Abstract
Patient registries are a valuable tool in the research of rare conditions such as pulmonary hypertension (PH). We report comprehensive hemodynamic and survival data of 174 patients with pulmonary arterial hypertension (PAH) and chronic thromboembolic pulmonary hypertension (CTEPH), included in the prospective Latvian PH registry over a period of > 9 years. In total, 130 adult PAH patients (75%) and 44 adult CTEPH patients (25%) were enrolled. The median follow-up period was 33 months for PAH and 18 months for CTEPH, P = 0.001. Latvian CTEPH patients had significantly higher plasma levels of B-type natriuretic peptide, higher pulmonary vascular resistance, and lower cardiac index than Latvian PAH patients. Calculated incidence of PAH and CTEPH in Latvia in 2016 was 13.7 and 5.1 cases per million inhabitants, calculated prevalence was 45.7 and 15.7 cases per million inhabitants, respectively. Survival rates at one, three, and five years for PAH patients was 88.0%, 73.3%, and 58.1%, and 83.8%, 59.0%, and 44.2% for CTEPH patients, respectively. We compared our study results with data from European adult PH registries. Latvian PAH patients had the fourth lowest and CTEPH patients the lowest one-year survival rate among European adult PH registries. As most PH registries in Europe are small, yet with equivalent patient inclusion criteria, it would be desirable to combine these registries to produce more reliable and high-quality study results.
Keywords: pulmonary arterial hypertension, chronic thromboembolic pulmonary hypertension, prevalence, incidence, survival
Patient registries are one of the key instruments to conduct research in rare diseases, such as pulmonary hypertension (PH), because low patient numbers limit the ability to achieve adequate sample size for epidemiological or clinical studies.1
There are several examples of successful multinational collaboration in the field of PH, e.g. the COMPERA registry and international chronic thromboembolic pulmonary hypertension (CTEPH) registry, proving that large-scale projects are feasible, albeit with financial support from the pharmaceutical industry.2,3
However, information about PH registries in Europe that could potentially collaborate in the future is somewhat limited. For example, in the latest Orphanet (an organization providing information about orphan drugs and rare diseases) report, only three European countries—France,4 Spain,5,6 and Sweden7—are reported as having a PH registry.8
To our knowledge, this is the first study to report comprehensive hemodynamic and transplant-free survival data from our national referral center (Latvian PH registry) (part of the data has been published before9–11). Additionally, to discuss possibility of future collaboration between PH centers/registries in Europe and to gain a better understanding of current state of PH registries in the region, we conducted a systematic literature search to identify prospective studies based on European adult PH registry data.
Methods
Data collection
This is a single-center prospective observational cohort study based on incident cases of pulmonary arterial hypertension (PAH) and CTEPH in the Latvian population. The Latvian PH registry was created in September 2007 at Pauls Stradins Clinical University Hospital (PSCUH), Riga.
Between 1 September 2007 and 31 December 2016 (9.3 years), 1239 consecutive patients with clinical suspicion of PH were referred to our center from all regions of Latvia. In total, 683 patients underwent diagnostic right heart catheterization (RHC) and PH was confirmed in 503 of them. Patients were eligible for enrolment if they met diagnostic criteria of PAH or CTEPH12 and were aged ≥ 18 years. The date of diagnostic RHC was considered the date of diagnosis. Patients included in the present study were followed until 1 July 2017. Patients who underwent lung transplantation or pulmonary endarterectomy (PEA) were censored at the time of the surgery; those lost to follow-up were censored at the time of their last visit. Based on the etiology of their PH, patients were divided into PAH subgroups. Diagnosis of CTEPH was based on contrast-enhanced computed tomography (CT) and not V/Q scanning because this method is not available in Latvia.13
Informed consent was obtained from each patient; the study protocol was in compliance with ethics guidelines of the Declaration of Helsinki.
The overall incidence and prevalence of PAH and CTEPH in Latvia was calculated using Latvian population data in 2016 from the Central Statistical Bureau of Latvia database (n = 1,969,000).14
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or as median with interquartile range (IQR; Q1–Q3) when data were not normally distributed. The Kolmogorov–Smirnov test was used to assess normality of data. Comparison of follow-up period was made using the Mann–Whitney U test. Categorical variables were expressed as frequencies and percentages. Survival was estimated using the Kaplan–Meier analysis. P values < 0.05 were considered significant.
