Abstract
Temporomandibular joint (TMJ) disorders, including degenerative TMJ disease, occur primarily in women of reproductive age. Previous studies showed elevated estrogen levels in subjects with TMJ disorders relative to controls and the presence of estrogen receptors α and β (ERα and ERβ) in TMJ fibrocartilage. Additionally, estrogen-induced overexpression of specific matrix metalloproteinases (MMPs), including MMP-9 and MMP-13, in TMJ fibrocartilage is accompanied by loss of extracellular matrices. However, the contribution of ERα and ERβ in estrogen-mediated induction of MMP-9 and MMP-13 and the signaling cascade leading to the upregulation of these MMPs have not been elucidated. Here, we show that specific siRNAs and selective ER antagonists effectively block ERα or ERβ expression in primary mouse TMJ fibrochondrocytes, but that only blockage of ERα suppresses MMP-9 and MMP-13 levels induced by 17β-estradiol (E2). Overexpression of ERα but not ERβ enhances E2-induced MMP-9. Using the same loss-of-function and gain-of-function approaches, we demonstrate that E2 stimulates ERK activation through ERα and that inhibition of ERK phosphorylation reduces E2-induced MMP-9. Furthermore, we reveal that E2 promotes NF-κB and ELK-1 activation through ERα/ERK signaling and that knockdown of either one decreases the respective activity of these signaling mediators and MMP-9 expression induced by E2, indicating that both contribute to E2/ERα/ERK-mediated MMP-9 upregulation. This is supported by findings in which mutated binding sites of either NF-κB or ELK-1 in the MMP-9 promoter lead to a significant reduction of E2-stimulated promoter activity. Our findings provide novel molecular mechanisms for the understanding of E2-mediated upregulation of MMPs, having implications to pathophysiologic TMJ cartilage matrix turnover that may yield therapeutic intervention targets for TMJ disorders.
Keywords: temporomandibular joint disorder, signal transduction, extracellular matrix, estrogen receptor beta, extracellular signal-regulated kinase (ERK), nuclear factor kappa B
Introduction
Estrogens, which belong to the steroid family of hormones, contribute to the development and function of the female reproductive system and modulate many biological activities in cardiovascular, central nervous, and musculoskeletal systems in males and females (Eyster 2016). The major naturally occurring estrogens are 17β-estradiol (E2), estrone, and estriol, among which E2 is the predominant one in women during the reproductive years. Within the musculoskeletal system, E2 is an important regulator of extracellular matrix (ECM) remodeling in bone and cartilage (Kapila et al. 2009; Khosla et al. 2012). While E2 has an anabolic effect in bone, it contributes to matrix loss in cartilaginous tissue and cells of the temporomandibular joint (TMJ; Naqvi et al. 2005; Hashem et al. 2006), which may predispose to degenerative changes in these joints. Degenerative TMJ disease is a common clinicopathologic finding within a highly prevalent spectrum of conditions known as TMJ disorders (TMJDs) that primarily afflict women during the reproductive years (Landi et al. 2005). The early onset of degenerative TMJ disease in women during the reproductive years, as opposed to similar degenerative conditions in systemic joints that largely afflict postmenopausal women, has implicated the role of female sex hormones, particularly E2, in this disorder (Landi et al. 2005; Maixner et al. 2011).
Most pathophysiologic functions of estrogens primarily involve estrogen receptors (ERs) α and β, whose cellular locations include the nucleus and cytoplasm (Levin and Hammes 2016). ERα and ERβ are localized in various cartilages, including long bone articular cartilage, pubic symphysis, knee meniscus, and TMJ fibrocartilages (Wang et al. 2009; Börjesson et al. 2013). These receptors are more highly expressed in female than male murine and primate TMJ fibrocartilages (Milam et al. 1987; Wang et al. 2009). While studies have shown that E2 contributes to matrix loss in fibrocartilage, it is not clear which of its receptors is responsible for ECM remodeling activities in these tissues. This distinction is important because, although the 2 receptors share 96% amino acid homology in their DNA binding domain, they have substantial differences in their ligand-binding region and activating function regions. Thus, activation of the 2 receptors can regulate different target genes and often result in varied and sometimes opposite downstream effects. ERβ was shown to repress transcriptional activity of ERα with the same ligand (Hall and McDonnell 1999). Given these distinctions in ER functions and the coexpression of both ERs in many tissues, including TMJ fibrocartilage, it is important to determine how the presence and relative levels of both receptors affect tissue responses to E2.
E2 contributes to ECM turnover primarily through its modulation of matrix metalloproteinases (MMPs), possibly by activating 1 or more of its receptors, which trigger unknown downstream signaling pathways. The effect of E2 on tissue turnover through modulation of MMPs appears to be dose dependent and tissue/cell specific (Hashem et al. 2006; Kapila et al. 2009). E2 induces several MMPs, predominantly MMP-9 and MMP-13 in TMJ fibrocartilage, leading to loss of collagen and glycosaminoglycans, which is mitigated by a pan-MMP inhibitor (Naqvi et al. 2005; Hashem et al. 2006; Kapila et al. 2009). However, the signals that mediate E2’s induction of MMPs by activation of 1 or both of its primary receptors and whether these responses occur via genomic or nongenomic actions have not been elucidated. This study was undertaken to determine the contributions of ERα and ERβ to the regulation of MMP-9 and MMP-13 by E2 and to elucidate the downstream signaling pathway involved in inducing MMP-9 in mouse TMJ fibrochondrocytes.
