Abstract
Background:
In the intensity-modulated radiotherapy (IMRT) era, the survival benefit of concurrent chemotherapy for locoregionally advanced nasopharyngeal carcinoma (LA-NPC) remains undetermined. This study aimed to evaluate the benefits of IMRT with concurrent chemotherapy compared with IMRT alone for LA-NPC patients with different plasma Epstein–Barr virus (EBV) DNA levels.
Methods:
Patients were identified from a prospectively maintained database in an endemic area between November 2002 and December 2013. Cox proportional hazards models, propensity score matching, and inverse probability weighting models were established for survival analysis. Stratification analysis was performed based on interaction effects analysis. Finally, sensitivity analysis was performed considering unmeasured confounders.
Results:
A total of 1357 eligible patients were enrolled (median follow up 62.4 months; range 3.5–155.8 months). No significant survival differences were observed between groups in the entire cohort. Notably, a significant interaction effect was observed between treatment regimens and EBV DNA levels. In patients with high EBV DNA levels (>4000 copies/ml), all three models showed that IMRT with concurrent chemotherapy significantly improved overall survival [hazard ratio (HR) 2.521, 95% confidence interval (CI) 1.218–5.216], disease-free survival (HR 2.168, 95% CI 1.349–3.483), and distant metastasis-free survival (HR 2.331, 95% CI 1.194–4.551) compared with IMRT alone. No differences were found in patients with low EBV DNA levels. Sensitivity analysis confirmed the robustness of the results.
Conclusion:
In the IMRT era, concurrent chemotherapy treatment of LA-NPC patients with high EBV DNA levels is reasonable. However, the optimal regimen for LA-NPC patients with low EBV DNA levels needs further validation in randomized clinical trials.
Keywords: concurrent chemotherapy, EBV DNA, intensity-modulated radiotherapy, interaction effect, nasopharyngeal carcinoma, propensity score analysis, stratification analysis, survival
Introduction
Nasopharyngeal carcinoma (NPC) is a distinct type of head and neck cancer originating from the epithelium of the nasopharynx with high metastatic and invasive potential. NPC is endemic in certain areas of southern China and Southeast Asia, with an incidence of 20–50/100,000.1 Radiotherapy (RT) with concurrent chemotherapy (CCRT) is recommended as the standard treatment for locoregionally advanced NPC (LA-NPC), which accounts for approximately 80% of NPC cases at the initial diagnosis.2 In the conventional two-dimensional RT (2DRT) era, CCRT was confirmed to increase the tumour control rate, reduce metastasis and ultimately improve the long-term survival of LA-NPC patients.3,4
However, CCRT is associated with enhanced severe acute and late toxicities compared with radiotherapy alone. For example, CCRT increased acute toxicities and impaired treatment compliance.5,6 With a relatively high cure rate of nonmetastatic NPC and a long survival time, CCRT patients present more generalized and severe late toxicities. Severe late toxicities can reduce the quality of life or even be life threatening. A meta-analysis based on randomized controlled trials indicated that CCRT was associated with a higher rate of late toxicities than RT alone.7
Over the last two decades, intensity-modulated RT (IMRT) has been widely utilized for the treatment of NPC. Compared with 2DRT, IMRT can significantly improve overall survival (OS) and reduce metastasis and the incidence of toxicities in organs at risk (OARs),8 especially for tumours located in a complex anatomic site, such as NPC. Previous studies have reported that local and regional control rates have exceeded 95% for NPC patients in the IMRT era.9,10 Recently, several studies have reported that combining concurrent chemotherapy with IMRT does not improve patient survival compared with IMRT alone,8,11,12 although one study reported a positive conclusion.13 Thus, a reasonable question is whether concurrent chemotherapy remains necessary and beneficial for the treatment of NPC in the IMRT era despite chemotherapy-induced toxicities in a real-world setting.
Quantification of pretreatment plasma Epstein–Barr virus (EBV) DNA levels has been shown to be a useful biomarker for risk stratification, monitoring and prediction of the survival of NPC patients.1 Based on previous studies, the plasma EBV DNA level has become a key complement for traditional TNM staging.14–17 This study aimed to investigate the benefit of CCRT for NPC patients with different plasma EBV DNA levels in the IMRT era.
Materials and methods
Study patients
This study was reviewed and approved by the Institutional Review Board and Ethics Committee of Sun Yat-sen University Cancer Center (SYSUCC), Guangzhou, China (approval number: GZR2016-210). Written informed consent for the use of clinical data and collected samples for future studies (including retrospective studies) was obtained when the patients were admitted to receive treatment as a general standard procedure for patients treated in our centre. All patient records were anonymous and de-identified before the analysis.
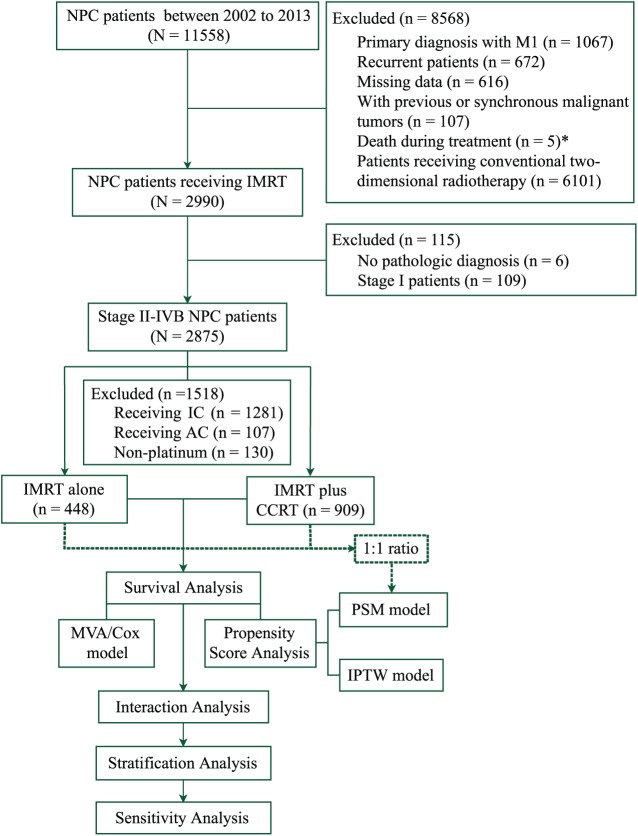
Using a prospectively created database, this study collected 11,558 consecutive patients with NPC at the SYSUCC between November 2002 and December 2013. The disease was restaged according to the International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) TNM classification (seventh edition, 2011) based on clinical and radiographic data. The inclusion criteria included the following: (a) histologically confirmed NPC; (b) disease classified as stages II–IVB; and (c) the radiation delivery technique was IMRT. The main exclusion criteria were as follows: (a) a previous malignancy or concomitant malignant disease; (b) the use of adjuvant or induction chemotherapy or additional concurrent systemic therapy; (c) the radiation delivery technique was 2DRT; and (d) missing or incomplete clinical data. A total of 2875 newly diagnosed NPC patients with stage II–IVB disease were identified. Figure 1 shows detailed information for the study patients. Ultimately, 1357 patients were enrolled in this study for the analysis.
Figure 1.
STROBE flow diagram. *Among the five patients who died during the treatment, three patients received two-dimensional conventional radiotherapy, one received induction chemotherapy, and one died from massive haemorrhage of the gastrointestinal tract after the 6th fraction of radiotherapy and one cycle of concurrent chemotherapy.
AC, adjuvant chemotherapy; CCRT, concurrent chemotherapy; IC, induction chemotherapy; IMRT, intensity-modulated radiotherapy; IPTW, inverse probability of treatment weighting; MVA/Cox model, multivariate analysis with a Cox proportional hazards model; NPC, nasopharyngeal carcinoma; PSM, propensity score matching.