Statistical analysis was made using IBM SPSS Statistics 23.0 (IBM Corp., Armonk, NY, USA).
Systematic literature search
We performed a systematic literature search to identify registry studies in Europe that included adult patients with PH, PAH, and/or CTEPH and reported registry design, patient hemodynamic characteristics, and survival. This was performed on 29 October 2017 in PubMed using the following search string: (“pulmonary hypertension” OR “pulmonary arterial hypertension” OR “chronic thromboembolic pulmonary hypertension”) AND (“registry” OR “cohort”) NOT (pediatric) [All Fields]. Time was restricted to studies published between January 2000 and October 2017. This search strategy identified 1499 publications. Studies were considered for inclusion by manual screening of article titles (and subsequently full articles) to identify prospective registry studies in relevant populations, published in English, and included survival data. Very small studies (i.e. < 100 patients with PH) were not included in the analysis. In case of multiple similar (i.e. describing the same PH group) studies based on the same registry, we reported the one with largest cohort. In addition to the three PH registries mentioned in Orphanet report,4–7 ten prospective European registry studies were in accord with our inclusion criteria.2,3,15–22 One single-center study (157 patients with PAH and 82 inoperable CTEPH patients) was excluded from our analysis as it mainly focused on the presence of atrial flutter and fibrillation and its effect on patients’ survival.15
Results
A total of 130 PAH and 44 CTEPH patients were enrolled in our registry by December 2016 and constituted the two main study groups. Most common PAH subtypes were: idiopathic PAH (IPAH) = 53 patients (41% of all PAH patients); PAH associated with connective tissue diseases (PAH-CTD) = 23 patients (18%); and PAH associated with congenital heart disease (PAH-CHD) = 49 patients (38%). Four patients had portopulmonary hypertension (PoPH) (3%) and one patient had drug-induced PAH.
Main demographic, hemodynamic, and other baseline characteristics in both patient groups are presented in Table 1. Overall, gender distribution, as well as age and BMI were similar in both PAH and CTEPH patient groups, with a majority of patients being women (73% and 61%, respectively), with median age at the time of diagnosis of 65 and 67 years, respectively. Both patient groups had elevated B-type natriuretic peptide (BNP) indicating advanced disease at the time of diagnosis. This was partially reflected in patients’ functional status; the majority of patients in both groups were in NYHA class III/IV (72% of patients in PAH group and 84% of those in CTEPH group) and had low 6-min walking distance (6MWD) (322 ± 122 m and 274 ± 111 m, respectively).
Table 1.
Baseline characteristics of Latvian PAH and CTEPH patients.
| Parameters | PAH | CTEPH |
|---|---|---|
| Patients (n (%)) | 130 (75) | 44 (25) |
| Female (n (%)) | 95 (73) | 27 (61) |
| Age median (years (IQR)) | 65 (47–71) | 67 (47–73) |
| BMI (kg/m2) | 28.1 ± 7.5 | 28.5 ± 7.3 |
| BNP (pg/mL (IQR)) | 204 (98–413) | 340 (181–756) |
| 6MWD (m) | 322 ± 122 | 274 ± 111 |
| NYHA functional class (n (%)) | ||
| I | 2 (2) | 0 (0) |
| II | 34 (26) | 7 (16) |
| III | 85 (65) | 33 (75) |
| IV | 7 (7) | 4 (9) |
| Hemodynamic parameters | ||
| RAP (mmHg) | 11 ± 7 | 13 ± 8 |
| mPAP mmHg) | 49 ± 18 | 51 ± 15 |
| PAWP (mmHg (IQR)) | 14 (9–15) | 12 (8-15) |
| PVR (WU (IQR)) | 6.6 (4.4–10.9) | 10.3 (6.9–13.8) |
| CI (L/min/m2) | 2.47 ± 0.73 | 1.93 ± 0.74 |
Values are expressed as mean ± SD or as median with interquartile range (IQR), where appropriate.
