Abstract
Objectives:
We assessed sociodemographic and health care factors of mothers and newborns during a 2015-2016 outbreak of microcephaly in Recife, Brazil, and we analyzed the spatial distribution and incidence risk of newborns with microcephaly in relation to socio-environmental indicators.
Methods:
We collected data from August 2015 through May 2016 from Brazil’s Live Birth Information System and Bulletin of Microcephaly Notification, and we geocoded the data by maternal residence. We constructed thematic maps of districts, according to socio-environmental and vector indicators. We identified spatial aggregates of newborns with microcephaly by using the Bernoulli model. We performed logistic regression analyses to compare the incidence risk of microcephaly within socio-environmental indicator groups.
Results:
We geocoded 17 990 of 19 554 (92.0%) live births in Recife, of which 202 (1.1%) newborns were classified as having microcephaly, based on a head circumference of ≥2 standard deviations below the mean. Larger proportions of newborns with microcephaly (compared with newborns without microcephaly) were born to mothers who delivered in a public hospital, did not attend college, were aged ≤19, or were black or mixed race. A higher risk of microcephaly (incidence rate ratio [IRR] = 3.90; 95% confidence interval [CI], 1.88-8.06) occurred in districts with the lowest (vs highest) Municipal Human Development Index (ie, an index that assesses longevity, education, and income). The risk of microcephaly was significantly higher where rates of larvae density (IRR = 2.31; 95% CI, 1.19-4.50) and larvae detection (IRR = 2.04; 95% CI, 1.05-4.00) were higher and rates of sewage system (IRR = 2.20; 95% CI, 1.16-4.18) and garbage collection (IRR = 1.96; 95% CI, 0.99-3.88) were lower. Newborns with microcephaly lived predominantly in the poorest areas and in a high-risk cluster (relative risk = 1.89, P = .01) in the north.
Conclusions:
The disproportionate incidence of microcephaly in newborns in poor areas of Recife reinforces the need for government and public health authorities to formulate policies that promote social equity and support for families and their children with microcephaly.
Keywords: ecological studies, environmental indicators, health inequalities, microcephaly, social indicators, Zika
During 2015 and 2016, the number of newborns with microcephaly in Brazil increased substantially. The highest rate was recorded in Pernambuco state; the rate of newborns with microcephaly increased from 0.06 per 100 000 live births in 2010-2014 to 4.61 per 100 000 live births in 2015.1 Toward the end of 2016, the number of newborns born with microcephaly declined in Brazil, but prevalence remained high in the city of Recife, the capital of Pernambuco, where 54 newborns with microcephaly were identified from June 2016 through February 2017.2
Although speculation about the possible causes of this epidemic varies, the most robust scientific evidence associates microcephaly with infection by the Zika virus.3–5 Whereas not all cases of Zika infection in pregnant women during the epidemic resulted in microcephaly,6,7 a temporal relationship between the increase in circulation of the Zika virus and the increase in reports of microcephaly existed.3
Historically, multiple factors have been implicated in the etiopathogenesis of microcephaly, including genetic causes, environmental elements, and gestational infections, all of which potentially affect the growth of the fetus’s brain.3 Environmental elements include the exposure of pregnant women to infectious agents (viruses and parasites), toxic substances, and radiation.6 Zika virus infection was added to this list of risk factors for microcephaly in 2016.5
From the beginning of the epidemic in the second half of 2015 through June 2016, surveillance data identified 7830 newborns in Brazil who were suspected to have microcephaly. Of these, 1551 newborns were confirmed to have microcephaly, with or without alteration of the central nervous system. Of these newborns with microcephaly, 1987 (25.4%) were located in Pernambuco state, which was the highest proportion among any of the 27 Brazilian states.8
Owing to the severity of microcephaly and its central nervous system sequelae, and the possibility of expanding vector propagation, the World Health Organization issued a warning in February 2016. In this announcement, the World Health Organization declared a state of international emergency, recommended surveillance of microcephaly and other neurological disorders in areas at risk of Zika transmission, and encouraged researchers to identify whether Zika causes microcephaly and whether other factors were involved in the epidemic.9
Since that time, much has been written and published about the association between Zika infection and microcephaly. However, more can be learned, including about the relationship during outbreaks between local socio-environmental factors and microcephaly. The objective of this study was to assess the maternal and newborn sociodemographic and health care factors involved in the microcephaly outbreak in Recife, Brazil, and to analyze the spatial distribution and incidence risk of newborns with microcephaly in relation to various socio-environmental indicators.
Methods
We conducted an ecological study of Recife by using secondary databases. Recife covers an area of 218.4 km2 and had a population density of 7039 inhabitants/km2 in 2010.10 In 2010, it had the highest Municipal Human Development Index (MHDI) in northeastern Brazil, calculated as 0.772.11 The MHDI is derived by adjusting the methodology used for the global human development index to the Brazilian context; it assesses levels of longevity, education, and income, and values range from 0 (lowest level) to 1 (highest level).
We used data from the Brazil Live Birth Information System (SINASC),12 which provides microdata on live births by sex, birthplace, birth weight, age, and residence of mother. We collected data for Recife from August 2015 through May 2016. Data on some variables in a small number of births were missing from the SINASC database. To fill in these gaps, we used data from the Bulletin of Notification of the Health Strategic Surveillance Information Center Platform of Recife (CIEVS/RECIFE).13 The bulletin is the compulsory notification instrument, established by the Ministry of Health of Brazil, for all suspected diseases, outbreaks, and events that are considered public health emergencies, including microcephaly. For our definition of microcephaly, we used the International Fetal and Newborn Growth Consortium for the 21st Century (Intergrowth-21st) criterion, which considers those with a head circumference of ≥2 standard deviations below the mean to have microcephaly.14 Using this definition, we reclassified all births with suspected microcephaly found in the SINASC and CIEVS/RECIFE data. We used the mother’s address to geocode the data (ie, convert addresses into geographic coordinates). We classified hospitals where births occurred as either public or private.
