Abstract
Interleukin-6 (IL-6) is a pleotropic cytokine that signals through the membrane-bound IL-6 receptor (mIL-6R) to induce anti-inflammatory (“classic-signaling”) responses. This cytokine also binds to the soluble IL-6R (sIL-6R) to promote inflammation (“trans-signaling”). mIL-6R expression is restricted to hepatocytes and immune cells. Activated T cells release sIL-6R into adjacent tissues to induce trans-signaling. These cellular actions require the ubiquitously expressed membrane receptor gp130. Reports show that IL-6 is produced by pulmonary arterial smooth muscle cells (PASMCs) exposed to hypoxia in culture as well as the medial layer of the pulmonary arteries in mice exposed to chronic hypoxia (CH), and IL-6 knockout mice are protected from CH-induced pulmonary hypertension (PH). IL-6 has the potential to contribute to a broad array of downstream effects, such as cell growth and migration. CH-induced PH is associated with increased proliferation and migration of PASMCs to previously non-muscularized vessels of the lung. We tested the hypothesis that IL-6 trans-signaling contributes to CH-induced PH and arterial remodeling. Plasma levels of sgp130 were significantly decreased in mice exposed to CH (380 mmHg) for five days compared to normoxic control mice (630 mmHg), while sIL-6R levels were unchanged. Consistent with our hypothesis, mice that received the IL-6 trans-signaling-specific inhibitor sgp130Fc, a fusion protein of the soluble extracellular portion of gp130 with the constant portion of the mouse IgG1 antibody, showed attenuation of CH-induced increases in right ventricular systolic pressure, right ventricular and pulmonary arterial remodeling as compared to vehicle (saline)-treated control mice. In addition, PASMCs cultured in the presence of IL-6 and sIL-6R showed enhanced migration but not proliferation compared to those treated with IL-6 or sIL-6R alone or in the presence of sgp130Fc. These results indicate that IL-6 trans-signaling contributes to pulmonary arterial cell migration and CH-induced PH.
Keywords: hypoxia, IL-6, pulmonary vasculature, sgp130, sIL-6R
Introduction
Interleukin 6 (IL-6) is a pleiotropic cytokine with a wide range of biologic activities in immune regulation, hematopoiesis, inflammation, and oncogenesis. IL-6 is secreted by a variety of cells including lymphocytes, macrophages, and smooth muscle cells, so much so that IL-6 is also considered a myokine.1 The role of IL-6 in homeostasis is mediated through the classic-signaling pathway, whereas pathology-associated responses are mediated by IL-6 trans-signaling.2 IL-6 association with the membrane-bound IL-6 receptor alpha subunit (mIL-6Rα) is referred to as the “classic-signaling” pathway, whereas with the soluble IL-6R (sIL-6R) is called “trans-signaling.” Classic IL-6 signaling is essential for defending the host against bacterial infections and mediates the activation of anti-inflammatory and regenerative epithelial pathways.3 In contrast, enhanced IL-6 trans-signaling is observed in chronic inflammatory disorders such as Crohn’s disease, atherosclerosis, and rheumatoid arthritis.3,4
IL-6 binding to the mIL-6Rα on target cells is of low affinity.5 Once IL-6 and mIL-6R have bound, this complex binds to the signal-transducing membrane glycoprotein 130 (gp130) to form a high-affinity, functional hexameric receptor complex of two IL-6, IL-6R, and gp130 initiating intracellular signaling via the janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway.6 Whereas robust mIL-6R expression is limited to neutrophils, naïve T cells, and hepatocytes, gp130 is ubiquitously expressed.7 The receptor gp130 has no affinity for IL-6, making cells that lack mIL-6R unresponsive to IL-6. Therefore, classical signaling involves IL-6 binding to mIL-6R only in lymphocytes and hepatocytes. IL-6R can be shed from mIL-6R-expressing cells through receptor cleavage or produced by alternative RNA splicing.8 This soluble form of IL-6 receptor (sIL-6R) has been found in urine and blood9 and binds with IL-6 with the same affinity as the mIL-6R.10 Again, this form of IL-6 signaling (trans-signaling) relies upon the formation of an IL-6/sIL-6R complex in solution, which binds to membrane gp130, activating the same downstream JAK/STAT signaling as does the classic IL-6 signaling pathway.11,12 A soluble form of gp130 has also been detected in the circulation;13 however, unlike sIL-6R, sgp130 has been found to be produced solely from alternatively spliced messenger RNA (mRNA) and inhibits trans-signaling by complexing with IL-6/sIL-6R.14 In summary, although classical IL-6 signaling appears to regulate the important homeostatic functions of IL-6, trans-signaling has been discovered to contribute to chronic inflammatory responses.7
Increases in lung and serum IL-6 have been associated with pulmonary hypertension (PH).15 Our group16 and another study17 has shown a rapid rise in lung IL-6 mRNA levels following exposure of mice to hypoxia, peaking at 24 h and remaining elevated for one week. Consistent with the increase in lung IL-6 mRNA, IL-6 immuno-labeling is increased in the medial layer of pulmonary arteries of mice exposed to five days of CH compared to normoxic controls.17 Interestingly, lung IL-6-overexpressing transgenic mice develop spontaneous PH with significant vascular remodeling and perivascular infiltration of T-cells but not B-cells, not due to hypoxia, indicating a close association between IL-6 levels and lung pathology.18 A study relying on IL-6 knockout (KO) mice has demonstrated a central role for IL-6 in the development of chronic hypoxia (CH)-induced PH.17 The study showed that IL-6 KO mice exhibit less severe PH through decreased right ventricular (RV) hypertrophy and a lower right ventricular systolic pressure (RVSP) compared to wild-type (WT) mice following exposure to CH. However, it is unclear whether IL-6 was acting through the classic or the trans-signaling pathway. Therefore, the present study tested the hypothesis that IL-6 trans-signaling contributes to CH-induced PH by promoting pulmonary arterial remodeling. Addressing this hypothesis has clinical implications because specific inhibition of IL-6 trans-signaling should only block the pro-inflammatory effects of IL-6 without inhibiting its important homeostatic properties.
Methods
Animals
C57BL/6 mice (male, 25 g; The Jackson Laboratory) were used in these studies. Protocols were approved by the Institutional Animal Care and Use Committee of the University of New Mexico Health Sciences Center.
Chronic hypoxia exposure
Mice exposed to CH were housed in a hypobaric chamber (∼380 mmHg, 5 or 21 days). Control mice were housed at ambient barometric pressure (normoxia, N; ∼630 mmHg, Albuquerque, NM, USA).
