Abstract
The objective of our experiments was to identify new therapeutic strategies to stimulate dentin formation in an adult tooth. To address this objective, we evaluated dentin production in 2 acute trauma models: one involving a pulp exposure and the other involving a superficial dentin injury. Molecular, cellular, and histologic analyses revealed that in response to a severe injury, where the pulp is exposed to the oral cavity, cell death is rampant and the repair response initiates from surviving pulp cells and, to a lesser extent, surviving odontoblasts. When an injury is superficial, as in the case of a dentin injury model, then disturbances are largely confined to pulp tissue immediately underneath the damaged dentin tubules. We found that the pulp remained vital and innervated; primary odontoblasts upregulated HIF1α; and the rate of mineralization was significantly increased. A tamoxifen-inducible Axin2CreERT2/+; R26RmTmG/+ reporter strain was then used to demonstrate that a population of long-lived Wnt-responsive odontoblasts, which secreted dentin throughout the life of the animal, were responsible for depositing new dentin in response to a superficial injury. Amplifying Wnt signaling in the pulp stimulates dentin secretion, and in the dentin injury model, we show that a liposomal formulation of human WNT3A protein passes through dentinal tubules and is capable of upregulating Wnt signaling in the pulp. These data provide strong proof of concept for a therapeutic pulp-capping material to stimulate Wnt signaling in odontoblasts and thus improve the pulp repair response.
Keywords: dental pulp, dentinogenesis, Wnt3A protein, cell death, regeneration, molar
Introduction
The dentin-pulp complex is capable of repair proportional to the severity of the trauma, and its primary response is to produce more dentin in an attempt to insulate the pulp from further damage. The type of new dentin formed can differ in its cellular origin (when it emerges), its microstructure, and its barrier function. Despite considerable debate about what to call this dentin that forms in response to an injury (Kuttler 1959), it is generally agreed that new dentin produced by odontoblasts has a tubular structure, while the dentin produced by pulp cells does not (Tomes 1878; Saito et al. 2016). The former process is considered an amplified version of the normal dentin secretion that occurs throughout life, while the latter process is more rapid and evoked by acute injury (Fish 1931).
The objective of our experiments was to identify new therapeutic strategies to stimulate dentin formation in an adult tooth. Therefore, we first needed to identify which cells were responsible for the new dentin formation after an injury. Pulp exposure models have been employed for decades to study how a tooth responds to acute injury. A small exposure is made through the dentin, which creates a direct communication between the pulp and oral cavity. While this lesion triggers a robust response for examination, investigators have been equally aware of its limitations. In 1931, Fish wrote that if an attempt is made to simulate a natural lesion by cutting an experimental cavity that penetrates into the pulp, “a very serious discrepancy . . . exists between the natural and the experimental lesion, in that the former [develops] gradually, while the latter lays open a large number of tubules to the mouth fluids. The effect of these fluids . . . may be much more severe than any reaction observed under early caries.”
We considered other injury models. The removal of enamel and dentin in one location, similar to a cavity preparation, allows the pulp cavity to remain intact, but it still elicits a repair response from the pulp (see, e.g., Murray, Kitasako, et al. 2002). There is, however, an inherent disadvantage to this method in that the experimental injury may be significantly less severe than natural lesions created by attrition and/or caries.
We considered the advantages and disadvantages of each model. With an array of molecular, cellular, and genetic tools at our disposal, we revisited the question of how the pulp responds to trauma by comparing, over time, the biological reaction to a pulp exposure versus a dentin injury. We discovered that the response of primary odontoblasts to superficial injury involves activation of the endogenous Wnt pathway, followed by the secretion of new dentin in an attempt to wall off the pulp to the noxious stimulus. A therapeutic strategy was tested whereby exogenous WNT3A was delivered via the dentinal tubules to augment the Wnt-based repair response of the pulp. Together these data provide insights into a therapeutic strategy to enhance the secretion of dentin by preexisting odontoblasts.
Methods and Materials
Animals
Procedures were approved by the Stanford Committee on Animal Research and conformed to the ARRIVE guidelines (Kerschnitzki et al. 2011). Axin2CreERT2/+; R26RmTmG/+ (018867 and 007576) mice were obtained from Jackson Lab.
Preparation of L-tamoxifen, L-WNT3A, and L-PBS
Tamoxifen (T5648; Sigma-Aldrich) was incorporated into a liposome to produce L-tamoxifen. In the other cases, tamoxifen was combined with WNT3A or phosphate-buffered saline (PBS) to create liposome-tamoxifen-WNT3A (L-tam-WNT3A) or liposome-tamoxifen-PBS (L-tam-PBS).
Surgery
To induce Cre expression, tamoxifen (4 mg per 25 g of body weight) was delivered intraperitoneally for 3 consecutive days following the superficial tooth injury or lineage tracing.
