Abstract
We show that hypoxia inducible factor 2α (HIF2α) is highly expressed in patients with pulmonary hypertension (PH). HIF2α is expressed in every patient with congenital diaphragmatic hernia, while only half of the controls express HIF2α. Our data suggest that HIF2α is a link between hypoxia and the development of PH.
Keywords: hypoxia inducible factor, pulmonary hypertension, pulmonary vascular development and epithelium
Introduction
Idiopathic pulmonary hypertension in newborns (PHN) is a life-threatening condition and requires intensive clinical support.1,2 PHN is frequently associated with congenital disorders, such as congenital heart diseases and congenital diaphragmatic hernia (CDH).2 The underlying causes of PHN are still largely unknown, although several potential genetic and epigenetic mechanisms have been described.2,3 Prolonged exposure to hypoxia causes pulmonary arterial hypertension (PAH), and hypoxia inducible factors (HIF) are the key component of the cellular response to hypoxia.4 HIFs are heterodimeric transcription factors composed of two subunits, a stable HIF1β and one of three oxygen-sensitive subunits HIF1α, HIF2α, or HIF3α.4
HIFs have been associated with lung development and PH.5 In humans, genome-wide studies showed a positive correlation of HIF2α with adaptation to hypobaric hypoxia in Tibetan highlanders.6,7 In rodents, both Hif1α and Hif2α appeared to modulate hypoxia-induced PH in gene ablation studies.5–12 Two reports described that endothelial-specific ablation of prolyl-4-hydroxylase 2 (PHD2), which targets HIFs for degradation by hydroxylating proline residues in HIF2α under normoxic conditions, results in a HIF2α-dependent adult PAH.10,11 Using an endothelial-specific inactivation of HIF2α, Cowburn et al. showed that HIF2α is involved in hypoxia-induced PAH.12 Since PAH is different from PHN, we examined HIF2α protein expression in a developmental series of normal lung tissue, as well as in the lungs of a cohort of CDH patients and patients with idiopathic PH.
Materials and methods
Human lung tissue collection
With the approval of the Erasmus MC Medical Ethical Committee and the informed consent of parents, lung tissue was obtained from the archives of the Department of Pathology, Erasmus MC (Rotterdam).
Immunohistochemistry
Tissue micro arrays were constructed as described.13 Paraffin embedded lung material was sectioned, blocked, and incubated overnight at 4℃ (HIF2α, Genetex). The Envision kit (Dako) was used and images were taken with a charge-coupled device camera attached to an Olympus BX41 microscope.
Results
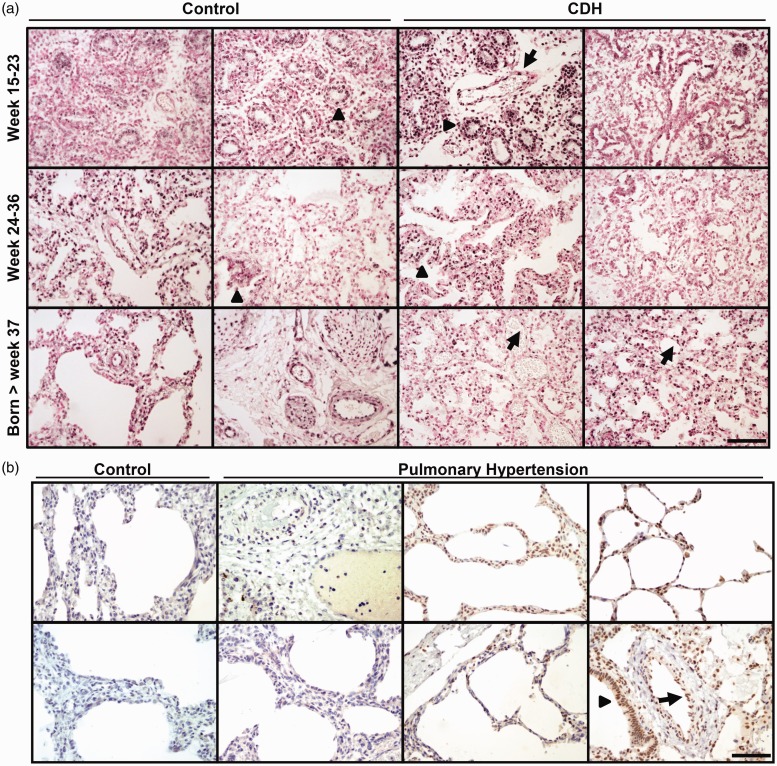
HIF2α was expressed in half of the fetal lungs that were analyzed at 15–36 weeks of gestation. In addition, lungs of neonates born after week 37 were also positive for HIF2α (Fig. 1a). HIF2α was localized in the nucleus of proximal and distal airway epithelial cells and alveolar epithelial cells (Fig. 1a, arrowheads). Hif2α is also highly expressed in alveolar type II cells in lungs as we and others have shown before in mouse lungs.9,14
Fig. 1.