For data analysis, we excluded 123 mothers with missing data on ethnicity, 34 mothers with missing data on type of pregnancy, 35 mothers who gave birth at home, 1 mother for whom data on location of delivery were unknown, and 5 newborns whose sex could not be identified because of malformations.
We used MHDI as a global indicator of socioeconomic status. To calculate MHDI, we used data from the Municipal Health Department Mortality Information System for 2009-201115 (for longevity) and from the Brazilian Institute of Geography and Statistics (IBGE) Demographic Census 201010 (for education and income). We determined the 2010 MHDI for individual census tracts using the methodology proposed by the United Nations Development Program.16 We then used the MHDI for each census tract to create an MHDI for each district, by averaging the MHDIs of any census tracts that intercepted the district, weighted by the relative proportions of the census tracts.
We also used data from the IBGE Demographic Census 201010 to calculate our sanitation indicators: sewage-system rate and garbage-collection rate. We defined these rates as the proportion of households that had sewage-system and garbage-collection services, respectively. As we did when performing our 2010 MHDI calculations, we calculated rates for sewage system and garbage collection for individual census tracts, and then we calculated these rates for each district by averaging the rates of the intercepting census tracts and applying weighting. For both the MHDI and sanitation indicator calculations, we used ArcGIS ArcInforelease 9.3.17
For the entomological surveillance analyses, we conducted a bimonthly survey of relevant 2014 and 2015 data from both the Antivectorial Service of the National Dengue Control Program (PNCD) (unpublished data, Secretaria de Saúde de Recife, PNCD, Registro Diário do Serviço Antivetorial, 2016) and the Aedes aegypti Infestation Rapid Survey (Levantamento Rápido do Índice de Infestação por Aedes aegypti [LIRA]) (unpublished data, LIRA, 2016). PNCD provides data on Aedes aegypti vector control, and LIRA provides data on Aedes aegypti vector infestation (density and detection). Both data sets are aggregated by district. Although we collected PNCD and LIRA data for 2014 and 2015, we used only the data from October through December 2014 for our analysis. We wanted to restrict our assessment of vector infestation and control to the spring and summer seasons in Recife, when vector proliferation tends to be highest. We also wanted our assessment to focus on the period that involved the beginning of gestation of the newborns analyzed in the study and that best represented the 9 months before onset of the outbreak.
The PNCD records a weekly summary of antivectorial, service, and entomological surveillance for the control of Aedes aegypti, and its bulletins include data on the number of homes visited and treated per district, as well as the amount of larvicide used per district. Using the data from these bulletins, we calculated the percentage of mosquito breeding sites treated with larvicide, which we defined as the number of deposits treated divided by the number of deposits identified in a household. We also calculated the larvicide consumption index, which we defined as the amount of larvicide used, in grams, divided by the number of deposits treated in a household. We subsequently determined a mean percentage of water deposits treated with larvicide and a mean larvicide consumption index for each district.
The LIRA records data on Aedes aegypti infestation of a random sample of households per district and reports these data bimonthly. Using LIRA data, we employed indicators previously used by others.18 We calculated the Breteau index, which is defined as the number of households with water tanks testing positive for Aedes aegypti divided by the number of households investigated, and the building infestation index, which is defined as the number of households infested by Aedes aegypti divided by the number of households investigated. We subsequently determined a mean Breteau index and a mean building infestation index for each district.
We compared the groups of newborns with microcephaly and newborns without microcephaly by newborn variables (gestational age, hospital of birth, sex, and weight) and by maternal variables (age, education level, self-reported race/ethnicity, number of previous pregnancies, number of prenatal visits, pregnancy type, and delivery type). We did not include the small amounts of missing data from the SINASC database in statistical testing. We used the Pearson χ2 test to compare the groups, and we considered P < .05 to be significant.
We verified the spatial dependence of the microcephaly data by using the spatial autocorrelation of residues, and we evaluated spatial clustering versus random spread by using Moran’s I test19 and a neighborhood matrix by contiguity. When we determined that spatial dependence was missing, we performed classical regression analysis, using a univariate negative binomial regression model, with results expressed as incidence rate ratios (IRRs) and 95% confidence intervals (CIs). For this model, we created 4 categories within each of the following socioeconomic and environmental variables: 2010 MHDI, Breteau index, building infestation index, sewage-system rate, garbage-collection rate, larvicide consumption index, and percentage of water deposits treated with larvicide. We used Stata release 1320 to conduct the negative binomial regression analyses and χ2 analyses. We used R version 3.3.121 to conduct Moran’s I test.
To construct thematic maps, we started with the cartographic base map of Recife, onto which we superimposed data on district microcephaly density. For each map, we then superimposed data on one of the following socio-environmental and vector presence indicators (using 4 results groups for each indicator) for the district: 2010 MHDI, garbage-collection rates, sewage-system rates, and Breteau index. In addition, on one of the microcephaly density data maps, we superimposed the map of communities of social interest (CSIs) of Recife for 2014,22 obtained from the Sanitation Authority of Recife. CSIs are areas with precarious infrastructure, characterized by owner-built homes and homes that are occupied by low-income families.
To determine the presence or absence of spatial aggregates of newborns with microcephaly, we scanned high-risk and low-risk areas of Recife by using the Bernoulli model, and we treated newborns without microcephaly as the controls. We defined the relative risk (RR) of microcephaly as the calculated risk of microcephaly within the cluster divided by the calculated risk of it outside the cluster, which we derived from the relationship between the numbers of observed and expected newborns with microcephaly.23 We tested significance by using the Monte Carlo replication method for 999 interactions, increasing the scanning power by risk areas, with P < .05 considered significant. We performed analyses using SaTScan version 9.4.24 This study was approved by the Institutional Review Board of Instituto de Medicina Integral Professor Fernando Figueira.