Adult mice (2 to 3 mo, 72 mice) were anesthetized with an intraperitoneal injection of ketamine (80 mg/kg) and xylazine (8 mg/kg). A 0.3-mm-diameter round bur (E0123; Dentsply Maillefer) was used to generate cavities on maxillary molars. We attempted to drill into the dental fossa to avoid injuring the pulp horns, but due to the number of cusps, the size of the drill, and the overall size of the mouse molar, it was inevitable that some drilling removed enamel near to or directly at the cusp tip. To produce pulp exposures, the same cavity was generated; then, a sterilized endo explorer (DG16; Hu-Friedy) was advanced until the pulp cavity was encountered. In some cases, after preparation of the injury site, L-tam-WNT3A or L-tam-PBS was swabbed onto the floor of the prepared cavity. In all surgery, Ketac Cem Easy Mix Glass Ionomer Cement (3M ESPE) was used to cover the cavity.
For sample preparation, processing, histology, histomorphometric assays, and micro–computed tomography analyses (Lim et al. 2014; Minear et al. 2010), see Appendix.
Statistical Analysis
Student’s t test was used to quantify differences between 2 groups; for multiple groups, 1-way analysis of variance, followed by Tukey’s multiple comparison test. P < 0.05 was significant. GraphPad Prism 7 (GraphPad Software, Inc.) was used for these analyses.
Results
Pulp Exposure Results in Necrosis and Widespread Persistent Inflammation
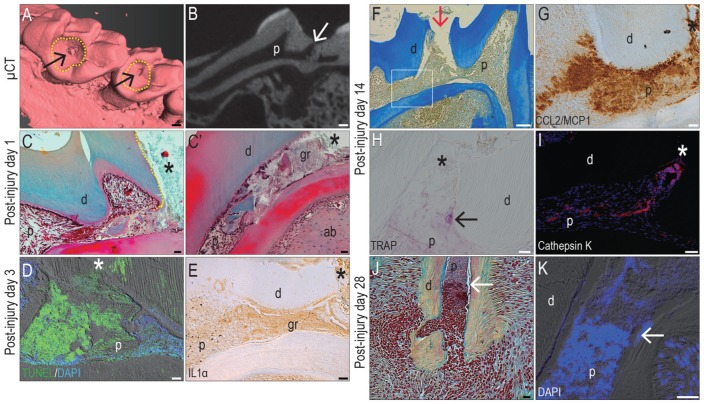
Pulp exposure models have been extensively employed to study how odontoblasts and pulp cells respond to acute injury (Byers and Taylor 1993). In this model, enamel and some dentin are removed from a healthy molar, followed by a pinpoint pulp exposure (arrows, Fig. 1A, B). Within 24 h, degenerated tissue accumulated near the point of exposure (Fig. 1C, C′). Extensive programmed cell death was evident throughout the pulp chamber, and this apoptotic response expanded with time (Fig. 1D, Appendix Fig. 1). Cell death was accompanied with rampant inflammation, as shown by widespread interleukin 1α expression (Fig. 1E).
Figure 1.
A pulp exposure model results in extensive cell death and prolonged inflammation. In adult maxillary molars, 3-dimensional rendering of µCT imaging identifies the sites of (A) enamel and partial dentin removal (dotted circles) and pulp exposure (arrow); (B) µCT sections verify that the pulp cavity has been accessed (arrow). Representative pentachrome-stained tissue sections on PID1 identify (C) cut dentin (dotted line) flanking the site of pulp exposure (hereafter indicated with an asterisk); (C′) adjacent to the exposure site, granulation tissue and debris accumulate. (D) On PID3, TUNEL activity is detectable throughout the pulp cavity and is associated with (E) a widespread inflammatory reaction, evidenced by immunostaining for IL1α. On PID14 (F), representative tissue sections of the pulp exposure site (arrow) show no evidence of new dentin formation; instead (G) inflammation persists, as evidenced by CCL2/MCP1 immunostaining. (H) TRAP activity (black arrow) and (I) cathepsin K immunostaining indicate resorption continues through PID14. On PID28, (J) pentachrome staining and (K) DAPI staining demarcate the boundary (white arrows) between the nonvital and vital pulp that remains in the roots. ab, alveolar bone; d, dentin; gr, granulation tissue; IL1α, interleukin 1 α; p, pulp; PID, postinjury day; µCT, micro–computed tomography. Scale bars: 100 µm (A, B, F), 50 µm (I), 25 µm (C, C′, D, E, G, H, J, K).
The inflammatory response was persistent; even on postinjury day 14 (PID14), most cells near the exposure (Fig. 1F) were immunopositive for CCL2/MCP1 (Fig. 1G). In the pulp chamber, TRAP activity (Fig. 1H) and cathepsin K expression (Fig. 1I) indicated that internal resorption had commenced. Although the exact location (or locations) of the degenerating versus surviving odontoblasts changed with each injury, a diffuse boundary could oftentimes be observed between surviving and dying odontoblasts, especially in the roots. For example, on PID28, pentachrome staining highlighted one such boundary: pyknotic nuclei of the nonvital pulp tissues (Fig. 1J) were distinguished from vital tissues by DAPI staining (Fig 1K).
