Abstract
Objectives:
The cost of direct-acting antiviral agents (DAAs) for hepatitis C virus (HCV) infection may contribute to treatment disparities. However, few data exist on factors associated with DAA initiation.
Methods:
We conducted a retrospective cohort study of HCV-infected Kaiser Permanente Northern California members aged ≥18 during October 2014 to December 2016, using Poisson regression models to evaluate demographic, behavioral, and clinical factors associated with DAA initiation.
Results:
Of 14 790 HCV-infected patients aged ≥18 (median age, 60; interquartile range, 53-64), 6148 (42%) initiated DAAs. DAA initiation was less likely among patients who were non-Hispanic black (adjusted rate ratio [aRR] = 0.7; 95% confidence interval [CI], 0.7-0.8), Hispanic (aRR = 0.8; 95% CI, 0.7-0.9), and of other minority races/ethnicities (aRR = 0.9; 95% CI, 0.8-1.0) than among non-Hispanic white people and among those with lowest compared with highest neighborhood deprivation index (ie, a marker of socioeconomic status) (aRR = 0.8; 95% CI, 0.7-0.8). Having maximum annual out-of-pocket health care costs >$3000 compared with ≤$3000 (aRR = 0.9; 95% CI, 0.8-0.9) and having Medicare (aRR = 0.8; 95% CI, 0.8-0.9) or Medicaid (aRR = 0.7; 95% CI, 0.6-0.8) compared with private health insurance were associated with a lower likelihood of DAA initiation. Behavioral factors (eg, drug abuse diagnoses, alcohol use, and smoking) were also significantly associated with a lower likelihood of DAA initiation (all P < .001). Clinical factors associated with a higher likelihood of DAA initiation were advanced liver fibrosis, HCV genotype 1, previous HCV treatment (all P < .001), and HIV infection (P = .007).
Conclusions:
Racial/ethnic and socioeconomic disparities exist in DAA initiation. Substance use may also influence patient or provider decision making about DAA initiation. Strategies are needed to ensure equitable access to DAAs, even in insured populations.
Keywords: hepatitis, racial disparities, substance abuse, treatment, health care delivery
As of 2014, more than 3 million people in the United States had chronic hepatitis C virus (HCV) infection.1–3 Interferon has historically been used to treat patients with HCV, but toxicity and low cure rates limited its use.4 The emergence of all-oral, interferon-free regimens using direct-acting antiviral agents (DAAs) has removed many of the clinical barriers to HCV treatment (eg, infection with HIV in the interferon era5), thus substantially increasing the number of HCV-infected patients for whom treatment can be tolerated and successful.6–9 However, the wholesale cost of DAA regimens can exceed $100 000 per patient,10 thereby potentially reducing access to treatment, which may in turn lead to disparities in uptake.
Studies from the interferon era identified racial/ethnic disparities in HCV treatment; treatment rates were lower among black patients than among white patients, even in settings with equal access to health care.11,12 Disparities in HCV treatment during the interferon era may have been driven by clinical factors, such as lower treatment efficacy among black patients than among white patients.13 Because DAAs are effective across racial/ethnic and other demographic subgroups,14,15 the clinical explanation for demographic disparities in HCV treatment no longer exists. However, such disparities may persist or even be exacerbated in the interferon-free treatment era because of the cost of treatment. Identifying subgroups with low rates of DAA initiation can inform efforts to promote equitable access to and initiation of HCV treatment, yet few studies have assessed predictors of DAA initiation in clinical practice settings.
We investigated the demographic, behavioral, and clinical factors associated with DAA initiation among Kaiser Permanente Northern California (KPNC) members in the DAA era. Our objective was to determine whether disparities exist in DAA uptake, independent of the clinical factors that drive HCV treatment decisions.
Methods
Study Setting, Population, and Design
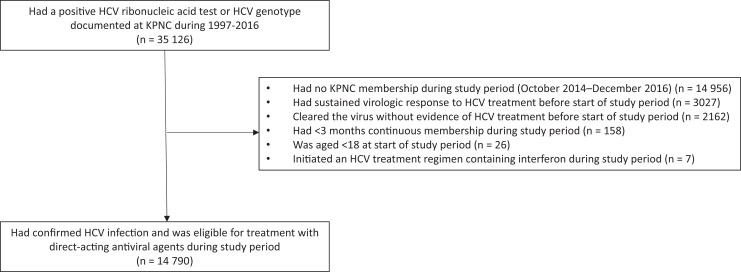
KPNC is a large, integrated health care system that provides comprehensive medical services to 4.1 million members, corresponding to approximately one-third of insured people in the surrounding population.16 Our study population included all KPNC members aged ≥18 who were eligible to receive DAAs during the study period, defined as October 2014 (month of approval of ledipasvir/sofosbuvir, when DAA use became widespread at KPNC) through December 2016 (Figure 1). To build this cohort, we first identified patients with confirmed HCV infection at any time during 1997-2016, defined as a positive HCV ribonucleic acid test or the presence of an HCV genotype (n = 35 126). We then excluded patients who were no longer KPNC members, had <3 months of continuous membership or were aged <18 during the study period, or were not eligible to receive DAAs because they had been previously treated or cleared the virus without evidence of treatment (n = 20 336). The final study population was 14 790 patients eligible to initiate DAAs. We defined the start of follow-up for each HCV-infected patient as October 1, 2014, or the earliest date thereafter of confirmed HCV infection and health plan enrollment. We followed HCV-infected patients until initiation of a DAA regimen, health plan disenrollment, death, or end of study (December 31, 2016), whichever occurred first. During the study period, KPNC recommended prioritization of patients with HCV who had advanced liver fibrosis for DAA initiation but did not place restrictions on DAA initiation for patients in early stages of disease. The institutional review board at KPNC approved this study with a waiver of written informed consent.
