Abstract
Objectives:
The care cascade, a method for tracking population-level progression from diagnosis to cure, is an important tool in addressing and monitoring the hepatitis C virus (HCV) epidemic. However, little agreement exists on appropriate care cascade steps or how best to measure them. The New York City (NYC) Department of Health and Mental Hygiene (DOHMH) sought to construct a care cascade by using laboratory surveillance data with clinically relevant categories that can be readily updated over time.
Methods:
We identified all NYC residents ever reported to the DOHMH surveillance registry with HCV through June 30, 2017 (n = 175 896). To account for outmigration, death, or treatment before negative RNA results became reportable to the health department, we limited the population to people with any test reported since July 1, 2014. Of these residents, we identified the proportion with a reported positive RNA test and estimated the proportion treated and cured since July 2014 by using DOHMH-developed surveillance-based algorithms.
Results:
Of 78 886 NYC residents ever receiving a diagnosis of HCV and tested since July 1, 2014, a total of 70 397 (89.2%) had ever been reported as RNA positive through June 30, 2017; 36 875 (46.7%) had initiated treatment since July 1, 2014, and 23 766 (30.1%) appeared cured during the same period.
Conclusion:
A substantial gap exists between confirming HCV infection and initiating treatment, even in the era of direct-acting antivirals. Using this cascade, we will monitor progress in improved treatment and cure of HCV in NYC.
Keywords: care cascade, hepatitis C, surveillance, treatment, cure
Hepatitis C virus (HCV) is a leading cause of cirrhosis, liver cancer, and death. Since late 2013, highly effective direct-acting antivirals, with rates of sustained virologic response (SVR; ie, cure) >90%,1 have been available for treatment of HCV, making the control and elimination of HCV achievable.
The care cascade is one method to conceptualize the path from diagnosis to cure, on both an individual level and a population level. Care cascades are used extensively for HIV by using surveillance data and are increasingly used for HCV and other diseases.2,3 However, currently available HCV care cascades are highly disparate; many focus on specific clinical populations4–6 or are based on proportions from smaller studies applied to larger populations, which may not reflect any one location or population.7 In addition, many care cascades use categories that are difficult to measure without access to clinical data (eg, prescribing information). The need for clinical information is a particular concern for health departments, where access to such data is limited. Additionally, few published care cascades offer direct-acting antiviral–era treatment or cure rates. Since 2006, the New York City (NYC) Department of Health and Mental Hygiene (DOHMH) has had near–real-time electronic laboratory reporting for all reportable conditions, including tests for HCV infection.8 In addition, in mid-2014, negative HCV RNA results became reportable.9 These features allow the DOHMH to measure HCV testing and develop surveillance-based algorithms that estimate HCV treatment and cure.10
Given the limited access to clinical data and the availability of a robust surveillance system, DOHMH developed an HCV care cascade by using surveillance data that encompassed the NYC population with an HCV diagnosis. We developed a cascade that presents the current state of HCV care, can be readily refreshed with the latest surveillance data, and can identify gaps and direct interventions to improve HCV diagnosis, treatment, and cure rates in NYC.
Methods
Surveillance Data
Mandated reporting for HCV in NYC includes all positive HCV antibody (enzyme immunoassay or recombinant immunoblot assay, excluding rapid tests), positive and negative RNA, and genotype tests among NYC residents. All tests from the same person are automatically de-duplicated by using a matching algorithm, with daily manual review for close matches.
Identifying People With an HCV Diagnosis
We identified all people ever reported to DOHMH with any positive HCV test performed through June 30, 2017. Positive tests included antibody, RNA, or genotype reports. We excluded anyone reported as currently living outside NYC on the basis of the address from their most recent laboratory report. Information on vital status is sporadically available in our surveillance database because chronic HCV cases are not routinely investigated and vital status information from the DOHMH Vital Statistics registry has not been fully incorporated into the HCV registry. However, when such information was available, we excluded HCV-positive people recorded as deceased. We also excluded anyone reported as antibody positive and only RNA negative (with no report of a positive RNA). With no history of a positive RNA test, these people were uninfected at the time of first report; however, we included people without an RNA report.