A Dentin Injury Model Preserves Pulp Vitality and Stimulates New Dentin Formation
We sought to replicate in a mouse model the clinically relevant scenario where the pulp is induced to form new dentin in response to superficial trauma (Murray et al. 2000). In this mouse model, enamel and dentin were removed (Fig. 2A). We attempted to place the injuries in the fossa and avoid injuring the pulp horns, although this was not always possible (Appendix Fig. 2). The pulp chamber was not accessed. The response of the pulp to this type of damage was investigated over the course of a month.
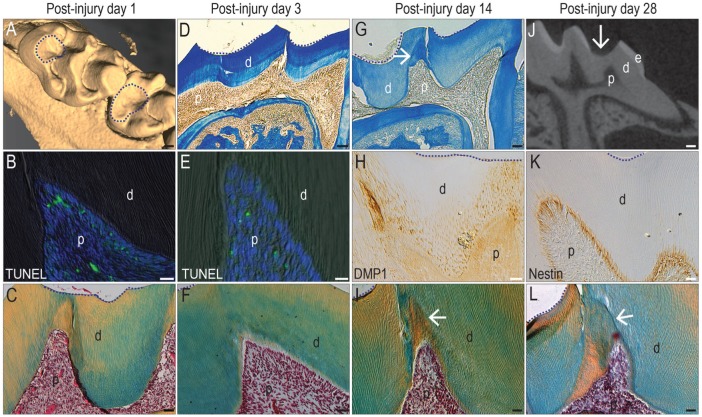
Figure 2.
A dentin injury model elicits a reparative response from odontoblasts. In adult maxillary molars, µCT imaging identifies sites of (A) enamel and some dentin removal but no pulp exposure (dotted circles). On PID1, (B) representative tissue sections stained with TUNEL show evidence of apoptosis. (C) On an adjacent pentachrome-stained tissue section, the pulp exhibits a normal morphology (in all panels, dotted line indicates the edge of the injury). On PID3, (D) aniline blue staining indicates a dentin bridge between the injury (dotted lines) and the pulp. (E) On an adjacent tissue section, TUNEL identifies few apoptotic cells, and (F) the pulp continues to exhibit a normal morphology. By PID14, (G) aniline blue staining identifies new dentin formation (arrow) underneath the injury site (dotted lines), and (H) DMP1 immunostaining identifies odontoblasts responsible for secreting the new matrix and (arrow, I) new dentin formed. By PID28, (J) µCT sections show that a dentin bridge separates the injury site from the pulp (arrow). (K) Odontoblasts underneath the injury site are nestin+ve, and (L) the new dentin formed by the surviving odontoblasts has protected the pulp from exposure (arrow). d, dentin; e, enamel; p, pulp; PID, postinjury day; µCT, micro–computed tomography. Scale bars: 100 µm (A, D, G, J), 25 µm (B, C, E, F, H, I, K, L).
On PID1, apoptotic odontoblasts were detected directly underneath the drill site (Fig. 2B, Appendix Fig. 3), demonstrating that even without a pulp exposure, the dentin injury caused some odontoblast. The extent of cell death, however, is significantly less than that observed in a pulp exposure model (Fig. 1D, Appendix Fig. 1). At this time point, no obvious histologic changes were noted (Fig. 2C). On PID3, the injury site remained separated from the pulp by a preexisting dentin bridge (Fig. 2D), and TUNEL staining continued to be minimal (Fig. 2E, Appendix Fig. 3). Again, no obvious histologic changes were observed (Fig. 2F). By PID14, new dentin had begun to accumulate (arrow, Fig. 2G). The sites of new matrix deposition corresponded to sites where primary odontoblasts strongly expressed DMP1 (Fig. 2H). The tubular structure of the dentin (Fig. 2I) indicated that the new matrix arose from primary odontoblasts (Goldberg and Smith 2004; Tziafas 2004).
By PID28, the preexisting dentin bridge still separated the pulp from the injury site (Fig. 2J). Underneath the bridge, odontoblasts were strongly nestin+ve (Fig. 2K), indicating their functionality (About et al. 2000). By PID28, new dentin had formed near the site of superficial injury (arrow, Fig. 2L), creating a thicker dentin bridge. Collectively, these data indicated that this dentin injury model caused a subpopulation of cells to undergo apoptosis; however, most pulp cells remained vital, and the reparative response was primarily due to increased matrix secretion by primary odontoblasts.