Results
From August 2015 through May 2016, we identified 19 554 live births in Recife, 17 990 (92.0%) of which we were able to geocode. Using the Intergrowth-21st criterion, we classified 202 (1.1%) geocoded live births as newborns with microcephaly (Table 1). The highest incidence of microcephaly occurred from October through December 2015 (n = 110). The incidence peaked in November 2015, when 47 newborns with microcephaly were identified, and then progressively declined until May 2016, when 8 newborns with microcephaly were identified.
Table 1.
Characteristics of newborns with microcephaly and without microcephaly, by maternal and newborn sociodemographic and health care variables, during an outbreak in Recife, Pernambuco, Brazil, August 2015–May 2016a
| Sociodemographic and Health Care Variables | No. (%) | P Valueb | |
|---|---|---|---|
| Newborns Without Microcephaly (n = 17 788) | Newborns With Microcephaly (n = 202) | ||
| Maternal age, y | .02 | ||
| ≤19 | 2667 (15.0) | 41 (20.3) | |
| 20-34 | 12 440 (69.9) | 142 (70.3) | |
| ≥35 | 2681 (15.1) | 19 (9.4) | |
| Maternal education | <.001 | ||
| Elementary school not completed | 760 (4.3) | 13 (6.4) | |
| Elementary, primary, and/or high school completed | 11 863 (66.7) | 170 (84.2) | |
| College completed | 5165 (29.0) | 19 (9.4) | |
| Self-reported maternal racec | <.001 | ||
| White | 5135 (29.1) | 31 (15.3) | |
| Black | 1068 (6.0) | 23 (11.4) | |
| Mixed | 11 462 (64.9) | 148 (73.3) | |
| No. of previous pregnancies | .27 | ||
| 0 | 8603 (48.4) | 108 (53.4) | |
| 1-3 | 8588 (48.3) | 86 (42.6) | |
| ≥4 | 597 (3.3) | 8 (4.0) | |
| No. of prenatal visits | .003 | ||
| ≤5 | 4519 (25.4) | 70 (34.6) | |
| ≥6 | 13 269 (74.6) | 132 (65.3) | |
| Pregnancy typed | .15 | ||
| Singleton | 17 326 (97.5) | 193 (96.0) | |
| Multiple | 429 (2.3) | 8 (4.0) | |
| Delivery typee | <.001 | ||
| Vaginal | 8010 (45.0) | 127 (62.9) | |
| Cesarean | 9733 (54.7) | 74 (36.6) | |
| Gestational age, wk | .04 | ||
| <37 | 3873 (21.8) | 59 (29.2) | |
| 37-41 | 13 043 (73.3) | 133 (65.8) | |
| >41 | 872 (4.9) | 10 (5.0) | |
| Hospital of birthf | <.001 | ||
| Public | 10 822 (60.8) | 178 (88.1) | |
| Private | 6930 (39.0) | 24 (11.9) | |
| Newborn sexg | <.001 | ||
| Female | 8666 (48.7) | 124 (61.4) | |
| Male | 9117 (51.3) | 78 (38.6) | |
| Newborn weight, g | <.001 | ||
| <2500 | 1507 (8.5) | 74 (36.6) | |
| ≥2500 | 16 281 (91.5) | 128 (63.4) | |
a Data sources: Brazil Live Birth Information System12 and the Health Strategic Surveillance Information Center Platform of Recife.13
b Using Pearson χ2 test, with P < .05 considered significant.
c Excludes 123 mothers for whom data on ethnicity were missing.
d Excludes 34 mothers for whom data on type of pregnancy were missing.
e Excludes 46 mothers for whom data on type of delivery were missing.
f Excludes 35 mothers who gave birth at home and 1 mother whose data on location of delivery were missing.
g Excludes 5 newborns whose sex could not be identified because of malformations.
Mothers aged ≤19 had a higher proportion of newborns with microcephaly (n = 41 of 202, 20.3%) than without microcephaly (n = 2667 of 17 788, 15.0%). Mothers with a college education had a lower proportion of newborns with microcephaly (n = 19, 9.4%) than without microcephaly (n = 5165, 29.0%). Mothers who were black or of mixed race had 171 (84.7%) newborns with microcephaly and 12 530 (70.9%) newborns without microcephaly. Compared with mothers of newborns without microcephaly, a significantly higher proportion of mothers of newborns with microcephaly had vaginal deliveries (n = 127 [62.9%] vs 8010 [45.0%]; P < .001), preterm births (n = 59 [29.2%] vs 3873 [21.8%]; P = .037), and ≤5 prenatal visits (n = 70 [34.7%] vs 4519 [25.4%]; P = .003). Compared with newborns without microcephaly, a significantly higher proportion of newborns with microcephaly were female (n = 124 [61.4%] vs 8666 [48.7%]; P < .001), of low birth weight (n = 74 [36.6%] vs 1507 [8.5%]; P < .001), and born in public hospitals (n = 178 [88.1%] vs 10 822 [60.8%]; P < .001) (Table 1).
In general, the risk of microcephaly was significantly higher as each socio-environmental and vector variable worsened (Table 2). The risk of microcephaly for each of the districts with a lower MHDI (when compared with districts with the highest MHDI) increased as MHDI decreased (P < .001), and the risk of microcephaly was highest for the districts with the lowest MHDI (IRR = 3.90; 95% CI, 1.88-8.06). When compared with districts with the lowest Breteau index (larvae density) and building infestation index (larvae detection), the risk of microcephaly increased as the Breteau index (P = .015) and building infestation index (P = .027) decreased, and the risk of microcephaly was highest in the districts with the highest Breteau index (IRR = 2.31; 95% CI, 1.19-4.50) and the second-highest building infestation index (IRR = 2.39; 95% CI, 1.24-4.64). When compared with districts with the highest sewage-system rates, the risk of microcephaly increased as the sewage-system rates of districts decreased (P = .023); however, when compared directly, the risk was higher in only the districts with the lowest sewage-system rates (IRR = 2.20; 95% CI, 1.16-4.18). When compared with districts with the highest garbage-collection rates, the risk of microcephaly increased as the garbage-collection rates decreased (P = .033); however, when compared directly, none of the individual differences was significant.