(a) Representative images of HIF2α protein expression in lungs of two independent control (left) and two CDH patients (right) at three different gestational ages (weeks 15–23, weeks 24–36, and born after week 37). HIF2α is expressed in half of the control lungs tested, although some expression is observed in the epithelial cells of the airways (arrowheads). In contrast, HIF2α is expressed in all examined lungs of the CDH patients, in both epithelial cells (arrowheads) and endothelial cells (arrows). (b) HIF2α is absent in age-matched control lungs, but prominently expressed in the lungs of some cases of PHN. Two representative images are shown for the control lungs and six images of PHN cases. HIF2α is expressed in alveolar type II cells (arrowheads) and in endothelial cells (arrows). Scale bar: 100 µm.
Next, we analyzed the expression of HIF2α in lungs from a cohort of CDH patients of different gestational ages until birth. HIF2α is prominently present in all CDH cases analyzed, contrasting the expression of HIF2α in normal, unaffected lungs (Fig. 1a, Table 1). The sites of HIF2α expression were in both alveolar epithelial cells (Fig. 1a, arrowheads) and all endothelial cells of the blood vessels (Fig. 1a, arrows). Moreover, in some CDH lungs, the level of HIF2α appeared very high as indicated by the intense staining (Fig. 1).
Table 1.
CDH patient characteristics and the age-matched control lungs.
| Developmental stage | Gestational age (weeks) | Postnatal age | Birth weight (g) | HIF2α-positive (n/total n) | VEGF-positive (n/total n) | |
|---|---|---|---|---|---|---|
| Control | Premature | 31.5 (15–36) | 1 h (0–24 h) | 2000 (57–2700) | 5/9 | 5/9 |
| Term | 39.5 (38–41) | 36 h (1 h–1 week) | 3220 (2490–3950) | 4/8 | 9/11 | |
| CDH | Premature | 31.5 (15–36) | 1 h (0–48 h) | 1032 (30–2515) | 7/7 | 6/6 |
| Term | 39 (37–40) | 7 h (1 h–3 days) | 2835 (2000–3800) | 6/6 | 9/11 |
Indicated are the average age in weeks, the average postnatal age, birth weight, and the number of HIF2α-positive and VEGF-positive samples per total samples tested.
We also analyzed 11 lungs of neonates with idiopathic PH and age-matched controls (Fig. 1b). In the neonatal control human lung, only few cells are positive for HIF2α (Fig. 1b, control). Clear staining patterns were observed in the endothelial cells of the vessels of patients with PHN (Fig. 1b, arrows) and in alveolar epithelial cells (Fig. 1b, arrowheads), although not all PHN cases expressed HIF2α (Fig. 1b). Our data suggest that expression of HIF2α is maintained in some of the clinical cases of PH, supporting the relative hypoxia of these patients. Finally, we also analyzed the expression of one of the HIF2α targets, VEGF, and found comparable numbers of positive samples as for the HIF2α staining (Table 1).
Discussion
Previously, we showed a gradual increase of HIF2α messenger RNA (mRNA) during gestation, but no differences were detected in expression levels between CDH patients and controls.15,16 Here, we report an increased expression of HIF2α protein in CDH patients compared to controls. Even before birth, we found significantly higher expression of HIF2α in CDH patients. Our data suggest that high levels of HIF2α correlate with PHN and CDH-associated PH. Since prolonged exposure to hypoxia results in PH,5 and the lungs of patients with PH are under constant hypoxic conditions, it may result in elevated levels of HIF2α. Although all CDH cases tested were clearly positive for HIF2α, not all cases of PHN expressed high levels of HIF2α. This most likely reflects the patient variability and the heterogeneity of PHN.
In all fetal CDH cases, HIF2α was expressed in the lung and at much higher levels than controls. Moreover, HIF2α is also highly expressed in the lungs of patients suffering from PHN. This suggests that early in gestation, the lungs of CDH patients are already intrinsically different from control lungs, and high levels of HIF2α may contribute to postnatal PH, which develops in a significant number of patients with CDH after birth.