Characterizing the Reparative Response of Primary Odontoblasts
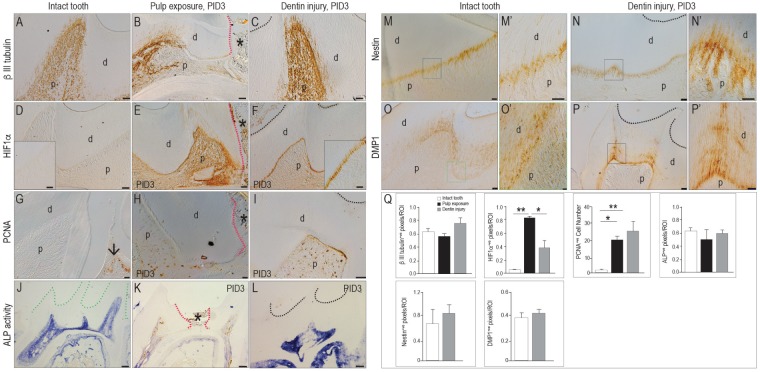
Our next objective was to identify the most prominent features distinguishing a reparative reaction in primary odontoblasts from a homeostatic response. We began with an analysis of βIII tubulin expression as a marker of pulp vitality (Marrelli et al. 2015). In an intact pulp, βIII tubulin is widely expressed in odontoblasts and pulp cells (Fig. 3A). In the pulp exposure model (Fig. 3B), βIII tubulin was undetectable at the exposure site, but at distant sites some innervated odontoblasts remained immunopositive (Fig. 3B). In the dentin injury group, βIII tubulin was strongly expressed in pulp cells, odontoblasts, and their processes underneath the injury site (Fig. 3C; quantified in Q).
Figure 3.
Expression of HIF1α and the extent of cell proliferation differentiate between a homeostatic and repair response from odontoblasts. Immunostaining for the neural marker βIII tubulin in an (A) intact molar versus PID3 following (B) a pulp exposure (indicated by an asterisk and red dotted lines) or (C) a dentin injury (where the floor of the cavity preparation is indicated with a black dotted line). Immunostaining for the hypoxia marker HIF1α in (D) an intact molar versus PID3 following (E) a pulp exposure or (F) a dentin injury. Immunostaining for PCNA to detect mitotically active cells in an (G) intact molar versus PID3 following (H) a pulp exposure or (I) a dentin injury. ALP activity shown in (J) an intact molar (green dotted line indicates the coronal dentin) versus PID3 following (K) a pulp exposure or (L) a dentin injury. Nestin is expressed in functional odontoblasts in (M, M′) an intact molar and (N, N′) odontoblasts underneath a dentin injury. DMP1 is expressed in secreting odontoblasts in (O, O′) an intact molar and (P, P′) odontoblasts underneath a dentin injury. (Q) Quantification of the immunostaining in an ROI defined by the injury. Values are presented as mean ± SE. *P < 0.05. **P < 0.01. ALP, alkaline phosphatase; d, dentin; p, pulp; PCNA, proliferating cell nuclear antigen; PID, postinjury day; ROI, region of interest; µCT, micro–computed tomography. Scale bars: 100 µm (J–L), 25 µm (A–F, I, N–P), 10 µm (G, H, M, M′, O′, S′, P′).
Injury often compromises blood supply, which produces a hypoxic environment; we therefore evaluated the pulp tissues for HIF1α expression. Under normoxic conditions, HIF1α is degraded (Nathan 2003), and its expression is undetectable in the intact pulp (Fig. 3D). After an injury, however, HIF1α accumulates to modulate a repair response (Nathan 2003; Zhang et al. 2015). As expected, HIF1α was strongly expressed by pulp cells in the pulp exposure cases (Fig. 3E). In dentin injury cases, HIF1α expression was restricted to primary odontoblasts (Fig. 3F, quantified in Q), indicating that this model specifically triggered a reparative response from primary odontoblasts.
We evaluated the pulp’s mitotic activity. The adult pulp is a largely quiescent tissue (Huang et al. 2016), and in keeping with this observation, PCNA (proliferating cell nuclear antigen) immunostaining was minimal in an intact pulp (arrow, Fig. 3G) and abundant in the adjacent gingiva (Fig. 3G). In the pulp exposure model (Fig. 3H) and dentin injury model (Fig. 3I, quantified in Q), pulp cells were mitotically active. These data indicate that injury triggers a proliferative response in an otherwise quiescent tissue.
Using alkaline phosphatase (ALP) activity to monitor active mineralization of the dentin matrix, we found moderate staining in intact pulp chambers and in the roots (Fig. 3J). In the pulp exposure model, ALP activity was weakly detected and then only at a distance from the exposure site (Fig. 3K). In contrast, odontoblasts and pulp cells in the dentin injury model were strongly ALP+ve throughout the cavity (Fig. 3L, quantified in Q).
Active odontoblasts are labeled by nestin and DMP1 (Goldberg and Smith 2004). As expected, odontoblasts were nestin+ve and DMP1+ve in the intact pulp (Fig. 3M–O′; quantified in Q). Nestin immunostaining was undetectable in the pulp exposure model (not shown), but primary odontoblasts underneath the dentin injury site were strongly nestin+ve and DMP1+ve (Fig. 3N–P′; quantified in Q). In summary, the cellular “hallmarks” of a reparative reaction from primary odontoblasts were upregulation of HIF1α and a notable increase in cell proliferation. Other features, including expression of βIII tubulin, nestin, and DMP1, were either shared with uninjured odontoblasts or found in severely damaged pulp.
