Abstract
Pulmonary arteriovenous malformations (PAVMs) often occur in children with hereditary hemorrhagic telangiectasia (HHT). A 14-year longitudinal study of PAVMs in children with HHT was undertaken to assess the prevalence, the clinical impact, and progression of these malformations. This was a retrospective, single-center study from May 2002 to December 2016 of 129 children with HHT diagnosed using Curacao criteria and/or confirmed by genetic testing. Transthoracic contrast echocardiography (TTCE) was the primary screening modality in all patients and PAVMs were diagnosed based on Barzilai criteria. Moderately positive TTCE (Barzilai criteria ≥ 2) was confirmed with subsequent contrast chest CT. New PAVMs were diagnosed with a positive TTCE after an initial negative TTCE. Embolization of PAVMs were performed according to HHT consensus guidelines. Of 129 children with HHT, 76 (59%) were found to have PAVMs. Sixty-seven (88%) were positive for PAVMs on initial screening. Of 63 children without PAVMs on initial screening, 31 were followed for >1 year. Nine of the 31 (29%) developed new PAVMs after initial negative study. Thirty-eight (50%) of the total 76 children with PAVMs had or developed lesions large enough to be treated with embolization. Nine patients with PAVMs initially too small to be treated with embolization, developed progression of disease and ultimately were treated with embolization over time. The majority, 60% (23/38), of the children with large PAVMs had no related clinical symptoms. After embolization, 21% (8/38), of patients underwent repeat interventions. Genetic diagnosis, age, and gender were not associated with risk of having PAVM nor with need for repeat interventions. Nearly 60% of children with HHT develop PAVMs. The risk for new PAVMs to develop, small PAVMs to become large, and previously embolized PAVMs to require further intervention remains throughout childhood. Thus, children with HHT require continued follow-up until adulthood.
Keywords: pediatric hereditary hemorrhagic telangiectasia, pulmonary arteriovenous malformation, embolization, pediatrics
Introduction
Hereditary hemorrhagic telangiectasia (HHT), also known as Rendu-Osler-Weber disorder, is an autosomal dominant disease affecting approximately 1:5000.1–5 The disease is characterized by mucocutaneous telangiectasia and arteriovenous malformations (AVMs) in several organs, but primarily affecting the lungs, gastrointestinal tract, and brain.6–10 The majority of cases of HHT are due to germline mutations in the endoglin (ENG) gene, activin receptor-like kinase1 (ALK1) gene, and SMAD family 4 (SMAD4) gene, referred to as HHT type 1 (HHT1), type 2 (HHT2), and type 3 (HHT3), respectively.11–14 All three genes are involved in TGFb signaling.
HHT is clinically diagnosed based on the Curaçao criteria, which include recurrent epistaxis, telangiectasia, visceral AVMs, and a first-degree family member with HHT.15 While epistaxis is the most common presenting symptom in adults, almost half of patients remain asymptomatic until adulthood, making diagnosis in childhood challenging.7,16–19 The more concerning clinical aspect of HHT is the presence of AVMs. Patients with large visceral AVMs may suffer significant morbidity and even death. Pulmonary AVMs (PAVMs) are the most common AVMs seen in patients with HHT and have been associated with debilitating and life-threatening complications such as stroke, cerebral abscess, massive hemoptysis, and hemothorax. While these complications have been reported predominantly in the adult population, they are also known to occur albeit less frequently in pediatric HHT cohorts20–24 where the prevalence of PAVMs approaches that in adults. Symptoms such as shortness of breath, exercise intolerance, clubbing, cyanosis, and hemoptysis may lead to the diagnosis of PAVMs in children; however, more than half (56%) of children diagnosed with PAVMs may be asymptomatic at the time of detection.19
While the prevalence of PAVMs reported in children approaches the rate reported in adults, questions regarding the natural history of PAVMs in children remain largely unknown: Are children with HHT at risk for developing PAVMs at any age and what identifiable risk factors might they have? If children have no or minimal PAVMs at initial diagnosis of their HHT, what risk do they have for the subsequent development of PAVMs and/or their worsening? Finally, how often do PAVMs progress to the point of needing intervention and perhaps even several interventions? The answers to these questions have obvious implications for counseling families and setting expectations regarding future follow-up care. This 14-year longitudinal study of 129 children with HHT is one of the largest and longest studies to date designed to help provide answers to these questions.