Dosing
Mice were administered sgp130Fc (0.5 mg/kg twice per week i.p.) or vehicle (saline) and exposed to either CH or normoxia for up to 21 days. Sgp130Fc was purchased from R&D Systems, Minneapolis, MN, USA. The dose and route of administration was based on previous reports demonstrating sgp130Fc efficacy in other disease models.19,20
Sgp130Fc is a Fc-dimerized version of sgp130, which specifically blocks IL-6 trans-signaling and is highly efficacious in diverse models of chronic inflammation.12,21,22 Importantly, dimerization increases the efficacy of blocking signaling via IL-6/sIL-6R by a factor of 10–100 compared with monomeric sgp130.22
Assessment of right ventricular systolic pressure and right ventricular hypertrophy
Peak right ventricular systolic pressure (RVSP) was assessed in isoflurane-anesthetized mice as previously described.23 An upper transverse laparotomy was performed to expose the diaphragm. A 25-gauge needle, connected to a pressure transducer (model P23 XL, Spectramed), was inserted into the right ventricle (RV) via a closed-chest trans-diaphragmatic approach, and the output amplified by a Gould Universal amplifier. All data were recorded and heart rate was calculated with a computer-based data acquisition system (AT-CODAS, DATAQ Instruments). After collecting hemodynamic data, the heart was isolated and the atria and major vessels were removed. The RV was dissected from the left ventricle (LV) and septum (S). RV hypertrophy (Fulton’s Index) was expressed as the percentage ratio of RV to left ventricle plus septum (LV+S) weight and RV to body weight (BW).
Circulating and lung levels of sIL-6R and sgp130
IL-6, sIL-6R, and sgp130 were measured in heparinized plasma and the supernatant of a suspension of dissociated lung cells using a Milliplex Map Kit (EMD Millipore) following the manufacturer’s recommendations. The cellular suspension was prepared by enzymatically digesting the lungs as previously reported.16 Lung IL-6, sIL-6R, and sgp130 levels were normalized to total protein determined by the Bradford method (Biorad).
Vascular morphometry
Pulmonary arterial remodeling was assessed using a modification of previously published methods.16 After collection of hemodynamic data, the lungs were perfused via the RV with ∼5 mL of modified physiological saline solution (HEPES-PSS, 134 mM NaCl, 6 mM KCl, 1 mM MgCl2, 10 mM HEPES, 2 mM CaCl2, 0.026 mM EDTA, and 10 mM glucose) containing heparin, 4% albumin (Sigma), and 10−4 M papaverine (Sigma), at 20 mmHg to maximally dilate and flush the circulation of blood. The heart, lungs, and trachea were removed in block, and the lungs inflated through the trachea with the same solution. The right lobes were tied off, removed, and preserved in RNA Later. The left lobe of the blood-cleared lung was fixed in 4% paraformaldehyde (Polyscience, Warrington, PA, USA) in phosphate-buffered saline (PBS) fixative.
Lung sections (5 µm) were stained with rabbit anti-smooth muscle α-actin (Abcam Ab5694, Cambridge, MA, USA) antibody or rabbit IgG control (negative) followed by DyLight 549-donkey anti-rabbit (Thermo Fisher Scientific). Sections were examined using a 20× or 40× objective on a Zeiss Axiovert 200 M scope, and images acquired with a Cool Snap EZ camera using NIS-Elements F 3.0 software. Images were analyzed with Image J (NIH, Bethesda, MD, USA). Vessels sectioned at oblique angles were excluded from analysis. Approximately ten images with 25 arteries/animal with an outer diameter of 20–50 µm were analyzed. Arterial wall thickness was calculated according to the following equation: % wall thickness = [(external diameter – luminal diameter)/external diameter] × 100. Diameters were calculated from the measured circumferences.
To determine percent muscularization, images were thresholded using Image J. Regions of interest (ROIs) were drawn around each artery. The percent thresholded area to total ROI area was calculated for each artery as the percent muscularization. Arterial outer diameter was calculated based on the circumference of the ROI and only arteries in the range of 20–40 µm were analyzed as previously described.25 Most arteries > 40 µm are fully muscularized, even under control conditions.
Immunofluorescence
Sections from paraffin-embedded lungs were deparaffinized, rehydrated, washed, subjected to antigen retrieval for 20 min ≥ 90℃ in a buffer containing 10 mM Tris, pH 9.0 + 1 mM EDTA in rice cooker, and blocked/permeabilized with 1× PBS + 2% normal goat serum + 0.1% Triton X-100.
For the detection of proliferating cells, lung sections were incubated with control IgG or with rabbit monoclonal anti-Ki67 SP6 clone (1:500, ThermoFisher Scientific RM-9106-S0)17 overnight at 4℃, followed by secondary antibody donkey DyLight 549 anti-rabbit.
For the detection of CD3+ RORγτ+ (TH17 cells), lung sections were incubated with IgG control or rat anti-CD3 primary antibody (1:100; clone CD3-12, AbCam ab11089, lot GR280475-8, stock 1.0 mg/mL) and rabbit anti-RORγτ (1:3000; clone EPR20006, AbCam ab207082, lot GR3179110-1, stock 0.658 mg/mL), followed by AlexaFluor 488 goat anti-rat F(ab′2) (1:800; Jackson ImmunoResearch 712-546-153, lot 128228, stock 1.4 mg/mL) and DyLight 549 donkey anti-rabbit (1:800 final; Jackson ImmunoResearch 711-505-152, lot 93339, stock 1.5 mg/mL).16
In all cases, sections were counter stained with AlexaFluor 633 hydrazide to label the elastic lamina. Images were acquired using a Leica TCS SP5 Spectral Confocal System. The number of Ki67+ cells in the arterial wall or CD3+ RORγτ+ in the perivascular region were normalized to outer diameter. Approximately ten images with 25 arteries/animal with an outer diameter of 20–50 µm were analyzed.
Pulmonary artery smooth muscle cell [Ca2+]i, migration, confluency, and proliferation assays
Primary pulmonary arterial smooth muscle cell (PASMC) cultures were established and cells authenticated as previously described.24,25 PASMCs were plated on gelatin-coated dishes and cultured in Smooth Muscle Cell Medium (Cell Biologics) containing 10% fetal bovine serum and antibiotic-antimycotic solution in a humidified atmosphere of 5% CO2-95% air at 37℃. Before experiments, PASMCs were cultured for at least 48 h in serum-free Smooth Muscle Cell Medium containing insulin, EGF, hydrocortisone, L-glutamine, and antibiotic-antimycotic solution (M2268SF Cell Biologics).