Wnt-Responsive Primary Odontoblasts Are Responsible for New Dentin Formation after Superficial Trauma
Amplifying Wnt signaling after a pulp exposure stimulates osteodentin formation (Hunter et al. 2015). We sought to identify which cells were responsive to a Wnt stimulus and to determine whether those Wnt-responsive cells were directly accountable for producing new dentin in a dentin injury model involving a tamoxifen-inducible Axin2CreERT2/+; R26RmTmG/+ reporter strain of mice. When tamoxifen is delivered, Axin2-expressing Wnt-responsive cells and their progeny express green fluorescent protein (GFP). This strategy genetically labels a population that is responsive to an endogenous Wnt signal and their descendants (Ransom et al. 2016).
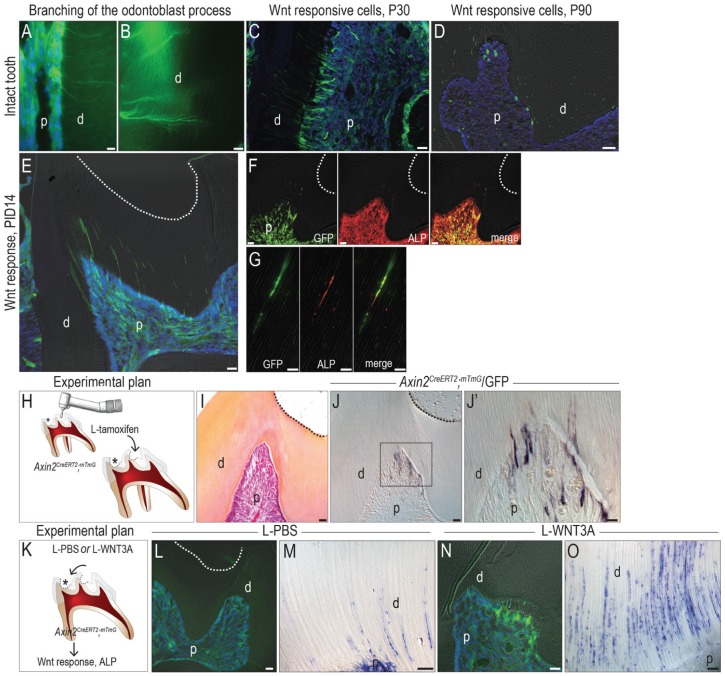
In an intact pulp, a fraction of pulp cells and almost all odontoblasts were responsive to Wnt (Fig. 4A). Their Wnt responsiveness was robust enough to observe the lateral branches of odontoblast processes (Fig. 4B). In young pups (e.g., postnatal day 30), a 14-d chase period resulted in labeling of nearly all the odontoblasts and their processes (Fig. 4C). In an adult, however, endogenous Wnt signaling was much lower; the same 14-d chase resulted in minimal odontoblast labeling (Fig. 4D). These data demonstrate that Wnt signaling in the pulp declines with age. It was in these adult mice that we subsequently performed a dentin injury.
Figure 4.
L-WNT3A amplifies endogenous Wnt signaling and the repair response from primary odontoblasts. In Axin2CreERT2/+; R26RmTmG/+ reporter mice, GFP immunofluorescence identifies (A) Wnt-responsive pulp cells and (B) odontoblasts and their processes. (C) In a juvenile, most odontoblasts are Wnt responsive, but (D) in an adult, very few odontoblasts remain Wnt responsive. (E) GFP immunofluorescence on PID14 demonstrates a significant increase in the number of Wnt-responsive cells in the pulp following a dentin injury. Coimmunostaining of GFP and ALP demonstrates that most Wnt-responsive pulp cells are strongly positive for ALP (F) in the cell body as well as (G) in the processes. (H) Schematic showing experimental plan, where a dentin injury (asterisk) is created in Axin2CreERT2/+; R26RmTmG/+ mice and then tamoxifen, as formulated in a liposome, is applied to the floor of the cavity preparation. (I) Pentachrome staining of the floor of the cavity preparation on PID14. (J, J′) GFP immunostaining on an adjacent section identifies Wnt-responsive odontoblasts underneath the dentin injury site. (K) Schematic showing experimental plan, where a dentin injury (asterisk) is created in Axin2CreERT2/+; R26RmTmG/+ mice and then either PBS or WNT3A protein, as formulated in liposomes, is applied to the floor of the cavity preparation. (L) Following L-tam-PBS treatment, GFP immunostaining identifies Wnt-responsive odontoblasts. (M) ALP activity in odontoblast processes on PID3. (N) Following L-tam-WNT3A treatment, GFP immunostaining identifies Wnt-responsive odontoblasts underneath the injury site. (O) Significantly more ALP activity was detected in odontoblast processes. ALP, alkaline phosphatase; d, dentin; GFP, green fluorescent protein; p, pulp; P30, postnatal day 30; P90, postnatal day 90; PBS, phosphate-buffered saline; PID, postinjury day. Scale bar: 25 µm (C–F, I, J, L, N), 10 µm (A, B, G, J′, M, O).