Figure 1.
Identification of hepatitis C virus (HCV)-infected adults aged ≥18 who were eligible to receive direct-acting antiviral agents, Kaiser Permanente Northern California (KPNC), October 2014–December 2016.
Study Measurements
We extracted data from the clinical and administrative databases that comprise KPNC’s electronic health record, including age; sex; race/ethnicity; health plan enrollment periods and insurance information; inpatient and outpatient diagnoses of drug abuse (International Classification of Diseases, Ninth Revision [ICD-9]17 codes 305.2-305.5; International Classification of Diseases, Tenth Revision [ICD-10]18 codes F11.xx-F14.xx, F16.xx, and F18.xx-F19.xx, where xx includes .10, .90, and .120), smoking/tobacco use (ICD-9 codes 305.1, V15, V65, 649, and social history codes internal to KPNC; ICD-10 codes: F17.200, F17.201, F17.210-211, F17.220-221, and F17.290-291), and hepatitis B virus (HBV) infection (ICD-9 code 070.32; ICD-10 code B18.1); number of alcoholic drinks per week, which has been systematically collected at KPNC since 201319; laboratory tests and results (ie, HCV genotype, platelets, aspartate aminotransferase, alanine transaminase); and pharmacy fills for HCV medications. We measured socioeconomic status by using the neighborhood deprivation index, which incorporates census tract–level measures of education, income and poverty, employment, housing, and occupation.20 We categorized the neighborhood deprivation index into quartiles, with the highest quartile (4) indicating the greatest neighborhood deprivation and the lowest quartile (1) indicating the least neighborhood deprivation. We extracted data on HIV status from the KPNC HIV registry, which includes all known HIV/AIDS cases since the early 1980s. HIV cases in the registry were confirmed by review of medical charts or medical center case lists. We ascertained dates of death from the electronic health record, California death certificates,21 and Social Security Administration data sets.22
We determined whether a patient had advanced liver fibrosis by using noninvasive measures, specifically transient elastography (ie, FibroScan), if available, or the Fibrosis-4 (FIB-4) index. We extracted transient elastography scores from a clinician-maintained database. FIB-4 is a serum biomarker comprising age and routine liver function test results, including platelets, alanine transaminase, and aspartate aminotransferase.23 We defined advanced liver fibrosis as a transient elastography score ≥9.5 kPa or as a FIB-4 score >3.25.
Statistical Analysis
The outcome of interest for this analysis was DAA initiation. Covariates of interest included age at start of follow-up (18-49, 50-59, 60-69, ≥70); sex (male/female); race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, Asian/Pacific Islander, non-Hispanic other, unknown); insurance type (private, Medicaid, Medicare, unknown); annual maximum individual out-of-pocket health care costs (≤$3000, >$3000, unknown); quartile of neighborhood deprivation index; number of alcoholic drinks per week (0, 1-7, 8-14, ≥15, unknown), using the most recent measurement before start of follow-up; liver fibrosis (early stage, advanced, unknown); HCV genotype (genotype 1, genotypes 2-6, unknown); HCV treatment before start of follow-up (yes/no); and drug abuse diagnosis, smoking, HIV infection, and HBV infection, all defined as ever or never through end of follow-up. We fixed all variables for analysis except for liver fibrosis, which we assessed at the start of follow-up and updated at 6-month intervals throughout follow-up.
We first described the demographic, behavioral, and clinical characteristics of HCV-infected patients at the start of follow-up. To assess trends in treatment disparities, we plotted the cumulative incidence of treatment from October 2014 through December 2016, stratified by race/ethnicity and neighborhood deprivation index and using the log-rank test to assess differences across strata. Finally, we obtained unadjusted rate ratios (RRs) and adjusted rate ratios (aRRs) for factors associated with DAA initiation from Poisson regression models, with the adjusted model including terms for age, sex, race/ethnicity, neighborhood deprivation index, annual maximum out-of-pocket health care costs, insurance type, number of alcoholic drinks per week, drug abuse diagnosis, smoking, HCV genotype, liver fibrosis, previous HCV treatment, HIV infection, and HBV infection.
We conducted all analyses by using SAS version 9.4.24 All statistical tests were 2-sided, and we considered P < .05 to be significant.