Materials and Methods
Animal Procedures and Cell Retrieval
All animal procedures were conducted on 12-wk-old female C57BL/6J mice (Charles River Laboratories) following approval from the Institutional Animal Care and Use Committee. TMJ fibrochondrocytes were retrieved and cultured in α-MEM supplemented with 10% fetal bovine serum, and the studies were performed as described previously (Ahmad et al. 2012). Passage 4 to 6 fibrochondrocytes were used for each experiment.
Methods for Gain and Loss of ER Function
The fibrochondrocytes were seeded at 1.0 × 106 cells per 6-cm dish overnight prior to initiating gain-of-function, loss-of-function, and chemical inhibitor studies. The doses of siRNA, cDNA, and signaling inhibitors and the optimal time frame for each experiment were determined by preliminary dose-response and time-course studies. Overexpression and suppression of ER were performed by transfection of 2 μg of ERα or ERβ or control cDNA vectors and 250pM ERα or ERβ or control siRNAs (sc-29306, sc-35326, and sc-37007; Santa Cruz Biotech), respectively, as described previously (Ahmad et al. 2012). The transfection efficiency was about 40% to 60%, as determined by pSV-β-galactosidase vector transfection with Effectene transfection reagent (according to the manufacturer’s instructions; Qiagen) in serum-free Opti-MEM media. After 6 h of incubation, the Opti-MEM was replaced with α-MEM containing 10% fetal bovine serum and maintained for 12 h. The cells were washed and maintained in serum-free medium (α-MEM with 0.2% lactalbumin hydrolysate) for 4 h, before being incubated in fresh serum-free medium with E2. After 48 h of incubation with E2, cell-conditioned media were retrieved for MMP assays and cell lysates collected for ER blots.
To determine the effect of ER signaling on MMP expression, the fibrochondrocytes were transfected with 250pM extracellular signal-regulated kinase 1/2 (ERK), E-26 transcription factor (ELK-1), or p65 nuclear factor kappa B (NF-κB; a major unit of NF-κB) or control siRNAs (sc-29859, sc-3529, sc-29411, and sc-37007; Santa Cruz Biotech) or treated with the following signaling inhibitors: 10μM U0126 (Santa Cruz Biotech), 1μM MPP for ERα, 1μM PHTPP for ERβ and 1μM ICI for ERα and ERβ (Tocris Biosciences) for 30 min prior to incubation with E2. On the basis of preliminary time-course studies, cell lysates were collected after 5 min for ERK and 10 min for ELK-1 and NF-κB assays, while the conditioned media were collected after 6 h for MMP-9 assay.
Western Blot
BCA protein assay (Pierce Biotech) and Western blots were performed as previously described (Ahmad et al. 2012). The membranes were incubated with each of the following primary antibodies: rabbit anti-ERα or ERβ and NF-κBp65, which was anti-phosphorylated at serine-536 NF-κBp65, an activated phosphorylated form of NF-κBp65 (Mattioli et al. 2004) that can be identified by Western blot antibodies (Yuan et al. 2017; all from Santa Cruz Biotech); rabbit anti-MMP-9 (Sigma Chemicals); rabbit anti-MMP-13 (Abcam Inc.); anti-phospho-ERK (Upstate Inc.); and rabbit anti-ERK, anti-phospho-ELK-1, and ELK-1 (from Cell Signaling). This was followed by incubation with a horseradish peroxidase–conjugated secondary antibody (Santa Cruz Biotech) and development of the blots. Equal loading of the cell lysate was confirmed by stripping the membranes, reprobing with a rabbit anti-actin antibody (Santa Cruz Biotech), and developing the blot as described earlier. The images were quantified by ImageJ (National Institutes of Health).
Immunofluorescence
Fibrochondrocytes (5 × 104 cells/chamber) were seeded into slide chambers and maintained in serum-containing α-MEM until 70% confluent, fixed with 75% ethanol, permeabilized, and washed. After blocking with 1% bovine serum albumin, cells were incubated with primary antibodies specific for phospho-ERK or MMP-13 or control IgG for 3 h and washed. Following incubation with a fluorescent-labeled secondary antibody (Invitrogen) for 1 h and staining with DAPI (4′-6-diamidino-2-phenylindole), the cells were observed under a fluorescent microscope (Nikon TS100).
Mutagenesis and Luciferase-Reporter Assay
The mutants in the –1,017 MMP-9 promoter-luciferase vector (Tacon et al. 2010) were made with the site-directed mutagenesis kit (Invitrogen) and confirmed by sequencing. Wild-type (WT) or mutated NF-κB or ELK-1 sites of MMP-9 promoter-luciferase constructs were transfected with a β-galactosidase vector (Clontech) into cells seeded at 1.0 × 106 cells per 6-cm dish for 16 h as described. The cells were washed and maintained in α-MEM with 0.2% lactalbumin hydrolysate for 4 h before being incubated in fresh serum-free medium with E2. After 6 h of incubation with E2, cell lysates were collected and assayed for luciferase activity (Promega Corp.) and β-galactosidase activity with Tropix assay reagent (Applied Biosystems).
Statistical Analysis
Data are presented as mean ± SD fold change and analyzed by 1-way analysis of variance, followed by Sidak’s multiple comparisons test, with GraphPad Prism 7.04 software. The experiments were repeated at least 3 times, and P values <0.05 were considered statistically significant.