Intracellular Ca2+ levels ([Ca2+]i) were measured in cells loaded at room temperature with HEPES-PSS containing the cell-permeable ratiometric Ca2+-sensitive fluorescent dye fura 2-acetoxymethyl ester (fura 2-AM; 2 µM; Life Technologies) and 0.02% pluronic acid (Life Technologies). Fura 2-AM-loaded cells were alternately excited at 340 and 380 nm at a frequency of 10 Hz with an IonOptix Hyperswitch dual-excitation light source and the respective 510 nm emissions were collected with a photomultiplier tube (F340/F380) at 37℃. After subtracting background fluorescence, emissions ratios were calculated with Ion Wizard software (IonOptix) and recorded continuously throughout the experiment.23
To measure cell migration or cell numbers, an IncuCyte (Essen Bioscience) live-cell imaging system was used. Images were captured every 2 h over a period of 60 h. A WoundMaker (Essen Bioscience) was used to produce a homogenous 700–800-µm wide scratch wound on a confluent monolayer of PASMCs as previously described.16 Relative wound density (%) was calculated by IncuCyte Scratch Wound Cell Migration Software Module (Essen Bioscience) as the cell density in the wound area expressed relative to the cell density outside of the wound area over time. This metric normalizes for changes in cell density caused by proliferation and/or pharmacological effects.
Cell proliferation was assessed, in part, by determining the confluency of the culture over time, divided by the initial confluency.16 Proliferation was also measured using a Click-IT EdU microplate assay (Invitrogen, C10214). The assay determines cell proliferation by measuring the amount of EdU (5-ethynyl-2’ deoxyuridine, a thymidine analogue in which a terminal alkyne group replaces the methyl group in the 5 position) incorporation into DNA during cellular DNA replication. The terminal alkyne group was detected with fluorescent azides using the Click-IT chemistry.
Cells were treated with IL-6 (500 ng/mL) or sIL-6R (500 ng/mL) alone or in combination and in the absence or presence of sgp130Fc (10 µg/mL), STAT3 Inhibitor III (1 µM, WP 1066, Santa Cruz Biotechnology), or 5 µM BAPTA AM (Thermofisher) with 10 µM cyclopiazonic acid (CPA, Sigma-Aldrich) and 50 µM diltiazem (Sigma-Aldrich). IL-6 was pre-incubated with sIL-6R or sIL-6R plus sgp130Fc at 37℃ before adding it to the cells.
RT-PCR
Total lung RNA was extracted using Direct-zol (Zymo Research) and reverse transcribed to complementary DNA using the High-Capacity cDNA Reverse Transcription Kit (Life Technologies). The Roche Universal Probe Library System was used for real- time detection of gp130 (5’ cccatgggcaggaatataga, 3’ cataatccaagattttcccattg, probe 97) and ACTB as a reference gene (5’ ctaaggccaaccgtgaaaag, 3’ accagaggcatacagggaca, probe 64). The normalized gene expression method (2-ΔΔCT) was used for relative quantification of gene expression using pooled samples as the calibrator.26
Statistics
Results were expressed as mean ± standard error. Statistical significance was tested at the 95% (P < 0.05) confidence level using an unpaired t-test or two-way ANOVA followed by Student-Newman-Keuls post-test using GraphPad Prism 7.
Results
Chronic hypoxia alters components of the IL-6 trans-signaling pathway
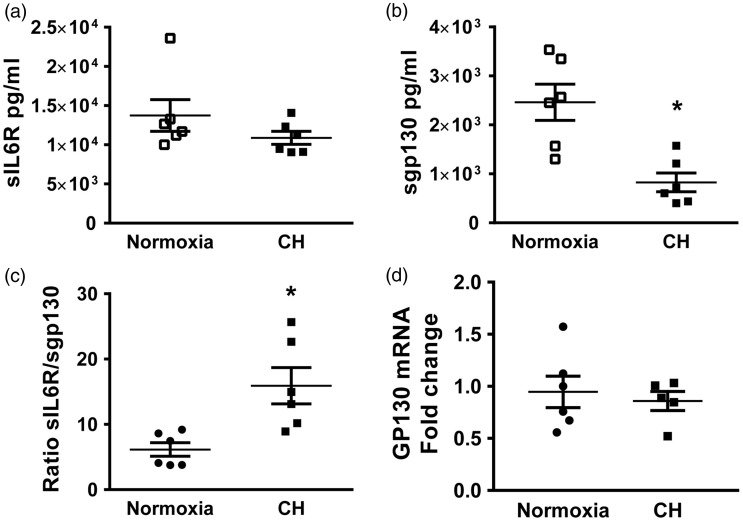
IL-6 trans-signaling is tightly controlled through the concentrations of sIL-6R and IL-6.7 Under normal (non-inflammatory) conditions, the levels of sIL-6R and sgp130 are roughly 1000 times higher than IL-6 levels.7 This, then, implies that once IL-6 is secreted, it forms the IL-6/sIL-6R complex, and this complex is neutralized by sgp130. Only under conditions in which IL-6 concentrations exceed that of sIL-6R, or under reduced levels of sgp130, can IL-6 act on target cells, such as in sepsis.7,27 Therefore, we examined the levels of these three molecules in the plasma, and the supernatant of lung digest of mice exposed to normoxia and CH. IL-6 levels were below the limit of detection in plasma samples. However, there was a significant increase in IL-6 levels in the supernatant of lung digests from mice exposed to CH versus normoxia (normoxia 0.0004 ± 0.0001 vs CH 0.0016 ± 0.0003 pg/µg of total protein, n = 6). This result is consistent with our previous report showing increased lung IL-6 mRNA levels and IL-6 immunostaining in the medial layer of pulmonary arteries from mice exposed to five days of CH compared to normoxic animals.17 There was no difference in plasma (Fig. 1a) or lung digest sIL-6R levels between normoxic and mice exposed to five days of CH (normoxia 0.086 ± 0.006 vs. CH 0.097 ± 0.010 pg/µg of total protein, n = 7). Interestingly, there was a significant decrease in sgp130 in the plasma of mice exposed to CH, as compared to normoxia (Fig. 1b) but no differences in lung sgp130 levels between groups (normoxia 0.007 ± 0.001 vs CH 0.005 ± 0.001 pg/µg of total protein, n = 7). As expected, the ratio of sIL-6R to sgp130 was increased in plasma from CH mice compared to controls (Fig. 1c). Furthermore, lung gp130 mRNA levels were not different between normoxic and CH mice (Fig. 1d).