Table 2.
Incidence rate ratios of newborns with microcephaly, by socio-environmental and vector variables, during an outbreak in Recife, Pernambuco, Brazil, August 2015–May 2016a
| Socio-environmental Variables | Incidence Rate Ratio (95% CI) | P Valueb |
|---|---|---|
| Municipal Human Development Index, 2010a | <.001 | |
| 0.710-0.809 (high/very high) | 1.00 [Reference] | |
| 0.692-0.709 (medium/high) | 2.69 (1.28-5.66) | |
| 0.671-0.691 (medium) | 3.67 (1.79-7.53) | |
| 0.494-0.670 (very low/medium) | 3.90 (1.88-8.06) | |
| Breteau indexc | .02 | |
| 0.0-1.3 | 1.00 [Reference] | |
| 1.4-2.5 | 1.70 (0.86-3.37) | |
| 2.6-4.5 | 2.03 (1.04-3.97) | |
| 4.6-9.3 | 2.31 (1.19-4.50) | |
| Building infestation indexd | .03 | |
| 0.0-1.3 | 1.00 [Reference] | |
| 1.4-2.5 | 1.62 (0.82-3.19) | |
| 2.6-3.7 | 2.39 (1.24-4.64) | |
| 3.8-8.1 | 2.04 (1.05-4.00) | |
| Sewage-system rate,e % | .02 | |
| 78.4-99.0 | 1.00 [Reference] | |
| 54.5-78.3 | 1.61 (0.84-3.10) | |
| 30.2-54.4 | 1.55 (0.78-3.09) | |
| 3.3-30.1 | 2.20 (1.16-4.18) | |
| Garbage-collection rate,f % | .03 | |
| 99.6-100.0 | 1.00 [Reference] | |
| 97.6-99.5 | 1.35 (0.69-2.62) | |
| 95.0-97.5 | 1.69 (0.88-3.24) | |
| 65.6-94.9 | 1.96 (0.99-3.88) | |
| Larvicide consumption indexg | .60 | |
| 0.9-2.7 | 1.00 [Reference] | |
| 2.8-4.3 | 0.68 (0.36-1.25) | |
| 4.4-6.5 | 1.04 (0.59-1.83) | |
| 6.6-27.0 | 0.99 (0.56-1.75) | |
| Percentage of water deposits treated with larvicide,h | .57 | |
| 1.2-19.5 | 1.00 [Reference] | |
| 19.6-29.3 | 2.38 (1.22-4.65) | |
| 29.4-40.5 | 1.81 (0.91-3.60) | |
| 40.6-76.2 | 1.86 (0.94-3.71) | |
a Assesses levels of longevity, education, and income, and its level ranges from 0 (lowest) to 1 (highest); data obtained from the Municipal Health Department Mortality Information System for 2009-201115 (for longevity) and from the Brazilian Institute of Geography and Statistics (IBGE) Demographic Census 201010 (for education and income).
b Using negative binomial regression models, with P < .05 considered significant.
c Defined as the number of water tanks testing positive for Aedes aegypti divided by the number of households investigated. Data source: Aedes aegypti Infestation Rapid Survey (unpublished data, Levantamento Rápido do Índice de Infestação por Aedes aegypti [LIRA], 2016).
d Defined as the number of households infested by Aedes aegypti divided by the number of households investigated. Data source: Aedes aegypti Infestation Rapid Survey (unpublished data, LIRA, 2016).
e Defined as the proportion of households with sewage systems. Data source: IBGE Demographic Census 2010.10
f Defined as the proportion of households that received garbage-collection services. Data source: IBGE Demographic Census 2010.10
g Defined as the amount of larvicide used, in grams, divided by the number of deposits treated in a household. Data sources: Antivectorial Service of the National Dengue Control Program (PNCD) (unpublished data, Secretaria de Saúde de Recife, PNCD, Registro Diário do Serviço Antivetorial, 2016) and Aedes aegypti Infestation Rapid Survey (unpublished data, LIRA, 2016). Only data from October 2014 through December 2014 (the period that involved the beginning of gestation of the newborns analyzed in the study) were used for this analysis.
h Defined as the number of deposits treated divided by the number of deposits identified in a household. Data sources: PNCD (unpublished data, Secretaria de Saúde, PNCD, Registro Diário do Serviço Antivetorial, 2016) and Aedes aegypti Infestation Rapid Survey (unpublished data, LIRA, 2016). Only data from October 2014 through December 2014 (the period that involved the beginning of gestation of the newborns analyzed in the study) were used for this analysis.
The risk for microcephaly did not follow a significant trend of increasing as larvicide consumption index and percentage of water deposits treated with larvicide increased (Table 2). The only exception was in districts in the second-to-lowest percentage of water deposits treated with larvicide group, which, when compared with districts in the lowest group, had a significantly higher risk of microcephaly (IRR = 2.38; 95% CI, 1.22-4.65). When we changed the model to use percentage of water deposits treated with larvicide as a dichotomous (high vs low) variable, the IRR was 0.96 (95% CI, 0.65-1.43).
In the multivariate regression model, of all the aforementioned variables, only MHDI continued to show a significantly increasing risk of microcephaly for districts in progressively lower MHDI categories (P = .036). These findings persisted in all models tested, including those tested with 2 or 3 variables (data not shown).