Real-time quantitative measurement of plasma EBV DNA
Pretreatment plasma EBV DNA was extracted and subjected to real-time quantitative polymerase chain reaction as described previously with the same measurement for all the measured patients.18,19 The pretreatment plasma EBV DNA levels were routinely assessed for 940 of the 1357 patients prior to treatment. Overall, two groups containing low or high levels (defined by a cut-off level of 4000 copies per ml) were analysed in this study. This cut-off level was chosen because it was previously confirmed to be a prognostic threshold in NPC studies using the same measurement system in the same endemic area.14,17,20 Meanwhile, four groups defined by magnitudes of 10 were used to objectively create the survival analysis curves provided in the Supplementary Material [Supplementary Figure 1(a–d)].
Treatments and follow up
The pretreatment evaluation is presented in the Supplementary Material. All NPC patients were treated with IMRT according to the principle of treatment for NPC at SYSUCC. The patients were examined every 3 months during the first 3 years and then every 6 months thereafter or until death after treatment. Detailed information for the treatment and follow up is presented in the Supplementary Materials.
Clinical outcome definitions
The primary endpoint was OS, which was calculated from the date of histological diagnosis to the date of death from any cause. The secondary endpoints were disease-free survival (DFS), which was calculated from the date of diagnosis to the date of death or relapse due to the tumour, and distant metastasis-free survival (DMFS) or locoregional relapse-free survival (LRFS), which was calculated from the date of diagnosis to the date of distant metastasis or locoregional failure, respectively.
Statistical analysis
The Chi-square test was used to assess differences between two groups. Survival rates were estimated using the Kaplan–Meier method with the log-rank test. We used the following four complementary approaches to adjust our comparison of survival rates among patients receiving two regimens based on differences in baseline characteristics: a standard Cox proportional hazards model; the propensity score matching (PSM) model; the inverse probability of treatment weighting (IPTW) model; and a sensitivity analysis. All statistical tests were two sided, and p < 0.05 was considered significant. All statistical analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria; version 3.2.2).
In the multivariable analysis (MVA) with standard Cox proportional hazards models using the forward likelihood ratio method, we included all observed variables with clinical significance or a significant association with survival.21 Then, we performed an interaction effect analysis between the treatment regimen and other variables. The stratification analysis was further performed based on the significant interaction effects.
Propensity score analysis
The propensity score analysis (PSA) adjusts for the bias induced by nonrandom treatment assignment by comparing patients who have a similar likelihood of receiving one treatment but who receive another treatment. We estimated the effect of treatment on survival using the following two PSA approaches: PSM and IPTW. Prior work has verified that both approaches allow for estimation of marginal hazard ratios (HRs) with minimal bias.22
For the PSM analysis, we matched each patient who received IMRT alone with one patient who received IMRT with concurrent chemotherapy using the logit of the propensity score, using callipers of a width equal to 0.2 of standard deviations of the logit of the estimated propensity score.23 To compare the groups, a marginal Cox model was applied using maximum partial likelihood estimates of regression parameters and a robust sandwich covariance matrix.24,25 The main limitation of PSM is that it limits the analysis to the matched cohort and thus reduces the statistical power. The IPTW model does not carry this limitation and instead uses the inverse probability of the treatment weights. For the IPTW model analysis, each observation was weighted using the inverse probability of receiving the treatment based on the propensity score. Then, a Cox proportional hazards model was fitted with the chemotherapy regimen as the only predictor variable. A robust sandwich variance estimator was used to account for the weighted nature of the sample.26
Sensitivity analysis
Although propensity scores can address biases caused by observed variables, unknown or unmeasured variables (i.e. performance status and economic status), may also be associated with the receipt of chemotherapy and the prognosis. We performed a sensitivity analysis for the unmeasured variables in the aforementioned analysis to measure their potential confounding effects on our results and to validate the robustness of our analysis.27 We varied the prevalence of a poor performance status and the adjusted mortality HRs in the two different regimens using estimates from prior studies of patients.28 Based on these studies, we assumed that a performance status of 2 or greater would be associated with an HR of 1.2–2. Using these data, we calculated adjusted HRs and 95% confidence intervals (CIs) for the IMRT-alone regimen.
Results
Patient characteristics and follow up
The characteristics of the 1357 NPC patients, including 909 patients (67%) who received IMRT with concurrent chemotherapy and 448 patients (33%) who received IMRT alone, are presented in Table 1. The percentages of patients with clinical stage II, III, and IVA–B tumours were 25.9%, 54.7%, and 19.4%, respectively. No significant differences were found in the sex, body mass index (BMI), education, smoking, drinking, and comorbidity distributions (all p > 0.05), but differences were observed in the ages, EBV DNA levels, tumour and node stages, clinical stages, and year of diagnosis. Older patients (p < 0.001) and patients with earlier-stage tumours (p < 0.001) were more likely to receive IMRT alone than CCRT.
Table 1.
Baseline patient characteristics according to treatment in the entire cohort and in the propensity score-matched cohort.