Fig. 1.
sgp130 plasma levels are decreased following exposure to CH. Mice were exposed to CH or normoxia for five days. (a) Plasma levels of sIL-6R and (b) sgp130; (c) ratio of sIL-6R/sgp130 and (d) lung gp130 mRNA fold change from calibrator. Values are means ± SEM; n = 6/group, *P < 0.05; analyzed with T-test.
IL-6 trans-signaling contributes to CH-induced PH
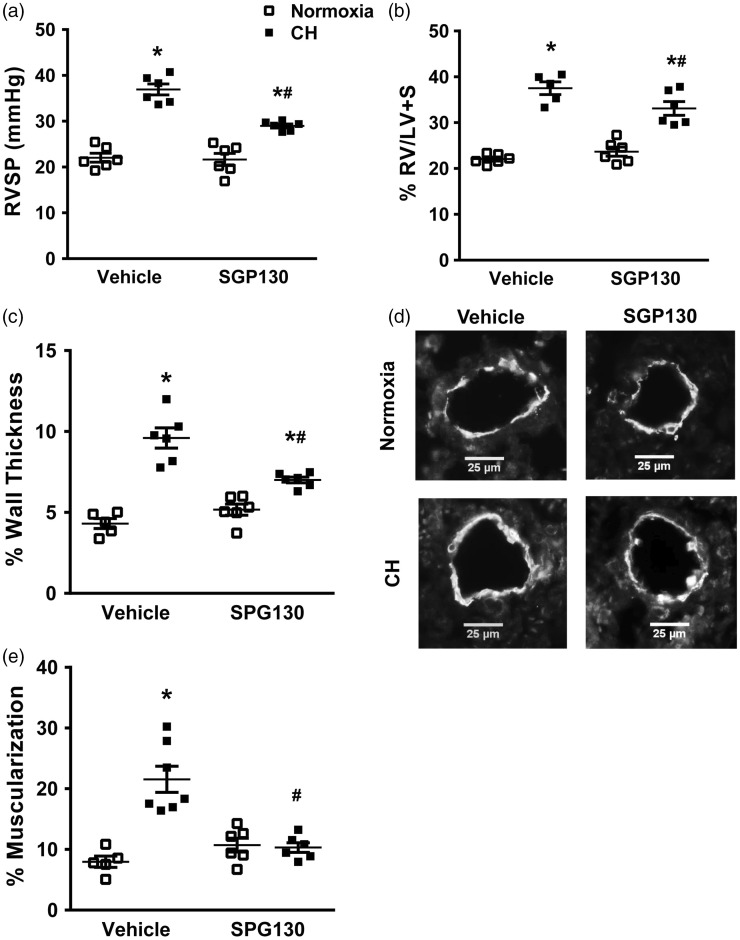
To determine the role of IL-6 trans-signaling on CH-induced PH, IL-6 trans-signaling was specifically inhibited by administering sgp130 Fc to mice exposed to normoxia or CH. When IL-6 is not elevated in plasma, sgp130Fc has no effect on classic IL-6 signaling, yet disrupts IL-6 trans-signaling making it an effective tool in dissecting the contribution of classic versus trans-signaling in various physiologic responses.7 Therefore, mice were given sgp130Fc (0.5 mg/kg twice per week i.p.)19,20 or vehicle (saline) control, and exposed to either CH or normoxia for up to 21 days. Mice treated with vehicle exhibited the expected significant increase in RVSP following CH, while mice receiving sgp130Fc demonstrated a significantly attenuated increase in RVSP as compared to vehicle control mice (Fig. 2a). Similar results were seen with regard to RV hypertrophy (Fig. 2b) and % arterial wall thickness (Fig. 2c, d).
Fig. 2.
Inhibition of IL-6 trans-signaling attenuates CH-induced increases in RV systolic pressure, RV hypertrophy, and pulmonary arterial remodeling. (a) Right ventricular systolic pressure (RVSP), (b) Fulton’s index (RV/LV+S weight), (c) % wall thickness of α-smooth muscle actin-stained pulmonary artery sections with external diameters of 20–50 µm from mice that received vehicle (saline) or sgp130Fc during exposure to normoxia or CH for 21 days. (d) Representative images of α-smooth muscle actin-stained pulmonary artery sections. (e) % Muscularization of arteries of < 40 µm from the same groups. Values are mean ± SEM; *P < 0.05 vs. normoxia vehicle, #P < 0.05 vs. CH vehicle n = 6 animals/group; analyzed with ANOVA and individual groups compared with the Student-Newman-Keuls test. At least 25 arteries/animal were analyzed and averaged.
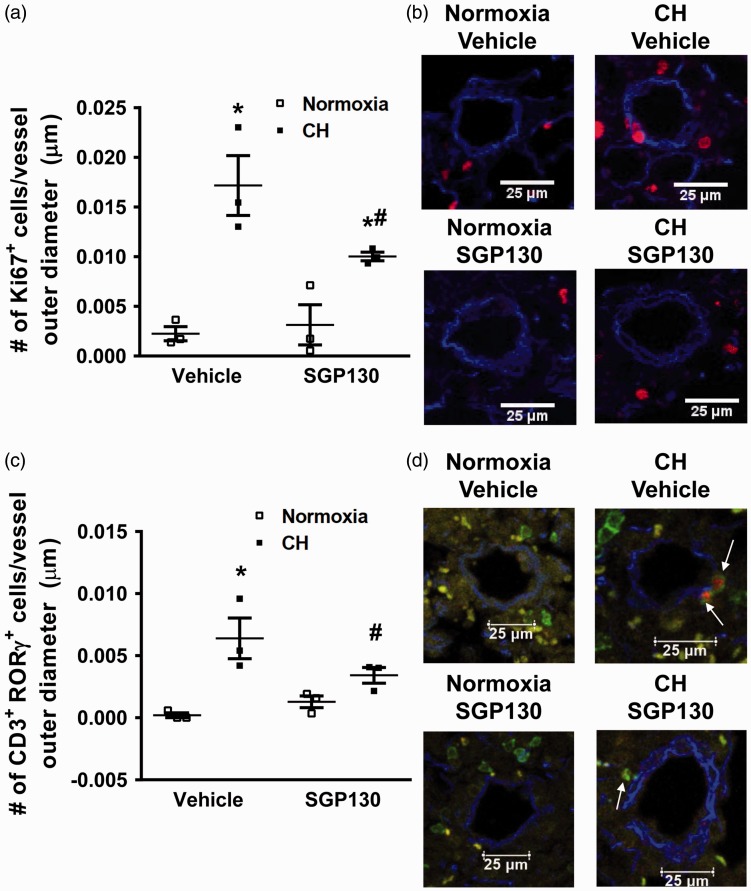
It is well accepted that pulmonary arterial remodeling is due, in part, to appearance of cells expressing smooth muscle specific markers, like SM-α-actin, in normally non-muscular small diameter vessels, resulting from proliferation and migration of pulmonary arterial smooth muscle cells (reviewed in Shimoda et al.28). Therefore, the % muscularization of small pulmonary arteries was also evaluated in this study. As expected,25 mice exposed to CH and treated with vehicle showed significantly increased % muscularization after 21 days but this response was significantly attenuated in mice that received sgp130Fc (Fig. 2e). As reported before,16 mice treated with vehicle showed a significant increase in the number of proliferating cells (Ki67+) in the walls of pulmonary arteries following five days of CH which was significantly attenuated by sgp130Fc administration (Fig. 3a, b). We have previously demonstrated that proliferation is no longer observed after seven days of CH exposure.29
Fig. 3.