Results
E2 Enhances MMP-9 and MMP-13 Expression via ERα but Not ERβ
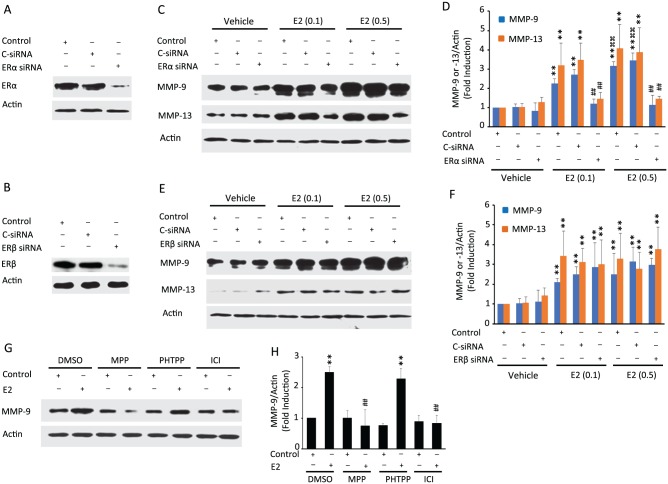
ERα and ERβ are highly expressed in primary TMJ fibrochondrocytes, and their expression was suppressed by ERα- and ERβ-specific siRNAs relative to their respective controls or control siRNA (Fig. 1A, B). With the doses of 0.1 and 0.5 ng/mL of E2 representing various serum concentrations in cycling women (Marsh et al. 2011), we found that E2 markedly increased the expression MMP-9 (molecular weight, 92-kDa proenzyme) and MMP-13 (molecular weight, 60-kDa proenzyme; Fig. 1C, D), with the higher concentration of E2 producing a more robust response than the lower dose. Knockdown of ERα led to a reduction in E2’s induction of MMP-9 and MMP-13 (Fig. 1C, D). In contrast, E2’s induction of MMP-9 and MMP-13 was not affected by the suppression of ERβ (Fig. 1E, F). We further confirmed the contribution of ERα but not ERβ to the modulation of MMPs by using ERα-specific inhibitor MPP, ERβ selective inhibitor PHTTP, and pan-ER inhibitor ICI (Dehghan et al. 2015) with cells stimulated with 0.5 ng/mL of E2. None of the inhibitors had any effect on basal MMP-9 levels (Fig. 1G, H). MPP and ICI almost completely blocked E2-induced MMP-9 expression, while inhibition of ERβ had no effect on E2’s induction of MMP-9. Similar suppressive effects of ERα inhibitors rather than the ERβ inhibitor on E2-induced MMP-13 were observed by immunofluorescence (Appendix Fig. 1).
Figure 1.
17β-estradiol (E2) upregulates matrix metalloproteinase (MMP) expression via estrogen receptor α (ERα) but not ERβ in mouse temporomandibular joint (TMJ) fibrochondrocytes. Untransfected cells (controls) or cells transiently transfected with control siRNA (C-siRNA) or siRNA to ERs were cultured in the absence or presence of E2. Western blot shows suppression of ERα (A) or ERβ (B) in TMJ fibrochondrocytes transfected with ERα- or ERβ-specific siRNAs, respectively. (C, D) Representative images and pooled data in the graph show that E2’s induction of MMP-9 and MMP-13 was abrogated in cells in which ERα expression was suppressed. (E, F) Representative images and pooled data in the graph demonstrate that inhibition of ERβ expression did not change E2-induced MMP-9 and MMP-13 levels. (G, H) The cells treated with pan-ER-antagonist (ICI) or ERα inhibitor (MPP) or ERβ inhibitor (PHTPP) and then stimulated with 0.5 ng/mL of E2 show that the induction of MMP-9 is inhibited in the presence of ICI and MPP but not by PHTPP. Actin was used as a loading control. **P < 0.01 vs. corresponding vehicle-treated control. ##P < 0.01 vs. control within E2 concentration treatment group. ⌘⌘P < 0.01 vs. corresponding E2 treatment group (0.1 ng/mL). Values are presented as mean ± SD fold change.
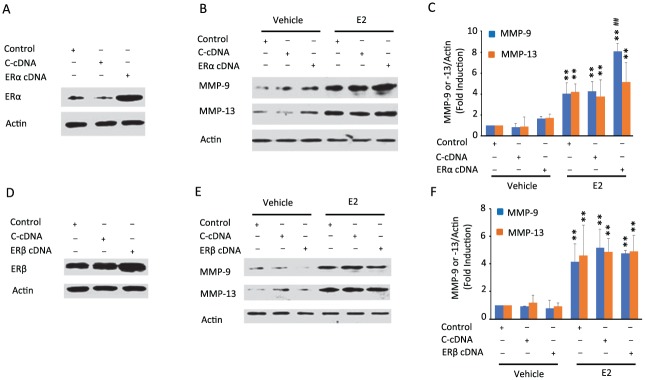
Next, we investigated whether ectopic expression of ERα or ERβ amplifies E2’s induction of MMP-9 and MMP-13 (Fig. 2). While overexpression of ERα or ERβ (Fig. 2A, B) alone did not modulate MMP-9 or MMP-13 (Fig. 2C–F), E2 increased expression of both MMPs, confirming previous observations. Importantly, ectopic expression of ERα but not ERβ amplified E2’s induction of MMP-9. In contrast, E2-induced MMP-13 was not amplified by either ERα or ERβ overexpression, suggesting a likely saturation of the response under the experimental conditions. Taken together, the data from loss-of-function and gain-of-function studies provide evidence that ERα but not ERβ is essential for E2-induced MMP-9 and MMP-13 expression in TMJ fibrochondrocytes.
Figure 2.