| Characteristics | The entire cohort |
p-value | Propensity score-matched cohort |
p-value | ||
|---|---|---|---|---|---|---|
| IMRT + CCRT n = 909 (%) |
IMRT alone n = 448 (%) |
IMRT + CCRT n = 326 (%) |
IMRT alone n = 326 (%) |
|||
| Age | <0.001 | 0.932 | ||||
| <40 | 283 (31.1) | 125 (27.9) | 96 (29.4) | 92 (28.2) | ||
| 40–49 | 315 (34.7) | 134 (29.9) | 102 (31.3) | 109 (33.4) | ||
| 50–59 | 208 (22.9) | 94 (21.0) | 72 (22.1) | 68 (20.9) | ||
| ⩾60 | 103 (11.3) | 95 (21.2) | 56 (17.2) | 57 (17.5) | ||
| Sex | 0.090 | 0.788 | ||||
| Male | 661 (72.7) | 345 (77.0) | 240 (73.6) | 244 (74.8) | ||
| Female | 248 (27.3) | 103 (23.0) | 86 (26.4) | 82 (25.2) | ||
| Body mass index, kg/m2 | 0.465 | 0.997 | ||||
| ⩽18.49 | 63 (6.9) | 18 (4.0) | 16 (4.9) | 15 (4.6) | ||
| 18.50–22.99 | 371 (40.8) | 188 (42.0) | 138 (42.3) | 137 (42.0) | ||
| 23.00–27.50 | 391 (43.0) | 206 (46.0) | 143 (43.9) | 145 (44.5) | ||
| >27.50 | 84 (9.3) | 36 (8.0) | 29 (8.9) | 29 (8.9) | ||
| Education | 0.281 | 0.876 | ||||
| Unschooled | 54 (5.9) | 27 (6.0) | 23 (7.1) | 22 (6.7) | ||
| Low | 283 (31.2) | 121 (27.0) | 100 (30.7) | 91 (27.9) | ||
| Middle | 308 (33.9) | 164 (36.6) | 111 (34.0) | 117 (35.9) | ||
| High | 264 (29.0) | 136 (30.4) | 92 (28.2) | 96 (29.4) | ||
| Smoking status | 0.438 | 0.870 | ||||
| Yes | 331 (36.4) | 173 (38.6) | 116 (35.6) | 119 (36.5) | ||
| No | 578 (63.6) | 275 (61.4) | 210 (64.4) | 207 (63.5) | ||
| Drinking status | 0.249 | 1.000 | ||||
| Yes | 121 (13.3) | 70 (15.6) | 46 (14.1) | 47 (14.4) | ||
| No | 788 (86.7) | 378 (84.4) | 280 (85.9) | 279 (85.6) | ||
| Charlson/Deyo comorbidity score | 0.214 | 0.909 | ||||
| 0 | 696 (76.6) | 331 (73.9) | 247 (75.8) | 245 (75.2) | ||
| 1 | 189 (20.8) | 96 (21.4) | 69 (21.2) | 69 (21.2) | ||
| ⩾2 | 24 (2.6) | 21 (4.7) | 10 (3.1) | 12 (3.7) | ||
| EBV DNA, copies/millilitre | ||||||
| By magnitude of 10 | <0.001 | 0.287 | ||||
| 0–999 | 353 (38.8) | 201 (44.9) | 155 (47.5) | 144 (44.2) | ||
| 1000–9999 | 130 (14.3) | 35 (7.8) | 38 (11.7) | 25 (7.7) | ||
| 10,000–99,999 | 133 (14.6) | 25 (5.6) | 37 (3.4) | 21 (6.4) | ||
| ⩾100,000 | 51 (5.6) | 12 (2.7) | 11 (3.4) | 10 (3.1) | ||
| By cut-off of 4000 | < 0.001 | 0.261 | ||||
| 0–4000 | 427 (47.0) | 221 (49.3) | 179 (54.9) | 158 (48.5) | ||
| >4000 | 240 (26.4) | 52 (11.6) | 62 (19.0) | 42 (12.9) | ||
| Unmeasured | 242 (26.6) | 175 (39.1) | 85 (26.1) | 126 (38.6) | ||
| Histology, WHO type | 0.878 | 0.292 | ||||
| I | 2 (0.2) | 1 (0.2) | 0 (0) | 1 (0.5) | ||
| II | 41 (4.5) | 23 (5.1) | 17 (7.1) | 9 (4.5) | ||
| III | 866 (95.3) | 424 (94.7) | 224 (92.9) | 190 (95.0) | ||
| Tumour stage | <0.001 | 0.567 | ||||
| 1 | 52 (5.7) | 52 (11.6) | 29 (8.9) | 38 (11.7) | ||
| 2 | 176 (19.4) | 215 (48.0) | 113 (34.7) | 116 (35.6) | ||
| 3 | 494 (54.3) | 148 (33.0) | 145 (44.5) | 140 (42.9) | ||
| 4 | 187 (20.6) | 33 (7.4) | 39 (12.0) | 32 (9.8) | ||
| Node stage | <0.001 | 0.935 | ||||
| 0 | 147 (16.2) | 179 (40.0) | 84 (25.8) | 89 (27.3) | ||
| 1 | 413 (45.4) | 202 (45.1) | 170 (52.1) | 171 (52.5) | ||
| 2 | 307 (33.8) | 62 (13.8) | 68 (20.9) | 62 (19.0) | ||
| 3 | 42 (4.6) | 5 (1.1) | 4 (1.2) | 4 (1.2) | ||
| Clinical stage | <0.001 | 0.434 | ||||
| II | 113 (12.4) | 238 (53.1) | 110 (33.7) | 125 (38.3) | ||
| III | 569 (62.6) | 174 (38.9) | 175 (53.7) | 166 (50.9) | ||
| IVA–B | 227 (25.0) | 36 (8.0) | 41 (12.6) | 35 (10.7) | ||
| Year of diagnosis | 0.047 | 0.859 | ||||
| 2002a–2006 | 168 (18.5) | 137 (30.6) | 81 (24.8) | 84 (25.8) | ||
| 2007–2009 | 361 (39.7) | 119 (26.6) | 89 (27.3) | 93 (28.5) | ||
| 2010–2013 | 380 (41.8) | 192 (42.8) | 156 (47.9) | 149 (45.7) | ||
The first patient who received IMRT in this study was occurred in the year of 2002.
CCRT, concurrent chemotherapy; EBV, Epstein–Barr virus; IMRT, intensity-modulated radiotherapy; WHO, World Health Organization.
With a median follow-up time of 62.4 months (range 3.5–155.8 months), 145 (10.7%) of the 1357 patients developed a distant metastasis, 100 (7.4%) patients developed local regional recurrence, and 159 (11.7%) patients died. The 5-year OS, DFS, DMFS and LRFS rates in the entire cohort were 89.9%, 80.0%, 89.7%, and 92.7%, respectively.
Prognostic value of the plasma EBV DNA level
The plasma EBV DNA level was routinely assessed for 940 of the 1357 patients prior to treatment. Weak positive correlations were found between the EBV DNA levels and primary tumours (Supplementary Table 1). Patients with high EBV DNA levels tended to receive CCRT rather than IMRT alone (p < 0.001; Table 1). Kaplan–Meier estimates showed that the differences in survival rates between these EBV DNA levels were significant. Patients with low and high EBV DNA levels had 5-year OS, DFS, DMFS and LRFS rates of 94.4% versus 4.3% (p < 0.001), 85.5% versus 67.1% (p < 0.001), 93.6% versus 82.2% (p < 0.001), and 94.1% versus 89.0% (p = 0.004), respectively [Supplementary Figure 1(e–h)].
Survival analysis based on the treatment regimens
In the unadjusted analysis, the following factors were all significant prognostic factors for poor OS: an older age, male sex, lower BMI, lower education, smoking, Charlson comorbidity score of 2 or greater, higher EBV DNA levels, higher tumour or node stage, and advanced clinical stage. The 5-year OS, DFS, DMFS, and LRFS rates for patients receiving IMRT alone compared with patients treated with CCRT were 91.8% versus 88.7% (HR 0.790, 95% CI 0.562–1.111, p = 0.175), 84.7% versus 77.8% (HR 0.814, 95% CI 0.630–1.051, p = 0.114), 92.6% versus 88.3% (HR 0.702, 95% CI 0.486–1.014, p = 0.059), and 94.7% versus 92.3% (HR 0.795, 95% CI 0.515–1.226, p = 0.300), respectively.
The results of the multivariate analysis using the standard Cox proportional hazards model are shown in Table 2. After adjusting for all observational factors, the survival rates were not significantly different between the CCRT and IMRT-alone groups in terms of OS (HR 1.371, 95% CI 0.946–1.988, p = 0.095), DFS (HR 1.108, 95% CI 0.832–1.475, p = 0.483), DMFS (HR 1.225, 95% CI 0.827–1.816, p = 0.312), and LRFS (HR 1.030, 95% CI 0.651–1.630, p = 0.898).
Table 2.