Blocking IL-6 trans-signaling attenuates CH-induced proliferation of cells in the arterial wall and perivascular TH17 cell infiltration. Mice were exposed to five days of normoxia or CH, treated with or without sgp130Fc. (a) Number of Ki67+ (red) cells/pulmonary artery outer diameter from mice exposed to five days of normoxia or CH. Ki67 is a marker of cell proliferation. Ki67+ cells were detected by immunofluorescence microscopy in the arterial wall. (b) Representative images. Ki67+ cells in red and elastic lamina in blue. (c) Number of perivascular CD3+ (green) RORγτ+ (red). The perivascular region was defined as external to the vessel media within the adventitia. (d) Representative images. CD3 in green, RORγτ in red, and elastic lamina in blue. Values are mean ± SEM; *P < 0.05 vs. normoxia vehicle, #P < 0.05 vs. normoxia sgp130, n = 6 mice/group; analyzed with ANOVA and individual groups compared with the Student-Newman-Keuls test. At least 25 arteries/animal were analyzed and averaged.
Treatment of mice with sgp130Fc did not affect heart rate (beats/min: normoxia vehicle 457 ± 8; normoxia sgp130Fc 407 ± 28; CH vehicle 383 ± 19; CH sgp130Fc 441 ± 14; n = 6) or the polycythemic response to CH (hematocrit %: normoxia vehicle 45.0 ± 1.3; normoxia sgp130Fc 45.5 ± 1.4; CH vehicle 59.3 ± 1.6; CH sgp130Fc 62.0 ± 0.9; n = 6).
It is generally thought that IL-6 trans-signaling is responsible for the pro-inflammatory actions of IL-6, while IL-6 classical-signaling is important for organism homeostasis and the cytokine’s regenerative properties.7 To elucidate the inflammatory effects of IL-6 trans-signaling on the lung vasculature following exposure to CH, we exposed mice to CH for five days while treating the mice with either vehicle or sgp130Fc. As previously demonstrated,16 we found again that five days’ exposure of mice to CH leads to perivascular infiltration of TH17 cells (CD3+ RORγτ+) while mice receiving sgp130Fc demonstrated a marked reduction in perivascular TH17 cells (Fig. 3c, d).
IL-6 trans-signaling enhances PASMC migration but not proliferation
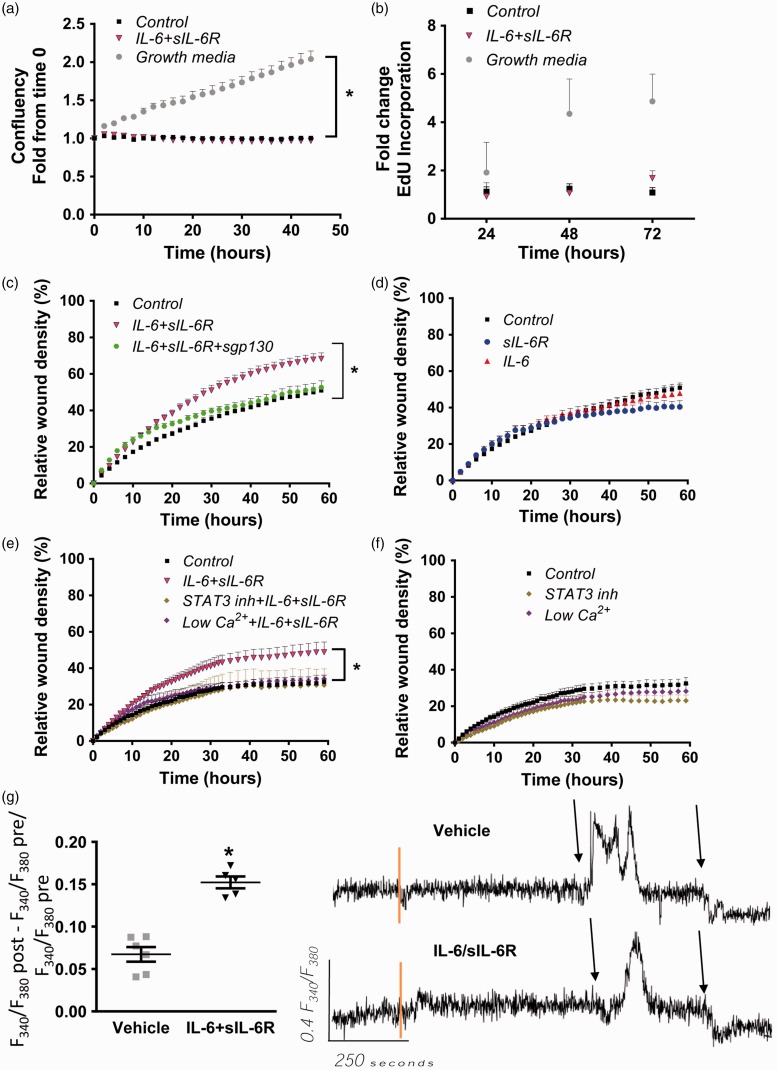
As described above, both PASMC proliferation and migration are major contributors to the development of pulmonary vascular remodeling in response to CH. IL-6 signaling has been reported to enhance human PASMC migration but not proliferation.17 To determine whether this effect could be involved in the pulmonary arterial remodeling observed in response to CH, the proliferative and migratory response to IL-6 trans-signaling activation was evaluated in mouse pulmonary artery smooth muscle cells (mPASMC) in culture. Cell culture confluency was not affected by treatment with IL-6/sIL-6R (Fig. 4a), neither was the proliferation rate of the cells assessed by EdU incorporation to DNA (Fig. 4b).