Results
Study Population
Of the 14 790 patients with HCV infection, the median age was 60 (interquartile range, 53-64), and 5804 (39%) patients were female (Table 1). By race/ethnicity, 7897 (53%) patients were non-Hispanic white, 2512 (17%) were non-Hispanic black, 2347 (16%) were Hispanic, 931 (6%) were Asian, and 789 (5%) were of other or unknown races/ethnicities. Most patients (n = 9765, 66%) were privately insured, and 5247 (35%) had annual maximum out-of-pocket health care costs >$3000. A total of 2571 (17%) patients reported some alcohol use, 3346 (23%) ever had a drug abuse diagnosis, and 7015 (47%) had ever smoked. Most patients (n = 9983, 68%) had HCV genotype 1 and had no previous HCV treatment (n = 13 164, 89%). Of the 6103 patients with available liver fibrosis measures at baseline, 1778 (29%) had advanced liver fibrosis. In total, 892 (6%) patients were coinfected with HBV and 446 (3%) were coinfected with HIV.
Table 1.
Baseline characteristics of HCV-infected adults aged ≥18 (n = 14 790), Kaiser Permanente Northern California, October 2014–December 2016
| Characteristics | Patients |
|---|---|
| Age at baseline, median (IQR), y | 60 (53-64) |
| Female, no. (%) | 5804 (39) |
| Race/ethnicity, no. (%) | |
| Non-Hispanic white | 7897 (53) |
| Non-Hispanic black | 2512 (17) |
| Hispanic | 2347 (16) |
| Asian | 931 (6) |
| Non-Hispanic other | 789 (5) |
| Unknown | 314 (2) |
| Insurance type, no. (%) | |
| Private | 9765 (66) |
| Medicare | 4113 (28) |
| Medicaid | 866 (6) |
| Unknown | 46 (<1) |
| Annual maximum out-of-pocket health care costs, no. (%) | |
| ≤$3000 | 9507 (64) |
| >$3000 | 5247 (35) |
| Unknown | 36 (<1) |
| No. of alcoholic drinks per week, no. (%) | |
| 0 | 10 335 (70) |
| 1-7 | 1782 (12) |
| 8-14 | 499 (3) |
| ≥15 | 290 (2) |
| Unknown | 1884 (13) |
| Ever had a drug abuse diagnosis, no. (%) | 3346 (23) |
| Ever smoked, no. (%) | 7015 (47) |
| HCV genotype, no. (%) | |
| Genotype 1 | 9983 (68) |
| Genotype 2 | 1555 (11) |
| Genotype 3 | 1243 (8) |
| Genotype 4 | 164 (1) |
| Genotype 5 | 3 (<1) |
| Genotype 6 | 192 (1) |
| Unknown | 1650 (11) |
| Never treated for HCV, no. (%) | 13 164 (89) |
| Advanced liver fibrosis, no. (%) | |
| Yes | 1778 (12) |
| No | 4325 (29) |
| Unknowna | 8687 (59) |
| HIV infection, no. (%) | 446 (3) |
| Hepatitis B virus infection, no. (%) | 892 (6) |
Abbreviations: HCV, hepatitis C virus; IQR, interquartile range.
a Although 8687 (59%) patients did not have liver fibrosis measurements at baseline, subsequent liver fibrosis measurements were included in time-updated analyses, and only 4017 (27%) did not have liver fibrosis measurements throughout follow-up.
Demographic Factors Associated With DAA Initiation
Of the 14 790 HCV-infected patients in the study cohort, 6148 (42%) initiated DAAs. Of these 6148 patients, 5099 (83%) patients received ledipasvir/sofosbuvir, 480 (8%) received sofosbuvir/velpatasvir, 395 (6%) received sofosbuvir alone, and 174 (3%) received other DAAs. Cumulative incidence of DAA initiation was lower among non-Hispanic black and Hispanic patients than among non-Hispanic white and Asian patients, as well as among patients with higher neighborhood-level deprivation than among patients with lower neighborhood-level deprivation. Racial/ethnic and socioeconomic disparities persisted throughout the study period (P < .001 by log-rank test for both) (Figure 2).
Figure 2.