Methods
Patient population and study design
This study was approved by the Washington University Institutional Review Board; given the retrospective nature of the review, informed consent was waived. The medical records of 197 pediatric patients referred for evaluation of HHT from May 2002 through December 2016 were reviewed. Patients diagnosed with definite HHT were included in the study. HHT was diagnosed clinically by fulfilling ≥ 3 Curaçao criteria and/or confirmed with genetic testing. Curaçao criteria include recurrent spontaneous epistaxis, cutaneous telangiectasia, visceral arteriovenous malformations, and a first-degree relative with confirmed HHT.15 Patients with suspected or unlikely clinical diagnosis (Curaçao criteria <3) were excluded. Genetic testing was offered to all patients with possible or suspected HHT.
A review of the medical record was performed to identify information regarding patient characteristics such as age at diagnosis of HHT, sex, genetic mutation, prevalence of pulmonary AVMs, and clinical symptoms. Primary outcomes evaluated included the prevalence of PAVMs on initial screening, freedom from PAVM over time, incidence of PAVM-related complications, and treatment with embolization.
Screening, diagnosis, and treatment of PAVMs
Transthoracic contrast echocardiography (TTCE) was the initial screening method for the presence of PAVMs in all patients in the study. PAVMs were diagnosed in the absence of intracardiac shunting based on Barzilai criteria.25 New PAVMs were diagnosed by a positive TTCE after a previous negative TTCE. A positive TTCE with a moderate degree of microcavitations (Barzilai criteria ≥ 2) was confirmed with contrast-enhanced chest CT. Once PAVMs were monitored with chest computed tomography (CT), this became the primary modality for serial evaluation.
Simple PAVMs were defined by one or more afferent feeding arteries originating from a single segmental pulmonary artery, and complex PAVMs were characterized by multiple afferent feeding arteries originating from several segmental arteries. PAVMs were considered large when the feeding artery diameter was ≥3 mm.
Patients with no or small PAVMs were followed with serial screening every five years. Large PAVMs were referred for transcatheter embolization. After embolization, follow-up assessments occurred annually or biannually, and included chest CT. When reperfusion of the PAVM was suspected, pulmonary angiography was performed.
Statistical analysis
Differences between the groups were assessed with Chi-squared analysis for discrete variables and T-test for continuous variables. P < 0.05 was considered statistically significant. Statistical analysis was performed with SPSS version 24.
Results
In total, 197 patients were evaluated for HHT between May 2002 and December 2016; of these, 129 were diagnosed with definite HHT. Fifty-five of the patients had at least one sibling included in the cohort. All patients were offered genetic testing; out of the 129 patients, 80 chose to undergo genetic testing. The primary reasons families chose to decline were lack of insurance coverage and fear of increased insurance premiums in the future do to a pre-existing medical condition. Of the 80 children genetically tested, 77 were found to have mutations consistent with HHT. Sixty-two patients had HHT 1 (ENG mutation), 11 HHT 2 (ALK1 mutation), and four had HHT 3 (SMAD4 mutation). The demographic and clinical features of the 129 patients with HHT are shown in Table 1.
Table 1.
Demographic and clinical features of pediatric patients with HHT (n = 129).
| Age at diagnosis (years) | 8.77 ± 5.02 |
| Sex ratio, males | 66 (51) |
| Genetically confirmed | 77 (60) |
| ENG mutation, HHT 1 | 62 (81) |
| ALK1 mutation, HHT 2 | 11 (14) |
| SMAD4 mutation, HHT 3 | 4 (5) |
| Curaçao criteria | |
| Epistaxis | 119 (92) |
| Telangiectasia | 103 (80) |
| Family history | 121 (94) |
| Visceral involvement | 81 (63) |
| Diagnosis based on | |
| Genetics | 17 (36) |
| Curaçao | 50(39) |
| Both | 62 (48) |
| Pulmonary involvement | |
| PAVMs* | 76 (59) |
| Small | 38 (29.5) |
| Large | 38 (29.5) |
| Simple | 60 (46.5) |
| Complex | 15 (11.6) |
| Symptomatic PAVMs† | 15 (11.6) |
| Respiratory symptoms (exercise intolerance, cyanosis, hemoptysis, chest pain, SOB) | 12 (9.3) |
| Cardiac symptoms (MI)‡ | 1 (0.8) |
| Neurologic symptoms (embolic stroke)‡ | 2 (1.6) |
| Embolotherapy§ | 38 (29.5) |
Continuous data are shown as mean ± SD, and categorical data as number (%).