Adjustments Based on Data Availability and Limitations
Mortality data in our surveillance system are limited. Likewise, information about when an individual moves out of NYC is not routinely or systematically reported. Unless we receive a report to the contrary, we assume that a person is alive and still an NYC resident; receipt of a new test result confirms that a person is still an NYC resident as of that test date. To reduce the likelihood of including people who were deceased or no longer living in NYC, we further limited the starting population to people with a report of an HCV test (of any result) since at least July 1, 2014. We chose this cut-off date to correspond with the start of negative RNA reporting. Because negative RNA results are required to estimate individual-level treatment and cure status, we wanted to ensure that all included people had the opportunity to have a negative RNA test reported to DOHMH and that those who were potentially treated and cured before negative RNA reporting (and so no longer tested) were not included.
The starting population for the care cascade comprised all people ever reported to DOHMH with a positive HCV test through June 30, 2017, and with a record of any test reported on or after July 1, 2014, who were not known to be deceased, living outside of NYC, or known to be uninfected at first report.
HCV RNA-Positive Population
We examined all reported RNA results for people included in the starting population. We identified all people with a record of any positive RNA result reported to DOHMH through June 30, 2017.
Treatment Initiation Population
We determined the treatment initiation status for all people in the starting population by using reported positive and negative RNA test results. Our program developed and validated an algorithm for identifying people in our surveillance system who likely initiated treatment.10 The algorithm required a person to have a negative RNA test result in 2014 or later, preceded by a high-positive (viral load ≥1000 IU/mL) RNA result.
Cured Population
Among all people initiating treatment, we applied a similarly developed cure algorithm to identify those achieving cure. The cure algorithm identifies each person’s first negative, low-positive (<1000 IU/mL), or indeterminate (positive, below the limit of detection) RNA test that occurred after a high-positive RNA test. Based on this anchor test, a person must have at least 1 additional negative RNA test at least 4 months later, and no subsequent high-positive RNA result, to be identified as cured.10 The algorithm considered positive RNA tests reported through June 30, 2017, and negative RNA tests reported between July 1, 2014, and June 30, 2017. We examined the test histories of people who appeared treated per the treatment algorithm but not cured per the cure algorithm to determine if there were likely reasons for being uncured.
People who initiate treatment may fail to appear cured if their most recent RNA result was a high positive, regardless of past negative results, or if the number of negative RNA results was insufficient, if at least 1 of their additional negative RNA results was not at least 4 months after their anchor test, or if their anchor test was within 4 months of June 30, 2017.
We completed all analyses by using SAS version 9.4.11 This analysis was classified as public health surveillance by the DOHMH Institutional Review Board.
Results
We identified 175 896 people with any positive HCV test who were not known to be deceased or residing outside of NYC as of June 30, 2017. Limiting the study population to those with any reported HCV test performed between July 1, 2014, and June 30, 2017, resulted in 78 886 people in the starting population.
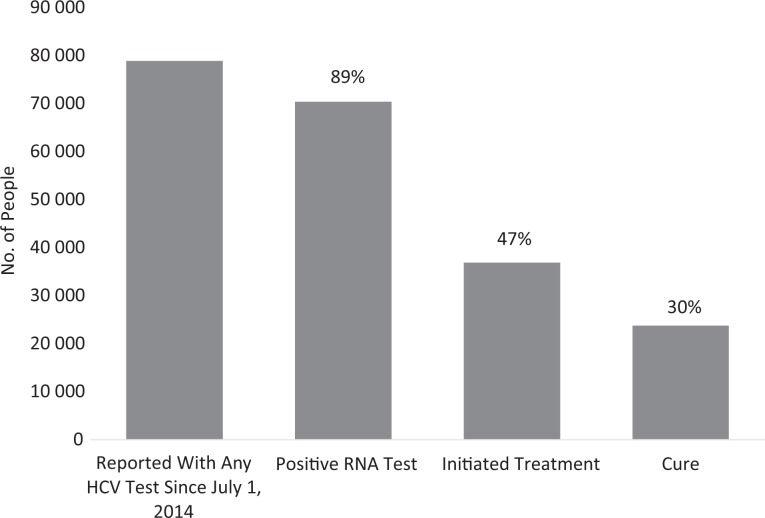
From this population, 70 397 (89.2%) people had ever had a positive RNA test through June 30, 2017; 36 875 (46.7%) people had initiated treatment between July 1, 2014, and June 30, 2017; and 23 766 (30.1%; 64.5% of those initiating treatment) people were cured between July 1, 2014, and June 30, 2017 (Figure).
Figure.