BMI, body mass index; BNP, B-type natriuretic peptide; CI, cardiac index; CTEPH, chronic thromboembolic pulmonary hypertension; NYHA, New York Heart Association; 6MWD, 6-min walking distance; RVSP, right ventricular systolic pressure; PAH, pulmonary arterial hypertension; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; WU, Wood units.
Apart from two IPAH patients (1% of all patients) who responded to acute vasodilator testing and received high-dose calcium channel blockers, all PAH and CTEPH patients received PH-specific monotherapy after the diagnosis, mainly sildenafil (n = 123, 71% of all patients). Thirty-one patients (18%) received bosentan and 18 patients (10%) received ambrisentan. None of the patients received initial combination or prostacyclin therapy, as it is currently not reimbursed in Latvia.
As PEA is not performed in Latvia, only seven Latvian CTEPH patients underwent PEA (performed in various PH centers abroad).
Survival
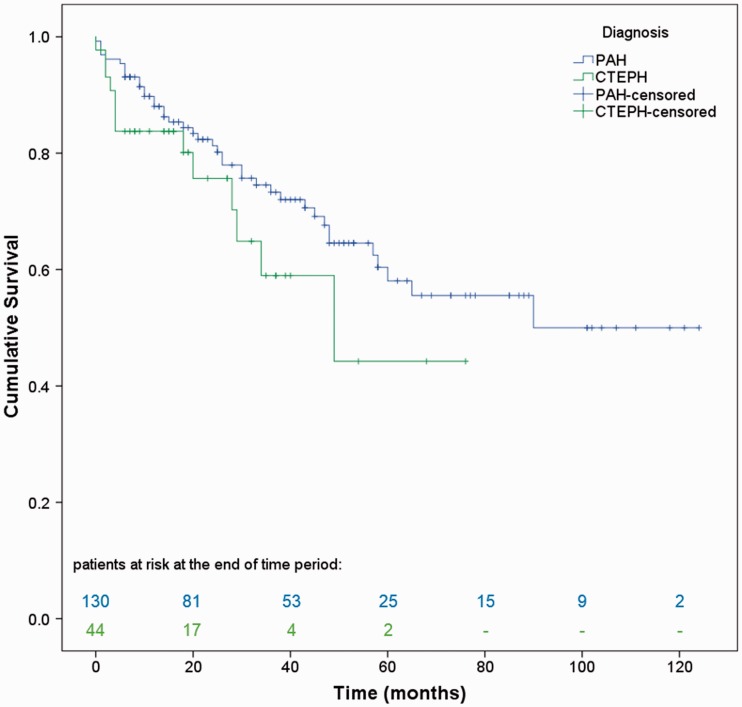
Median follow-up period was 33 months (range = 14–54 months) for PAH and 18 months (range = 8–34 months) for CTEPH, P = 0.001. Survival rates at one, three, and five years were 88.0%, 73.3%, and 58.1% for PAH patients, and 83.8%, 59.0%, and 44.2% for CTEPH patients, respectively. By the end of the study, 40 patients in the PAH group (30.8%) and 14 in the CTEPH group (31.8%) had died. Two PAH patients were lost to follow-up and one patient underwent lung transplantation. Seven CTEPH patients (16%) underwent PEA. The Kaplan–Meier curve for both patient groups is illustrated in Fig. 1.
Fig. 1.
Kaplan–Meier estimates of cumulative survival from date of diagnosis in Latvian PAH and CTEPH patients.
PH registries in Europe
A total of 14 prospective studies based on 12 national and multinational PH registries were included in our literature review, representing 18 European countries (17 of them are member states of the European Union) and Canada (one center as a part of international CTEPH registry). The main characteristics of these 12 registries are shown in Table 2.2–7,16–22 Four of the registries were single-center, the number of centers in other registries ranged from five in the Portuguese registry to 31 in the Spanish registry (REHAP).
Table 2.