Dentin injury triggered a dramatic increase in endogenous Wnt signaling, demonstrated by widespread distribution of Wnt-responsive cells on PID14. Underneath the injury site, GFP fluorescence was detected in odontoblast processes and in the pulp chamber (Fig. 4E), much like the pattern observed in the young pulp (Fig. 4C). The Wnt-responsive odontoblasts were responsible for the increased ALP activity that we had observed following dentin injury (Fig. 3L). We used immunostaining for GFP and ALP to demonstrate their colocalization in the pulp cavity (Fig. 4F) and odontoblast processes (Fig. 4G). These data indicate that the pulp responds to injury by upregulating endogenous Wnt signaling and that the repair response is subsequently mediated by these Wnt-activated cells.
We tested if supplying exogenous WNT3A to a dentin injury would enhance its repair response. First, we aimed to demonstrate if a WNT-based medication could be delivered to the pulp via dentinal tubules. To do this, we prepared a superficial injury in Axin2CreERT2/+; R26RmTmG/+ mice and then delivered tamoxifen via a novel method: we encapsulated the hydrophobic molecule in a liposome and swabbed the solution onto the floor of prepared cavity (Fig. 4H, I). GFP is expressed only by Wnt-responsive cells exposed to tamoxifen, and the only source of tamoxifen was via the dentinal tubules; consequently, the detection of GFP+ve cells in the pulp of the Axin2CreERT2/+; R26RmTmG/+ mice proved that L-tamoxifen could penetrate tubular dentin. On PID14, GFP+ve odontoblasts were found underneath—and only underneath—the dentin injury site (Fig. 4J, J′).
As a second step, we produced the same dentin injuries in Axin2CreERT2/+; R26RmTmG/+ mice and then treated the cavity preparations with either L-tam-PBS or L-tam-WNT3A (see Methods; Fig. 4K). Compared with L-tam-PBS-treated dentin injuries (Fig. 4L), those treated with L-tam-WNT3A showed more GFP staining (Fig. 4N). When compared with the ALP activity elicited by dentin injury (Fig. 4N), the L-tam-WNT3A-treated dentin injuries showed considerably broader ALP activity (Fig. 4O), demonstrating that the liposome-packaged WNT3A could amplify the reparative response observed by primary odontoblasts.
Discussion
If they survive the trauma, odontoblasts generate new dentin matrix in an attempt to protect the pulp. The monikers used to describe this new dentin may change (reviewed in Tomes 1878; Kuttler 1959; Tziafas 2004), but what is invariant is the microstructure of the new dentin: when pulp cells secrete dentin, the resulting material has a bone-like characteristic; however, when preexisting odontoblasts secrete dentin, the resulting material has a tubular structure (About et al. 2001; Ricucci et al. 2014).
Here, our goal—like many before us (Fish 1931)—was to distinguish the reparative response of primary odontoblasts from the reparative response of pulp cells, for a relatively straightforward reason: the majority of clinical cases do not involve intentional exposure of an otherwise healthy pulp. Instead, most clinical cases that progress to the stage where a root canal is needed begin with superficial trauma to the pulp (Bjørndal 2002). If primary odontoblasts respond robustly to this trauma and secrete copious amounts of new dentin, then the pulp generally retains its vitality (Kawashima and Okiji 2016). When that repair response is attenuated, then the result is typically chronic inflammation, followed by pulp necrosis (Cooper et al. 2010). Our goal here was to simulate this clinically relevant scenario and, in doing so, gain insights into how the reparative response could be amplified.
Pulp exposures (Fig. 1) were compared with the equivalent of a deep cavity preparation that purposefully avoided pulp exposure (Fig. 2). The most important distinction was that, in contrast to a pulp exposure, the dentin injury model resulted in the formation of new dentin by preexisting odontoblasts. Apoptosis was detected but not past PID3; inflammation was short-lived; and the pulp remained vital (Fig. 2). In contrast, the pulp exposure model resulted in widespread cell death, rampant and prolonged inflammation, and the survival of small amounts of pulp tissue remaining in the root tips (Fig. 1). In neither case was the pulp exposure associated with a bacterial contamination, as would be expected in a carious lesion. This can be considered a limitation of almost all rodent models of pulp injury because, without contamination, some part of the pulp will remain vital. Since infection and uncontrolled inflammation are barriers to a repair response (Bergenholtz 2001), these sterile models likely reveal the maximum pulp repair response in an otherwise healthy young animal.
So how might the presence of a bacterial infection affect the pulp repair response? Despite many advantages, rodent models are not readily amenable to this line of inquiry. The reason is that the genomic response to acute inflammatory stresses (e.g., pulp exposure) correlate poorly with analogous human conditions and also with one another (Seok et al. 2013).