Estrogen receptor α (ERα) overexpression enhances matrix metalloproteinase 9 (MMP-9) expression but does not modulate MMP-13 expression by 17β-estradiol (E2) in mouse temporomandibular joint (TMJ) fibrochondrocytes, while ERβ overexpression does not affect E2-induced MMP-9 or MMP-13 expression. Untransfected cells (controls) or cells transiently transfected with pcDNA vector or ERα or ERβ cDNA constructs were cultured in the absence or presence of E2. Western blots of TMJ cell lysates showed overexpression of ERα (A) or ERβ (B) following transfection of cells with their respective pcDNA vectors. (C, D) Representative images and pooled graphical data show that overexpression of ERα increases the expression of MMP-9 induced by E2, with no effect on E2’s modulation of MMP-13 levels. (E, F) Representative images and pooled graphical data demonstrate that overexpression of ERβ does not modulate MMP-9 or MMP-13 expression by E2. Actin was used as an internal control. **P < 0.01 vs. corresponding vehicle-treated control. ##P < 0.01 vs. corresponding within E2 treatment controls. Values are presented as mean ± SD fold change.
Estrogen Induces Expression of MMP-9through the ERα/ERK Pathway
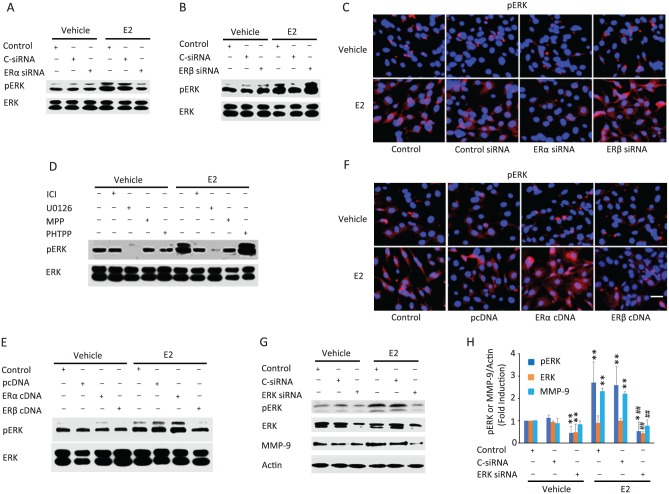
While E2 is known to activate ERK (Zhang et al. 2002), it is unknown whether this signaling pathway participates in E2-induced MMP-9 expression. We found that treatment of the fibrochondrocytes with E2 for 5 minutes resulted in ERK activation (Fig. 3A–D). Knockdown of ERα or ERβ did not influence basal phosphorylated ERK (Fig. 3A–C). Silencing of ERα but not ERβ reduced E2-induced ERK activation. The rapidity of this response also suggests that it occurs through nongenomic activity involving ERα. The concept is supported by findings that inhibition of ERα by MPP and ICI decreased E2-induced phosphorylated ERK responses similar to those found with MEK (a direct upstream signaling molecule of ERK) inhibitor U0126 action (Fig. 3D). Additional support for these findings is provided by experiments in which forced expression of ERα but not ERβ modestly increased E2-stimulated ERK activation (Fig. 3E, F). Together, the data provide evidence that E2 modulates rapid phosphorylation of ERK likely via its binding to ERα. Interestingly, PHTTP or ERβ siRNA increased while ERβ cDNA decreased E2-stimulated ERK activation, suggesting that ERβ has an opposite effect to that of ERα on ERK activation.
Figure 3.
Estrogen receptor α (ERα) and ERβ differentially regulate 17β-estradiol (E2)–induced phosphorylation of the ERK, which mediates matrix metalloproteinase 9 (MMP-9) induction in temporomandibular joint (TMJ) fibrochondrocytes. (A, B) Knockdown of ERα diminishes, and knockdown of ERβ enhances, E2-stimulated phosphorylation levels of ERK. (C) Immunofluorescence for pERK showing that E2 stimulation resulted in increased pERK staining (red), which was diminished in cells transfected with ERα siRNA and increased in cells transfected with ERβ siRNA relative to untransfected or C-siRNA-transfected controls. (D) E2-stimulated phosphorylation of ERK was inhibited by ICI, MPP, and pERK activation inhibitor U0126 but augmented by PHTPP. (E, F) Overexpression of ERα increased whereas overexpression of ERβ reduced the phosphorylation of ERK following stimulation with E2, as determined by Western blots and immunofluorescence. White bar in the lower right image in panel F represents 100 µm. (G, H) Western blot and pooled graphical data demonstrate that suppression of ERK by its siRNA abolished E2-induced upregulation of MMP-9. Actin was used as an internal control. *P < 0.05 and **P < 0.01 vs. corresponding vehicle-treated controls. ##P < 0.01 vs. corresponding within E2 treatment control and C-siRNA controls. Values are presented as mean ± SD fold change.
To determine any link between ERα-induced ERK activation and MMP-9 upregulation by E2, we assayed for MMP-9 expression following knockdown of ERK. Silencing of ERK resulted in the loss of E2-mediated ERK phosphorylation and subsequently impaired E2-mediated MMP-9 expression (Fig. 3G, H), indicating a mechanistic link between activation of ERK and increased MMP-9 level. The same pathway is involved in E2’s induction of MMP-13 (Appendix Fig. 1). Interestingly, our results also show that ERK phosphorylation is not critical to basal MMP-9 expression levels, implying that constitutively expressed MMP-9 may be regulated by other potential compensatory pathways, as demonstrated previously for MMP-2 (Kuo et al. 2006).