Care cascade for New York City residents with a diagnosis of hepatitis C through June 30, 2017, who were reported to the New York City Department of Health and Mental Hygiene (NYC DOHMH) with any hepatitis C virus (HCV) test since July 1, 2014 (n = 78 886). Data source: NYC DOHMH Viral Hepatitis Surveillance Program.
We examined the 13 109 people who appeared treated but uncured to provide likely interpretations for how failures to meet cure algorithm requirements translated into real-world clinical scenarios (Table). For 5573 (42.5%) of those who were uncured, the anchor test was >4 months before June 30, 2017, but no subsequent RNA tests had been performed since the anchor test, suggesting that at least some patients may not be returning for SVR testing. An additional 3592 (27.4%) people had follow-up testing within 4 months of their anchor test but no test after 4 months, even though enough time had passed for additional testing; some of these people might have completed their treatment regimen and were awaiting SVR testing. For 3055 (23.3%) people, <4 months had passed between their anchor test and the end of the study period, suggesting that these people may have only recently started treatment. Excluding those without enough follow-up time, the cure rate among those who were treated was 70.3% (23 766 of 33 820). Only 889 (6.8%) people who were treated but uncured had a subsequent positive RNA test after their anchor test; for 794 of them, that positive test was within 1 year of their last negative RNA result, suggesting treatment relapse or failure. Ninety-five people had a positive test reported >1 year after their last negative result, suggesting late treatment relapse, late detection of an earlier treatment relapse, or reinfection.
Table.
Summary of reasons and interpretations for New York City residents reported with a hepatitis C test to the New York City Department of Health and Mental Hygiene between July 1, 2014, and June 30, 2017, identified as treated per the Viral Hepatitis Program treatment algorithm but uncured per the cure algorithm (n = 13 109)a
| Reason Uncured | No. (%) | Interpretation |
|---|---|---|
| Anchor test is ≥120 days before June 30, 2017. | ||
| No testing since anchor test. | 5573 (42.5) | Patient not returning for follow-up/SVR testing |
| All tests are within 120 days of anchor test. | 3592 (27.4) | Patient awaiting SVR test; ceased follow-up testing |
| Anchor test is <120 days before June 30, 2017. | 3055 (23.3) | Patient recently started treatment |
| Most recent RNA test is a high positive (viral load ≥1000 IU/mL). | ||
| Positive test is <1 year after last negative result. | 794 (6.1) | Treatment relapse; early reinfection |
| Positive test is ≥1 year after last negative result. | 95 (0.7) | Late treatment relapse; late detection of early treatment relapse; reinfection |
Abbreviation: SVR, sustained virologic response.
aTreatment algorithm definition: a negative RNA test result in 2014 or later preceded by a high-positive (viral load ≥1000 IU/mL) RNA test result. Cure algorithm definition: a negative RNA test result at least 4 months after an anchor RNA test (the first negative, low-positive [viral load <1000 IU/mL] RNA test after the most recent high-positive RNA test), with no subsequent high-positive RNA test result through June 30, 2017. Data source: New York City Department of Health and Mental Hygiene, Viral Hepatitis Program. Treatment and cure status assessed among 78 886 people diagnosed with hepatitis C and reported to the New York City Department of Health and Mental Hygiene with any hepatitis C test since July 1, 2014.
Discussion
The DOHMH HCV care cascade is one of few to estimate treatment and cure on a population level in the direct-acting antiviral era. The goal of this cascade is to provide a practical tool, grounded in surveillance data, that can be used to direct resources toward improving HCV treatment and cure rates. Although cross-sectional, the cascade can be used for monitoring because its metrics are easily updated to reflect the current state of the HCV epidemic in NYC. We are also able to follow cohorts as they move through the care cascade.
Our care cascade showed a sizable gap between confirmed infection and treatment initiation, indicating challenges both in connecting people to HCV-related care and treatment and in providing clinicians with the knowledge and tools to initiate treatment. Administrative barriers also likely contributed substantially to this gap; when direct-acting antivirals were first introduced, private insurers and Medicaid programs, including those in New York State, introduced various restrictions in prescribing HCV medications, including limiting treatment to only those with advanced liver disease, requiring screening and/or abstinence from drugs and alcohol, and limiting the type of provider that can prescribe treatment.12 Beginning in 2016, most insurers in New York State removed restrictions based on liver disease stage, and other restrictions were removed or reduced depending on the insurance plan.12,13 Despite this progress, prior authorization requirements and prescriber restrictions remain substantial barriers to accessing treatment.