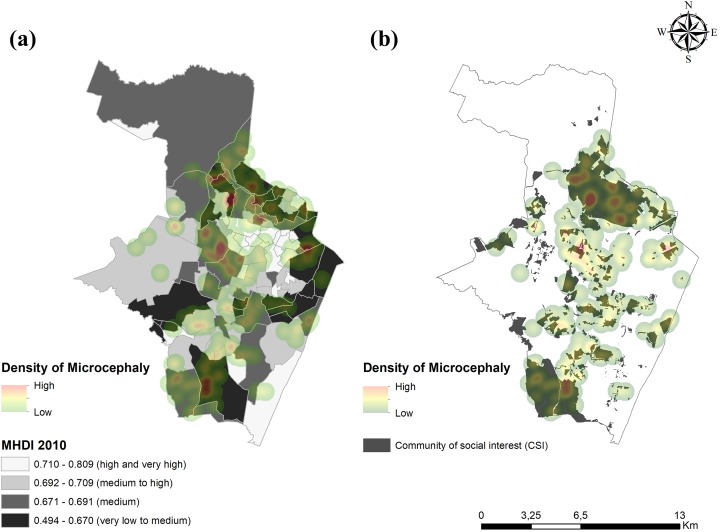
Thematic maps involving MHDI and CSIs illustrate the predominance of newborns with microcephaly in the poorest areas of the city (Figure 1). Of the 202 newborns with microcephaly, 147 (72.8%) were located in areas with MHDI <0.7. In addition, 141 (70.0%) newborns with microcephaly were located in a CSI.
Figure 1.
Spatial distributions of newborns with microcephaly during an outbreak in the districts of Recife, Pernambuco, Brazil, August 2015–May 2016, by (a) Municipal Human Development Index (MHDI) 2010 and (b) community of social interest (CSI). The MHDI assesses levels of longevity, education, and income, and it ranges from 0 (low) to 1 (high). Data sources: Municipal Health Department Mortality Information System 2009-201115 (for longevity) and Brazilian Institute of Geography and Statistics Demographic Census 201010 (for education and income). CSIs are areas with precarious infrastructure (ie, homes are self-built and occupied by low-income families). Data source: Sanitation Authority of Recife for 2014.22
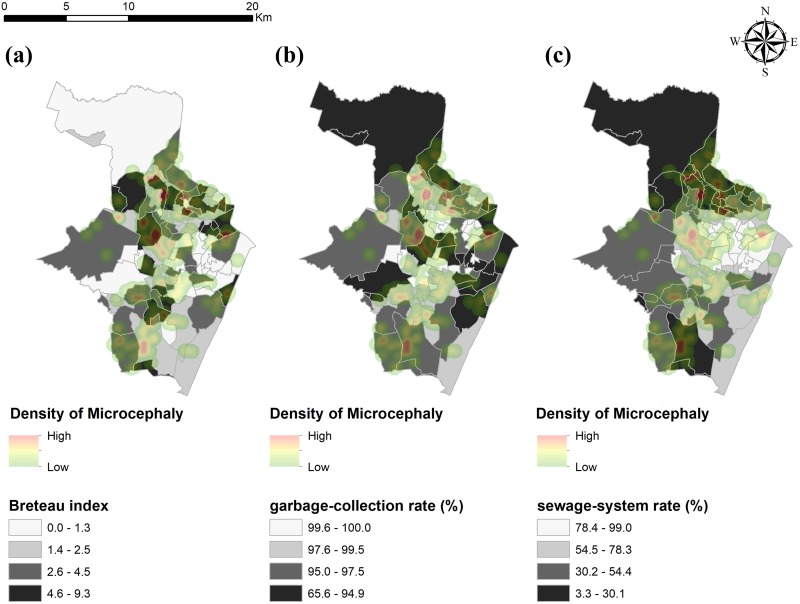
Thematic maps involving vector presence and sanitation indicators illustrate the spatial distribution of newborns with microcephaly in Recife districts with varying Breteau indexes, garbage-collection rates, and sewage-system rates (Figure 2). Of the 202 newborns with microcephaly, 135 (66.8%) were located in districts with a Breteau index (density of larvae) higher than the median (2.5). Most newborns with microcephaly were located in districts with lower garbage-collection and sewage-system rates.
Figure 2.
Spatial distributions of newborns with microcephaly during an outbreak in districts of Recife, Pernambuco, Brazil, August 2015–May 2016, by (a) Breteau index, (b) garbage-collection rate, and (c) sewage-system rate. The Breteau index is the number of water tanks testing positive for Aedes aegypti divided by the number of households investigated. A higher Breteau index indicates more households with water tanks testing positive for Aedes aegypti. Data source: Aedes aegypti Infestation Rapid Survey, October 2014 through December 2014 (unpublished data, Levantamento Rápido do Índice de Infestação por Aedes aegypti, 2016). The garbage-collection rate is defined as the proportion of households that receive garbage-collection services. The sewage- system rate is defined as the proportion of households with sewage systems. Data source: Brazilian Institute of Geography and Statistics Demographic Census 2010.10
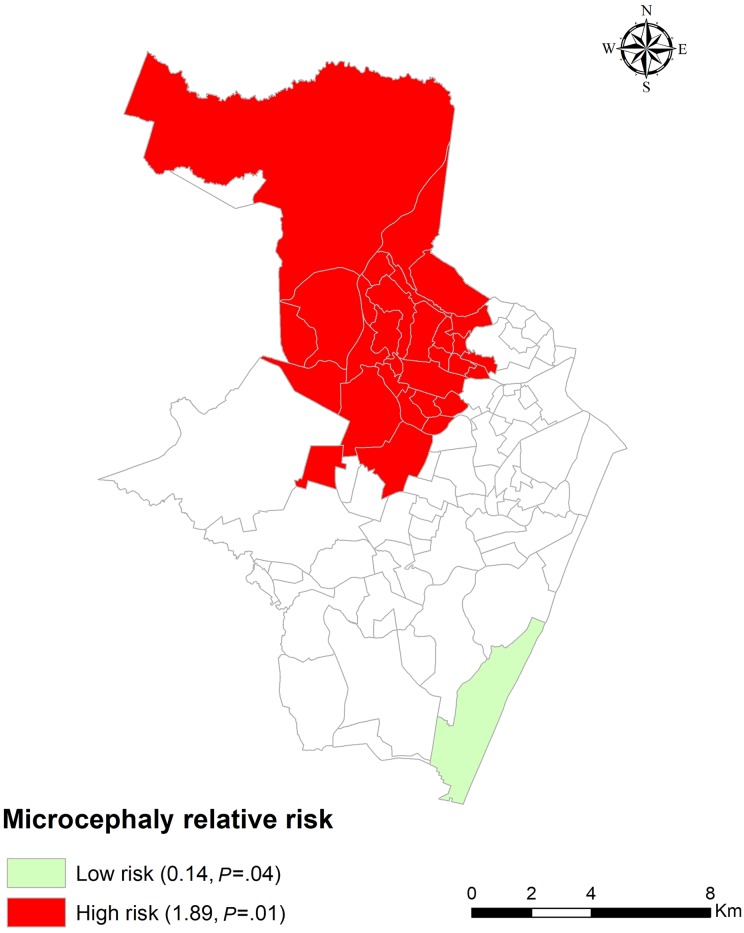
Finally, another thematic map shows spatial clusters where the risk of microcephaly was low or high (Figure 3). A low-risk region in the south of Recife had an RR of microcephaly of 0.14 (P = .04); 2 (1.0%) newborns were observed with microcephaly, and 13 newborns were expected to have microcephaly. A high-risk cluster in the north of the municipality had an RR of microcephaly of 1.89 (P = .01); 86 (42.6%) newborns were observed with microcephaly, and 57 newborns were expected to have microcephaly. On the map, the low-risk cluster was in a region with a high MHDI, and the high-risk cluster was in a region with a low MHDI.