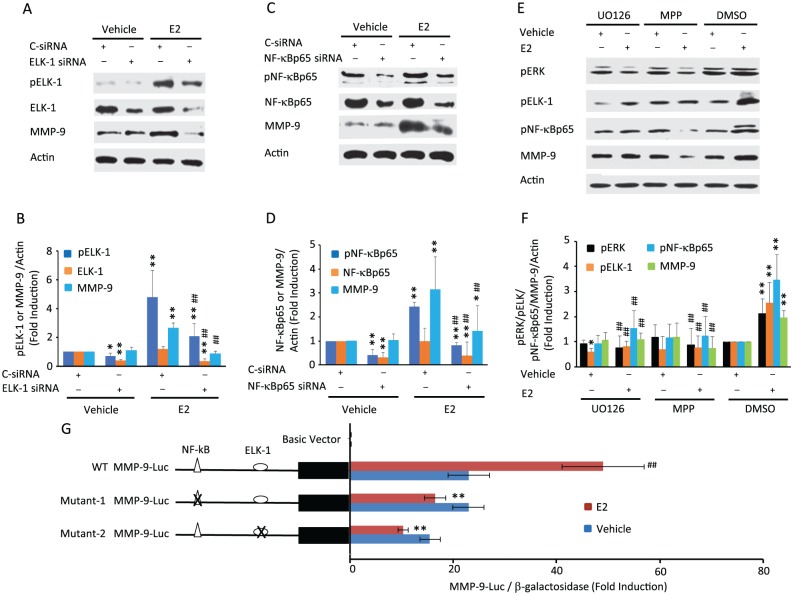
NF-κB and ELK-1 Are the Downstream Targets of the ERK Pathway for E2/ERα-Induced MMP-9 Expression
Since MMP-9 promoter contains NF-κB and ELK-1 binding sites and both are ERK downstream effectors (Eberhardt et al. 2000; Hsieh et al. 2008; Chen et al. 2016), we next examined whether E2 increases NF-κB and ELK-1 phosphorylation and what their possible contribution is to increased MMP-9 expression. E2 did not affect NF-κBp65 and ELK-1 levels but markedly increased phosphorylation of ELK-1 (Fig. 4A, B) and NF-κBp65 (Fig. 4C, D). Knockdown of ELK-1 and NF-κB by their respective siRNAs reduced E2-induced NF-κBp65 and ELK-1 phosphorylation. This was accompanied by the impairment of E2-induced MMP-9 expression, indicating that NF-κBp65 and ELK-1 activation is essential for E2’s induction of MMP-9.
Figure 4.
Inhibition of ELK-1 and NF-κB by their siRNAs or ERK and estrogen receptor α (ERα) with their respective inhibitors diminishes the 17β-estradiol (E2)–modulated phosphorylation of ELK-1 and/or NF-κBp65 with a corresponding decrease in matrix metalloproteinase 9 (MMP-9) induction. Additionally, mutagenesis of NF-κB or ELK-1 binding sites leads to a loss of E2-induced MMP-9 promoter activity. Western blots with quantified analysis show that (A, B) ELK-1 siRNA suppresses basal and E2-stimulated ELK-1 expression and phosphorylation and (C, D) NF-κB siRNA decreases basal and E2-stimulated expression and phosphorylation of NF-κBp65, with both resulting in the inhibition of E2’s induction of MMP-9. *P < 0.05 and **P < 0.01 vs. corresponding vehicle-treated controls. ##P < 0.01 vs. C-siRNA within E2 treatment groups. (E, F) ERK inhibitor, U0126, and ERα inhibitor MPP block E2-induced phosphorylation of ELK-1 and pNF-κBp65 and induction of MMP-9. Actin was used as an internal control. *P < 0.05 and **P < 0.01 vs. corresponding vehicle-treated DMSO controls. ##P < 0.01 vs. corresponding E2 treatment DMSO controls. (G) Studies with MMP-9 promoter-luciferase (MMP-9-Luc) constructs shows E2’s induction of wild-type (WT) MMP-9 promoter activity in fibrochondrocytes, which was abrogated when promoters were used with mutated NF-κB or ELK-1 binding sites in the promoter. **P < 0.01 vs. corresponding E2-treated WT MMP-9 promoter-luciferase construct. ##P < 0.01 vs. vehicle-treated wild-type MMP-9 promoter-luciferase construct. Values are presented as mean ± SD fold change.
To determine whether phosphorylated NF-κBp65 and ELK-1 are downstream mediators of E2/ERα-activated ERK signaling, we treated the fibrochondrocytes with U0126 or MPP in the presence or absence of E2. Treatment with U0126 and MPP resulted in an almost complete ablation of E2-induced NF-κBp65 and ELK-1 activation, subsequently leading to a reduction of E2-mediated MMP-9 induction (Fig. 4E, F), suggesting that NF-κBp65 and ELK-1 mediate E2/ERα/ERK-induced MMP-9 expression. Finally, to investigate whether activated NF-κB and ELK-1 are essential for MMP-9 transcription, we mutated the binding sites of NF-κB and ELK-1 in the MMP-9 promoter, which was confirmed by sequencing. Using these constructs, we showed that E2 significantly increased MMP-9 wild-type promoter activity, while the mutation of either NF-κB or ELK-1 binding sites resulted in a loss of basal and E2-induced MMP-9 promoter activity as compared with vehicle-treated control (Fig. 4G). These findings confirmed that NF-κB and ELK-1 binding sites are essential for E2-induced MMP-9 transcription.
Discussion
We elucidated the molecular mechanism by which E2 upregulates MMP-9 and MMP-13 expression in TMJ fibrochondrocytes. MMP-9 and MMP-13 are key enzymes responsible for cartilage ECM breakdown (Yang et al. 2008; Troeberg and Nagase 2012), which are also upregulated in TMJDs (Leonardi et al. 2008; Loreto et al. 2013). Our data show that E2 induces these MMPs specifically through ERα but not via ERβ. Furthermore, we show that E2’s induction of MMP-9 involves the MEK/ERK/NF-κBp65 and ELK-1 signaling pathways. The rapid activation of ERK by E2/ERα interactions within a few minutes, with the fact that MMP-9 and MMP-13 promoters lack estrogen response elements (Thaler et al. 2014), supports the concept that E2 drives MMP-9 and MMP-13 transcription through a nongenomic pathway.