A large gap in the care cascade exists between those who are treated and those who are cured, especially relative to the high cure rates of direct-acting antivirals shown in clinical trials. Investigating this gap, we found that approximately one-quarter of people may have only recently initiated treatment and had not had time to achieve cure. Although many people may go on to achieve cure at a later date, and despite a higher cure rate when excluding these people (70.3%) than when including them (64.5%), we presented the cascade as a cross-section, capturing each person’s disease state at a particular point in time, including if the person had begun treatment but not achieved SVR as of our cut-off date. Investigating other reasons for appearing uncured reveals the limitation of relying on laboratory testing to determine cure—people who do not return for testing, as may be the case for at least 42.5% of uncured people (or 15.1% of all those initiating treatment) in our cohort, cannot be confirmed as cured regardless of their true SVR status. Likewise, although it appears that a relatively small proportion of people are failing treatment, and even fewer may be reinfected, we cannot make definitive conclusions about these people without clinical data.
One goal of this care cascade was to use readily measurable categories that were also clinically relevant to achieving cure. The chosen categories are clear steps that must be completed to achieve cure. However, we realize that many additional steps occur between RNA confirmation and treatment initiation that are not presented in this cascade. In addition to the administrative barriers associated with prior authorization, many patient- and provider-specific steps are needed to initiate treatment, and the steps that are needed may differ from one person to another. Identifying and describing these steps will be important for any program that attempts to help link people to care and access treatment; however, these steps are also likely largely not measurable by using surveillance test data alone and, as such, are not presented in this cascade.
Many cascades start with an estimate that includes both people with and people without an HCV diagnosis.2,3,14–16 We restricted our cascade to people reported through surveillance to increase its use as a monitoring tool. Including people with no diagnosis in the care cascade reduces our ability to see progress in addressing gaps, because we include potentially thousands of people in the denominator whose main problem is not failure to complete the cascade, but failure to initiate the cascade. Additionally, estimates of people with no diagnosis are not easily updated and, therefore, cannot be changed to reflect progress in increasing the number of diagnoses. However, a cascade based on prevalent HCV cases can still be useful for resource allocation for programs and initiatives that integrate screening and linkage-to-care efforts.
One possible concern about the care cascade is that the estimate of treated people might include an unknown number of people who spontaneously resolved their infections. About 20% of newly infected people are able to clear the infection without treatment, usually within the first 6 months of infection.17 The testing history of people who spontaneously clear infection may resemble that of people receiving treatment if they are tested in the time period when they transition from RNA positive to RNA negative. However, we do not think that spontaneous clearance was a substantial problem in our cascade. Because most new HCV infections are asymptomatic, few newly infected people are likely to be tested in this window of RNA clearance. To test this assumption, we investigated a small, random sample of people with a new diagnosis of HCV whose RNA test histories suggested either spontaneous clearance or treatment; we found that only 4% (2 of 50) had spontaneous resolution (NYC DOHMH, unpublished data). Excluding the 4% of people in this cascade who could potentially have spontaneously cleared (ie, those who were RNA positive for <6 months before testing RNA negative) would decrease our estimate of the treatment rate by only 0.4%.
Limitations
Our care cascade had several limitations. First, we did not have comprehensive data on deaths or outmigration in our surveillance population.3 Therefore, an unknown proportion of people appear stalled in the cascade and would be unable to progress through all stages. Starting our care cascade with people who had a relatively recent test reported to DOHMH likely excluded many people who were deceased or had moved. We also likely excluded people who were treated and cured during the interferon era, before negative RNA reporting at DOHMH, although we expect this number to be low.7 The trade-off is erroneously excluding people who are alive, residing in NYC, but untested and disconnected from care. However, given the data available, we believe our approach was the best way to capture the current state of the HCV epidemic in NYC. We hope to improve our ability to identify death or outmigration in the future through increased case investigation and more routine matching to the DOHMH Vital Statistics registry, thereby improving the accuracy of our care cascades going forward.
Another limitation of this care cascade model was that it may not be replicable by other health jurisdictions that do not have as extensive a surveillance system as available to the DOHMH; HCV surveillance and reporting requirements across the United States vary, and few other jurisdictions have negative RNA reporting.18 Given the importance of being able to measure and monitor this epidemic across the country, advocating for funding and developing more robust surveillance in other jurisdictions should be a priority, and we hope that future and continued engagement with federal, state, and possibly academic institutions will build this capacity.
