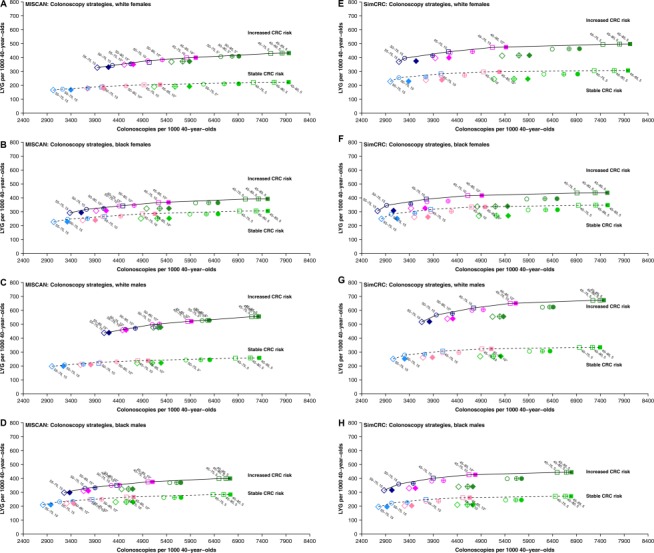
Lifetime number of colonoscopies and LYG for colonoscopy screening strategies under 2 scenarios for CRC risk by model and demographic subgroup. Colors reflect the screening interval (blue, 15 years; pink, 10 years; green, 5 years), symbols reflect the starting age (diamonds, 55 years; circles, 50 years; squares, 45 years), and the filling of the symbols reflects the end age (empty, 75 years; crossed, 80 years; and full, 85 years). Efficient and near‐efficient strategies are labeled, with efficiency assessed among all evaluated colonoscopy‐based screening strategies. In the stable‐risk scenario, the risk within each age‐, race‐, and sex‐specific demographic subgroup was assumed to have remained stable over time since the early screening phase in the United States (1975‐1979 for SimCRC and 1990‐1994 for MISCAN). In the increased‐risk scenario, the CRC risk was increased proportionally to observed trends in CRC incidence among adults younger than 40 years. Estimated incidence rate ratios were 1.80 to 1.90 for white females (range across models), 1.24 to 1.27 for black females, 2.07 to 2.13 for white males, and 1.41 to 1.56 for black males. CRC indicates colorectal cancer; LYG, life‐years gained; MISCAN, Microsimulation Screening Analysis; SimCRC, Simulation Model of Colorectal Cancer.
Compared with the scenario of stable CRC risk, the scenario of increased CRC risk was predicted to result in lower ERs because of increased LYG from screening; a smaller total ER range across models, evaluated strategies, and population subgroups (0.1‐992.8); and a further expanded set of near‐efficient strategies in MISCAN‐Colon.
Model‐Recommendable Strategies
In the scenario of stable age‐specific CRC risk, the 2 models differed in their recommended ages to start screening and their recommended colonoscopy screening intervals. Among all colonoscopy strategies deemed efficient or near‐efficient in MISCAN‐Colon, the optimal (most effective) strategy meeting both the imposed benefit and incremental burden‐to‐benefit criteria (ie, providing sufficient LYG and having an ER < 50) was colonoscopy every 10 years from the ages of 50 to 75 years for white males and females and colonoscopy every 10 years from the ages of 45 to 75 years for black males and females (Table 3 and Supporting Table 7). In SimCRC, colonoscopy screening every 15 years from the ages of 45 to 75 years was model‐recommendable, regardless of race or sex. Among other screening strategies with the same start and stop ages, recommended strategies by both models included FIT every year and CTC every 5 years. From the stool‐based screening modalities, HSgFOBT and FIT‐DNA were not model‐recommendable because of their inefficiency from higher false‐positive rates and, in the case of FIT‐DNA, also because of an unfavorable balance of burden and benefit (a minimum of 63.3 additional colonoscopies per LYG for annual FIT‐DNA vs annual FIT). SIG was not model‐recommendable because of a failure to provide at least 90% of the benefit of colonoscopy screening.