Large PAVM: at least one large PAVM (feeding artery ≥ 3 mm). Small PAVM: feeding artery ≤ 3 mm. Simple PAVM: one or more afferent feeding arteries originating from a single segmental pulmonary artery. Complex PAVM: multiple afferent feeding arteries originating from several segmental arteries.
All of the symptomatic patients had large PAVMs.
MI and embolic stroke were the presenting symptoms for 1 and 2 patients, respectively.
Treatment was only considered for large PAVMs.
PAVM, pulmonary AVM; MI, myocardial infarction; CVM, cerebrovascular malformation; ICH, intracranial hemorrhage; SOB, shortness of breath.
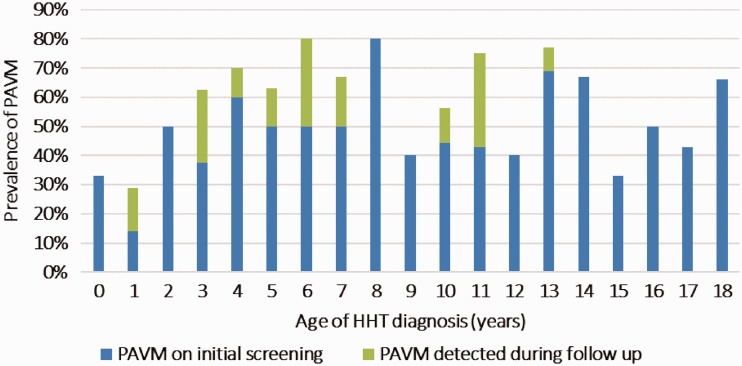
Fifty-nine percent (76/129) of patients had evidence of PAVMs either at the time of their HHT diagnosis or upon subsequent follow-up. The mean age of PAVM detection was approximately nine years. There was no significant trend in detection with age, suggesting that PAVMs may arise at any age (Fig. 1). Half of the 76 children diagnosed with PAVMs had their lesions characterized as small, the other half large. None of the 38 children with small PAVMs were symptomatic from their lesions. Interestingly, 23 (60%) of the children diagnosed with large PAVMs were also asymptomatic. The other 15 (40%) children found to have large PAVMs had symptoms secondary to their vascular lesions, which included exercise intolerance, cyanosis, hemoptysis, chest pain, shortness of breath, embolic strokes, and cardiac ischemia. All 38 children with large PAVMs underwent embolization therapy. Thus, PAVMs can occur at any age and even those with large lesions may have no symptoms.
Fig. 1.
Prevalence of pulmonary arteriovenous malformation by age at diagnosis of HHT.
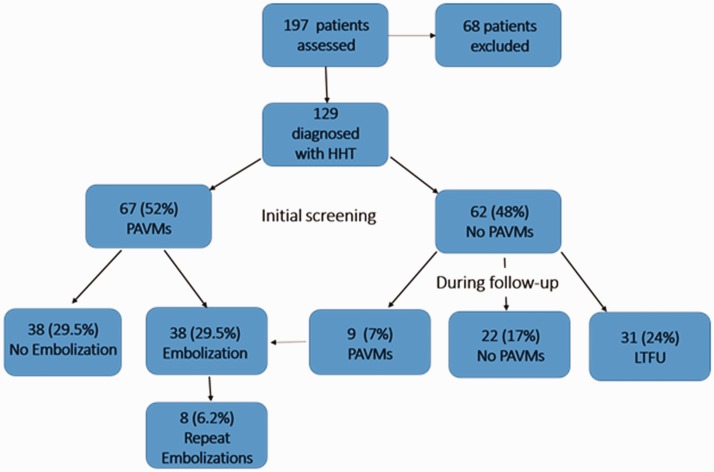
As noted above and shown in Fig. 2, the overall prevalence of PAVMs in the 129 children with HHT followed over our 14-year observational period was ∼ 60% (n = 76). Almost 90% (67) of the children with PAVMs in this study were diagnosed at the time of initial screening. Of the 62 children without PAVMs on initial screening, 31 were followed for > 1 year. Notably, nine of these 31 children (29%) developed evidence of a new PAVM after the initial negative study (mean time to detection = 5.6 years). The characteristics of the nine children who developed new PAVMs during follow-up are shown in Table 2. New PAVMs were diagnosed by a positive TTCE after a previous negative TTCE, and a moderately positive TTCE (Barzilai criteria ≥ 2) was confirmed with contrast-enhanced chest CT. On average, these patients were younger, with a mean age at diagnosis of 5.3 years compared to the overall mean age at time of detection of approximately nine years. All were asymptomatic at the time of detection and only one had large PAVMs. Thus, while most HHT children who develop PAVMs will have their PAVMs discovered at the same time as their HHT diagnosis, ∼ 30% will develop PAVMs at a later date.