Multivariable analysis of prognostic factors for survival.
| Characteristics | OS |
DFS |
DMFS |
LRFS |
||||
|---|---|---|---|---|---|---|---|---|
| HR(95%CI) | p | HR(95%CI) | p | HR(95%CI) | p | HR(95%CI) | p | |
| IMRT alone versus IMRT + CCRT (ref.) | ||||||||
| PSA/PSM model | 1.338 (0.821–2.179) | 0.242 | 1.255 (0.877–1.796) | 0.214 | 1.091 (0.670–1.778) | 0.726 | 1.215 (0.687–2.149) | 0.503 |
| PSA/IPTW model | 1.254 (0.816–1.925) | 0.302 | 1.207 (0.866–1.684) | 0.267 | 1.276 (0.792–2.053) | 0.316 | 1.077 (0.615–1.885) | 0.795 |
| MVA/Cox model | 1.371 (0.946–1.988) | 0.095 | 1.108 (0.832–1.475) | 0.483 | 1.225 (0.827–1.816) | 0.312 | 1.030 (0.651–1.630) | 0.898 |
| Age | <0.001 | <0.001 | 0.780 | 0.116 | ||||
| <40 | Reference | Reference | Reference | Reference | ||||
| 40–49 | 1.433 (0.898–2.286) | 0.131 | 1.169 (0.840–1.627) | 0.356 | 0.982 (0.637–1.514) | 0.936 | 1.332 (0.777–2.281) | 0.297 |
| 50–59 | 1.406 (0.836–2.365) | 0.200 | 1.512 (1.067–2.143) | 0.020 | 0.995 (0.616–1.608) | 0.983 | 1.758 (0.997–3.100) | 0.051 |
| ⩾60 | 3.605 (2.275–5.713) | <0.001 | 1.850 (1.281–2.672) | 0.001 | 1.080 (0.642–1.818) | 0.771 | 1.453 (0.739–2.853) | 0.279 |
| Sex | ||||||||
| Male | Reference | Reference | Reference | Reference | ||||
| Female | 0.603 (0.382–0.953) | 0.030 | 0.553 (0.393–0.778) | <0.001 | 0.444 (0.269–0.731) | 0.001 | 0.935 (0.563–1.550) | 0.793 |
| Body mass index | <0.001 | <0.001 | 0.028 | 0.049 | ||||
| ⩽18.49 | Reference | Reference | Reference | Reference | ||||
| 18.50–22.99 | 0.675 (0.398–1.144) | 0.144 | 0.755 (0.481–1.186) | 0.223 | 1.288 (0.637–2.063) | 0.482 | 0.604 (0.300–1.219) | 0.159 |
| 23.00–27.50 | 0.452 (0.263–0.776) | 0.004 | 0.576 (0.364–0.911) | 0.018 | 0.943 (0.461–1.927) | 0.871 | 0.459 (0.224–0.942) | 0.033 |
| >27.50 | 0.321 (0.132–0.779) | 0.012 | 0.419 (0.220–0.800) | 0.008 | 0.484 (0.170–1.377) | 0.174 | 0.450 (0.174–1.164) | 0.099 |
| Education | 0.026 | 0.154 | 0.021 | 0.607 | ||||
| Unschooled | Reference | Reference | Reference | Reference | ||||
| Low | 1.056 (0.551–2.023) | 0.871 | 0.807 (0.505–1.290) | 0.371 | 0.777 (0.424–1.424) | 0.414 | 0.565 (0.259–1.235) | 0.153 |
| Middle | 0.810 (0.419–1.565) | 0.530 | 0.758 (0.478–1.202) | 0.238 | 0.580 (0.315–1.068) | 0.080 | 0.803 (0.384–1.681) | 0.561 |
| High | 0.637 (0.316–1.286) | 0.209 | 0.694 (0.426–1.130) | 0.142 | 0.525 (0.273–1.011) | 0.054 | 0.802 (0.372–1.731) | 0.574 |
| Smoking status | ||||||||
| Yes | 1.012 (0.694–1.476) | 0.951 | 0.936 (0.703–1.246) | 0.650 | 0.998 (0.674–1.476) | 0.990 | 0.854 (0.520–1.405) | 0.534 |
| No | Reference | Reference | Reference | Reference | ||||
| Drinking status | ||||||||
| Yes | 0.738 (0.455–1.196) | 0.217 | 0.919 (0.642–1.315) | 0.643 | 1.053 (0.658–1.686) | 0.829 | 0.900 (0.467–1.736) | 0.753 |
| No | Reference | Reference | Reference | Reference | ||||
| Charlson/Deyo comorbidity score | <0.001 | 0.034 | 0.685 | 0.281 | ||||
| 0 | Reference | Reference | Reference | Reference | ||||
| 1 | 1.294 (0.898–1.863) | 0.167 | 1.082 (0.810–1.445) | 0.593 | 0.904 (0.595–1.373) | 0.636 | 0.867 (0.516–1.458) | 0.591 |
| ⩾2 | 3.669 (1.980–6.796) | <0.001 | 2.284 (1.334–3.911) | 0.003 | 1.806 (0.781–4.177) | 0.167 | 2.692 (1.204–6.019) | 0.016 |
| EBV DNAa, copies/millilitre | 0.009 | <0.001 | 0.010 | 0.052 | ||||
| 0–999 | Reference | Reference | Reference | Reference | ||||
| 1000–9999 | 1.771 (0.968–3.241) | 0.063 | 1.636 (1.086–2.464) | 0.018 | 1.630 (0.921–2.886) | 0.094 | 1.741 (0.915–3.313) | 0.091 |
| 10,000–99,999 | 1.824 (1.038–3.207) | 0.036 | 1.644 (1.101–2.459) | 0.015 | 1.952 (1.146–3.326) | 0.013 | 1.143 (0.803–1.627) | 0.458 |
| ⩾100,000 | 2.296 (1.172–4.497) | 0.015 | 3.150 (2.006–4.947) | <0.001 | 2.666 (1.417–5.019) | 0.002 | 1.494 (1.182–1.888) | 0.001 |
| Unmeasured | 1.965 (1.167–3.310) | 0.011 | 1.835 (1.268–2.654) | 0.001 | 1.730 (1.108–2.703) | 0.016 | 1.032 (0.910–1.171) | 0.624 |
| High level versus low levelb | 2.004 (1.252–3.183) | 0.003 | 2.200 (1.598–3.030) | <0.001 | 2.031 (1.305–3.163) | 0.002 | 1.887 (1.119–3.182) | 0.017 |
| Histology, WHO type | ||||||||
| I–II | Reference | Reference | Reference | Reference | ||||
| III | 0.778 (0.308–1.966) | 0.596 | 0.863 (0.604–1.233) | 0.417 | 1.070 (0.771–1.487) | 0.685 | 0.785 (0.491–1.254) | 0.311 |
| Tumour stage | 0.606 | 0.128 | 0.145 | 0.005 | ||||
| 1 | Reference | Reference | Reference | Reference | ||||
| 2 | 1.056 (0.459–2.429) | 0.899 | 1.547 (0.782–3.062) | 0.210 | 1.224 (0.529–2.834) | 0.637 | 1.627 (0.475–5.579) | 0.439 |
| 3 | 1.224 (0.542–2.762) | 0.627 | 1.808 (0.917–3.566) | 0.088 | 1.630 (0.717–3.708) | 0.244 | 1.867 (1.037–3.361) | 0.037 |
| 4 | 1.157 (0.389–3.439) | 0.793 | 1.831 (0.745–4.502) | 0.187 | 2.039 (0.667–6.231) | 0.211 | 1.422 (0.921–2.195) | 0.112 |
| Node stage | <0.001 | <0.001 | <0.001 | 0.712 | ||||
| 0 | Reference | Reference | Reference | Reference | ||||
| 1 | 1.700 (1.062–2.723) | 0.006 | 1.511 (1.029–2.356) | 0.035 | 1.938 (1.111–3.382) | 0.020 | 1.545 (0.923–2.587) | 0.098 |
| 2 | 2.131 (1.315–3.452) | <0.001 | 1.658 (1.166–2.356) | 0.004 | 2.632 (1.481–4.678) | <0.001 | 0.839 (0.425–1.656) | 0.612 |
| 3 | 4.253 (2.157–8.386) | <0.001 | 2.990 (1.659–5.388) | <0.001 | 5.733 (2.611–12.59) | <0.001 | 1.367 (0.328–5.704) | 0.668 |
| Clinical stage | <0.001 | <0.001 | <0.001 | 0.011 | ||||
| II | Reference | Reference | Reference | Reference | ||||
| III | 1.647 (0.979–2.773) | 0.060 | 1.489 (1.013–2.187) | 0.043 | 1.831 (1.057–3.173) | 0.031 | 1.623 (0.647–4.070) | 0.302 |
| IVA–B | 3.690 (2.136–6.375) | <0.001 | 2.573 (1.686–3.929) | <0.001 | 3.364 (1.860–6.086) | <0.001 | 1.611 (1.072–2.421) | 0.022 |
| Year of diagnosis | 0.427 | 0.106 | 0.309 | 0.822 | ||||
| 2002–2006 | Reference | Reference | Reference | Reference | ||||
| 2007–2009 | 0.874 (0.564–1.354) | 0.547 | 0.713 (0.505–1.008) | 0.056 | 1.095 (0.628–1.909) | 0.750 | 0.935 (0.570–1.536) | 0.792 |
| 2010–2013 | 0.794 (0.467–1.351) | 0.396 | 0.778 (0.584–1.037) | 0.087 | 1.308 (0.722–2.369) | 0.376 | 0.909 (0.508–1.625) | 0.747 |
Comparison was given in four groups for more readable results. bThe cut-off point between the high and low levels of EBV DNA is 4000 copies/ml.