Table 3.
Model‐Recommendable Screening Strategies for 2 Scenarios of CRC Risk
| Model | Test Class | Scenario 1: Stable CRC Riska | Scenario 2: Increased CRC Riskb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| White Females | Black Females | White Males | Black Males | White Females | Black Females | White Males | Black Males | ||
| MISCAN | COL | 50‐75, 10 | 45‐75, 10 | 50‐75, 10 | 45‐75, 10 | 45‐75, 10 | 45‐75, 10 | 45‐75, 5 | 45‐75, 10 |
| Stool | FIT 50‐75, 1 | FIT 45‐75, 1 | FIT 50‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | — | FIT 45‐75, 1 | |
| SIG | — | — | — | — | 45‐75, 5 | 45‐75, 5 | — | 45‐75, 5 | |
| CTC | 50‐75, 5 | 45‐75, 5 | 50‐75, 5 | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | — | 45‐75, 5 | |
| SimCRC | COL | 45‐75, 15 | 45‐75, 15 | 45‐75, 15 | 45‐75, 15 | 45‐75, 10 | 45‐75, 10 | 45‐75, 10 | 45‐75, 10 |
| Stool | FIT 45‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | FIT 45‐75, 1 | |
| SIG | — | — | — | — | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | |
| CTC | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | 45‐75, 5 | |
Abbreviations: —, no model‐recommendable strategy within this class; COL, colonoscopy; CRC, colorectal cancer; CTC, computed tomographic colonography; FIT, fecal immunochemical testing; MISCAN, Microsimulation Screening Analysis; SIG, flexible sigmoidoscopy; SimCRC, Simulation Model of Colorectal Cancer.
The numbers in each field of the table successively represent the recommended age to start screening, the recommended age to stop, and the recommended interval, all in years. For the class of stool‐based screening modalities, the model‐recommendable modality is also included (ie, FIT).
The risk within each age‐, race‐, and sex‐specific demographic subgroup was assumed to have remained stable over time since the early screening period in the United States (1975‐1979 for SimCRC and 1990‐1994 for MISCAN).
The CRC risk was increased proportionally to observed trends in CRC incidence among adults younger than 40 years. Estimated incidence rate ratios were 1.80 to 1.90 for white females (range across models), 1.24 to 1.27 for black females, 2.07 to 2.13 for white males, and 1.41 to 1.56 for black males.
With assumed increased age‐specific CRC risk, both models recommended screening between the ages of 45 and 75 years for all 4 demographic subgroups, with colonoscopy recommended every 10 years, FIT annually, SIG every 5 years, and CTC every 5 years, except for white males, for whom MISCAN‐Colon recommended only colonoscopy every 5 years. SIG every 5 years was added to the list of model‐recommendable strategies for other population subgroups because of its higher comparative effectiveness with more assumed distal tumors.
Sensitivity Analysis
Model recommendations were influenced by alternative assumptions for test performance, the minimum acceptable percentage of LYG for alternative strategies in comparison with colonoscopy screening, and a more stringent acceptance threshold for the burden‐to‐benefit ratio (Supporting Tables 8‐11). Most notably, under worst‐case performance assumptions, MISCAN‐Colon no longer recommended CTC or SIG screening (Supporting Table 9); with a 75% acceptance threshold for LYG rather than 90% in comparison with colonoscopy screening, both MISCAN‐Colon and SimCRC included SIG in the set of model‐recommendable strategies, regardless of the scenario for background risk (Supporting Table 10); and with a burden‐to‐benefit threshold of a maximum of 40 additional colonoscopies per LYG rather than 50, MISCAN‐Colon no longer recommended earlier screening for blacks in the stable CRC risk scenario (Supporting Table 11).