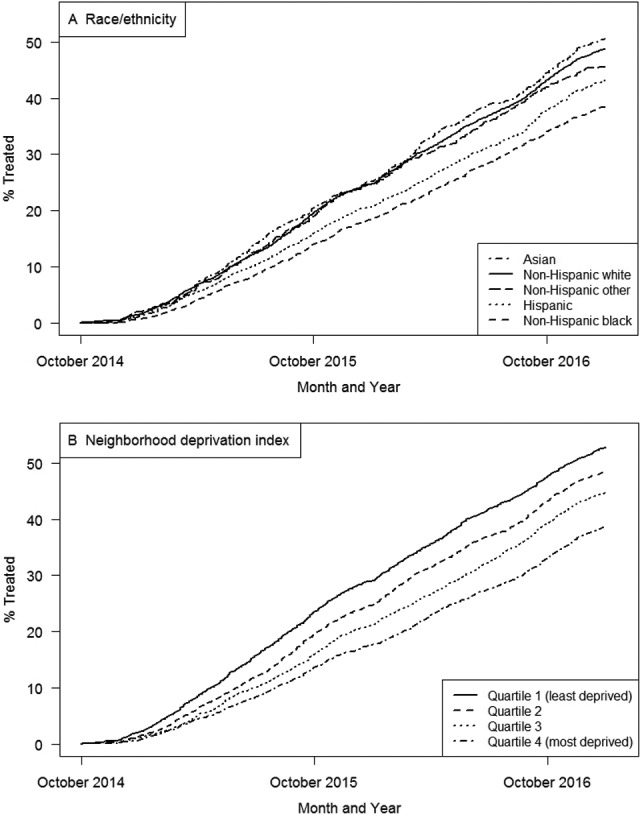
Cumulative incidence of direct-acting antiviral agent (DAA) initiation among hepatitis C virus (HCV)-infected adults aged ≥18 (n = 14 790), by year, stratified by (A) race/ethnicity and (B) neighborhood deprivation index, Kaiser Permanente Northern California, October 2014–December 2016. The neighborhood deprivation index is a measure of socioeconomic status that incorporates census tract–level measures of education, income and poverty, employment, housing, and occupation.20 The highest quartile (4) indicates the greatest neighborhood deprivation, and the lowest quartile (1) indicates the lowest neighborhood deprivation. Differences in DAA initiation by race/ethnicity and neighborhood deprivation index were significant (both P < .001 by log-rank test).
In unadjusted models, age, race/ethnicity, neighborhood deprivation index, and insurance characteristics were associated with DAA initiation (Table 2). In adjusted analysis, compared with patients aged 18-49, patients aged ≥70 were less likely to initiate DAAs (aRR = 0.6; 95% confidence interval [CI], 0.5-0.6). Compared with DAA initiation among non-Hispanic white patients, DAA initiation was less likely among patients who were non-Hispanic black (aRR = 0.7; 95% CI, 0.7-0.8), Hispanic (aRR = 0.8; 95% CI, 0.7-0.9), and of other minority races/ethnicities (aRR = 0.9; 95% CI, 0.8-1.0). Greater neighborhood deprivation was also associated with a reduced likelihood of DAA initiation in adjusted models. DAA initiation was less likely among Medicare (aRR = 0.8; 95% CI, 0.8-0.9) and Medicaid (aRR = 0.7; 95% CI, 0.6-0.8) enrollees compared with privately insured patients and among those with maximum annual out-of-pocket health care costs >$3000 compared with ≤$3000 (RR = 0.9; 95% CI, 0.8-0.9). Sex was not associated with DAA initiation in unadjusted or adjusted analyses.
Table 2.
Characteristics associated with initiation of direct-acting antiviral agents for HCV infection among adults aged ≥18 (n = 14 790), Kaiser Permanente Northern California, October 2014–December 2016
| Characteristics | Initiation of Direct-Acting Antiviral Agents, Unadjusted RR (95% CI) | P Value | Initiation of Direct-Acting Antiviral Agents, Adjusted RRa (95% CI) | P Valueb |
|---|---|---|---|---|
| Age, y | ||||
| 18-49 | 1.00 [Reference] | 1.00 [Reference] | ||
| 50-59 | 1.1 (1.0-1.2) | .04 | 1.0 (0.9-1.1) | .93 |
| 60-69 | 1.1 (1.0-1.2) | .006 | 1.0 (0.9-1.1) | .55 |
| ≥70 | 0.6 (0.5-0.6) | <.001 | 0.6 (0.5-0.6) | <.001 |
| Sex | ||||
| Male | 1.00 [Reference] | 1.00 [Reference] | ||
| Female | 1.0 (0.9-1.0) | .15 | 1.0 (0.9-1.0) | .08 |
| Race/ethnicity | ||||
| Non-Hispanic white | 1.00 [Reference] | 1.00 [Reference] | ||
| Non-Hispanic black | 0.7 (0.7-0.8) | <.001 | 0.7 (0.7-0.8) | <.001 |
| Hispanic | 0.8 (0.8-0.9) | <.001 | 0.8 (0.7-0.9) | <.001 |
| Asian | 1.0 (0.9-1.2) | .35 | 1.1 (1.0-1.2) | .21 |
| Non-Hispanic other/unknown | 0.9 (0.8-1.0) | .037 | 0.9 (0.8-1.0) | .02 |
| Neighborhood deprivation index quartilec | ||||
| 1 | 1.00 [Reference] | 1.00 [Reference] | ||
| 2 | 0.9 (0.8-0.9) | <.001 | 0.9 (0.8-1.0) | .005 |
| 3 | 0.8 (0.7-0.8) | <.001 | 0.9 (0.8-0.9) | <.001 |
| 4 | 0.7 (0.6-0.7) | <.001 | 0.8 (0.7-0.8) | <.001 |
| Insurance type | ||||
| Private | 1.00 [Reference] | 1.00 [Reference] | ||
| Medicare | 0.7 (0.7-0.8) | <.001 | 0.8 (0.8-0.9) | <.001 |
| Medicaid | 0.6 (0.6-0.7) | <.001 | 0.7 (0.6-0.8) | <.001 |
| Annual maximum out-of-pocket health care costs >$3000 (reference: ≤$3000) | 0.8 (0.8-0.9) | <.001 | 0.9 (0.8-0.9) | <.001 |
| No. of alcoholic drinks per week | ||||