Figure 3.
Spatial clustering of low-risk and high-risk districts for newborns with microcephaly, Recife, Pernambuco, Brazil, August 2015–May 2016. The relative risk of microcephaly was defined as the calculated risk of microcephaly within a cluster divided by the calculated risk of it outside the cluster, which was derived from the relationship between numbers of observed and expected newborns with microcephaly.23 Data obtained from the Brazil Live Birth Information System12 and the Health Strategic Surveillance Information Center Platform of Recife.13
Discussion
In our study, we found that microcephaly occurred disproportionately in the poorest populations, particularly those residing in CSIs (indicating precarious urban infrastructure) and in areas with low MHDI (indicating low levels of longevity, education, and income).
The significantly different patterns of incidence of newborn microcephaly that we identified among various maternal races, education levels, and access to health care services provide evidence of the socioeconomic inequality involved in this microcephaly outbreak. In our study, the proportion of newborns with microcephaly born to black and mixed-race mothers in Recife (84.6%) was substantially higher than the proportions of black or mixed-race women in both the general Pernambuco state (61.8%) and Brazil (50.7%) populations.10 We also observed disparately low levels of education, high levels of use of public health care services, and high levels of CSI residence in the mothers of newborns with microcephaly. In a broader study, women in Brazil and Latin America compared with women in developed countries were found to have lower education levels and other socioeconomic inequities as a result of the intergenerational transmission of poverty and the lack of public policies that adequately protect their rights.25 These socioeconomic disparities are likely to increase the challenges of the families of children with microcephaly to accept these children and to offer them compassionate care and lives of dignity.
Our findings also validate the findings of Lesser and Kitron,26 who argued that the Zika virus crisis is just another indicator of the social inequality that persists in contemporary Brazil. In contending that Aedes aegypti is not a democratic mosquito, they also showed that the Zika virus crisis and resulting microcephaly outbreak were embedded primarily in the pockets of poverty and precarious sanitation that exist in parts of Brazil.
We are not the first to report socioeconomic disparities in Recife. Intraurban inequalities in infant mortality and communicable diseases in Recife were the subject of other epidemiological studies that examined the effect of various environmental and socioeconomic factors on these outcomes.27–29 In addition, the WATERLAT-GOBACIT Network Working Papers, published in 2016, discussed the occurrence of microcephaly in 59 children from poor areas of Recife and drew attention to the social inequalities in the spatial distribution of microcephaly in the city.30 Furthermore, although in the literature the relationships between Aedes aegypti infestation indicators (eg, Breteau index and building infestation index) and socioeconomic factors, urban infrastructure levels, and dengue incidence are inconclusive, factors such as demographic density, population mobility, sanitation, and mosquito infestation affect the risk of other diseases, especially dengue.31–33
For this study, we used the Intergrowth-21st criterion to identify newborns as having microcephaly.14 Victora et al34 also used the Intergrowth-21st criterion, along with gold-standard imaging of newborns with microcephaly from the Brazilian surveillance system, and they established that this criterion had a sensitivity of 85% and specificity of 97.8% for newborns with microcephaly in Brazil. As a result, they recommended the use of the Intergrowth-21st criterion for the classification of microcephaly in epidemics, stressing the importance in times of uncertainty of maintaining high specificity without sacrificing sensitivity. However, a cohort study of pregnant Brazilian women questioned the use of the cephalic perimeter as a single criterion to identify congenital Zika virus infections.7 The authors of the study found unfavorable outcomes, including other substantial neurological abnormalities, in 41.9% of newborns of mothers exposed to the Zika virus, despite the fact that only 3.4% of the newborns were diagnosed with microcephaly. These findings suggest the possibility that focusing only on microcephaly may lead to a large underreporting of the unfavorable outcomes related to Zika infection, of which microcephaly appears to be only one. With this in mind, whereas the number of newborns with microcephaly identified in our study may accurately reflect the microcephaly epidemic that affected Recife during the study period, it may represent only the tip of the iceberg of the overall morbidity caused by congenital Zika virus infection in the city.
Of the 19 554 live births that we identified in Recife during the study period, about 1% were newborns with microcephaly. At about the same time, the Latin-American Collaborative Study of Congenital Malformations estimated a historical microcephaly prevalence in Brazil of 0.02%.35 Whereas our data suggest the presence of an epidemic in Recife during the study period, Butler36 questioned whether there was in fact a microcephaly epidemic in Brazil, and whether there was a clear causal relationship between maternal infection with Zika and children born with microcephaly (or whether it may also be related to the environmental exposure of pregnant women to teratogenic agents). These questions point to the need to expand our analysis and perform more extensive prospective studies. Our results also point to the need for increased efforts at entomological surveillance and at measuring Aedes aegypti control in cities such as Recife.