DISCUSSION
The results from this modeling study suggest that CRC screening should be considered from the age of 45 years in average‐risk black Americans. The recommended age to begin screening among whites varied across models, with one model suggesting that screening should begin at the age of 50 years in a scenario of stable age‐specific CRC risk and with the other suggesting that screening should begin at the age of 45 years. If lifetime risk increases proportionally to trends observed at younger ages, both models support recommending screening from the age of 45 years for all population subgroups. Within blacks and whites, recommendable strategies generally did not differ for men and women. Although men are at higher risk for CRC than women, the higher potential benefit from screening is partly offset by their lower life expectancy. Model‐recommendable strategies generally included colonoscopy screening every 10 or 15 years, FIT screening every year, and CTC every 5 years through the age of 75 years. SIG was not consistently model‐recommendable because of its inability to meet the minimum benefit criterion, and HSgFOBT and FIT‐DNA were not recommendable because of inefficiency.
Model‐based recommendations were dependent on assumptions for CRC risk. The models used in this study were calibrated to data from a period in which guideline‐adherent screening was uncommon to avoid serious contamination from either prevention of disease or an earlier diagnosis. By making this assumption, the models implicitly assumed that the current underlying age‐specific risk of CRC in the absence of screening would be the same as that observed in the prescreening era. However, SEER data indicate that incidence has risen for every subsequent generation born since the 1950s,34 and this suggests that the projected underlying lifetime risk for current 40‐ to 50‐year‐olds may be elevated in comparison with earlier birth cohorts. To reflect this uncertainty, we considered 2 scenarios for lifetime CRC risk: one in which age‐, race‐, and sex‐specific risks were assumed to remain stable over time and another in which risks increased proportionally to trends observed in young‐onset cases. As we showed, screening should be considered as early as the age of 45 years in both white and black men and women if the lifetime risk is increasing. This stems from converging risks in white and black adults younger than 40 years26, 37 and is consistent with the recommendation in an accompanying article by Peterse et al.10 The article by Peterse et al. discusses the potential increase in disease risk in more detail, including possible causal mechanisms other than increased adenoma onset.
There were some discrepancies in screening recommendations across the 2 models. Under the first scenario of no increase in age‐, race‐, and sex‐specific CRC risk over time, MISCAN‐Colon recommended screening for black adults from the age of 45 years and for white adults from the age of 50 years, both at 10‐year intervals for colonoscopy. In contrast, SimCRC recommended both white and black adults begin screening at the age of 45 years with longer recommended colonoscopy intervals of 15 years. These differences reflect the differences in the dwell time of adenomas (ie, the time from adenoma onset to symptom‐detected cancer in the absence of screening among individuals with a CRC diagnosis)38 and were observed in previous analyses for the USPSTF.13 SimCRC has longer adenoma dwell times, and this suggests that screening can be deferred longer after a negative previous screening result. In addition, models were calibrated to different time periods from the early‐screening era (1975‐1979 for SimCRC and 1990‐1994 for MISCAN‐Colon). Although there may have been some screening during the more recent period used to inform MISCAN‐Colon, this was to a large extent low‐sensitivity guaiac‐based fecal occult blood testing with limited presumed influence on CRC incidence.39 CRC incidence was similar in blacks and whites until the mid‐1980s, but it has since been higher among blacks than whites for screening‐eligible ages26; this may partly explain why MISCAN‐Colon recommended differential screening by race. Although colonoscopy every 15 years between the ages of 45 and 75 years was not considered efficient in MISCAN‐Colon for white adults in the stable‐risk scenario, the colonoscopy requirement and the predicted number of LYG were close to those for colonoscopy every 10 years from the age of 50 to 75 years. This suggests that the former strategy might be considered an option if uniformity in starting ages across demographic population subgroups were desired. To date, younger recommended start ages for screening black individuals by some organizations6, 7 have not led to higher screening rates among blacks in comparison with whites aged 45 to 49 years according to National Health Interview Survey data.40 Conversely, there is no evidence that race‐specific recommendations negatively affect screening uptake among whites aged 50 to 54 years.