| 0 | 1.00 [Reference] | 1.00 [Reference] | ||
| 1-7 | 0.9 (0.8-0.9) | <.001 | 0.8 (0.8-0.9) | <.001 |
| 8-14 | 0.8 (0.7-0.9) | <.001 | 0.8 (0.6-0.9) | <.001 |
| ≥15 | 0.6 (0.5-0.7) | <.001 | 0.6 (0.5-0.8) | <.001 |
| Drug abuse diagnosis | 0.7 (0.7-0.8) | <.001 | 0.8 (0.7-0.8) | <.001 |
| Smoking | 0.7 (0.7-0.7) | <.001 | 0.8 (0.7-0.9) | <.001 |
| HCV genotype 1 | 1.5 (1.4-1.6) | <.001 | 1.5 (1.4-1.6) | <.001 |
| Advanced liver fibrosis | 1.3 (1.2-1.4) | <.001 | 1.3 (1.3-1.4) | <.001 |
| Never treated for HCV | 0.5 (0.5-0.5) | <.001 | 0.7 (0.6-0.7) | <.001 |
| HIV infection | 1.4 (1.2-1.6) | <.001 | 1.2 (1.1-1.4) | .007 |
| Hepatitis B virus infection | 1.0 (0.9-1.1) | .48 | 0.9 (0.8-1.0) | .07 |
Abbreviations: HCV, hepatitis C virus; RR, rate ratio.
a Adjusted RRs were obtained from a Poisson model including age, sex, race/ethnicity, neighborhood deprivation index, HCV genotype, time-updated liver fibrosis, previous HCV treatment, alcohol use, drug abuse diagnosis, smoking, HIV infection, and hepatitis B virus infection.
b P values were obtained from Pearson's χ2 test, with P < .05 considered significant.
c The neighborhood deprivation index is a measure of socioeconomic status that incorporates census tract–level measures of education, income and poverty, employment, housing, and occupation.20 The highest quartile (4) indicates the greatest neighborhood deprivation and the lowest quartile (1) indicates the lowest neighborhood deprivation.
Behavioral Factors Associated With DAA Initiation
In unadjusted analysis, alcohol use, drug abuse diagnosis, and smoking were associated with DAA initiation. All behavioral factors were associated with treatment in adjusted analysis, with a lower likelihood of DAA initiation among those with more alcoholic drinks per week (aRR = 0.6; 95% CI, 0.5-0.8), those who ever had a drug abuse diagnosis (aRR = 0.8; 95% CI, 0.7-0.8), and those who ever smoked (aRR = 0.8; 95% CI, 0.7-0.9) (Table 2).
Clinical Factors Associated With DAA Initiation
In unadjusted analysis, HCV genotype, stage of liver fibrosis, not having previous HCV treatment, and being infected with HIV were associated with DAA initiation. All clinical factors were associated with DAA initiation in adjusted analysis. The likelihood of DAA initiation was higher among patients with HCV genotype 1 compared with other genotypes (aRR = 1.5; 95% CI, 1.4-1.6), those with advanced liver fibrosis compared with early stage liver fibrosis (aRR = 1.3; 95% CI, 1.3-1.4), and HIV-infected patients compared with HIV-uninfected patients (aRR = 1.2; 95% CI, 1.1-1.4). The likelihood of DAA initiation was lower among patients who had no previous HCV treatment compared with those previously treated (aRR = 0.7; 95% CI, 0.6-0.7). HBV infection was not associated with treatment in unadjusted or adjusted analysis (Table 2).
Discussion
In this cohort of HCV-infected adults in a large health care system, more than 40% initiated DAAs during the 2-year period of initial availability. We observed disparities in DAA initiation by race/ethnicity, socioeconomic status, and cost-sharing burden that persisted even after adjusting for clinical factors, including HCV genotype, liver fibrosis, and previous HCV treatment. Furthermore, we found that behavioral factors, including alcohol use and drug abuse diagnosis, were independently associated with a reduced likelihood of DAA initiation. Overall, our results highlight the need for strategies to ensure more widespread DAA initiation among non-Hispanic black, Hispanic, socioeconomically disadvantaged, and substance-using patients with HCV infection.