Fig. 4.
IL-6/sIL-6R enhances cell migration and [Ca2+] but not proliferation. (a) Confluency of mPASMC was determined using IncuCyte in cells cultured in differentiation media in the presence or absence of IL-6/sIL-6R complex. (b) EdU incorporation in cells treated as in (a). (c) Scratch wound assay in mPASMC treated with IL-6/sIL-6R, IL-6/sIL-6R ± sgp130 or (d) IL-6, sIL-6R, and sgp130 alone. (e) Same as (c) but with IL-6/sIL-6R ± STAT3 inhibitor or in the presence of low [Ca2+]i or (f) vehicle ± STAT3 inhibitor or in the presence of low [Ca2+]i. (g) Change in fura-2 fluorescence ratio (F340/F380) from baseline in cells incubated in the absence (vehicle) or presence of IL-6/sIL-6R. Representative traces are shown on the right. Pre = before vehicle or IL-6/sIL-6R, post = after vehicle or IL-6/sIL-6R. Vertical orange lines indicate the addition of vehicle or IL-6/sIL-6R. Black arrows indicate the addition of 1 μM ionomycin and superfusion of Ca2+-free PSS. Values are mean ± SEM; *P < 0.05 vs. control, n = 3–8/group; analyzed with repeated measures ANOVA.
mPASMC incubated in the presence of IL-6/sIL-6R showed a significant increase in cell migration compared to cells grown in control media (mPASMC differentiation media) (Fig. 4c), cells incubated with IL-6/sIL-6R plus sgp130 Fc (Fig. 4c), IL-6 alone, or sIL-6R alone (Fig. 4d).
In the process of IL-6 trans-signaling, gp130 forms a homodimer, recruiting Jak/STAT proteins,2 specifically STAT3, and initiating downstream signaling. To verify that STAT3 activation contributes to IL-6/sIL-6R-induced enhanced mPASMC migration, cells were pre-incubated with the STAT3 Inhibitor III WP 1066 and the rate of cell migration was measured as described above observing a significant attenuation in the enhanced migratory response (Fig. 4e). STAT3 inhibition alone did not affect the migration in response to the wound in the absence of IL-6/sIL-6R (Fig. 4f).
In fibroblasts, it has been shown that IL-6 trans-signaling also increases intracellular Ca2+ concentration ([Ca2+]i) but this has not been reported in PASMC.30 A rise in [Ca2+]i is a key stimulus for PASMC proliferation and migration. Ca2+ chelation together with inhibition of Ca2+ influx through voltage-dependent Ca2+ channels and inhibition of endoplasmic reticulum Ca2+-ATPase prevented IL-6/sIL-6R-induced mPASMC migration (Fig. 4e) without affecting migration in response to the wound in the absence of IL-6/sIL-6R (Fig. 4f). Consistent with this finding, IL-6/sIL-6R caused a significant increase in the change in fura-2 fluorescence ratio from baseline compared to vehicle (Fig. 4g). A representative trace of fura-2 fluorescence ratio is included in Fig. 4g, also depicting the response to the Ca2+ ionophore ionomycin (1 μM) followed by superfusion with Ca2+-free HEPES-PSS.
Discussion
In this study, we have demonstrated an effect of CH exposure in mice to disrupt the balance between lung IL-6 and circulating levels of sIL-6R and sgp130. Inhibition of IL-6 trans-signaling with sgp130Fc attenuated indices of CH-induced PH, including elevated RVSP, RV remodeling and pulmonary arterial remodeling, and proliferation of cells in the arterial wall. Furthermore, CH-induced perivascular TH17 cell infiltration was inhibited in mice treated with sgp130Fc. Finally, the IL-6/sIL-6R complex enhanced PASMC migration, but not proliferation, through a pathway that requires increases in [Ca2+]i and activation of STAT3. Taken together, this study reports the novel finding that IL-6 contributes to CH-induced PH through the trans-signaling pathway.
It has been previously demonstrated that cytokines contribute to the pathogenesis of PH in both human and animal studies. IL-6 has been implicated in PH in multiple studies, from patients with chronic obstructive pulmonary disease (COPD) showing elevated levels of plasma IL-6,31 to several experimental studies showing an active role for IL-6 on pulmonary vascular remodeling and hypoxic PH in mice.19 Furthermore, IL-6 mRNA levels rise rapidly in the lungs following hypoxic exposure, along with IL-6 protein. Our group16 along with others have demonstrated,17 via immunohistochemistry, that IL-6 levels are strongly increased in PASMCs of the vessel media. Savele et al.17 further demonstrated that hypoxia resulted in a marked increase in lung expression of IL-6R and gp130. In this study, we found no changes in lung gp130 mRNA expression or lung protein levels of sgp130, the latter of which acts as a natural inhibitor of IL-6 trans-signaling by binding IL-6/sIL-6R in solution, thereby preventing the complex from interacting with gp130 on the cell surface. In contrast, we observed a significant increase in lung IL-6 peptide levels and a decrease in circulating sgp130 levels with no change in plasma sIL-6R. Together, these finding suggest that IL-6 trans-signaling is elevated after CH exposure based on locally increased levels of IL-616 and decreased circulating sgp130.
Therapeutic monoclonal antibodies are currently being used to treat patients, and these antibodies bind various sites on the IL-6 signaling complex, interfering with IL-6 signaling.32 For example, Tocilizumab (Actemra, Hoffmann-La Roche), an anti-human IL-6R monoclonal antibody, blocks the interaction of IL-6 and IL-6R by binding a specific site on the receptor, whereas olokizumab (R-Pharm-UCB), an anti-human IL-6 monoclonal antibody, blocks hexamer formation by targeting another specific site on IL-6R. These antibodies are believed to provide blockade of IL-6 signaling in an indiscriminate way, i.e. both classic and trans-signaling. Recent data support the hypothesis that these two IL-6 signaling pathways lead to different biological sequela. The contribution of trans-signaling to disease has recently been advanced through development of the engineered variant of soluble gp130, sgp130Fc.22
Administration of an antibody against IL-6R attenuates CH-induced PH in mice33 and monocrotaline- and SU 5416/hypoxia-induced PH in rats,34 but again this antibody does not discriminate between the membrane and the soluble form of the IL-6R blocking both classic and IL-6 trans-signaling.35 In the present study, we explored the direct effect of IL-6 trans-signaling using the specific inhibitor sgp130Fc. Using this novel approach, we found that IL-6 trans-signaling contributes to CH-induced PH. A dissociation between RVSP and RV remodeling was observed in Fig. 2. While it has been widely believed that sustained pressure overload is sufficient to cause adaptive hypertrophy of the heart, this and other studies3,15,39 suggest hypoxia itself is a major contributor to RV remodeling through mechanisms that are partially independent of pressure overload. The mechanism that underlies the dissociation between RVSP and RV remodeling is currently unknown.