The results of our study emphasize several ethical concerns, as previously identified by Rego and Palácios.37 In their article, they focused on 3 ethical issues that pertained to the Zika virus outbreak in Brazil. First, they noted that Zika infection should be considered a pandemic disease in a world without boundaries, and they suggested that cooperative international teams be set up to address Zika infection. Second, they identified the need to maintain balance between individual rights and state intervention. Third, they emphasized the importance of improved access to comprehensive sexual and reproductive health care services, including contraception and abortion, as well as access to ongoing support and care for the children and families affected by Zika infection. The neurological impairment of children with microcephaly demands the provision of round-the-clock care by their mothers, who often have other children and are the sole providers in their homes. In addition, the situations of these women are frequently aggravated by being poor and having difficulty accessing specialized health services, both of which amplify the social injustice.
Limitations
This study had several limitations. First, we were unable to geocode 8% of the newborns identified in the SINASC and CIEVS/RECIFE databases because of missing data on residential addresses of mothers. However, we were able to geocode all newborns with microcephaly in those databases. We believe that the geocoding rate of 92% was acceptable for this type of study, and that the exclusion of 8% of non-microcephaly births from our analysis should not have substantially influenced the results. In fact, because we analyzed environmental variables by district (the smallest administrative unit for which data were available), the strength of the association between these variables and microcephaly may have been masked. Recife, like other Brazilian capitals, is characterized by differences in internal infrastructure and intraurban socio-environmental inequalities, where areas with high socioeconomic status border areas with low socioeconomic status. It is a challenge to find and study areas that are more environmentally and socioeconomically homogenous.
Second, we did not use water supply as one of the socio-environmental variables for our study. Recife has a water supply network that covers 87% of its population.38 However, sporadic supply and the lack of water storage infrastructure in poorer areas mean that this percentage may not accurately reflect the realities of access to clean water or water storage alternatives. Owing to the sporadic water supply in Recife, the population is forced to develop strategies for storing water in reservoirs of all kinds and without adequate protection, facilitating the reproduction of Aedes aegypti.26 Because of the concern that data on the available water supply network may not accurately reflect the reality in Recife, and the fact that databases provide no additional information about sporadic supply or other storage and consumption alternatives, we did not analyze this variable in our study.
Third, because the IBGE Demographic Census 2010 data included sewage systems that discharge directly into stormwater drains as being equivalent to systems that provide more adequate sewage collection and treatment, the sewage-system rates used in our study may have overestimated the rates for quality sewage systems. As a result, the risk of microcephaly in our study in areas with lower sewage-system rates may have been underestimated. Finally, the SINASC database lacked data for some socio-environmental variables in a small number of live births. However, given the large size of the non-microcephaly study population and that the small amount of missing data was only within this population, we think it unlikely that these missing data substantially influenced our results.
Conclusions
The microcephaly outbreak in Recife disproportionately affected the newborns of women who were of black or mixed race, had low levels of education, were dependent on public health care services, and lived in the poorest areas. The concentration of newborns with microcephaly was highest in areas of Recife with more precarious urban infrastructure. These disparities in microcephaly incidence expose the level of socio-environmental inequality that exists in Recife, and they reinforce the need for government and public health authorities to formulate policies that promote both social equity and support for families and their children with microcephaly.
Acknowledgments
The authors thank the Municipal Health Secretariat of Recife for the availability of data, especially the sectors team: (1) the Information System on Live Births (SINASC)/Information Sector of Health Surveillance/Epidemiological Surveillance Unit/Executive Board of Health Surveillance/Health Secretariat of Recife; (2) the Center for Strategic Information in Health Surveillance/Epidemiological Surveillance Unit/Executive Board of Health Surveillance/Health Secretariat of Recife; and (3) the Center of Environmental Surveillance.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Foundation for Support of Science and Technology of Pernambuco (FACEPE), Brazil (grant No. APQ-0051-4.06/16).
References
- 1. McNeil DG., Jr Zika: The Emerging Epidemic. New York: WW Norton & Company; 2016. [Google Scholar]
- 2. RECIFE. Secretaria de Saúde. Informe Epidemiológico—Síndrome Congênita associada à Infecção pelo Vírus Zika (SCZ) [Epidemiological report—congenital syndrome associated with Zika virus infection (SCZ)]. 2017. https://cievsrecife.files.wordpress.com/2012/08/boletim-scz-se01-2017.pdf. Accessed May 9, 2018.
- 3. Nunes ML, Carlini CR, Marinowic D, et al. Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil. J Pediatr (Rio J). 2016;92(3):230–240. [DOI] [PubMed] [Google Scholar]
- 4. Souza BS, Sampaio GL, Pereira CS, et al. Zika virus infection induces mitosis abnormalities and apoptotic cell death of human neural progenitor cells. Sci Rep. 2016;6:39775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Possible association between Zika virus infection and microcephaly—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(3):59–62. [DOI] [PubMed] [Google Scholar]
- 6. Slavov SN, Otaguiri KK, Kashima S, Covas DT. Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Braz J Med Biol Res. 2016;49(5):e5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brasil P, Pereira JP, Jr, Moreira ME, et al. Zika virus infection in pregnant women in Rio de Janeiro. N Engl J Med. 2016;375(24):2321–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ministério da Saúde, Brasil. Monitoramento Dos Casos de Microcefalia No Brasil. 2016. http://combateaedes.saude.gov.br/images/pdf/Informe-Epidemiologico-n57-SE-52_2016-09jan2017.pdf. Accessed May 9, 2018.
- 9. World Health Organization. WHO statement on the first meeting of the International Health Regulations (2005) (IHR. 2005) Emergency Committee on Zika Virus and observed increase in neurological disorders and neonatal malformations. February 2016 http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en. Accessed May 9, 2018.
- 10. Instituto Brasileiro de Geografia e Estatística. Censo 2010. 2010. http://censo2010.ibge.gov.br. Accessed May 9, 2018.