CCRT, concurrent chemotherapy; CI, confidence interval; DFS, disease-free survival; DMFS, distant metastasis-free survival; EBV, Epstein–Barr virus; HR, hazard ratio; IMRT, intensity-modulated radiotherapy; LRFS, locoregional relapse-free survival; MVA/Cox model, multivariate analysis with Cox proportional hazards model; OS, overall survival; PSA/IPTW, propensity score analysis by inverse probability of treatment weighting; PSA/PSM, propensity score analysis with matching method; WHO, world health organization.
PSA
Propensity score matching and weighted models with observational confounders were established to confirm and recalculate the effects of the two regimens using various algorithms to reduce confounding. For the matched analysis, 652 patients were identified. This analysis eliminated differences in all of the observed baseline characteristics in the larger cohort (Table 1) and revealed similar survival benefits for the two regimens. The PSA/IPTW model results were consistent with the multivariate analysis results obtained using the Cox proportional hazards model (Table 2).
Stratification analysis based on interaction effects
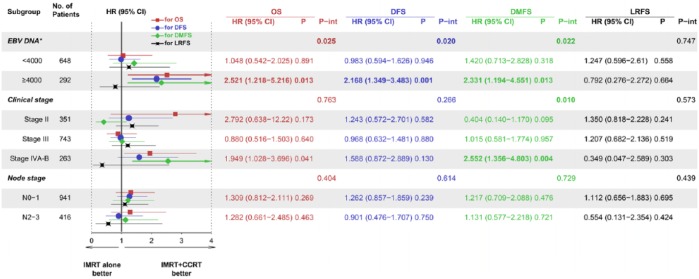
The interaction effects were examined for the EBV DNA level (categorized by a cut-off of 4000 copies per ml), N stage (categorized as N0–1 or N2–3), and clinical stage using standard Cox regression analysis of the treatment groups (Figure 2). A significant interaction was found for the EBV DNA level (p = 0.025) but not for the N stage or clinical stage (p = 0.404 and p = 0.763, respectively). The stratification analysis demonstrated the following differences between the treatment regimens: the mortality risk was more pronounced in the patients with higher EBV DNA copy numbers who received IMRT alone (Figure 3).
Figure 2.
Forest plots for the interaction and stratification analyses based on the EBV DNA levels, clinical stages, and node stages for OS, DFS, DMFS, and LRFS.
*The EBV DNA subgroup analysis was based on the 940 patients whose EBV DNA levels were measured. The HRs and 95% CIs were calculated based on the multivariable Cox analysis.
CCRT, concurrent chemotherapy; CI, confidence interval; DFS, disease-free survival; DMFS, distant metastasis-free survival; EBV, Epstein–Barr virus; HR, hazard ratio; IMRT, intensity-modulated radiotherapy; LRFS, locoregional relapse-free survival; OS, overall survival.
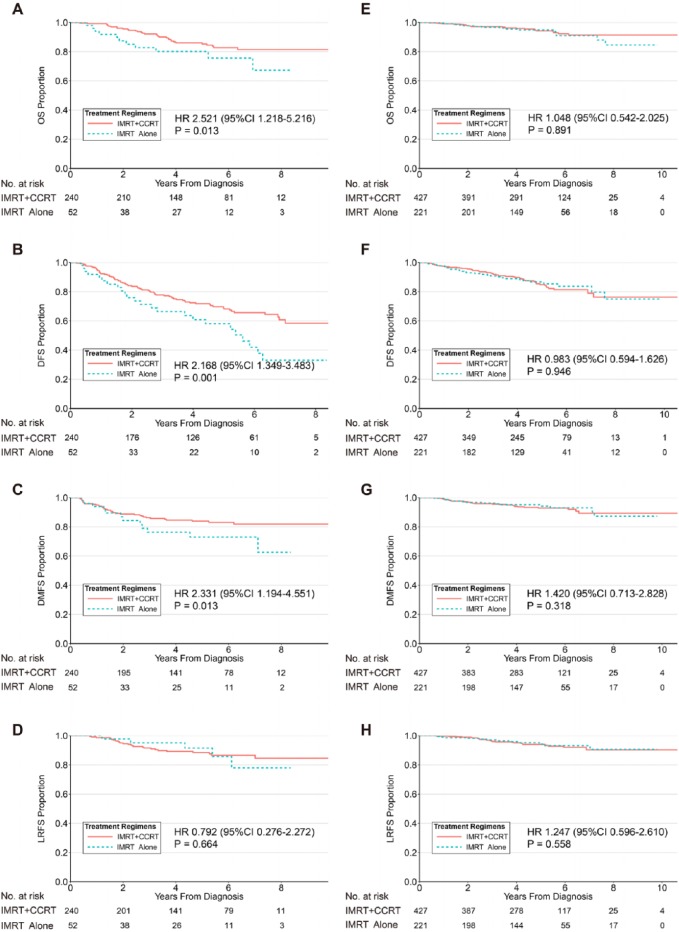
Figure 3.
Kaplan–Meier survival curves for OS, DFS, DMFS, and LRFS for treatment regimens in NPC patients with high EBV DNA levels (A, B, C, and D, respectively) and low EBV DNA levels (E, F, G, and H, respectively). A cut-off of 4000 copies per millilitre was selected.
CCRT, concurrent chemotherapy; CI, confidence interval; DFS, disease-free survival; DMFS, distant metastasis-free survival; EBV, Epstein–Barr virus; HR, hazard ratio; IMRT, intensity-modulated radiotherapy; LRFS, locoregional relapse-free survival; OS, overall survival.
Based on the strong significant interaction effect for DMFS between the treatment regimens and the clinical stages (p = 0.010; Figure 2), a stratification analysis of the treatment regimens was utilized to analyse the clinical stage (categorized as clinical stage II–III or IVA–B) with the EBV DNA level (Table 3). Compared with the CCRT group, significantly worse DMFS was found for the IMRT-alone group in stage IVA–B patients (HR 2.552, 95% CI 1.356–4.803, p = 0.004). A further stratification analysis found that stage IVA–B patients with high EBV DNA levels contributed to the difference in DMFS between the two regimens (HR 4.134, 95% CI 1.586–10.778, p = 0.004), whereas no difference was found in stage IVA–B patients with low EBV DNA levels (HR 2.340, 95% CI 0.418–13.087, p = 0.333). Additionally, no difference was found in patients with stages II–III regardless of the EBV DNA level. Similar results were obtained for OS and DFS but not for LRFS regardless of the nonsignificant interaction effects between the treatment regimens and the clinical stages (Table 3). Similar stratification results were obtained using the IPTW and PSM methods (Supplementary Table 2).