Fig. 2.
Flow chart depicting PAVM prevalence and treatment over time.
Table 2.
Nine patients who developed PAVMs after initial negative screen.
| Age at diagnosis (years) | Gender | Genetics | Time to PAVM detection (years) | Embolotherapy | Age at embolization (years) | Duration of follow-up (years) |
|---|---|---|---|---|---|---|
| 3 | M | None found | 3 | No | 10 | |
| 6 | M | - | 1 | No | 4 | |
| 4 | M | ENG | 1 | No | 7 | |
| 1 | F | ENG | 7 | Yes | 8 | 12 |
| 6 | F | ENG | 3 | No | 3 | |
| 7 | F | ENG | 4 | No | 9 | |
| 13 | F | None found | 3 | No | 3 | |
| 3 | M | - | 6 | No | 6 | |
| 5 | F | ENG | 1 | No | 1 |
M, male; F, female; ENG, endoglin mutation; PAVM, pulmonary arteriovenous malformation.
Having a genetic diagnosis, age at HHT diagnosis, and gender were not associated with risk of having PAVM (Table 3). The majority of patients with HHT type 2 did not have PAVMs, and this difference was statistically significant (P = 0.005). Of note, none of the children developed indirect of evidence for pulmonary hypertension based on screening echocardiograms.
Table 3.
Comparison of demographic variables between patients who developed PAVMs.
| PAVMs (n = 76) | No PAVMs (n = 54) | P value | |
|---|---|---|---|
| Age (years) | 8.7 ± 4.7 | 9.7 ± 5.49 | 0.916 |
| Male sex | 40/76 (53) | 24/54 (44) | 0.85 |
| Genetics | |||
| HHT 1 | 40/76 (53) | 24/54 (39) | 0.12 |
| HHT 2 | 2/76 (3) | 9/54 (16) | 0.005* |
| HHT 3 | 1/76 (1) | 3/54 (6) | 0.017 |
Continuous data are shown as mean ± SD, and categorical data as number (%).
P value < 0.05 considered statistically significant.
Of the 76 children diagnosed with PAVMs, 38 (50%) had lesions described as large and all were treated with embolization. Median age at embolization was 10 ± 5 years. Most (n = 29) of these 38 children were treated after their initial screening for HHT. The other nine (24%), however, had PAVMs that were too small to treat at the time of their HHT diagnosis but subsequently grew large enough to be treated with embolization at a later time (mean time from PAVM diagnosis to embolization = 3.4 years). After embolization, 21% (8/38) underwent repeat interventions (mean time between embolizations = 4.5 years). The characteristics of the eight patients who were treated with multiple interventions are shown in Table 4. All of these patients had complex PAVMs. The primary reason for repeat intervention was reperfusion to the previously embolized PAVMs. No children suffered complications from having a PAVM or having them embolized. Thus, small PAVMs can become large with time and even those embolized have the potential to reoccur or re-vascularize.
Table 4.