The cancer registry data used to inform the models in this study did not allow the simulation of races/ethnicities other than black and white. Although recommendations depend on patterns in risk across a person's lifetime and on other‐cause mortality, we expect that model recommendations for other races/ethnicities except Alaskan Natives would be closer to those for whites than those for blacks because of the relatively similar observed CRC mortality risks.1
To our knowledge, this is the first time that race and sex differences have been formally considered for model‐based screening recommendations. The approach and conclusions from the first stable age‐specific risk scenario in this study were similar to a study performed by Lansdorp‐Vogelaar et al.41 Our model‐recommended strategies differ from the 2016 USPSTF screening recommendations, which suggested offering screening from the ages of 50 to 75 years to all adults at average risk.8 Consistent with 2009 American College of Gastroenterology6 and 2017 US Multi‐Society Task Force guidelines,7 our models recommend 45 years as the preferred starting age for blacks. Previous modeling studies have suggested that personalizing the age to stop screening may result in more efficient use of resources and help to reduce potential harms from screening.42 However, tailoring screening recommendations to different subgroups may complicate the promotion of screening in the primary care setting, and this may hamper guideline‐consistent adherence. More research is needed to assess the performance of personalized screening programs before wide application in practice.
There are some general limitations to the approach of this study for selecting model‐recommendable screening strategies. First, we predicted the potential benefit of screening under the assumption of 100% adherence to provide the best possible recommendation for patients who adhere to screening. In practice, some forms of screening may be less acceptable to people than others,43 and preferences may vary by setting, race, and sex and over time.44, 45, 46 These preferences are an important determinant of the success of any screening approach and should be considered in practice. A test that is predicted to have higher performance in the model in comparison with other tests under the assumption of full adherence may have lower population‐based performance because of lower acceptance. Second, to measure the burden of screening, we used the required number of colonoscopies. This ruled out a direct comparison of all strategies because the burden from tests other than colonoscopy was not explicitly considered. In practice, the potential burden from the primary screening method, such as low‐dose radiation exposure in CTC, should also be considered when one is recommending any of the evaluated strategies. Finally, there are no objective or widely accepted standards for the decision criteria applied in this study to narrow down the set of potentially recommendable screening strategies. We used similar acceptance thresholds for the degree of efficiency (proximity to the efficient frontier), minimum number of LYG, and maximum number of colonoscopies per LYG as applied in analyses performed for the USPSTF.13 As we showed in sensitivity analyses, sigmoidoscopy may be added to recommended screening modalities with a more relaxed benefit criterion of at least 75% of LYG in comparison with colonoscopy‐based screening. Earlier ages to start screening may not be acceptable with more stringent burden‐to‐benefit thresholds.
In conclusion, using an established decision‐analytic modeling approach, we suggest that screening for CRC should be considered between the ages 45 and 75 years for black adults in the United States and also for whites, particularly if lifetime risks have increased similarly to trends observed under the age of 40 years. Colonoscopy every 10 to 15 years, FIT every year, and CTC every 5 years were predicted to generate similar overall LYG with an acceptable colonoscopy burden. Our findings differ from previous model recommendations for the USPSTF, in which no distinction was made between blacks and whites, but are consistent with recent recommendations by the US Multi‐Society Task Force.7 In recommending any particular screening strategy, policymakers and physicians should consider patient preferences.
FUNDING SUPPORT
Supported by the American Cancer Society (ACS). The development of the Microsimulation Screening Analysis‐Colon was supported by grant U01‐CA199335 from the National Cancer Institute (NCI) as part of the Cancer Intervention and Surveillance Modeling Network (CISNET) and by grant P30‐CA008748. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the ACS and the NCI. The ACS receives partial funding from the Centers for Disease Control and Prevention to support the National Colorectal Cancer Roundtable, of which Dr. Robert A. Smith is the co‐chair, to support initiatives related to colorectal cancer screening outside of the current study.