Table 3.
Stratification analysis between treatment regimens and EBV DNA Levels with Clinical Stagea.
| Factor | Clinical stage II–IVA–B |
Clinical stage II–III |
Clinical stage IVA–B |
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| EBV DNA level, copies/ml | ||||||
| Distant metastasis-free survival | ||||||
| All measured patients | 1.225 (0.827–1.816) | 0.312 | 0.937 (0.521–1.684) | 0.828 | 2.552 (1.356–4.803) | 0.004 |
| <4000 cohort | 1.420 (0.713–2.828) | 0.318 | 0.846 (0.355–2.013) | 0.705 | 2.340 (0.418–13.087) | 0.333 |
| ⩾4000 cohort | 2.331 (1.194–4.551) | 0.013 | 1.161 (0.471–2.864) | 0.746 | 4.134 (1.586–10.778) | 0.004 |
| Overall survival | ||||||
| All measured patients | 1.371 (0.946–1.988) | 0.095 | 1.375 (0.727–2.601) | 0.328 | 1.949 (1.028–3.696) | 0.041 |
| <4000 cohort | 1.048 (0.542–2.025) | 0.891 | 1.148 (0.557–2.367) | 0.708 | 1.031 (0.125–8.489) | 0.977 |
| ⩾4000 cohort | 2.521 (1.218–5.216) | 0.013 | 1.827 (0.592–5.637) | 0.295 | 5.232 (2.114–12.946) | <0.001 |
| Disease-free survival | ||||||
| All measured patients | 1.108 (0.832–1.475) | 0.483 | 1.418 (0.926–2.171) | 0.108 | 1.588 (0.872–2.889) | 0.130 |
| <4000 cohort | 0.983 (0.594–1.626) | 0.946 | 0.917 (0.553–1.520) | 0.736 | 2.015 (0.658–6.169) | 0.220 |
| ⩾4000 cohort | 2.168 (1.349–3.483) | 0.001 | 2.052 (1.131–3.721) | 0.018 | 3.696 (1.634–8.359) | 0.002 |
| Locoregional relapse-free survival | ||||||
| All measured patients | 1.030 (0.651–1.630) | 0.898 | 1.316 (0.699–2.478) | 0.395 | 0.349 (0.047–2.589) | 0.303 |
| <4000 cohort | 1.247 (0.596–2.610) | 0.558 | 1.500 (0.695–3.238) | 0.302 | NSb | − b |
| ⩾4000 cohort | 0.792 (0.276–2.272) | 0.664 | 1.343 (0.492–3.667) | 0.565 | NS | − |
There were significant interaction effects between treatment regimens and EBV DNA levels with clinical stage for distant metastasis-free survival, but not for overall, disease-free, or locoregional relapse-free survivals, and detailed interaction information was presented in Figure 3.
Due to small numbers of cases in these subgroups, CIs were extremely wide but NS.
CI, confidence interval; EBV, Epstein–Barr virus; HR, hazard ratio; NS, nonsignificant.
Sensitivity analysis
For patients with a high EBV DNA level, the advantage of CCRT was relatively robust for the survival benefit of patients with a poor performance status (Table 4). For instance, assuming an HR of 1.2, a poor performance status could not eliminate the significant survival benefit of CCRT even if we assumed that none of the patients presented with a poor performance status in the CCRT group and 100% of the patients presented with a poor performance status in the IMRT-alone group. However, assuming an HR of 2, the performance status eliminated the significant survival benefit of CCRT. The sensitivity analysis indicated that our analysis results were robust.
Table 4.
Sensitivity analysis for HR of all-cause mortality adjusted for poor performance status in patients with high EBV DNA level*.
| Prevalence of poor performance status |
HR |
95% CI | ||
|---|---|---|---|---|
| IMRT + CCRT | IMRT alone | Poor performance | IMRT alone (adjusted for poor performance) | |
| 0.1 | 0.8 | 1.2 | 2.216 | 1.071–4.586 |
| 0.1 | 0.9 | 1.2 | 2.179 | 1.052–4.508 |
| 0 | 0.9 | 1.2 | 2.136 | 1.032–4.420 |
| 0 | 1 | 1.2 | 2.100 | 1.015–4.346 |
| 0.1 | 0.3 | 2 | 2.133 | 1.030–4.413 |
| 0.1 | 0.4 | 2 | 1.980 | 0.957–4.098 |
NOTE: Bold font indicates situations where poor performance status was strong enough to influence significance of rules (i.e. lower bound of 95% CI crossed 1). Values based on multivariate analysis in patients with high EBV DNA level adjusted all-cause mortality HR of 2.521 (95% CI, 1.218–5.216).
CCRT, concurrent chemotherapy; CI, confidence interval; EBV, Epstein–Barr virus; IMRT, intensity-modulated radiotherapy; HR, hazard ratio; OS, overall survival.
Discussion
To the best of our knowledge, this study is the first to show that CCRT significantly improves the survival of LA-NPC patients with high plasma EBV DNA levels compared with IMRT alone based on a stratification analysis in a large endemic LA-NPC cohort. Conversely, CCRT and IMRT alone have similar benefits for patients with low plasma EBV DNA levels. This study confirms that the plasma EBV DNA level is a strong prognostic factor for NPC patients and suggests that this measure should be a significant supplement for the clinical stage and an individualized treatment marker for NPC in clinical practice. The combination of concurrent chemotherapy and RT is a recommended treatment regimen for patients with LA-NPC based on evidence from the 2DRT era. However, the benefit of CCRT for LA-NPC patients in the IMRT era may require further validation.
The Intergroup-0099 (INT-0099) trial was the first study to show that adding chemotherapy to RT increased the OS of NPC patients compared with RT alone.3 However, considering the poor survival of the RT group and the well-differentiated nature of the carcinoma cases, initially there were doubts regarding whether these practices were applicable in endemic areas. Several subsequent studies that directly compared CCRT with RT alone confirmed the effects of this regimen.29–31 CCRT was reported to have potentially advantageous effects, including a reduction in the local tumour burden, elimination of subclinical distant metastases and synergy with RT. The Taiwan-93 trial showed that CCRT improved local control and potentially reduced the rate of distant metastasis.29 In contrast, no significant differences in the occurrence of distant metastases were found in a study reported by Chan and colleagues30 Additionally, three meta-analyses primarily containing studies performed in the 2DRT era consistently showed that CCRT improved OS, locoregional control and DMFS compared with RT alone.7,32,33
Notably, CCRT studies restricted to the IMRT era reported that patients receiving IMRT plus concurrent chemotherapy failed to achieve survival benefits compared with patients receiving IMRT alone,11,12,34–36 with the exception of a report by Sun and colleagues13 However, this positive result was obtained from a small sample of patients with advanced N stages. Although this study was retrospective in design, more than 1000 cases were included, and multiple multivariate analysis methods were conducted simultaneously to confirm the robustness of the results. A key prognostic biomarker (the EBV DNA level) was adjusted in the analysis. Moreover, given that we found no interaction effects between the stage and the treatment regimen, the results from the above positive CCRT study in advanced N stage patients must be interpreted with caution. In our study, a significant interaction effect was observed between treatment regimens and differential EBV DNA levels, indicating that NPC treatment regimens should be more precisely individualized according to the EBV DNA levels in the IMRT era.