We observed disparities in DAA initiation by demographic characteristics that were independent of clinical and behavioral factors. Compared with non-Hispanic white patients, patients who were non-Hispanic black, Hispanic, and of other minority races/ethnicities were less likely to initiate DAAs, as were those with greater neighborhood-level deprivation compared with lower neighborhood-level deprivation. Our results are consistent with those of a large observational study conducted by the US Veterans Administration, which found lower rates of DAA initiation among black patients than among white patients during 2013-2015,25 and with the results of a study of a population-based cohort in Canada, which found that HCV-infected people with greater compared with lower neighborhood-level deprivation were less likely to initiate DAAs through 2015.26 Racial/ethnic and socioeconomic disparities in DAA initiation may be driven in part by the medication’s high cost. Although all patients in our cohort had access to health care, cost sharing for DAAs varied by health plans within Kaiser Permanente, and higher copayments and out-of-pocket health care costs have been associated with reduced adherence to other costly medications among Kaiser Permanente members.27,28 Our study found that patients with higher maximum out-of-pocket health care costs were less likely to initiate DAAs than patients with lower maximum out-of-pocket health care costs, indicating that cost is a barrier to treatment. Similarly, the Chronic Hepatitis Cohort Study found that lower compared with higher income was associated with lower rates of DAA initiation among insured patients with HCV.29 Racial/ethnic and neighborhood-level socioeconomic disparities in treatment persisted in our analysis even after adjusting for cost sharing; this finding suggests that other barriers to health care utilization, such as medical mistrust,30 perceived discrimination,31 or other contributors to overall financial burden,32 may play a role in these observed disparities.
We found that drug abuse diagnoses and reporting more alcoholic drinks per week were independently associated with a reduced likelihood of DAA initiation. Smoking, which may be a marker for recreational drug and alcohol use,33 was also associated with a lower likelihood of DAA initiation. The lower likelihood of DAA initiation we observed among substance-using patients may be partly driven by the effect of substance use on patient decision making about HCV treatment.34 Providers may also continue to be reluctant to initiate DAAs in patients with evidence of substance use because of concerns about nonadherence, even with shorter courses of therapy in the DAA era.35,36 Given evidence that HCV-infected people who use substances have similar rates of adherence and treatment efficacy compared with the overall HCV-infected population,37,38 HCV treatment guidelines currently state that substance use is not a contraindication to treatment and that pretreatment screening for substance use creates an unnecessary barrier to treatment among patients who may benefit from therapy.15 Efforts may be needed to facilitate linkage to substance use treatment for patients with HCV who report drug or alcohol use and to improve providers’ willingness to care for substance-using patients seeking HCV treatment.
Several clinical factors were independently associated with DAA initiation in our cohort, including advanced liver fibrosis, HCV genotype, HIV infection, and previous HCV treatment. Although DAAs are highly effective regardless of liver fibrosis stage, access to treatment has been limited by the high cost of DAAs.39 During the study period, KPNC recommended prioritization of patients with HCV who had advanced liver fibrosis for DAA initiation. However, unlike other settings such as state Medicaid programs,40 KPNC did not restrict access to DAAs among patients in earlier stages of disease. Patients who have advanced liver fibrosis may also be more motivated to seek treatment than patients who have mild liver fibrosis and are asymptomatic, and providers lack consensus about whether the clinical benefits of treatment during early stage disease outweigh the costs.41,42 We also found that patients with HCV genotype 1 were more likely to initiate DAAs than patients with other genotypes, which is consistent with the initial approval of ledipasvir/sofosbuvir for patients with HCV genotype 1. The higher likelihood of DAA initiation among HIV-infected patients may be attributable to the deferral of treatment until the emergence of more effective and better-tolerated therapies, as well as prioritization of this high-risk population by treating clinicians. Finally, patients who were previously treated with interferon-based therapies and, thus, already engaged in HCV specialty care were more likely than those not already engaged in care to initiate DAAs, which is consistent with findings from the Chronic Hepatitis Cohort Study.29
Limitations
This study had several limitations. First, although liver biopsy is considered the gold standard for measuring stage of liver fibrosis, it is not routinely conducted; thus, we used a combination of transient elastography and the FIB-4 index to measure stage of liver fibrosis. Although measuring liver fibrosis using transient elastography and FIB-4 may have resulted in some misclassification, these noninvasive measures are shown to be highly specific for advanced liver fibrosis,23,43 and we updated measurements every 6 months to account for potential changes in measurement during study follow-up. Furthermore, although data on liver fibrosis were available only for one-third of the cohort at baseline, 73% had at least 1 liver fibrosis measurement at baseline or during follow-up, and these subsequent measurements were included in time-updated analyses. Second, some patients may have spontaneously cleared their HCV infection during study follow-up and, thus, no longer should have been considered eligible for DAA initiation; however, we expect spontaneous clearance to have been rare, given that most spontaneous resolutions occur within 6 months of initial infection.44 Third, annual individual maximum out-of-pocket health care costs may not have fully captured the actual amount each patient would have had to pay for treatment; rather, they were a proxy for cost-sharing burden. Finally, because behavioral factors such as drug abuse diagnosis and smoking were collected from the electronic health record, some factors may have been misclassified, and the timing of these risk factors with respect to DAA initiation could not be analyzed in detail.