CONFLICT OF INTEREST DISCLOSURES
Rebecca L. Siegel is employed by the American Cancer Society, which received a grant from Merck, Inc, for intramural research outside the submitted work; however, her salary is solely funded through American Cancer Society funds. Dennis J. Ahnen reports personal fees from Ambry Genetics and Cancer Prevention Pharmaceuticals outside the submitted work.
AUTHOR CONTRIBUTIONS
Reinier G. S. Meester: Formal analysis, investigation, data curation, methodology, software, visualization, and writing–original draft. Elisabeth F. P. Peterse: Validation, methodology, and writing–review and editing. Amy B. Knudsen: Methodology, visualization, software, and writing–review and editing. Anne C. de Weerdt: Formal analysis and data curation. Jennifer C. Chen: Data curation and project administration. Anna P. Lietz: Data curation, software, and writing–reviewing and editing. Andrea Dwyer: Conceptualization and writing–review and editing. Dennis J. Ahnen: Conceptualization and writing–review and editing. Rebecca L. Siegel: Methodology, data curation, and writing–review and editing. Robert A. Smith: Conceptualization and writing–review and editing. Ann G. Zauber: Conceptualization, methodology, writing–review and editing, and supervision. Iris Lansdorp‐Vogelaar: Methodology, software, writing–review and editing, and supervision.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Supporting Information 1
See companion article on pages http://onlinelibrary.wiley.com/doi/10.1002/cncr.31543/full, this issue.
REFERENCES
- 1. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177‐193. [DOI] [PubMed] [Google Scholar]
- 2. Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112:594‐642. [DOI] [PubMed] [Google Scholar]
- 3. Eddy D. ACS report on the cancer‐related health checkup. CA Cancer J Clin. 1980;30:193‐240. [PubMed] [Google Scholar]
- 4. Arias E, Heron M, Jiaquan X. United States life tables, 2013. Natl Vital Stat Rep. 2017;66:1‐64. [PubMed] [Google Scholar]
- 5. Saini SD, van Hees F, Vijan S. Smarter screening for cancer: possibilities and challenges of personalization. JAMA. 2014;312:2211‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739‐750. [DOI] [PubMed] [Google Scholar]
- 7. Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: recommendations for physicians and patients from the U.S. Multi‐Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153:307‐323. [DOI] [PubMed] [Google Scholar]
- 8. Bibbins‐Domingo K, Grossman DC, Curry SJ, et al; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2564‐2575. [DOI] [PubMed] [Google Scholar]
- 9. Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi‐Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130‐160. [DOI] [PubMed] [Google Scholar]
- 10. Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murphy CC, Lund JL, Sandler RS. Young‐onset colorectal cancer: earlier diagnoses or increasing disease burden? Gastroenterology. 2017;152:1809‐1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zauber AG, Lansdorp‐Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:659‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: modeling study for the US Preventive Services Task Force. JAMA. 2016;315:2595‐2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. Preventive Services Task Force . Modeling report. https://www.uspreventiveservicestaskforce.org/Page/Document/modeling-report1/colorectal-cancer-screening2. Accessed March 6, 2017.
- 15. Cancer Intervention and Surveillance Modeling Network . Colorectal cancer model profiles. https://cisnet.cancer.gov/colorectal/profiles.html/. Accessed January 1, 2017.