Conclusion
We aimed to create a surveillance-based HCV care cascade in which data at each step could be readily updated and interpreted for people with an HCV diagnosis in NYC and that could be replicated or modified by other jurisdictions with HCV surveillance and laboratory reporting. This care cascade is our best approximation of the true progress of people with an HCV diagnosis in NYC and can be used to identify gaps and inform interventions designed to help people move toward cure. We plan to use this tool to evaluate and direct our efforts toward achieving hepatitis C elimination in NYC.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded through city tax levies.
References
- 1. Bansal S, Singal AK, McGuire BM, Anand BS. Impact of all oral anti-hepatitis C virus therapy: a meta-analysis. World J Hepatol. 2015;7(5):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Medland NA, McMahon JH, Chow EP, Elliott JH, Hoy JF, Fairley CK. The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc. 2015;18(1):20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perlman DC, Jordan AE, Nash D. Conceptualizing care continua: lessons from HIV, hepatitis C virus, tuberculosis and implications for the development of improved care and prevention continua. Front Public Health. 2017;4:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jonas MC, Rodriguez CV, Redd J, Sloane DA, Winston BJ, Loftus BC. Streamlining screening to treatment: the hepatitis C cascade of care at Kaiser Permanente mid-Atlantic states. Clin Infect Dis. 2016;62(10):1290–1296. [DOI] [PubMed] [Google Scholar]
- 5. Noska AJ, Belperio PS, Loomis TP, O’Toole TP, Backus LI. Engagement in the hepatitis C care cascade among homeless veterans, 2015. Public Health Rep. 2017;132(2):136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mera J, Vellozzi C, Hariri S, et al. Identification and clinical management of persons with chronic hepatitis C virus infection—Cherokee nation, 2012-2015. MMWR Morb Mortal Wkly Rep. 2016;65(18):461–466. [DOI] [PubMed] [Google Scholar]
- 7. Yehia BR, Schranz AJ, Umscheid CA, Lo Re V., III The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS One. 2014;9(7):e101554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nguyen TQ, Thorpe L, Makki HA, Mostashari F. Benefits and barriers to electronic laboratory results reporting for notifiable diseases: the New York City Department of Health and Mental Hygiene experience. Am J Public Health. 2007;97(suppl 1): S142–S145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. NYC Health Code Article 13. https://www1.nyc.gov/assets/doh/downloads/pdf/about/healthcode/health-code-article13.pdf. Accessed April 14, 2017.
- 10. Moore MS, Bocour A, Jordan L, et al. Development and validation of surveillance-based algorithms to estimate hepatitis C treatment and cure in New York City [published online ahead of print December 7, 2017]. J Public Health Manag Pract. [DOI] [PubMed] [Google Scholar]
- 11. SAS Institute, Inc. SAS Version 9.4. Cary, NC: SAS Institute, Inc; 2014. [Google Scholar]
- 12. National Viral Hepatitis Roundtable. Hepatitis C: the state of Medicaid access—2017 national summary report. https://stateofhepc.org/wp-content/uploads/2017/10/State-of-HepC_2017_FINAL.pdf. Published 2017. Accessed February 1, 2018.
- 13. A.G. Schneiderman announces major agreement with seven insurers to expand coverage of chronic hepatitis C treatment for nearly all commercial health insurance plans across New York State [press release]. New York: New York State Office of the Attorney General; April 26, 2016 https://ag.ny.gov/press-release/ag-schneiderman-announces-major-agreement-seven-insurers-expand-coverage-chronic. Accessed February 1, 2018. [Google Scholar]
- 14. Janjua NZ, Kuo M, Yu A, et al. The population level cascade of care for hepatitis C in British Columbia, Canada: the BC Hepatitis Testers Cohort (BC-HTC). EBioMedicine. 2016;12:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maier MM, Ross DB, Chartier M, Belperio PS, Backus LI. Cascade of care for hepatitis C virus infection within the US Veterans Health Administration. Am J Public Health. 2016;106(2):353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuncio D. The Hepatitis C Cascade of Care in Philadelphia. Washington, DC: National Hepatitis Technical Assistance Meeting; 2015. [Google Scholar]
- 17. Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13(1):34–41. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. State Reporting Requirements for Viral Hepatitis. Atlanta, GA: Centers for Disease Control and Prevention; 2015. [Google Scholar]