PH can result from exposure to high altitude or chronic lung diseases that lead to global hypoxia, such as COPD and interstitial lung diseases. Over time, alveolar hypoxia likely contributes to “fixed” components of PH, such as structural remodeling of the pulmonary arterial bed leading to increased arterial resistance.36 Pulmonary arterial remodeling is characterized by thickening of the muscular medial layer of arteries and the extension of muscle into small previously non-muscular pre-capillary arterioles, likely mediated by PASMC proliferation and migration.37,38 Consistently, sgp130Fc administration also attenuated CH-induced increases in pulmonary arterial wall thickness, muscularization of small pulmonary arteries, and proliferation of cells in the arterial wall. However, a previous study examining the direct effect of IL-6 on systemic vascular smooth muscle cell (VSMC) migration and proliferation has demonstrated that IL-6 stimulates VSMC migration but not proliferation.39 This study showed that IL-6 caused the assembly of actin stress fibers, driving VSMC migration based on reorganization of the cytoskeleton. A more recent study looking specifically at the migration and proliferation of human PASMCs demonstrated a similar response to IL-6 regarding cell migration and proliferation.17 The addition of IL-6 to the cells promoted migration; however, when the PASMCs were cultured with IL-6 combined with sIL-6R, the effects on migration were even further enhanced, while proliferation was unchanged. These findings are consistent with a report showing that human VSMCs express very low levels of IL-6R under basal conditions, and therefore do not respond well to stimulation with IL-6 alone.40 Our results are in agreement with these previous findings in cultured human PASMCs. However, we found that primary mPASMC do not respond to IL-6 alone. IL-6 must be complexed with sIL-6R to enhance migration of mPASMC. In addition, this effect was completely blocked by the addition of sgp130Fc suggesting that the mIL-6R is either not expressed or not active in mPASMC. These previous findings, in combination with our own data, indicate that IL-6 exerts direct pro-migratory effects on PASMC but does not directly affect cell proliferation. Therefore, additional mechanisms (discussed below) might account for the inhibition of CH-induced proliferation of cells in the medial layer of small pulmonary arteries in animals in which IL-6 trans-signaling was inhibited with sgp130Fc.
Consistent with established effects of both IL-6 classic and trans-signaling to induce STAT3 activation,11,12 we found that inhibition of STAT3 prevented the effect of IL-6/sIL-6R to enhanced mPASMC migration without affecting the basal migratory response to the scratch wound. However, little is known about Ca2+ as an intracellular messenger in the IL-6 trans-signaling pathway. Our study shows that IL-6 trans-signaling causes a small but significant and consistent increase in [Ca2+]i levels in mPASMC in culture suggesting that this pathway might contribute to the enhanced basal [Ca2+]i reported in pulmonary arteries from PH mice.41,42 In addition, this increase in [Ca2+]i levels seems to contribute to the enhanced migration observed in response to IL-6/sIL-6R.
IL-6 bioactivity has been tightly linked to the localization and migration of T cells.43 We have recently shown that CH leads to the perivascular recruitment of CD3+ T cells to pulmonary arteries, specifically CD3+ CD4+, IL-17+ TH17 cells.16 Given the dependence of TH17 cells on IL-6,33,44 we were interested in examining the effect of sgp130 on TH17 cells infiltration of the perivascular space following CH. Again, we observed an increase in perivascular TH17 cells following CH, which was attenuated with the inhibition of IL-6 trans-signaling. This result is in accordance with previous studies showing that IL-6 trans-signaling plays an important role in chemokine release.43 Our data suggest that IL-6, locally increased in the lungs following CH,16 acting largely through trans-signaling, leads to the enhanced chemokine-mediated trafficking of TH17 cells to the perivascular space.
Interestingly, T cells, upon activation, greatly reduce IL-6R expression on their surface, indicating that classical IL-6 signaling is required for initial activation and polarization of naïve T cells, yet IL-6 trans-signaling may play a crucial role in maintaining an ongoing pro-inflammatory state45 by governing secretion of various chemokines and expression of chemokine receptors on TH17 cells.45
Given the importance of IL-6 trans-signaling in the tissue maintenance of TH17 cells,45 it would seem likely that it could contribute to a TH17-mediated pro-inflammatory environment. TH17 cells secrete IL-17A, which we have previously shown directly enhances migration without affecting PASMC proliferation.16 However, TH17 cells may indirectly contribute to PASMC proliferation through the production of IL-21, M2 macrophage skewing, and subsequent CXCL12 production33 or other unknown mechanisms. This might explain why IL-6 trans-signaling contributes to CH-induced proliferation of cells in the arterial wall in vivo but does not affect mPASMC proliferation in culture.
In summary, we conclude that IL-6 trans-signaling contributes to CH-induced pulmonary hypertension, at least partially though enhanced PASMC migration and perivascular infiltration of TH17 cells.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received funding from NIH NHLBI F30HL123109 to L.D.M, AHA 15GRNT25090038 to L.V.G.B., NIH T32 HL007736, NIH R01 HL111084 to N.L.J. and NIH R01 HL132883 to T.C.R.