E2’s modulation of MMPs appears to be highly cell and tissue specific (Afratis et al. 2017). Our findings on the upregulation of MMP-9 and MMP-13 by E2 in TMJ fibrochondrocytes concur with those of others in mesangial and endometrial (Nishi et al. 2013) cells. Interestingly, E2-mediated ECM loss, which is directly related to MMP activity, is also highly cell specific, including that in diverse types of fibrocartilaginous tissues (Hashem et al. 2006). The basis for the divergent responses of these tissues and cell types to E2 is not well understood, but several possible reasons may explain these observations. One explanation is that since E2 can have a bimodal effect (Lindheim et al. 1993), the use of varied E2 concentrations in these studies contributes to different MMP-inductive responses of E2. Another plausible reason is the relative cellular levels of ERα versus ERβ that can affect net cell responses because ERβ is known to repress ERα transcriptional activity by the same ligand and because these receptors often have opposing downstream effects (Hall and McDonnell 1999; Tee et al. 2004). Indeed, our study provides evidence that activation of ERα versus ERβ has opposite effects on ERK activation, supporting previous observations of the divergent responses by E2’s activation of its 2 primary receptors. We also demonstrate that ERα is specifically responsible for phosphorylation of ERK that further leads to activation of downstream targets triggering upregulation of MMP-9. This implies that despite the presence of ERα and ERβ in various cartilaginous tissues (Yamada et al. 2003; Kapila et al. 2009), the global effects of E2 on these tissues are likely mediated by the relative activities of both receptors that in turn are dictated by their relative expression levels.
Activation of NF-κB drives the expression of its proinflammatory target genes, such as interleukins 6 and 1β, leading to inflammation (Hoesel and Schmid 2013). As with its modulation of MMPs, E2’s effects on NF-κB activity are cell specific and partially dependent on E2 concentrations (Ghisletti et al. 2005; Hirano et al. 2007). Our studies not only confirm E2’s stimulation of NF-κB but also demonstrate that E2 does this via ERα and MEK/ERK phosphorylation in mouse TMJ fibrochondrocytes. More important, the blockage of NF-κB and mutated NF-κB binding site in the MMP-9 promoter diminishes E2-induced MMP-9 expression, suggesting that the NF-κB site is essential for E2-induced MMP-9 transcription. Pertinent to our studies, E2-activated NF-κB is a critical mediator of TMJ inflammation (Kou et al. 2011). Collectively, these findings suggest that NF-κB is an important convergence point for E2’s proinflammatory as well as MMP-9-inductive and matrix-degradative responses in the TMJ fibrocartilage. Therefore, NF-κB could serve as a potential therapeutic target against TMJ inflammation and degeneration caused by estrogen and increased ERα levels or activity.
Because ELK-1 is a direct target of ERK and has been shown to be important for MMP-9 induction in brain astrocytes (Wang et al. 2010), we also determined its role in E2’s induction of MMP-9 in TMJ fibrochondrocytes. We found that the phosphorylation status of the ELK-1 is enhanced by E2 treatment and is inhibited with a chemical inhibitor and an ERα-specific antagonist. Thus, our systematic analysis with specific chemical inhibitors identified the requirement of ELK-1 and NF-κB multiple regulatory elements for E2-mediated induction of MMP-9 that was earlier completely unknown. These results are further substantiated by knockdown of ERK, ELK-1, and NF-κB, leading to abrogation of E2’s upregulation of MMP-9. While the relative roles of NF-κB and ELK-1 in MMP-9 transcriptional activation by E2 are not known, the loss of E2-induced MMP-9 promoter activity by mutation of either NF-κB or ELK-1 binding sites suggests that both transcriptional factors are essential for E2-induced MMP-9 transcription. Further studies are warranted to identify whether the DNA fragment in the MMP-9 promoter forms a loop that is required for the binding of the 2 transcriptional factors (Tam et al. 2017) or whether the 2 factors participate together as a transcriptional complex in the MMP-9 promoter.
The pathophysiologic relevance of our findings for E2’s potential role in TMJ fibrocartilage turnover and TMJDs is supported by several previous studies. First, ERs have been localized to the human TMJ (Abubaker et al. 1993). Second, males and females with TMJDs have higher systemic levels of E2 than asymptomatic controls (Landi et al. 2005). Finally, single-nucleotide polymorphisms in human ERα gene that upregulate ERα expression (Herrington et al. 2002) are associated with the prevalence and severity of human TMJD and pain (Stemig et al. 2015), suggesting a possible mechanism by which ERα single-nucleotide polymorphisms may contribute to TMJDs through MMP induction and increased cartilage degradation, as shown in cells forced to overexpress ERα in our studies.
In summary, we conclusively identified novel pathways showing that ERα mediates E2’s induction of MMP-9 and MMP-13 in TMJ fibrochondrocytes and that induction of MMP-9 occurs through activation of ERK and NF-κB/ELK-1. Our observations and those of others on the higher levels of ERs in female versus male TMJs (Milam et al. 1987; Wang et al. 2009) may provide a possible rationale for the preponderance of TMJD in women of reproductive age. The results of this study suggest that at a molecular level, an aberrant modulation of ECM turnover by E2-induced MMP expression in TMJDs may be dependent on not only the systemic levels of E2 but also the receptor profiles in the target tissue. Understanding the signal transduction of MMP-9 and MMP-13 transcription induced by E2 will lead to better comprehension of clinical action of E2, ERα polymorphisms, and NF-κB activation in the pathogenesis of TMJDs. It is possible that manipulating differential signaling targets will yield therapeutic interventions for human TMJDs.