The effects of the plasma EBV DNA levels on treatment outcomes have been investigated for prognostic risk assessment, surveillance and treatment stratification.14,15,37–41 Leung and colleagues showed that plasma EBV DNA had better prognostic value when combined with the TNM staging system in the pre-IMRT era and appeared to be a more significant prognostic factor than clinical staging.17,42 Several recent studies have confirmed that the plasma EBV DNA level is a strong prognostic factor for patients with NPC when complemented with TNM staging in the IMRT era.14–16 These data also showed that the plasma EBV DNA level had a higher predictive value than clinical staging.15,16 However, the subgroup analysis results in previous studies need to be interpreted with caution due to the lack of interaction analyses.43 Our study performed a stratification analysis based on an interaction analysis for the plasma EBV DNA level and clinical staging to objectively obtain homogeneously stratified cohorts and showed that the plasma EBV DNA level appeared to have greater prior treatment stratification value than clinical staging. Based on our study, CCRT benefited NPC patients with high pretreatment plasma EBV DNA copy numbers, whereas IMRT alone was more suitable for patients with low EBV DNA levels. The ongoing NRG-HN001 trial is designed to compare the outcomes of NPC patients who receive different individualized regimens according to their EBV DNA levels; we anticipate that the results of this trial will set an example for individualized treatment guided by EBV DNA levels.
Considering that a high distant metastasis rate is a specific feature of NPC1 and based on the significant interaction effects on DMFS, we conducted an additional stratification analysis of DMFS to explore the effects of treatment regimens with the clinical stages and EBV DNA levels. Our results showed that clinical stage IVA–B NPC patients with high EBV DNA levels significantly benefited from CCRT with a decreased risk of distant metastasis, whereas CCRT might not benefit the DMFS of stage IVA–B patients with low EBV DNA levels and stage II–III patients.
The limitations of 2DRT, such as failure to achieve adequate coverage and poor OAR sparing, have resulted in unsatisfactory local control and survival rates.44,45 IMRT has overcome the limitations of 2DRT technology46 and can deliver a higher dose to the tumour while minimizing the exposure of adjacent tissues or OARs, which achieves excellent locoregional control. Naturally, the margin of potential survival benefits gained from additional concurrent chemotherapy in some patient subsets may be reduced in the IMRT era. Furthermore, several studies have shown that CCRT significantly increases acute toxicity reactions and impairs treatment compliance compared with IMRT alone.7,11,12,35,36 Therefore, the benefit of CCRT in LA-NPC patients remains uncertain and further prospective investigations are needed.
We acknowledge that limitations exist in our study. First, the main limitation of our study is its retrospective and single-institute nature, although the cases were extracted from a prospectively maintained database. To minimize bias, we not only adopted different models to confirm our analysis but also performed a sensitivity analysis to estimate the effects of unmeasured confounders and validate the robustness of our results. Second, the quantitative cut-off for plasma EBV DNA levels was selected based on the results of our previous studies conducted in the same epidemic area. However, a cut-off point of more than 6000 copies per ml has also been suggested.1 Therefore, we objectively analysed four groups using magnitudes of 10 and two groups with conventional cut-off levels in the endemic area. Third, we observed no interaction between the stage and regimens. However, a significant difference in OS was found between regimens in MVA in patients with clinical stage IV, possibly due to an insufficient number of cases. Thus, we concede that this result should be interpreted with caution. Fourth, although we regarded plasma EBV DNA as an extremely significant complement to the clinical staging system, use of this measure in combination with other important biomarkers (i.e. circulating fibrinogen20) could help provide more precise treatment choices. Lastly, EBV DNA has been shown to be an important factor for prediction of the prognosis of NPC patients. However, approximately 30% of patients lacked an EBV DNA measurement in this study due to its retrospective nature. Therefore, a prospective study is necessary to validate our findings.
In conclusion, this study is the first to combine an interaction effect analysis and stratification by plasma EBV DNA levels and show that CCRT can significantly improve the survival rates of NPC patients with high plasma EBV DNA levels compared with IMRT alone; however, CCRT does not significantly benefit patients with low plasma EBV DNA levels. CCRT may benefit DMFS for clinical stage IVA–B NPC patients with high EBV DNA levels but not those with low EBV DNA levels and clinical stage II–III NPC patients regardless of their EBV DNA levels. A well-designed prospective study with a large cohort is required to confirm the role of concurrent chemotherapy in the IMRT era and to explore individualized regimens for various LA-NPC patient subgroups with different plasma EBV DNA levels.
Supplemental Material
Supplemental material, T-Supplementary_Material for The plasma Epstein–Barr virus DNA level guides precision treatment for nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a large population-based cohort study from an endemic area by Hu Liang, Xing Lv, Lin Wang, Yi-Shan Wu, Rui Sun, Yan-Fang Ye, Liang-Ru Ke, Qin Yang, Ya-Hui Yu, Wen-Ze Qiu, Guo-Ying Liu, Xin-Jun Huang, Wang-Zhong Li, Shu-Hui Lv, Xiang Guo, Yan-Qun Xiang and Wei-Xiong Xia in Therapeutic Advances in Medical Oncology
Acknowledgments
Hu Liang, Xing Lv, and Lin Wang contributed equally to this work. The authors greatly thank Professor Qing Liu and Can-hong Wen, PhD, for statistical assistance during the preparation of this manuscript. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit (RDD) public platform (www.researchdata.org.cn) with the approval RDD number as RDDA2018000422.
Footnotes
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (grant numbers 81472525, 81572665, and 81672680).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Hu Liang, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Xing Lv, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Lin Wang, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Yi-Shan Wu, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Rui Sun, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Yan-Fang Ye, Clinical Trial Design Division, Clinical Research Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China.
Liang-Ru Ke, Collaborative Innovation Center for Cancer Medicine, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, China Department of Radiology, Sun Yat-sen University Cancer Center, Guangzhou, China.
Qin Yang, Collaborative Innovation Center for Cancer Medicine, State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, China.
Ya-Hui Yu, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Wen-Ze Qiu, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Guo-Ying Liu, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Xin-Jun Huang, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Wang-Zhong Li, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Shu-Hui Lv, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Xiang Guo, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Yan-Qun Xiang, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
Wei-Xiong Xia, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine; Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China; Department of Nasopharyngeal Carcinoma, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, China.