- 16. Arminski TC, McLean DW. Incidence and distribution of adenomatous polyps of the colon and rectum based on 1,000 autopsy examinations. Dis Colon Rectum. 1964;7:249‐261. [DOI] [PubMed] [Google Scholar]
- 17. Blatt LJ. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum. 1961;4:277‐282. [Google Scholar]
- 18. Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61:1472‐1476. [DOI] [PubMed] [Google Scholar]
- 19. Chapman I. Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clark JC, Collan Y, Eide TJ, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large‐bowel cancer. Int J Cancer. 1985;36:179‐186. [DOI] [PubMed] [Google Scholar]
- 21. Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33:1508‐1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24:799‐806. [DOI] [PubMed] [Google Scholar]
- 23. Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43:1847‐1857. [DOI] [PubMed] [Google Scholar]
- 24. Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49:819‐825. [DOI] [PubMed] [Google Scholar]
- 25. Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23:835‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Surveillance, Epidemiology, and End Results Program . SEER*Stat Database: incidence—SEER 9 regs research data, Nov 2013 sub (1973‐2011) <Katrina/Rita population adjustment>—linked to county attributes—total U.S., 1969‐2012 counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission. https://seer.cancer.gov/data/. Accessed May 25, 2016.
- 27. Rutter CM, Johnson EA, Feuer EJ, Knudsen AB, Kuntz KM, Schrag D. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst. 2013;105:1806‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849‐857. [DOI] [PubMed] [Google Scholar]
- 29. Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population‐based study. J Natl Cancer Inst. 2003;95:230‐236. [DOI] [PubMed] [Google Scholar]
- 30. van Hees F, Habbema JD, Meester RG, Lansdorp‐Vogelaar I, van Ballegooijen M, Zauber AG. Should colorectal cancer screening be considered in elderly persons without previous screening?: a cost‐effectiveness analysis. Ann Intern Med. 2014;160:750‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rutter CM, Knudsen AB, Marsh TL, et al. Validation of models used to inform colorectal cancer screening guidelines: accuracy and implications. Med Decis Making. 2016;36:604‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial. JAMA. 2014;312:606‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Segnan N, Armaroli P, Bonelli L, et al. Once‐only sigmoidoscopy in colorectal cancer screening: follow‐up findings of the Italian randomized controlled trial—SCORE. J Natl Cancer Inst. 2011;103:1310‐1322. [DOI] [PubMed] [Google Scholar]
- 34. Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal cancer incidence patterns in the United States, 1974‐2013. J Natl Cancer Inst. 2017;109:djw322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reumkens A, Rondagh EJ, Bakker CM, Winkens B, Masclee AA, Sanduleanu S. Post‐colonoscopy complications: a systematic review, time trends, and meta‐analysis of population‐based studies. Am J Gastroenterol. 2016;111:1092‐1101. [DOI] [PubMed] [Google Scholar]
- 36. Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2015;110:72‐90. [DOI] [PubMed] [Google Scholar]
- 37. Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age‐related incidences of colon and rectal cancers in the United States, 1975‐2010. JAMA Surg. 2015;150:17‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuntz KM, Lansdorp‐Vogelaar I, Rutter CM, et al. A systematic comparison of microsimulation models of colorectal cancer: the role of assumptions about adenoma progression. Med Decis Making. 2011;31:530‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ransohoff DF, Lang CA. Screening for colorectal cancer with the fecal occult blood test: a background paper. American College of Physicians. Ann Intern Med. 1997;126:811‐822. [DOI] [PubMed] [Google Scholar]
- 40. National Center for Health Statistics . 2010, 2013, and 2015 National Health Interview Survey. http://www.cdc.gov/nchs/nhis.htm. Accessed January 26, 2018.
- 41. Lansdorp‐Vogelaar I, van Ballegooijen M, Zauber AG, et al. Individualizing colonoscopy screening by sex and race. Gastrointest Endosc. 2009;70:96‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Hees F, Saini SD, Lansdorp‐Vogelaar I, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149:1425‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012;172:575‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liang PS, Dominitz JA. Bowel preparation: is fair good enough? Am J Gastroenterol. 2014;109:1725‐1727. [DOI] [PubMed] [Google Scholar]
- 45. Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: a retrospective cohort study. Ann Intern Med. 2016;164:456‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Winawer SJ, Fischer SE, Levin B. Evidence‐based, reality‐driven colorectal cancer screening guidelines: the critical relationship of adherence to effectiveness. JAMA. 2016;315:2065‐2066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Supporting Information 1