Characteristics of the eight patients who required multiple embolizations.
| Age at PAVM diagnosis (years) | Genetics | Age at initial embolization (years) | Age at re-intervention (years) | Type of PAVM* | Reason for re-intervention† | Total embolizations (n) | Duration of follow-up (years) |
|---|---|---|---|---|---|---|---|
| 7 | – | 8 | 12 | Diffuse bilateral, including several complex PAVMs | Progression and reperfusion through new collateral formation | 4 | 7 |
| 2 | – | 3 | 8 | Diffuse bilateral, including complex LLL PAVM | Reperfusion through new collateral formation | 3 | 6 |
| 2 | ENG | 2 | 6 | Diffuse right lung, including complex RML PAVM | Progression | 3 | 9 |
| 5 | Alk1 | 5 | 6 | Diffuse bilateral, including complex RUL and LLL PAVMs | Reperfusion through new collateral formation | 3 | 10 |
| 5 | – | 5 | 16 | Complex RML AVM | Reperfusion through new collateral formation | 2 | 11 |
| 6 | ENG | 6 | 10 | Complex RUL PAVM | Reperfusion through new collateral formation | 2 | 4 |
| 6 | – | 6 | 13 | Complex RML PAVM | Reperfusion through new collateral formation and through previously placed coils | 3 | 12 |
| 5 | ENG | 7 | 15 | Complex RLL PAVM | Reperfusion through new collateral formation | 2 | 14 |
Complex PAVM: multiple afferent feeding arteries originating from several segmental arteries.
Progression: increase in size of feeding vessel diameter of existing PAVMs. Reperfusion: recanalization of previously embolized PAVM.
PAVM, pulmonary arteriovenous malformation; LLL, left lower lobe; RML, right middle lobe; RUL, right upper lobe; RLL, right lower lobe.
Discussion
In this 14-year longitudinal study of 129 children with HHT, it was found that PAVMs occur at practically any age with a prevalence of nearly 60%. While most PAVMs were diagnosed at the same time as their initial HHT screening, this study demonstrates that ∼30% of children who were initially negative for PAVMs subsequently developed them within five years. The PAVM prevalence of 60% is slightly higher than that reported in other pediatric studies to date (22–52%),14,26–32 which may be secondary to the longitudinal nature of our study and that fact that new PAVMs do, in fact, develop over time. Also, a referral bias may be at play in patients seen at an HHT Center. In addition, it was noted that ∼25% of children whose PAVMs were considered too small to require embolization at initial screening went on to develop lesions large enough for intervention within 3–5 years. Finally, ∼ 20% of those children who underwent successful embolization received further transcatheter treatment within five years of the original intervention. Reintervention was primarily due to recanalization via collateral vessel formation. All of these observations emphasize the importance that children of any age diagnosed with HHT should be screened for potential PAVMs and that subsequent follow-up is critical regardless of whether PAVMs are absent, small, or embolized. The Washington University HHT Center routinely screens all children every five years for PAVMs using TTCE as the initial imaging modality of choice. In skilled hands, TTCE can be easily obtained on children under the age of five years; while the benefits of intervention in this age group have not been proven, detection of PAVMs is certainly achievable which has potential implications for future management.
The finding that nearly 60% of children with large PAVMs were asymptomatic at the time of initial screening suggests that relying on clinical findings alone may not identify children at risk for significant morbidity. This observation further argues for the importance of PAVM screening in all children with HHT. Using the current practice of screening with TTCE, we have not had any child develop severe complications from undiagnosed PAVMs such as embolic stroke, brain abscess, hemoptysis, or hemothorax. Interestingly, Hosman et al. recently reported similar outcomes with a less invasive approach31 that involves screening with pulse oximetry and chest radiography, utilizing chest CT only for those cases with heightened suspicion for PAVM. They found this approach was similarly successful in preventing serious PAVM-related sequelae.
Compared to other pediatric studies of HHT, not only did we find a higher prevalence of PAVMs in our pediatric cohort of 129 children, but also a higher rate of embolization: 29% of all patients with HHT underwent embolization. Comparatively, in the French series by Soysal32 where all pediatric patients with HHT were screened for PAVM with chest CT (59 patients), only 7% of all children with HHT underwent embolization. Furthermore, the Dutch study of Hosman et al.,31 using a more conservative screening approach designed to detect large PAVMs, reported only a 22% prevalence of PAVMs in 175 children with HHT compared to 60% in our study. Not surprisingly, their overall rates of embolization were also lower than in our series, as embolization was performed in 19% of all patients with HHT.
Limitations of this study include the retrospective nature of the review. The high incidence of PAVM detection may be related to recruitment bias, particularly in symptomatic patients. However, only 11% of our patients were symptomatic at the time of PAVM detection which suggests this effect may be minimal.