Wnt-Responsive Odontoblasts Are Responsible for the Secretion of New Dentin after a Subacute Injury
In nongrowing teeth, such as murine and human molars, new “odontoblast-like” cells are responsible for the secretion of new dentin following trauma (Murray, Kitasako, et al. 2002; Babb et al. 2017; reviewed by Goldberg 2011). Here we demonstrated that these primary odontoblasts, which normally secrete dentin throughout life and generate new dentin after superficial injury, are all derived from a Wnt-responsive population (Figs. 2, 4).
The Wnt-responsive status of primary odontoblasts changes with age. In the young pulp, almost all odontoblasts are Wnt responsive, but with aging, only a subset maintains their Wnt-responsive status. The sources of the endogenous Wnt signal are unknown but may be the odontoblast itself (Babb et al. 2017). Why the Wnt-responsive status declines with age is also unknown, but this diminution in endogenous Wnt signaling follows the well-described age-related loss in reparative potential (Murray et al. 2000; Murray, Matthews, et al. 2002; Feng et al. 2013).
One potential therapeutic strategy to stimulate the repair potential of an aged pulp would be to enhance Wnt signaling back to levels seen in youth. Other investigators attempted to activate Wnt signaling with a similar goal, albeit a different strategy, in mind. Here, we used recombinant WNT protein, whereas Ishimoto et al. (2015) used lithium chloride to activate Wnt signaling; nonetheless, the collective results point to an ability to stimulate odontoblasts to increase secretion of dentin.
Whether a WNT-based treatment strategy could be used to stimulate new dentin formation by odontoblasts remains an open question. We validated that a drug can be delivered to the pulp via the dentinal tubules (Fig. 4), but while the dose was sufficient to activate a recombination event (e.g., the expression of GFP in a Wnt reporter strain of mice), it is unclear whether the dosage of L-WNT3A was sufficient to elicit a robust repair response. Therein lies a limitation to this study: the dentin injury and, indeed, a pulp exposure are both created in an otherwise healthy tooth in an otherwise healthy adult animal. These characteristics do not match most patients undergoing endodontic care. Consequently, it will be of considerable importance to test such therapeutic strategies in animal models that fully mimic the patient population.
Author Contributions
Y. Zhao, contributed to data acquisition, analysis, and interpretation, drafted the manuscript; X. Yuan, B. Liu, U.S. Tulu, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; J.A. Helms, contributed to conception, design, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518763151 for Wnt-Responsive Odontoblasts Secrete New Dentin after Superficial Tooth Injury by Y. Zhao, X. Yuan, B. Liu, U.S. Tulu and J.A. Helms in Journal of Dental Research
Acknowledgments
We thank Luis Cordova, Benny Coyac, Masaki Anioka, and Brian Patrick Leahy for their invaluable suggestions on data collection.
Footnotes
This research project was supported by grants from the National Institutes of Health (R01DE024000-11) to J.A.H., the National Natural Science Foundation of China (81500835) to Y.Z., and the Fundamental Research Funds for the Central Universities (lzujbky-2015-36 and lzujbky-2015-290) to Y.Z.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
ORCID iDs: X. Yuan  https://orcid.org/0000-0002-8063-9431
https://orcid.org/0000-0002-8063-9431
J.A. Helms  https://orcid.org/0000-0002-0463-396X
https://orcid.org/0000-0002-0463-396X
References
- About I, Laurent-Maquin D, Lendahl U, Mitsiadis TA. 2000. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am J Pathol. 157(1):287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- About I, Murray PE, Franquin JC, Remusat M, Smith AJ. 2001. The effect of cavity restoration variables on odontoblast cell numbers and dental repair. J Dent. 29(2):109–117. [DOI] [PubMed] [Google Scholar]
- Babb R, Chandrasekaran D, Carvalho Moreno Neves V, Sharpe PT. 2017. Axin2-expressing cells differentiate into reparative odontoblasts via autocrine Wnt/β-catenin signaling in response to tooth damage. Sci Rep. 7(1):3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergenholtz G. 2001. Factors in pulpal repair after oral exposure. Adv Dent Res. 15:84. [DOI] [PubMed] [Google Scholar]
- Bjørndal L. 2002. Dentin and pulp reactions to caries and operative treatment: biological variables affecting treatment outcome. Endodontic Topics. 2(1):10–23. [Google Scholar]
- Byers MR, Taylor PE. 1993. Effect of sensory denervation on the response of rat molar pulp to exposure injury. J Dent Res. 72(3):613–618. [DOI] [PubMed] [Google Scholar]
- Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ. 2010. Inflammation-regeneration interplay in the dentine-pulp complex. J Dent. 38(9):687–697. [DOI] [PubMed] [Google Scholar]
- Feng X, Xing J, Feng G, Sang A, Shen B, Xu Y, Jiang J, Liu S, Tan W, Gu Z, et al. 2013. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/β-catenin signaling. Cell Mol Neurobiol. 33(8):1023–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW. 1931. The reaction of the dental pulp to peripheral injury of the dentine. Proc Roy Soc London Series B. 108(756):196–208. [Google Scholar]
- Goldberg M. 2011. Pulp healing and regeneration: more questions than answers. Adv Dent Res. 23(3):270–274. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Smith AJ. 2004. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 15(1):13–27. [DOI] [PubMed] [Google Scholar]
- Huang L, Salmon B, Yin X, Helms JA. 2016. From restoration to regeneration: periodontal aging and opportunities for therapeutic intervention. Periodontol 2000. 72(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, Bardet C, Mouraret S, Liu B, Singh G, Sadoine J, Dhamdhere G, Smith A, Tran XV, Joy A, et al. 2015. Wnt acts as a prosurvival signal to enhance dentin regeneration. J Bone Miner Res. 30(7):1150–1159. [DOI] [PubMed] [Google Scholar]
- Ishimoto K, Hayano S, Yanagita T, Kurosaka H, Kawanabe N, Itoh S, Ono M, Kuboki T, Kamioka H, Yamashiro T. 2015. Topical application of lithium chloride on the pulp induces dentin regeneration. PLoS One. 10(3):e0121938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima N, Okiji T. 2016. Odontoblasts: specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom (Kyoto). 56(4):144–153. [DOI] [PubMed] [Google Scholar]
- Kerschnitzki M, Wagermaier W, Roschger P, Seto J, Shahar R, Duda GN, Mundlos S, Fratzl P. 2011. The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. J Struct Biol. 173(2):303–311. [DOI] [PubMed] [Google Scholar]
- Kuttler Y. 1959. Classification of dentine into primary, secondary, and tertiary. Oral Surg Oral Med Oral Pathol. 12(8):996–999. [DOI] [PubMed] [Google Scholar]
- Lim WH, Liu B, Cheng D, Hunter DJ, Zhong Z, Ramos DM, Williams BO, Sharpe PT, Bardet C, Mah SJ, et al. 2014. Wnt signaling regulates pulp volume and dentin thickness. J Bone Miner Res. 29(4):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli M, Paduano F, Tatullo M. 2015. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J Dent Res. 94(6):843–852. [DOI] [PubMed] [Google Scholar]
- Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. 2010. Wnt proteins promote bone regeneration. Sci Transl Med. 2(29):29ra30. [DOI] [PubMed] [Google Scholar]
- Murray PE, About I, Lumley PJ, Franquin JC, Remusat M, Smith AJ. 2000. Human odontoblast cell numbers after dental injury. J Dent. 28(4):277–285. [DOI] [PubMed] [Google Scholar]
- Murray PE, Kitasako Y, Tagami J, Windsor LJ, Smith AJ. 2002. Hierarchy of variables correlated to odontoblast-like cell numbers following pulp capping. J Dent. 30(7–8):297–304. [DOI] [PubMed] [Google Scholar]
- Murray PE, Matthews JB, Sloan AJ, Smith AJ. 2002. Analysis of incisor pulp cell populations in Wistar rats of different ages. Arch Oral Biol. 47(10):709–715. [DOI] [PubMed] [Google Scholar]
- Nathan C. 2003. Immunology: oxygen and the inflammatory cell. Nature. 422(6933):675–676. [DOI] [PubMed] [Google Scholar]
- Ransom RC, Hunter DJ, Hyman S, Singh G, Ransom SC, Shen EZ, Perez KC, Gillette M, Li J, Liu B, et al. 2016. Axin2-expressing cells execute regeneration after skeletal injury. Sci Rep. 6:36524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricucci D, Loghin S, Lin LM, Spangberg LS, Tay FR. 2014. Is hard tissue formation in the dental pulp after the death of the primary odontoblasts a regenerative or a reparative process? J Dent. 42(9):1156–1170. [DOI] [PubMed] [Google Scholar]
- Saito K, Nakatomi M, Ida-Yonemochi H, Ohshima H. 2016. Osteopontin is essential for type I collagen secretion in reparative dentin. J Dent Res. 95(9):1034–1041. [DOI] [PubMed] [Google Scholar]
- Seok J, Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, et al. 2013. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 110(9):3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomes CS. 1878. On the structure and development of vascular dentine. Phil Trans R Soc Lond. 169(1878):25–47. [Google Scholar]
- Tziafas D. 2004. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 38(3):314–320. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Strehin I, Bedelbaeva K, Gourevitch D, Clark L, Leferovich J, Messersmith PB, Heber-Katz E. 2015. Drug-induced regeneration in adult mice. Sci Transl Med. 7(290):290ra292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518763151 for Wnt-Responsive Odontoblasts Secrete New Dentin after Superficial Tooth Injury by Y. Zhao, X. Yuan, B. Liu, U.S. Tulu and J.A. Helms in Journal of Dental Research