References
- 1. Chua ML, Wee JT, Hui EP, et al. Nasopharyngeal carcinoma. Lancet (London, England) 2016; 387: 1012–1024. [DOI] [PubMed] [Google Scholar]
- 2. Wei KR, Zheng RS, Zhang SW, et al. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin J Cancer 2014; 33: 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol 1998; 16: 1310–1317. [DOI] [PubMed] [Google Scholar]
- 4. Chan AT, Teo PM, Ngan RK, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol 2002; 20: 2038–2044. [DOI] [PubMed] [Google Scholar]
- 5. Wee J, Tan EH, Tai BC, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol 2005; 23: 6730–6738. [DOI] [PubMed] [Google Scholar]
- 6. Lee AW, Lau WH, Tung SY, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol 2005; 23: 6966–6975. [DOI] [PubMed] [Google Scholar]
- 7. Du CR, Ying HM, Kong FF, et al. Concurrent chemoradiotherapy was associated with a higher severe late toxicity rate in nasopharyngeal carcinoma patients compared with radiotherapy alone: a meta-analysis based on randomized controlled trials. Radiat Oncol (London, England) 2015; 10: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol 2014; 110: 398–403. [DOI] [PubMed] [Google Scholar]
- 9. Tham IW, Hee SW, Yeo RM, et al. Treatment of nasopharyngeal carcinoma using intensity-modulated radiotherapy: the national cancer centre Singapore experience. Int J Radiat Oncol Biol Phys 2009; 75: 1481–1486. [DOI] [PubMed] [Google Scholar]
- 10. Lin S, Pan J, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the 3-year outcome of a prospective series. Int J Radiat Oncol Biol Phys 2009; 75: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 11. Su Z, Mao YP, Tang J, et al. Long-term outcomes of concurrent chemoradiotherapy versus radiotherapy alone in stage II nasopharyngeal carcinoma treated with IMRT: a retrospective study. Tumour Biol 2016; 37: 4429–4438. [DOI] [PubMed] [Google Scholar]
- 12. Cao CN, Luo JW, Gao L, et al. Concurrent chemotherapy for T4 classification nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy. PloS One 2015; 10: e0119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun X, Zeng L, Chen C, et al. Comparing treatment outcomes of different chemotherapy sequences during intensity modulated radiotherapy for advanced N-stage nasopharyngeal carcinoma patients. Radiat Oncol (London, England) 2013; 8: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang L, Tang LQ, Chen QY, et al. Plasma Epstein-Barr viral DNA complements TNM classification of nasopharyngeal carcinoma in the era of intensity-modulated radiotherapy. Oncotarget 2016; 7: 6221–6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tang LQ, Li CF, Li J, et al. Establishment and Validation of Prognostic Nomograms for Endemic Nasopharyngeal Carcinoma. J Natl Cancer Inst 2016; 108: pii: djv291. [DOI] [PubMed] [Google Scholar]
- 16. Lu L, Li J, Zhao C, et al. Prognostic efficacy of combining tumor volume with Epstein-Barr virus DNA in patients treated with intensity-modulated radiotherapy for nasopharyngeal carcinoma. Oral Oncol 2016; 60: 18–24. [DOI] [PubMed] [Google Scholar]
- 17. Leung SF, Zee B, Ma BB, et al. Plasma Epstein–Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24: 5414–5418. [DOI] [PubMed] [Google Scholar]
- 18. An X, Wang FH, Ding PR, et al. Plasma Epstein–Barr virus DNA level strongly predicts survival in metastatic/recurrent nasopharyngeal carcinoma treated with palliative chemotherapy. Cancer 2011; 117: 3750–3757. [DOI] [PubMed] [Google Scholar]
- 19. Shao JY, Zhang Y, Li YH, et al. Comparison of Epstein–Barr virus DNA level in plasma, peripheral blood cell and tumor tissue in nasopharyngeal carcinoma. Anticancer Res 2004; 24: 4059–4066. [PubMed] [Google Scholar]
- 20. Tang LQ, Chen QY, Guo SS, et al. The impact of plasma Epstein–Barr virus DNA and fibrinogen on nasopharyngeal carcinoma prognosis: an observational study. Br J Cancer 2014; 111: 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cox DR. Regression models and life-tables. In: Kotz S, Johnson NL. (eds) Breakthroughs in statistics: methodology and distribution. New York, NY: Springer New York, 1992, pp.527–541. [Google Scholar]
- 22. Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013; 32: 2837–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011; 10: 150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gayat E, Resche-Rigon M, Mary JY, et al. Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat 2012; 11: 222–229. [DOI] [PubMed] [Google Scholar]
- 25. Qin J, Ning J, Liu H, et al. Maximum likelihood estimations and EM algorithms with length-biased data. J Am Stat Assoc 2011; 106: 1434–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin DY WL-J. The robust inference for the Cox proportional hazards model. J Am Stat Assoc 1989; 84: 1074–1078. [Google Scholar]
- 27. Lin DY, Psaty BM, Kronmal RA. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998; 54: 948–963. [PubMed] [Google Scholar]
- 28. Correa GT, Bandeira GA, Cavalcanti BG, et al. Analysis of ECOG performance status in head and neck squamous cell carcinoma patients: association with sociodemographical and clinical factors, and overall survival. Support Care Cancer 2012; 20: 2679–2685. [DOI] [PubMed] [Google Scholar]
- 29. Lin JC, Jan JS, Hsu CY, et al. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol 2003; 21: 631–637. [DOI] [PubMed] [Google Scholar]
- 30. Chan AT, Leung SF, Ngan RK, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 2005; 97: 536–539. [DOI] [PubMed] [Google Scholar]
- 31. Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst 2011; 103: 1761–1770. [DOI] [PubMed] [Google Scholar]
- 32. Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol 2015; 16: 645–655. [DOI] [PubMed] [Google Scholar]
- 33. Yan M, Kumachev A, Siu LL, et al. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: a Bayesian network meta-analysis. Eur J Cancer 2015; 51: 1570–1579. [DOI] [PubMed] [Google Scholar]
- 34. Junlin Y, Li G, Xiaodong H, et al. Nasopharyngeal carcinoma treated by intensity-modulated radiotherapy: long-term results of 416 patients. Chin J Radiat Oncol 2012; 21: 196–200. [Google Scholar]
- 35. Lin S, Lu JJ, Han L, et al. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer 2010; 10: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cao CN, Luo JW, Gao L, et al. Update report of T4 classification nasopharyngeal carcinoma after intensity-modulated radiotherapy: an analysis of survival and treatment toxicities. Oral Oncol 2015; 51: 190–194. [DOI] [PubMed] [Google Scholar]
- 37. Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. J Clin Oncol 2013; 31: 2861–2869. [DOI] [PubMed] [Google Scholar]
- 38. Wang WY, Twu CW, Chen HH, et al. Long-term survival analysis of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA levels. Cancer 2013; 119: 963–970. [DOI] [PubMed] [Google Scholar]
- 39. Leung SF, Chan KC, Ma BB, et al. Plasma Epstein-Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol 2014; 25: 1204–1208. [DOI] [PubMed] [Google Scholar]
- 40. Hong RL, Lin CY, Ting LL, et al. Comparison of clinical and molecular surveillance in patients with advanced nasopharyngeal carcinoma after primary therapy: the potential role of quantitative analysis of circulating Epstein-Barr virus DNA. Cancer 2004; 100: 1429–1437. [DOI] [PubMed] [Google Scholar]
- 41. Chan KCA, Woo JKS, King A, et al. Analysis of Plasma Epstein-Barr Virus DNA to Screen for Nasopharyngeal Cancer. N Engl J Med 2017; 377: 513–522. [DOI] [PubMed] [Google Scholar]
- 42. Leung SF, Chan AT, Zee B, et al. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 2003; 98: 288–291. [DOI] [PubMed] [Google Scholar]
- 43. Altman DG, Matthews JN. Statistics notes. Interaction 1: Heterogeneity of effects. BMJ 1996; 313: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waldron J, Tin MM, Keller A, et al. Limitation of conventional two-dimensional radiation therapy planning in nasopharyngeal carcinoma. Radiother Oncol 2003; 68: 153–161. [DOI] [PubMed] [Google Scholar]
- 45. Cheng JC, Chao KS, Low D. Comparison of intensity modulated radiation therapy (IMRT) treatment techniques for nasopharyngeal carcinoma. Int J Cancer 2001; 96: 126–131. [DOI] [PubMed] [Google Scholar]
- 46. Kam MK, Chau RM, Suen J, et al. Intensity-modulated radiotherapy in nasopharyngeal carcinoma: dosimetric advantage over conventional plans and feasibility of dose escalation. Int J Radiat Oncol Biol Phys 2003; 56: 145–157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, T-Supplementary_Material for The plasma Epstein–Barr virus DNA level guides precision treatment for nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: a large population-based cohort study from an endemic area by Hu Liang, Xing Lv, Lin Wang, Yi-Shan Wu, Rui Sun, Yan-Fang Ye, Liang-Ru Ke, Qin Yang, Ya-Hui Yu, Wen-Ze Qiu, Guo-Ying Liu, Xin-Jun Huang, Wang-Zhong Li, Shu-Hui Lv, Xiang Guo, Yan-Qun Xiang and Wei-Xiong Xia in Therapeutic Advances in Medical Oncology