Conclusion
PAVMs are present in up to 60% of children with HHT. Most PAVMs can be detected on initial screening with TTCE, but nearly 30% of children with HHT can develop new PAVMs over time. Furthermore, up to 25% of children initially diagnosed with small PAVMs can develop larger PAVMs requiring embolization. The majority of children with large PAVMs have no symptoms from their lesions. Finally, nearly 20% of those children who had successful embolization therapy needed repeat intervention. Children diagnosed with HHT need continued assessment for PAVMs throughout childhood.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Presentation
This paper was presented at the 2017 International HHT Scientific Conference in Dubrovnik, Croatia.
References
- 1.Bideau A, Plauchu H, Brunet G, et al. Epidemiological investigation of rendu-osler disease in France: It’s geographical distribution and prevalence. Popul 1989; 44: 3–22. [PubMed] [Google Scholar]
- 2.Guttmacher AE, McKinnon WC, Upton MD. Hereditary hemorrhagic telangiectasia: a disorder in search of the genetics community. Am J Med Genet 1994; 52: 252–253. [DOI] [PubMed] [Google Scholar]
- 3.Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med 1999; 245: 31–39. [DOI] [PubMed] [Google Scholar]
- 4.Dakeishi M, Shioya T, Wada Y, et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat 2002; 19: 140–148. [DOI] [PubMed] [Google Scholar]
- 5.Westermann CJ, Rosina AF, De Vries V, et al. The prevalence and manifestations of hereditary hemorrhagic telangiectasia in the afro-Caribbean population of the Netherlands Antilles: a family screening. Am J Med Genet A 2003; 116A: 324–328. [DOI] [PubMed] [Google Scholar]
- 6.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary hemorrhagic telangiectasia. J Med Genet 2009; 48: 73–87. [DOI] [PubMed] [Google Scholar]
- 7.Plauchu H, de Chadarevian JP, Bideau A, et al. Age-related clinical profile of hereditary hemorrhagic telangiectasia in an epidemiologically recruited population. Am J Med Genet 1989; 32: 291e7. [DOI] [PubMed] [Google Scholar]
- 8.Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev 2010; 24: 203–219. [DOI] [PubMed] [Google Scholar]
- 9.Velthuis S, Vorselaars VM, van Gent MW, et al. Role of transthoracic contrast echocardiography in the clinical diagnosis of hereditary hemorrhagic telangiectasia. Chest 2013; 144: 1876–1882. [DOI] [PubMed] [Google Scholar]
- 10.Mei-Zahav M, Letarte M, Faughnan ME, et al. Symptomatic children with hereditary hemorrhagic telangiectasia: a pediatric center experience. Arch Pediatr Adolesc Med 2006; 160: 596–601. [DOI] [PubMed] [Google Scholar]
- 11.Lesca G, Olivieri C, Burnichon N, et al. Genotype–phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French–Italian HHT network. Genet Med 2007; 9: 14–22. [DOI] [PubMed] [Google Scholar]
- 12.Bayrak-Toydemir P, McDonald J, Markewitz B, et al. Genotype–phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A 2006; 140: 463–470. [DOI] [PubMed] [Google Scholar]
- 13.Letteboer TG, Mager JJ, Snijder RJ, et al. Genotype–phenotype relationship in hereditary haemorrhagic telangiectasia. J Med Genet 2006; 43: 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giordano P, Nigro A, Lenato GM, et al. Screening for children from families with Rendu–Osler–Weber disease: from geneticist to clinician. J Thromb Haemost 2006; 4: 1237–1245. [DOI] [PubMed] [Google Scholar]
- 15.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (rendu-osler-weber syndrome). Am J Med Genet 2000; 91: 66–67. [DOI] [PubMed] [Google Scholar]
- 16.Shah RK, Dhingra JK, Shapshay SM. Hereditary hemorrhagic telangiectasia: a review of 76 cases. Laryngoscope 2002; 112: 767–773. [DOI] [PubMed] [Google Scholar]