- 11. Profeitura do Recife, Secretaria Municipal de Saúde. Plano Municipal de Saúde 2014-2017. 2014. http://www2.recife.pe.gov.br/sites/default/files/plano_municipal_de_saude_2015_revisado_menor.pdf. Accessed May 9, 2018.
- 12. Ministério da Saúde, Brasil. SINASC: Sistema de Informações de Nascidos Vivos. http://datasus.saude.gov.br/sistemas-e-aplicativos/eventos-v/sinasc-sistema-de-informacoes-de-nascidos-vivos. Accessed May 9, 2018.
- 13. CIEVSPE. Centro de Informações Estratégicas de Vigilância em Saúde de Pernambuco. Síndrome congênita associada a infecção pelo vírus Zika (SCZ). https://www.cievspe.com/microcefalia. Accessed May 9, 2018.
- 14. Villar J, Cheikh Ismail L, Victora CG, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. [DOI] [PubMed] [Google Scholar]
- 15. Ministério da Saúde, Departamento de Informática do SUS. DATASUS: informações de saúde. http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sim/cnv/obt10pe.def. Accessed May 9, 2018.
- 16. United Nations Development Program. Atlas of human development in Brazil. 2013. http://atlasbrasil.org.br/2013/en/o_atlas/idhm. Accessed May 9, 2018.
- 17. Environmental Systems Research Institute. ArcGIS Release 9.3. Redlands, CA: ESRI; 2009. [Google Scholar]
- 18. World Health Organization. Monitoring and evaluation: dengue control—vector surveillance. http://www.who.int/denguecontrol/monitoring/vector_surveillance/en. Accessed May 9, 2018.
- 19. Bivand RS, Pebesma E, Gómez-Rubio V. Applied Spatial Data Analysis With R. New York: Springer; 2013. [Google Scholar]
- 20. StataCorp. Stata Release 13. College Station, TX: StataCorp; 2013. [Google Scholar]
- 21. R Development Core Team. R Statistical Software. Vienna: R Development Core Team; 2016. [Google Scholar]
- 22. SANEAR—Sanitation Authority of Recife. Map the social interest communities. 2014. http://mundosafari.com.br/projetos/2015/prefeitura-atlas/en/sobre. Accessed May 9, 2018.
- 23. Kulldorff M. A spatial scan statistic. Commun Stat Theory Methods. 1997;26(6):1481–1496. [Google Scholar]
- 24. Kulldorff M. SaTScan Version 9.4. Boston, MA: Information Management Services; 2015. [Google Scholar]
- 25. Vélez AC, Diniz SG. Inequality, Zika epidemics, and the lack of reproductive rights in Latin America. Reprod Health Matters. 2016;24(48):57–61. [DOI] [PubMed] [Google Scholar]
- 26. Lesser J, Kitron U. A geografia social do zika no Brasil. Estud Avançados. 2016;30(88):167–175. [Google Scholar]
- 27. da Costa GN. Perinatal Mortality, Biological, Maternal-Infant Health Care, and Socioeconomic Determinants: An Analysis of the Inequalities Among the Districts of Recife [dissertation]. Recife, Brazil: Fundação Oswaldo Cruz, Centro de Pesquisas Aggeu Magalhães; 2008. https://www.arca.fiocruz.br/handle/icict/3885. Accessed May 9, 2018. [Google Scholar]
- 28. Guimarães MJB, Marques NM, Melo Filho DA, Szwarcwald CL. Condição de vida e mortalidade infantil: diferenciais intra-urbanos no Recife, Pernambuco, Brasil. Cad Saúde Pública. 2003;19(5):1413–1424. [DOI] [PubMed] [Google Scholar]
- 29. de Oliveira DS. Desigualdades Intraurbanas de Leptospirose no Recife [dissertation] Recife, Brazil: Fundação Oswaldo Cruz, Centro de Pesquisas Aggeu Magalhães; 2009. [Google Scholar]
- 30. Costa AM. A Determinação Social da Microcefalia/Zika No Brasil. Newcastle Upon Tyne: Desenvolvimento; 2016. [Google Scholar]
- 31. Mondini A, Chiaravalloti Neto F. Variáveis socioeconômicas e a transmissão de dengue. Rev Saúde Pública. 2007;41(6):923–930. [DOI] [PubMed] [Google Scholar]
- 32. Teurlai M, Menkès CE, Cavarero V, et al. Socio-economic and climate factors associated with dengue fever spatial heterogeneity: a worked example in New Caledonia. PLoS Negl Trop Dis. 2015;9(12):e0004211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vargas WP, Kawa H, Sabroza PC, Soares VB, Honório NA, de Almeida AS. Association among house infestation index, dengue incidence, and sociodemographic indicators: surveillance using geographic information system. BMC Public Health. 2015;15:746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Victora CG, Schuler-Faccini L, Matijasevich A, Ribeiro E, Pessoa A, Barros FC. Microcephaly in Brazil: how to interpret reported numbers? Lancet. 2016;387(10019):621–624. [DOI] [PubMed] [Google Scholar]
- 35. Poletta FA, Gili JA, Castilla EE. Latin American Collaborative Study of Congenital Malformations (ECLAMC): a model for health collaborative studies. Public Health Genomics. 2014;17(2):61–67. [DOI] [PubMed] [Google Scholar]
- 36. Butler D. Zika virus: Brazil’s surge in small-headed babies questioned by report [published erratum appears in Nature. 2016. Corrections]. Nature. 2016;530(7588):13–14. [DOI] [PubMed] [Google Scholar]
- 37. Rego S, Palácios M. Ética, saúde global e a infecção pelo vírus Zika: uma visão a partir do Brasil. Rev bioét. 2016;24(3):430–434. [Google Scholar]
- 38. COMPESA. Companhia Pernambucana de Saneamento. Índice de Atendimento Urbano de Água e Esgoto por município—2016. 2016. http://servicos.compesa.com.br/documentos-publicacoes-e-folhetos. Accessed June 21, 2017.