General information on national and multinational PH registries in Europe.
| PH registry | Centers (n) | Reported registry size (n) | Study cohort | Study design and time period | Study population (n) | Diagnosis, n (%) |
Incidence / Prevalence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PAH |
CTEPH | |||||||||||
| IPAH* | CTD* | CHD* | Other* | Total | ||||||||
| Latvian | 1 | 503 | PAH and CTEPH, age ≥ 18 years | Prospective, 2007–2016 | 174, 100% incident | 53 (41) | 23 (18) | 49 (38) | 5 (4) | 130 (75) | 44 (25) | PAH: 13.7 / 45.7 cases/MI; IPAH: 7.6 / 18.3 cases/MI; CTEPH: 5.1 / 15.7 cases/MI |
| Portuguese16 | 5 | 188 | PAH and CTEPH, age > 14 years | Prospective, 2008–2010 | 79, 100% incident | 17 (37) | 12 (26) | 10 (22) | 7 (15) | 46 (58) | 33 (42) | PAH: 1.5–2.2 / – cases/MI |
| Spanish (REHAP)5,6 | 31 | 1162 | PAH and CTEPH, age > 14 years | Retrospective, 1998–2006; Prospective, 2007–2008 | 1028, 16% incident | 314 (36) | 157 (18) | 167 (19) | 228 (27) | 866 (84) | 162 (16) | PAH: 3.7 / 16 cases/MAI; IPAH: 1.2 / 5.6 cases/MAI; CTEPH: 0.9 / 3.2 cases/MAI |
| CTEPH, age > 14 years | Retrospective, Jan–Dec 2006; Prospective, 2007–2013 | 391, incident and prevalent | – | – | – | – | – | 391 (100) | – | |||
| Swedish (SPAHR)7 | 7 | 944 | PAH and CTEPH, age ≥ 18 years | Prospective, 2008–2014 | 640, 100% incident | 227† (50) | 140 (31) | 61 (13) | 29 (6) | 457 (71) | 183 (29) | PAH: 8 / 49 cases/MI; IPAH, HPAH: 5 / 25 cases/MI; CTEPH: 2 / 19 cases/MI |
| Danish17 | 1 | 1142 | PAH | Prospective, 2000–2012 | 134, 100% incident | 43 (32) | 31 (23) | 48 (36) | 12 (9) | 134 (100) | – | – |
| Swiss18 | 13 | 1119 | All PH groups, age ≥ 18 years | Prospective, 1998–2012 | 960, 97% incident | 308 (60) | 94 (18) | 41 (8) | 74 (14) | 517 (67)‡ | 249 (33)‡ | – |
| Giessen (Gi-PH-Reg)19 | 1 | 2067 | All PH groups | Prospective, 1993–2011 | 2067, 90% incident | 294 (43) | 145 (21) | 91 (13) | 155 (23) | 685 (60)‡ | 459 (40)‡ | – |
| ASPIRE20 | 1 | 1344 | All PH groups, age ≥ 18 years | Prospective, 2001–2010 | 1344, 100% incident | 175 (29) | 188 (31) | 198 (33) | 39 (7) | 600 (71)‡ | 242 (29)‡ | PAH§: 0.9–6.1 / –cases/MI; IPAH§: 0.3–2.1 / – cases/MI; CTEPH§: 0.3–3.7 / –cases/MI |
| UK and Ireland21,22 | 8 | 500 | IPAH, HPAH, anorexigen-associated PAH, age ≥ 18 years | Prospective, 2001–2009 | 482, 100% incident | 448 (93) | – | – | 34 (7) | 482 (100) | – | IPAH, HPAH, and anorexigen-associated PAH: 0.7–1.1 / 6.6 cases/MI |
| 880 | CTEPH, age ≥ 18 years | Prospective, 1997–2012 | 880, 100% incident | – | – | – | – | – | 880 (100) | |||
| French4 | 17 | 674 | PAH, age ≥ 18 years | Prospective, 2002–2003 | 674, 18% incident | 264 (39) | 103 (16) | 76 (11) | 231 (34) | 674 (100) | – | PAH: 2.4 / 15 cases/MAI; IPAH: 1.0 / 5.9 cases/MAI |
| COMPERA2 | 28 | 2654 | IPAH, age ≥ 18 years | Prospective, 2007–2011 | 587, 100% incident | 587 (100) | – | – | – | 587 (100) | – | – |
| International CTEPH3 | 27 | 679 | CTEPH, age ≥ 18 years | Prospective, 2007–2009 | 679, 100% incident | – | – | – | – | – | 679 (100) | – |
The parameter values are shown as frequencies and proportions (%).