Strengths
Our study also had several strengths. First, the sample population was one of the largest cohorts of HCV-infected patients observed in a real-world setting, offering insights into the translation of DAA treatment from clinical trials into clinical practice. Second, in contrast to other large observational cohorts of HCV-infected patients that are almost entirely male,24 nearly 40% of our cohort was female, which strengthened the generalizability of our results. Third, rather than relying on diagnosis codes for alcohol abuse, we evaluated a self-reported measure of alcohol use that, although subject to underreporting, allowed us to observe a reduced likelihood of treatment even at relatively low levels of consumption. Fourth, because our study population was insured, we were able to assess disparities in DAA initiation in a setting in which all patients have broad access to health care, and we could also assess the effect of cost sharing on those disparities. Finally, the KPNC membership mirrors the age, sex, and race/ethnicity distributions of the surrounding population.16 Thus, our results are likely to be generalizable to other insured adults in California.
Conclusions
More than 40% of patients with HCV initiated DAAs during the initial 2 years of availability in this insured population. However, racial/ethnic and socioeconomic disparities exist in DAA initiation that were not explained by the clinical factors that drove treatment decisions. Drug abuse diagnoses and alcohol use also appeared to affect patient or provider decision making about HCV treatment, resulting in a lower likelihood of DAA initiation among substance-using patients with HCV. Efforts to increase affordability of DAA initiation and facilitate HCV treatment for substance-using patients may result in more equitable dissemination of HCV treatment in the interferon-free era.
Footnotes
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.L.M. reports research grant support from Merck. M.J.S. and C.P.Q. report research grant support from Pfizer and Merck. M.P.P. reports research grant support from Merck and Gilead.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Kaiser Permanente Delivery Science Research Program and the National Institute of Allergy and Infectious Diseases (K01 122 853 to J.L.M.).
References
- 1. Centers for Disease Control and Prevention. Disease burden from viral hepatitis A, B, and C in the United States. 2016. http://www.cdc.gov/hepatitis/HCV/StatisticsHCV.htm. Accessed November 6, 2017.
- 2. Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31(8):1090–1101. [DOI] [PubMed] [Google Scholar]
- 3. Mahajan R, Xing J, Liu SJ, et al. Mortality among persons in care with hepatitis C virus infection: the Chronic Hepatitis Cohort Study (CHeCS), 2006-2010 [published correction appears in Clin Infect Dis. 2014;58(12):1792]. Clin Infect Dis. 2014;58(8):1055–1061. [DOI] [PubMed] [Google Scholar]
- 4. Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55(9):1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mehta SH, Lucas GM, Mirel LB, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20(18):2361–2369. [DOI] [PubMed] [Google Scholar]
- 6. Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879–1888. [DOI] [PubMed] [Google Scholar]
- 7. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594–1603. [DOI] [PubMed] [Google Scholar]
- 8. Sulkowski MS, Naggie S, Lalezari J, et al. Sofosbuvir and ribavirin for hepatitis C in patients with HIV coinfection [published correction appears in JAMA. 2014;312(18):1932]. JAMA. 2014;312(4):353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. [DOI] [PubMed] [Google Scholar]
- 10. Hepatitis C Online. Ledipasvir-Sofosbuvir (Harvoni). https://www.hepatitisc.uw.edu/page/treatment/drugs/ledipasvir-sofosbuvir. Accessed September 27, 2017.
- 11. Kanwal F, Hoang T, Spiegel BM, et al. Predictors of treatment in patients with chronic hepatitis C infection—role of patient versus nonpatient factors. Hepatology. 2007;46(6):1741–1749. [DOI] [PubMed] [Google Scholar]
- 12. Rousseau CM, Ioannou GN, Todd-Stenberg JA, et al. Racial differences in the evaluation and treatment of hepatitis C among veterans: a retrospective cohort study. Am J Public Health. 2008;98(5):846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muir AJ, Bornstein JD, Killenberg PG; Atlantic Coast Hepatitis Treatment Group. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites [published correction appears in N Engl J Med. 2004;351(12):1268]. N Engl J Med. 2004;350(22):2265–2271. [DOI] [PubMed] [Google Scholar]
- 14. Wilder JM, Jeffers LJ, Ravendhran N, et al. Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: a retrospective analysis of phase 3 data. Hepatology. 2016;63(2):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org. Accessed September 27, 2017. [DOI] [PMC free article] [PubMed]
- 16. Gordon NP. Similarity of the adult Kaiser Permanente membership in Northern California to the insured and general population in Northern California: statistics from the 2011 California Health Interview Survey. Oakland, CA: Kaiser Permanente Northern California Division of Research; 2015. https://divisionofresearch.kaiserpermanente.org/projects/memberhealthsurvey/SiteCollectionDocuments/chis_non_kp_2011.pdf. Accessed September 27, 2017. [Google Scholar]
- 17. National Center for Health Statistics, Centers for Disease Control and Prevention. International Classification of Diseases, Ninth Revision (ICD-9). 2015. https://www.cdc.gov/nchs/icd/icd9.htm. Accessed March 22, 2018.
- 18. National Center for Health Statistics, Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision (ICD-10). 2016. https://www.cdc.gov/nchs/icd/icd10.htm. Accessed March 22, 2018.
