Abstract
Pharmacokinetic studies with oral treprostinil demonstrate that three times daily (TID) dosing reduces peak-to-trough plasma trepostinil fluctuations compared with twice daily (BID) dosing. TID dosing may allow for faster titration, higher total daily doses, and potentially improve the tolerability of oral trepostinil. This analysis, which looks at the real-world dosing of oral treprostinil, supports the utility of TID dosing.
Keywords: treprostinil, oral treprostinil, dosing, real-world use
Introduction
Oral treprostinil was approved by the U.S. Food and Drug Administration in December 2013 as the first oral prostacyclin analogue for the treatment of pulmonary arterial hypertension (PAH). The recommended starting dose is 0.25 mg twice daily (BID) or 0.125 mg three times daily (TID), with dose titrations of 0.25–0.5 mg BID or 0.125 mg TID every 3–4 days to the highest tolerated dose.1 The initial pivotal clinical trials2–4 evaluated a BID dosing regimen but generated interest for a TID dosing strategy. A long-term, open-label extension study evaluated both BID and TID regimens, yielding dosing, tolerability, and safety data over a period of up to 8.5 years.5 Pharmacokinetic data suggest that TID dosing reduces peak-to-trough plasma treprostinil fluctuations compared to BID dosing and may improve overall tolerability.6,7
Given the flexibility in dosing, the purpose of this analysis is to characterize the “real-world” dosing of oral treprostinil in clinical practice, including total daily doses (TDDs) achieved, rates of titration for BID and TID dosing, and the impact of previous exposure to treprostinil.
Methods
Medication shipment records were collected from patients who received at least one shipment of oral treprostinil from their specialty pharmacy service (SPS) between June 2014 (when first commercially available) and October 2016. Patients who switched between BID and TID dosing, patients who transferred between SPS providers, and patients in clinical studies were excluded. From the complete dataset of eligible patients, 76% of patients were prescribed TID dosing and 24% of patients were prescribed BID dosing.
For this study, a representative sample of 1600 patients was randomly selected for further analyses. This sample included 1200 patients receiving TID dosing and 400 patients receiving BID dosing, to reflect similar frequencies of BID and TID dosing from the complete dataset of eligible patients. Patients were further segmented as “de novo” (i.e. those who never received treatment with treprostinil) and “transition” (i.e. those who received intravenous/subcutaneous (IV/SC) or inhaled treprostinil shipments up to six months before being transitioned to oral treprostinil), resulting in the following patient subsets: TID-de novo (n = 758); TID-transition (n = 442); BID-de novo (n = 320); and BID-transition (n = 80).
Dosing was reported as shown in the SPS shipment records. If a patient received multiple shipments in a given month, then the TDD was based on the highest dose reported. Titration rates were calculated for de novo patients based on the dose acceleration rate (DAR), calculated by measuring the slope of dose increase from month 1 to month 6. The DARs were not reported for transition patients, as these patients likely received both formulations (oral and IV/SC or inhaled) during the transition period. Comparisons in DAR were made using the Wilcoxon test.
Results
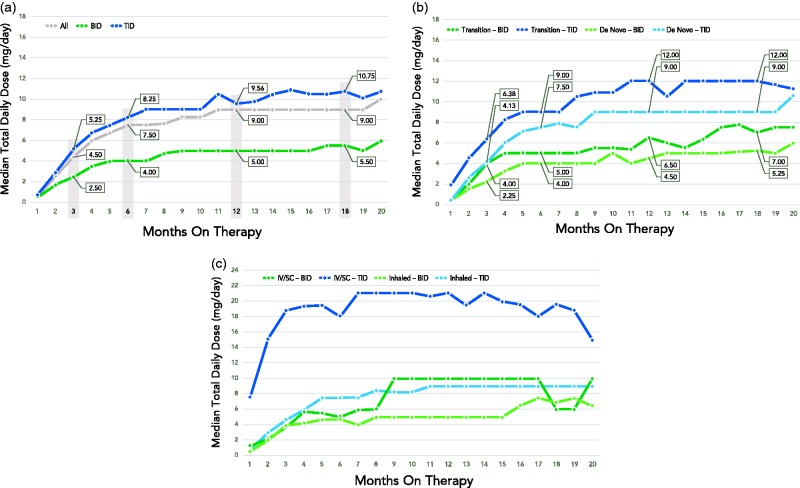
In the overall TID dosing group from the representative sample (n = 1200), the median TDD achieved was 5.3 mg, 8.3 mg, 9.6 mg, and 10.8 mg at 3, 6, 12, and 18 months, respectively (Fig. 1a). In the BID dosing group (n = 400), the median TDD was 2.5 mg, 4.0 mg, 5.0 mg, and 5.5 mg at 3, 6, 12, and 18 months, respectively. The median DARs were 1.2 mg/month for the TID-de novo group (n = 336) and 0.6 mg/month for the BID-de novo group (n = 122). The difference in DARs between groups was statistically significant (P < 0.0001).