Author Contributions
N. Ahmad, W. Wang, contributed to design and data acquisition, drafted and critically revised the manuscript; S. Chen, contributed to conception, data analysis, and interpretation, drafted and critically revised the manuscript; S. Kapila, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518767108 for 17β-estradiol Induces MMP-9 and MMP-13 in TMJ Fibrochondrocytes via Estrogen Receptor α by N. Ahmad, S. Chen, W. Wang and S. Kapila in Journal of Dental Research
Acknowledgments
We thank Dr. Leigh from the Health Research Innovation Centre, Calgary, Canada, for providing the MMP-9 promoter-luciferase construct.
Footnotes
A supplemental appendix to this article is available online.
This study was supported by the National Institutes of Health / National Institute of Dental and Craniofacial Research (grant R01 DE018455 to S.K.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abubaker AO, Raslan WF, Sotereanos GC. 1993. Estrogen and progesterone receptors in temporomandibular joint discs of symptomatic and asymptomatic persons: a preliminary study. J Oral Maxillofac Surg. 51(10):1096–1100. [DOI] [PubMed] [Google Scholar]
- Afratis NA, Bouris P, Skandalis SS, Multhaupt HA, Couchman JR, Theocharis AD, Karamanos NK. 2017. IGF-IR cooperates with ERα to inhibit breast cancer cell aggressiveness by regulating the expression and localisation of ecm molecules. Sci Rep. 7:40138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Wang W, Nair R, Kapila S. 2012. Relaxin induces matrix-metalloproteinases-9 and -13 via RXFP1: induction of MMP-9 involves the PI3K, ERK, Akt and PKC-ζ pathways. Mol Cell Endocrinol. 363(1–2):46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börjesson AE, Lagerquist MK, Windahl SH, Ohlsson C. 2013. The role of estrogen receptor α in the regulation of bone and growth plate cartilage. Cell Mol Life Sci. 70(21):4023–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Liu J, Ho TT, Ding X, Mo YY. 2016. ERK-mediated NF-κB activation through ASIC1 in response to acidosis. Oncogenesis. 5(12):e279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan F, Yusof A, Muniandy S, Salleh N. 2015. Estrogen receptor (ER)-α, β and progesterone receptor (PR) mediates changes in relaxin receptor (RXFP1 and RXFP2) expression and passive range of motion of rats’ knee. Environ Toxicol Pharmacol. 40(3):785–791. [DOI] [PubMed] [Google Scholar]
- Eberhardt W, Huwiler A, Beck KF, Walpen S, Pfeilschifter J. 2000. Amplification of IL-1 beta-induced matrix metalloproteinase-9 expression by superoxide in rat glomerular mesangial cells is mediated by increased activities of NF-kappa B and activating protein-1 and involves activation of the mitogen-activated protein kinase pathways. J Immunol. 165(10):5788–5797. [DOI] [PubMed] [Google Scholar]
- Eyster KM. 2016. The estrogen receptors: an overview from different perspectives. Methods Mol Biol. 1366:1–10. [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, Vegeto E. 2005. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol. 25(8):2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. 1999. The estrogen receptor beta-isoform (ERbeta) of the human estrogen receptor modulates ERalpha transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 140(12):5566–5578. [DOI] [PubMed] [Google Scholar]
- Hashem G, Zhang Q, Hayami T, Chen J, Wang W, Kapila S. 2006. Relaxin and beta-estradiol modulate targeted matrix degradation in specific synovial joint fibrocartilages: progesterone prevents matrix loss. Arthritis Res Ther. 8(4):R98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington DM, Howard TD, Brosnihan KB, McDonnell DP, Li X, Hawkins GA, Reboussin DM, Xu J, Zheng SL, Meyers DA, et al. 2002. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on e-selectin but not c-reactive protein. Circulation. 105(16):1879–1882. [DOI] [PubMed] [Google Scholar]
- Hirano S, Furutama D, Hanafusa T. 2007. Physiologically high concentrations of 17beta-estradiol enhance NF-kappaB activity in human T cells. Am J Physiol Regul Integr Comp Physiol. 292(4):R1465–R1471. [DOI] [PubMed] [Google Scholar]
- Hoesel B, Schmid JA. 2013. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 12:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HL, Wu CY, Yang CM. 2008. Bradykinin induces matrix metalloproteinase-9 expression and cell migration through a PKC-delta-dependent ERK/Elk-1 pathway in astrocytes. Glia. 56(6):619–632. [DOI] [PubMed] [Google Scholar]
- Kapila S, Wang W, Uston K. 2009. Matrix metalloproteinase induction by relaxin causes cartilage matrix degradation in target synovial joints. Ann N Y Acad Sci. 1160:322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S, Oursler MJ, Monroe DG. 2012. Estrogen and the skeleton. Trends Endocrinol Metab. 23(11):576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou XX, Wu YW, Ding Y, Hao T, Bi RY, Gan YH, Ma X. 2011. 17β-estradiol aggravates temporomandibular joint inflammation through the NF-κB pathway in ovariectomized rats. Arthritis Rheum. 63(7):1888–1897. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chang HC, Leu TH, Maa MC, Hung WC. 2006. Src oncogene activates MMP-2 expression via the ERK/Sp1 pathway. J Cell Physiol. 207(3):729–734. [DOI] [PubMed] [Google Scholar]