- 19. Kaiser Permanente. Heading off and helping with unhealthy alcohol use. 2014. https://share.kaiserpermanente.org/article/heading-off-and-helping-with-unhealthy-alcohol-use. Accessed August 11, 2017.
- 20. Messer LC, Laraia BA, Kaufman JS, et al. The development of a standardized neighborhood deprivation index. J Urban Health. 2006;83(6):1041–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. California Department of Public Health. Death data files. 2016. https://archive.cdph.ca.gov/data/dataresources/requests/Pages/DeathDataFiles.aspx. Accessed September 27, 2017.
- 22. National Technical Information Service, US Department of Commerce. Death master file monthly updates. 2016. https://dmf.ntis.gov/monthly. Accessed September 27, 2017.
- 23. Holmberg SD, Lu M, Rupp LB, et al. Noninvasive serum fibrosis markers for screening and staging chronic hepatitis C virus patients in a large US cohort. Clin Infect Dis. 2013;57(2):240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. SAS Institute. SAS Version 9.4. Cary, NC: SAS Institute; 2013. [Google Scholar]
- 25. Kanwal F, Kramer JR, El-Serag HB, et al. Race and gender differences in the use of direct acting antiviral agents for hepatitis C virus. Clin Infect Dis. 2016;63(3):291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Janjua NZ, Islam N, Wong J, et al. Shift in disparities in hepatitis C treatment from interferon to DAA era: a population-based cohort study. J Viral Hepat. 2017;24(8):624–630. [DOI] [PubMed] [Google Scholar]
- 27. Marcus JL, Hurley LB, Hare CB, et al. Preexposure prophylaxis for HIV prevention in a large integrated health care system: adherence, renal safety, and discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Karter AJ, Parker MM, Solomon MD, et al. Effect of out-of-pocket cost on medication initiation, adherence, and persistence among patients with type 2 diabetes: the Diabetes Study of Northern California (DISTANCE). Health Serv Res. 2018;53(2):1227–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spradling PR, Xing J, Rupp LB, et al. Uptake of and factors associated with direct-acting antiviral therapy among patients in the Chronic Hepatitis Cohort Study, 2014 to 2015 [published online June 5, 2017]. J Clin Gastroenterol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boulware LE, Cooper LA, Ratner LE, LaVeist TA, Powe NR. Race and trust in the health care system. Public Health Rep. 2003;118(4):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Houtven CH, Voils CI, Oddone EZ, et al. Perceived discrimination and reported delay of pharmacy prescriptions and medical tests. J Gen Intern Med. 2005;20(7):578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel MR, Nelson BW, Id-Deen E, Caldwell CH. Beyond co-pays and out-of-pocket costs: perceptions of health-related financial burden in managing asthma among African American women. J Asthma. 2014;51(10):1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daskalopoulou M, Rodger A, Phillips AN, et al. Recreational drug use, polydrug use, and sexual behaviour in HIV-diagnosed men who have sex with men in the UK: results from the cross-sectional ASTRA study. Lancet HIV. 2014;1(1):e22–31. [DOI] [PubMed] [Google Scholar]
- 34. Mehta SH, Genberg BL, Astemborski J, et al. Limited uptake of hepatitis C treatment among injection drug users. J Community Health. 2008;33(3):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rogal SS, McCarthy R, Reid A, et al. Primary care and hepatology provider-perceived barriers to and facilitators of hepatitis C treatment candidacy and adherence. Dig Dis Sci. 2017;62(8):1933–1943. [DOI] [PubMed] [Google Scholar]
- 36. King A, Bornschlegel K, Johnson N, Rude E, Laraque F. Barriers to treatment among New York City residents with chronic hepatitis C virus infection, 2014. Public Health Rep. 2016;131(3):430–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dimova RB, Zeremski M, Jacobson IM, Hagan H, Des Jarlais DC, Talal AH. Determinants of hepatitis C virus treatment completion and efficacy in drug users assessed by meta-analysis. Clin Infect Dis. 2013;56(6):806–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aspinall EJ, Corson S, Doyle JS, et al. Treatment of hepatitis C virus infection among people who are actively injecting drugs: a systematic review and meta-analysis. Clin Infect Dis. 2013;57(suppl 2):S80–S89. [DOI] [PubMed] [Google Scholar]
- 39. Jayasekera CR, Arora S, Ahmed A. Hepatitis C treatment delivery mandates optimizing available health care human resources: a case for task shifting. JAMA. 2016;315(18):1947–1948. [DOI] [PubMed] [Google Scholar]
- 40. Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med. 2015;163(3):226–228. [DOI] [PubMed] [Google Scholar]
- 41. Hezode C. Why I do not treat patients for mild disease. Liver Int. 2016;36(suppl 1):13–20. [DOI] [PubMed] [Google Scholar]
- 42. Calvaruso V, Craxi A. Why do I treat my patients with mild hepatitis C? Liver Int. 2016;36(suppl 1):7–12. [DOI] [PubMed] [Google Scholar]
- 43. Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128(2):343–350. [DOI] [PubMed] [Google Scholar]
- 44. Grebely J, Page K, Sacks-Davis R, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59(1):109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]