Fig. 1.
Median total daily dose of a representative sample of oral treprostinil patients: (a) overall, TID, and BID (TID: n = 840, 602, 338, and 171 at 3, 6, 12, and 18 months; BID: n = 250, 179, 89, and 44 at 3, 6, 12, and 18 months); (b) by TID and BID, transition vs. de novo (Transition – BID: n = 58, 43, 26, and 9 at 3, 6, 12, and 18 months; Transition – TID: n = 335, 241, 143, and 82 at 3, 6, 12, and 18 months; De Novo – BID: n = 192, 136, 63, and 35 at 3, 6, 12, and 18 months; De Novo – TID: n = 505, 361, 195, and 89 at 3, 4, 12, and 18 months); and (c) by TID and BID for transition patients only, based on previous treatment (IV/SC vs. inhaled). Representative sample data were captured between June 2014 and October 2016; duration on therapy is dependent on start date and treatment discontinuation, as applicable, during this period.
TID-transition patients achieved the highest median TDD, followed by TID-de novo patients, BID-transition patients, and BID-de novo patients (Fig. 1b). Among transition patients, the median dose of IV/SC treprostinil was 44.0 ng/kg/min and the median time on parenteral therapy before transitioning was 20.9 months. The median dose of inhaled treprostinil was nine breaths four times daily (QID) and the median time on therapy before transitioning was 15.3 months. In the TID-transition patients, the median TDD achieved was 6.4 mg, 9.0 mg, 12.0 mg, and 12.0 mg at 3, 6, 12, and 18 months, respectively. In the BID-transition patients, the median TDD was 4.0 mg, 5.0 mg, 6.5 mg, and 7.0 mg at 3, 6, 12, and 18 months, respectively. TID-transition patients who transitioned from IV/SC treprostinil received the highest median starting TDD of 7.5 mg and achieved the highest median TDD of 21 mg (Fig. 1c).
Discussion
These data indicate that TID dosing is three times more prevalent than BID dosing in the real-world clinical setting. Up-titration during the first six months of therapy was twice as fast with TID versus BID dosing.
We speculate the more prevalent use of TID dosing and the ability to reach higher TDDs may reflect an improved tolerability profile as compared with BID dosing. In an open-label trial, a pharmacokinetic analysis of a cohort of patients transitioning from BID to TID dosing found that TID reduced the peak-to-trough ratio (increased Cmin) and was associated with improved tolerability.6 In a separate study that evaluated patients transitioning from parenteral to oral treprostinil, both BID and TID dosing showed systemic exposures similar to the parenteral formulations; however, the TID regimen reduced the peak-to-trough fluctuations compared with BID dosing.7 A more stable plasma concentration may reduce the frequency and severity of adverse events, facilitating dose up-titration.
It is interesting to contrast the oral treprostinil doses achieved in our analysis relative to those achieved in clinical trials. The mean doses achieved were 3.0 mg and 3.1 mg BID at 16 weeks in two studies that evaluated oral treprostinil added on to background PAH therapies,2,3 and 3.4 mg BID at 12 weeks in a treatment-naïve population.4 An open-label extension study reported mean doses of 3.6 ± 2.7 mg, 4.2 ± 3.1 mg, and 5 ± 3.7 mg BID at 6, 12, and 24 months, respectively.1,8
In our analysis, median TDDs were 2.5 mg with BID dosing and 5.3 mg with TID dosing at 12 weeks. Up-titration continued, such that at the end of our 18-month analysis, TDDs were 5.5 mg with BID dosing and 10.8 mg with TID dosing. Thus, the TDDs achieved with BID dosing in the real world were generally lower when compared to those achieved with BID dosing in the controlled clinical trials. However, the TDDs achieved with TID dosing in the real world were generally higher when compared to doses from clinical trials. This is important as it has been suggested that doses achieved using a BID dosing strategy in the controlled clinical trials may be insufficient.9,10 Data from a recent post-hoc analysis of the studies that evaluated oral treprostinil added on to background PAH therapies suggest a dose response effect, with 6-min walk distance (6MWD) improving steadily in patients achieving higher doses.11 In a recent consensus document developed using the “Delphi Process” methodology, investigators strongly recommended TID dosing versus BID dosing, and also set target doses of 4 mg TID at 3 months, 6 mg TID at 6 months, and 8 mg TID at 1 year.9
An appropriate dose of oral treprostinil required in an early, low-risk patient is likely lower than the dose required in a patient transitioning from parenteral prostacyclins. For patients transitioning from IV/SC treprostinil, a higher dose of oral treprostinil is likely necessary for similar treprostinil exposure. In this analysis, patients on IV/SC treprostinil before transitioning to oral treprostinil achieved a median dose of 44.0 ng/kg/min. In a retrospective analysis, Benza et al.12 reported that approximately 40 ng/kg/min of parenteral treprostinil is associated with improved long-term survival. If ∼1 mg TID of oral treprostinil provides a treprostinil exposure similar to a 6-ng/kg/min infusion in a 70-kg patient,7 ∼20 mg (up to 7 mg TID) may be pharmacokinetically equivalent to 40-ng/kg/min infusion. Notably, in our analysis, we found the subset of patients who transitioned from IV/SC treprostinil to TID dosing with oral treprostinil achieved the highest median TDD (total doses >20 mg per day). These data seem to suggest that a strategy of “priming” with IV/SC treprostinil treatment may enable initiating oral therapy at a higher starting dose and allow patients to achieve higher TDDs. An ongoing prospective study is underway to test this hypothesis.