- Landi N, Lombardi I, Manfredini D, Casarosa E, Biondi K, Gabbanini M, Bosco M. 2005. Sexual hormone serum levels and temporomandibular disorders: a preliminary study. Gynecol Endocrinol. 20(2):99–103. [DOI] [PubMed] [Google Scholar]
- Leonardi R, Loreto C, Barbato E, Caltabiano R, Lombardo C, Musumeci G, Lo Muzio L. 2008. MMP-13 (collagenase 3) localization in human temporomandibular joint discs with internal derangement. Acta Histochem. 110(4):314–318. [DOI] [PubMed] [Google Scholar]
- Levin ER, Hammes SR. 2016. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 17(12):783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindheim SR, Presser SC, Ditkoff EC, Vijod MA, Stanczyk FZ, Lobo RA. 1993. A possible bimodal effect of estrogen on insulin sensitivity in postmenopausal women and the attenuating effect of added progestin. Fertil Steril. 60(4):664–667. [DOI] [PubMed] [Google Scholar]
- Loreto C, Leonardi R, Musumeci G, Pannone G, Castorina S. 2013. An ex vivo study on immunohistochemical localization of MMP-7 and MMP-9 in temporomandibular joint discs with internal derangement. Eur J Histochem. 57(2):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maixner W, Diatchenko L, Dubner R, Fillingim RB, Greenspan JD, Knott C, Ohrbach R, Weir B, Slade GD. 2011. Orofacial pain prospective evaluation and risk assessment study—the OPPERA study. J Pain. 12(11 Suppl):T4–T11.e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh EE, Shaw ND, Klingman KM, Tiamfook-Morgan TO, Yialamas MA, Sluss PM, Hall JE. 2011. Estrogen levels are higher across the menstrual cycle in African-American women compared with Caucasian women. J Clin Endocrinol Metab. 96(10):3199–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, Kracht M, Schmitz ML. 2004. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol. 172(10):6336–6344. [DOI] [PubMed] [Google Scholar]
- Milam SB, Aufdemorte TB, Sheridan PJ, Triplett RG, Van Sickels JE, Holt GR. 1987. Sexual dimorphism in the distribution of estrogen receptors in the temporomandibular joint complex of the baboon. Oral Surg Oral Med Oral Pathol. 64(5):527–532. [DOI] [PubMed] [Google Scholar]
- Naqvi T, Duong TT, Hashem G, Shiga M, Zhang Q, Kapila S. 2005. Relaxin’s induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants. Arthritis Res Ther. 7(1):R1–R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi H, Kuroda M, Isaka K. 2013. Estrogen and estrogen receptor induce matrix metalloproteinase-26 expression in endometrial carcinoma cells. Oncol Rep. 30(2):751–756. [DOI] [PubMed] [Google Scholar]
- Stemig M, Myers SL, Kaimal S, Islam MS. 2015. Estrogen receptor-alpha polymorphism in patients with and without degenerative disease of the temporomandibular joint. Cranio. 33(2):129–133. [DOI] [PubMed] [Google Scholar]
- Tacon CE, Wiehler S, Holden NS, Newton R, Proud D, Leigh R. 2010. Human rhinovirus infection up-regulates MMP-9 production in airway epithelial cells via NF-{kappa}B. Am J Respir Cell Mol Biol. 43(2):201–209. [DOI] [PubMed] [Google Scholar]
- Tam KT, Chan PK, Zhang W, Law PP, Tian Z, Fung Chan GC, Philipsen S, Festenstein R, Tan-Un KC. 2017. Identification of a novel distal regulatory element of the human neuroglobin gene by the chromosome conformation capture approach. Nucleic Acids Res. 45(1):115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. 2004. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 15(3):1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JD, Achari Y, Lu T, Shrive NG, Hart DA. 2014. Estrogen receptor beta and truncated variants enhance the expression of transfected MMP-1 promoter constructs in response to specific mechanical loading. Biol Sex Differ. 5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troeberg L, Nagase H. 2012. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim Biophys Acta. 1824(1):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, Hsieh HL, Wu CY, Yang CM. 2010. Oxidized low-density lipoprotein-induced matrix metalloproteinase-9 expression via PKC-delta/p42/p44 MAPK/Elk-1 cascade in brain astrocytes. Neurotox Res. 17(1):50–65. [DOI] [PubMed] [Google Scholar]
- Wang W, Hayami T, Kapila S. 2009. Female hormone receptors are differentially expressed in mouse fibrocartilages. Osteoarthritis Cartilage. 17(5):646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Nozawa-Inoue K, Kawano Y, Kohno S, Amizuka N, Iwanaga T, Maeda T. 2003. Expression of estrogen receptor alpha (ER alpha) in the rat temporomandibular joint. Anat Rec A Discov Mol Cell Evol Biol. 274(2):934–941. [DOI] [PubMed] [Google Scholar]
- Yang Y, Lu N, Zhou J, Chen ZN, Zhu P. 2008. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford). 47(9):1299–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Zhao L, Rakariyatham K, Han Y, Gao Z, Muinde Kimatu B, Hu Q, Xiao H. 2017. Isolation of a novel bioactive protein from an edible mushroom Pleurotus eryngii and its anti-inflammatory potential. Food Funct. 8(6):2175–2183. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Maier B, Santen RJ, Song RX. 2002. Membrane association of estrogen receptor alpha mediates estrogen effect on MAPK activation. Biochem Biophys Res Commun. 294(5):926–933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518767108 for 17β-estradiol Induces MMP-9 and MMP-13 in TMJ Fibrochondrocytes via Estrogen Receptor α by N. Ahmad, S. Chen, W. Wang and S. Kapila in Journal of Dental Research