Prolonged hypoxia, which is also observed in PHN patients, results in structural changes of the pulmonary vasculature characterized by a thickening of the vascular wall of small pulmonary arteries leading to an increased vascular resistance and a worsening of gas exchange.2 We recently showed that the vascular smooth muscle cells in the developing CDH lung had a different expression pattern of contractile components compared to control lungs, suggesting that these cells prematurely differentiate.17 Furthermore, the expression of VEGF-A mRNA was increased in the lungs from CDH patients at the canalicular stage, while a significant decrease in the expression of VEGF-A mRNA was observed in the alveolar stage of lung development in CDH patients. However, the spatial distribution of VEGF-A was not different between control and CDH lungs.16,18 Here, we report that HIF2α is expressed in more CDH lungs than in control lungs, and that the expression of VEGF appears to correlate with HIF2α expression. The site of expression does not differ between control and CDH lungs, as previously described. Additionally, we previously showed that a significant increase in Hif2α in epithelial alveolar type II cells did not induce an increased expression of Vegf-A.14
Although the mechanisms of HIF2α activity and its role in the development of PH are still incompletely understood, it may be a putative target for future therapies. In this respect, it is interesting to investigate the potential of the Hif2α-specific competitor, FM19G11.19
In summary, we showed high expression levels of HIF2α in the lungs of all CDH patients at different gestational ages and after birth, while only half of the age-matched controls showed expression of HIF2α. From these results, we suggest that HIF2α is associated with the development of PH in CDH, and possibly also with PHN.
Declaration of conflicting interests
The author(s) declare that there is no conflict of interest.
Funding
This study was supported in part by the Sophia Foundation for Medical Research (grant nos. 482 [YH] and 531 [IS]).
References
- 1.Sluiter I, Veenma D, van Loenhout R, et al. Etiological and pathogenic factors in congenital diaphragmatic hernia. Eur J Pediatr Surg 2012; 22: 345–354. [DOI] [PubMed] [Google Scholar]
- 2.Kool H, Mous D, Tibboel D, et al. Pulmonary vascular development goes awry in congenital lung abnormalities. Birth Defects Res C 2014; 102: 343–358. [DOI] [PubMed] [Google Scholar]
- 3.Donahoe PK, Longoni M, High FA. Polygenic causes of congenital diaphragmatic hernia produce common lung pathologies: a multimodal war on congenital diaphragmatic hernia. Am J Pathol 2016; 186: 2532–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 2014; 9: 47–71. [DOI] [PubMed] [Google Scholar]
- 5.Shimoda LA, Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med 2011; 183: 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beall CM, Cavalleri GL, Deng L, et al. Natural selection on EPAS1 (HIF2alpha) associated with low hemoglobin concentration in Tibetan highlanders. Proc Natl Acad Sci U S A 2010; 107: 11459–11464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonson TS, Yang Y, Huff CD, et al. Genetic evidence for high-altitude adaptation in Tibet. Science 2010; 329: 72–75. [DOI] [PubMed] [Google Scholar]
- 8.Yu AY, Shimoda LA, Iyer NA, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999; 103: 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Compernolle V, Brusselmans K, Acker T, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 2002; 8: 702–710. [DOI] [PubMed] [Google Scholar]
- 10.Dai Z, Li M, Wharton J, et al. Prolyl-4 hydroxylase 2 (PHD2) deficiency in endothelial cells and hematopoietic cells induces obliterative vascular remodeling and severe pulmonary arterial hypertension in mice and humans through hypoxia-inducible factor-2alpha. Circulation 2016; 133: 2447–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapitsinou PP, Rajendran G, Astleford L, et al. The endothelial prolyl-4-hydroxylase domain 2/hypoxia-inducible factor 2 axis regulates pulmonary artery pressure in mice. Mol Cell Biol 2016; 36: 1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cowburn AS, Crosby A, Macias D, et al. HIF2alpha-arginase axis is essential for the development of pulmonary hypertension. Proc Natl Acad Sci U S A 2016; 113: 8801–8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4: 844–847. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Kempen MB, Munck AB, et al. Hypoxia-inducible factor 2alpha plays a critical role in the formation of alveoli and surfactant. Am J Respir Cell Mol Biol 2012; 46: 224–232. [DOI] [PubMed] [Google Scholar]
- 15.Rajatapiti P, van der Horst IW, de Rooij JD, et al. Expression of hypoxia-inducible factors in normal human lung development. Pediatr Dev Pathol 2008; 11: 193–199. [DOI] [PubMed] [Google Scholar]
- 16.van der Horst IW, Rajatapiti P, van der Voorn P, et al. Expression of hypoxia-inducible factors, regulators, and target genes in congenital diaphragmatic hernia patients. Pediatr Dev Pathol 2011; 14: 384–390. [DOI] [PubMed] [Google Scholar]
- 17.Sluiter I, van der Horst I, van der Voorn P, et al. Premature differentiation of vascular smooth muscle cells in human congenital diaphragmatic hernia. Exp Mol Pathol 2013; 94: 195–202. [DOI] [PubMed] [Google Scholar]
- 18.de Rooij JD, Hosgor M, Ijzendoorn Y, et al. Expression of angiogenesis-related factors in lungs of patients with congenital diaphragmatic hernia and pulmonary hypoplasia of other causes. Pediatr Dev Pathol 2004; 7: 468–477. [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Manzano V, Rodríguez-Jiménez FJ, Aceña-Bonilla JL, et al. FM19G11, a new hypoxia-inducible factor (HIF) modulator, affects stem cell differentiation status. J Biol Chem 2010; 285: 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]