The major limitation of our study is that the data were collected from shipment records. Consequently, we were not able to provide any qualitative insights on the use of TID versus BID dosing such as adverse events or efficacy data to correspond with the dose titrations and TDDs achieved. We also were unable to collect any patient-level baseline data to help characterize treatment patterns. Another important limitation is that a selection bias exists, as only data for patients who were able to stay on therapy were analyzed.
In summary, our analysis suggests that prescribers favor TID dosing over BID dosing of oral treprostinil in real-world clinical practice. We believe this is related to an improved tolerability profile, especially during up-titration, and an ability to achieve higher TDDs for potentially greater clinical benefit. We note that a treatment strategy that incorporates use of IV/SC treprostinil before oral TID dosing may be a reasonable approach to achieve higher TDDs.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Orenitram® (treprostinil) extended-release tablets [Prescribing Information]. Research Triangle Park, NC: United Therapeutics Corp.
- 2.Tapson VF, Torres F, Kermeen F, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (the FREEDOM-C study): a randomized controlled trial. Chest 2012; 142: 1383–1390. [DOI] [PubMed] [Google Scholar]
- 3.Tapson VF, Jing ZC, Xu KF, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients on background endothelin receptor antagonist and/or phosphodiesterase type 5 inhibitor therapy (The FREEDOM-C2 study): a randomized controlled trial. Chest 2013; 144: 952–958. [DOI] [PubMed] [Google Scholar]
- 4.Jing ZC, Parikh K, Pulido T, et al. Efficacy and safety of oral treprostinil monotherapy for the treatment of pulmonary arterial hypertension: a randomized, controlled trial. Circulation 2013; 127: 624–633. [DOI] [PubMed] [Google Scholar]
- 5.Data on file. Research Triangle Park, NC: United Therapeutics Corp.
- 6.White RJ, Frutiger K, Theuer A, et al. A pharmacokinetic and tolerability comparison in subjects transitioning from twice daily to three times daily dosing of oral treprostinil. Chest 2014; 146(4_MeetingAbstracts): 865A. [Google Scholar]
- 7.Chakinala MM, Feldman JP, Rischard F, et al. Transition from parenteral to oral treprostinil in pulmonary arterial hypertension. J Heart Lung Transplant 2017; 36: 193–201. [DOI] [PubMed] [Google Scholar]
- 8.Kumar P, Thudium E, Laliberte K, et al. A Comprehensive review of treprostinil pharmacokinetics via four routes of administration. Clin Pharmacokinet 2016; 55: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahaghi FF. Recommendations for the use of oral treprostinil in clinical practice: a Delphi consensus project pulmonary circulation. Pulm Circ 2017; 7(1): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White RJ, Torres F, Allen R, et al. Pharmacokinetics of oral treprostinil sustained release tablets during chronic administration to patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol 2013; 61(6): 474–481. [DOI] [PubMed] [Google Scholar]
- 11.White RJ, Rao Y. Novel analysis of the oral treprostinil combination therapy trial data. Am J Respir Crit Care Med 2016; 193(12): 1434–1436. [DOI] [PubMed] [Google Scholar]
- 12.Benza RL, Gomberg-Maitland M, Naeije R, et al. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trial. J Heart Lung Transplant 2011; 30: 982–989. [DOI] [PubMed] [Google Scholar]