References
- 1.Muñoz-Cánoves P, Scheele C, Pedersen BK, et al. Interleukin-6 myokine signaling in skeletal muscle: A double-edged sword? FEBS J 2013; 280: 4131–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol 2015; 16(5): 448–457. [DOI] [PubMed] [Google Scholar]
- 3.Scheller J, Chalaris A, Schmidt-Arras D, et al. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011; 1813: 878–888. [DOI] [PubMed] [Google Scholar]
- 4.Jones SA, Richards PJ, Scheller J, et al. IL-6 transsignaling: the in vivo consequences. J Interferon Cytokine Res 2005; 25(5): 241–253. [DOI] [PubMed] [Google Scholar]
- 5.Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol 2014; 10(12): 720–727. [DOI] [PubMed] [Google Scholar]
- 6.Saito M, Yoshida K, Hibi M, et al. Molecular cloning of a murine IL-6 receptor-associated signal transducer, gp130, and its regulated expression in vivo. J Immunol 1992; 148(12): 4066–4071. [PubMed] [Google Scholar]
- 7.Rose-John S. IL-6 trans-signaling via the soluble IL-6 receptor: Importance for the proinflammatory activities of IL-6. Int J Biol Sci 2012; 8(9): 1237–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briso EM, Dienz O, Rincon M. Cutting Edge: Soluble IL-6R Is Produced by IL-6R Ectodomain Shedding in Activated CD4 T Cells. J Immunol 2008; 180: 7102–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novick D, Engelmann H, Wallach D, et al. Soluble cytokine receptors are present in normal human urine. J Exp Med 1989; 170(October): 1409–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rose-John S, Heinrich PC. Soluble receptors for cytokines and growth factors: generation and biological function. Biochem J 1994; 300(Pt 2): 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich PC, Behrmann I, Muller-Newen G, et al. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J 1998; 334(Pt 2): 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol 2014; 26: 2–12. [DOI] [PubMed] [Google Scholar]
- 13.Narazaki M, Yasukawa K, Saito T, et al. Soluble forms of the interleukin-6 signal-transducing receptor component gp130 in human serum possessing a potential to inhibit signals through membrane-anchored gp130. Blood 1993; 82(4): 1120–1126. [PubMed] [Google Scholar]
- 14.Diamant M, Hansen MB, Rieneck K, et al. Differential interleukin-6 (IL-6) responses of three established myeloma cell lines in the presence of soluble human IL-6 receptors. Leuk Res 1996; 20(4): 291–301. [DOI] [PubMed] [Google Scholar]
- 15.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 16.Maston LD, Jones DT, Giermakowska W, et al. Central role of T helper 17 cells in chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2017; 312(5): L609–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 2009; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraakman MJ, Kammoun HL, Allen TL, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab 2015; 21(3): 403–416. [DOI] [PubMed] [Google Scholar]
- 20.Goumas FA, Holmer R, Egberts JH, et al. Inhibition of IL-6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int J Cancer 2015; 137(5): 1035–1046. [DOI] [PubMed] [Google Scholar]
- 21.Garbers C, Aparicio-Siegmund S, Rose-John S. The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Curr Opin Immunol 2015; 34: 75–82. [DOI] [PubMed] [Google Scholar]
- 22.Jostock T, Müllberg J, Özbek S, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem 2001; 268(1): 160–167. [DOI] [PubMed] [Google Scholar]
- 23.Ramiro-Diaz JM, Nitta CH, Maston LD, et al. NFAT is required for spontaneous pulmonary hypertension in superoxide dismutase 1 knockout mice. Am J Physiol Lung Cell Mol Physiol 2013; 304(9): L613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jernigan NL, Paffett ML, Walker BR, et al. ASIC1 contributes to pulmonary vascular smooth muscle store-operated Ca(2+) entry. Am J Physiol Lung Cell Mol Physiol 2009; 297(2): L271–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nitta CH, Osmond DA, Herbert LM, et al. Role of ASIC1 in the development of chronic hypoxia-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol 2014; 306(1): H41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008; 3(6): 1101–1108. [DOI] [PubMed] [Google Scholar]
- 27.Waage A, Brandtzaeg P, Halstensen A, et al. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med 1989; 169(1): 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J Mol Med (Berl) 2013; 91(3): 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierer R, Nitta CH, Friedman J, et al. NFATc3 is required for chronic hypoxia-induced pulmonary hypertension in adult and neonatal mice. Am J Physiol Lung Cell Mol Physiol 2011; 301(6): L872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spörri B, Müller KM, Wiesmann U, et al. Soluble IL-6 receptor induces calcium flux and selectively modulates chemokine expression in human dermal fibroblasts. Int Immunol 1999; 11(7): 1053–1058. [DOI] [PubMed] [Google Scholar]
- 31.Eddahibi S, Chaouat A, Tu L, et al. Interleukin-6 gene polymorphism confers susceptibility to pulmonary hypertension in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3(6): 475–476. [DOI] [PubMed] [Google Scholar]
- 32.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest 2011; 121: 3375–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto-Kataoka T, Hosen N, Sonobe T, et al. Interleukin-6/interleukin-21 signaling axis is critical in the pathogenesis of pulmonary arterial hypertension. Proc Natl Acad Sci U S A 2015; 112: E2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartelds B, Van Loon RLE, Mohaupt S, et al. Mast cell inhibition improves pulmonary vascular remodeling in pulmonary hypertension. Chest 2012; 141(3): 651–660. [DOI] [PubMed] [Google Scholar]
- 35.Garbers C, Thaiss W, Jones GW, et al. Inhibition of classic signaling is a novel function of soluble glycoprotein 130 (sgp130), which is controlled by the ratio of Interleukin 6 and soluble Interleukin 6 receptor. J Biol Chem 2011; 286: 42959–42970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stenmark KR, McMurtry IF. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension: a time for reappraisal? Circ Res 2005; 97: 95–98. [DOI] [PubMed] [Google Scholar]
- 37.Stenmark KR, Meyrick B, Galie N, et al. Animal models of pulmonary arterial hypertension: the hope for etiological discovery and pharmacological cure. Am J Physiol Lung Cell Mol Physiol 2009; 297(6): L1013–1032. [DOI] [PubMed] [Google Scholar]
- 38.Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest 2000; 106(6): 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z, Newman WH. Smooth muscle cell migration stimulated by interleukin 6 is associated with cytoskeletal reorganization. J Surg Res 2003; 111(2): 261–266. [DOI] [PubMed] [Google Scholar]
- 40.Klouche M, Bhakdi S, Hemmes M, et al. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J Immunol 1999; 163: 4583–4589. [PubMed] [Google Scholar]
- 41.Shimoda LA, Sham JS, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 2000; 49: 549–560. [PubMed] [Google Scholar]
- 42.Ramiro-Diaz JM, Nitta CH, Maston LD, et al. NFAT is required for spontaneous pulmonary hypertension in superoxide dismutase 1 knockout mice. Am J Physiol Lung Cell Mol Physiol 2013; 304(9): L613–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McLoughlin RM, Jenkins BJ, Grail D, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci U S A 2005; 102: 9589–9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol 2010; 40: 1830–1835. [DOI] [PubMed] [Google Scholar]
- 45.Jones GW, McLoughlin RM, Hammond VJ, et al. Loss of CD4+ T cell IL-6R expression during inflammation underlines a role for IL-6 trans signaling in the local maintenance of Th17 cells. J Immunol 2010; 184: 2130–2139. [DOI] [PubMed] [Google Scholar]