- 17.Porteous ME, Burn J, Proctor SJ. Hereditary haemorrhagic telangiectasia: a clinical analysis. J Med Genet 1992; 29: 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OS AA, Friedman CM, White RI., Jr The natural history of epistaxis in hereditary hemorrhagic telangiectasia. Laryngoscope 1991; 101: 977–980. [DOI] [PubMed] [Google Scholar]
- 19.Gefen AM, White AJ. Asymptomatic pulmonary arteriovenous malformations in children with hereditary hemorrhagic telangiectasia. Ped Pulm 2017; 52: 1194–1197. [DOI] [PubMed] [Google Scholar]
- 20.Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 1998; 158: 643–661. [DOI] [PubMed] [Google Scholar]
- 21.Mager JJ, Overtoom TT, Blauw H, et al. Embolotherapy of pulmonary arteriovenous malformations: long-term results in 112 patients. J Vasc Interv Radiol 2004; 15: 451–456. [DOI] [PubMed] [Google Scholar]
- 22.Moussouttas M, Fayad P, Rosenblatt M, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology 2000; 55: 959–964. [DOI] [PubMed] [Google Scholar]
- 23.Pollak JS, Saluja S, Thabet A, et al. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol 2006; 17: 35–44. [DOI] [PubMed] [Google Scholar]
- 24.Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982–1997. Mayo Clin Proc 1999; 74: 671–680. [DOI] [PubMed] [Google Scholar]
- 25.Barzilai B, Waggoner AD, Spessert C, et al. Two-dimensional contrast echocardiography in the detection and follow-up on congenital pulmonary arteriovenous malformations. Am J Cardiol 1991; 68: 1507–1510. [DOI] [PubMed] [Google Scholar]
- 26.Karam C, Sellier J, Mansencal N, et al. Reliability of contrast echocardiography to rule out pulmonary arteriovenous malformations and avoid CT irradiation in pediatric patients with hereditary hemorrhagic telangiectasia. Echocardiography 2015; 32: 42–48. [DOI] [PubMed] [Google Scholar]
- 27.Al-Saleh S, Dragulescu A, Manson D, et al. Utility of contrast echocardiography for pulmonary arteriovenous malformation screening in pediatric hereditary hemorrhagic telangiectasia. J Pediatr 2012; 160: 1039–1043. [DOI] [PubMed] [Google Scholar]
- 28.Al-Saleh S, Mei-Zahav M, Faughnan ME, et al. Screening for pulmonary and cerebral arteriovenous malformations in children with hereditary haemorrhagic telangiectasia. Eur Respir J 2009; 34: 875–881. [DOI] [PubMed] [Google Scholar]
- 29.Latino GA, Al-Saleh S, Carpenter S, et al. The diagnostic yield of rescreening for arteriovenous malformations in children with hereditary hemorrhagic telangiectasia. J Pediatr 2014; 165: 197–199. [DOI] [PubMed] [Google Scholar]
- 30.Giordano P, Lenato GM, Suppressa P, et al. Hereditary hemorrhagic telangiectasia: arteriovenous malformations in children. J Pediatr 2013; 163: 179–186. [DOI] [PubMed] [Google Scholar]
- 31.Hosman AE, Gussem EM, Balemans WAF, et al. Screening children for pulmonary arteriovenous malformations: Evaluation of 18 years of experience. Pediatr Pulmonol 2017; 52: 1206–1211. [DOI] [PubMed] [Google Scholar]
- 32.Soysal N, Eyries M, Verlhac S, et al. Non-invasive CT screening for pulmonary arteriovenous malformations in children with confirmed hereditary hemorrhagic telangiectasia: results from two pediatric centers. Pediatr Pulmonol 2017; 52: 624–629. [DOI] [PubMed] [Google Scholar]
- 33.Lyle MA, Fenstad ER, McGoon MD, et al. Pulmonary hypertension in hereditary hemorrhagic telangiectasia. Chest 2016; 15: 362–371. [DOI] [PubMed] [Google Scholar]
- 34.Vorselaars VMM, Velthuis S, Snijder RJ, et al. Pulmonary hypertension in hereditary haemorrhaic telangiectasia. World J Cardiol 2015; 7(5): 230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sopena B, Perez-Rodriguez T, Portela D, et al. High prevalence of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. Eur J Int Med 2013; 24: e30–34. [DOI] [PubMed] [Google Scholar]
- 36.Chadha D, Handa A, Kumar A. Pulmonary hypertension in a patient with hereditary hemorrhagic telangiectasia. BMJ Case Rep 2013; 2013: bcr2012008352. [DOI] [PMC free article] [PubMed] [Google Scholar]