Relative percentage of all PAH patients shown.
IPAH and HPAH.
Relative percentage of combined PAH and CTEPH patient population.
Assuming a stable referral population of 15 million, between 2001 and 2009.
CHD, congenital heart disease; CTD, connective tissue disease; other abbreviations as in Table 1.
The combined PH patient study population was 10,109 (5164 PAH and 3326 CTEPH patients). Three largest studies represented 44% of combined study population.
All registries had prospective design with inclusion criteria mainly based on international guidelines requiring confirmation of PH by RHC: mean pulmonary artery pressure (mPAP) ≥ 25 mmHg at rest; and (for PAH and CTEPH) pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg. Only the international CTEPH registry permitted inclusion of patients with mPAP ≥ 30 mmHg during exercise, a diagnostic criterion, which due to the lack of reliable data was abandoned by expert consensus in 2009 and not reintroduced in the recent PH guidelines.23,24
When analyzing studies by the study cohort, four studies described only PAH (or its subgroup), three studies described only CTEPH, four studies described both PAH and CTEPH patients, and three studies included all five PH groups. The majority of studies included only adult patients (aged ≥ 18 years); however, the Portuguese and REHAP registries included patients aged > 14 years, and both Denmark and Giessen (Gi-PH-Reg) registries did not specify patient age in their inclusion criteria.
Most studies, being prospective, included only incident patients; however, REHAP, French, Swiss, and Gi-PH-Reg registries reported a proportion (range = 3–84%) of patients to whom diagnosis had been established before enrollment in the registry. Data collection in several registries (e.g. Gi-PH-Reg, UK, and Ireland registries) was started in the 1990s; however, most registries were established in the 2000s. Study duration was in the range of 1–18.6 years.
In seven studies that included PAH and CTEPH groups, more patients had PAH than CTEPH. The proportion of PAH patients in studies was variable, from 58% in the Portuguese registry to 84% in REHAP. When analyzing 11 studies which included patients with PAH subgroups, IPAH was the most prevalent subgroup in all studies, with some registries (e.g. COMPERA) focusing exclusively on IPAH. The second and third most prevalent PAH subgroups were PAH-CTD and PAH-CHD. It is important to mention that in REHAP, Gi-PH-Reg and French registries, > 20% of all PAH patients did not belong to any of the three subgroups. In these registries, there was a significant proportion of PoPH patients. However, one can only speculate whether these differences in PAH subgroup distribution can be explained by referral or diagnostic biases or they show true epidemiological differences between different registries.
PAH incidence ranged from 0.9 cases in the ASPIRE registry to 13.7 cases per million inhabitants in Latvia. The lowest PAH prevalence was reported in France with 15 cases per million adult inhabitants (MAI); however, the French registry study reported data obtained over a period of only one year, thereby considerably underestimating true PAH prevalence in France. The highest PAH prevalence was observed in Sweden (49 cases/MI) and Latvia (45.7 cases/MI).
Only four registries reported incidence and/or prevalence of CTEPH. The incidence of CTEPH was approximately three times lower than that of PAH, ranging from 0.3–3.7 in the ASPIRE registry to 5.1 cases/MI in Latvia. The lowest CTEPH prevalence was reported in Spain (3.2 cases/MI) and the highest in Sweden (19 cases/MI).
PAH patients
Out of 12 analyzed PH registries, 11 included patients with PAH or its subgroup. The main patient characteristics of these 11 registries are presented in Table 3. Female gender was predominant in all registries, constituting as much as 73% of PAH patients in Latvian registry. Most patients were diagnosed with reduced functional capacity: NYHA class III/IV constituted from 69% of all PAH patients in REHAP to 94% of patients in COMPERA. Only two registries did not report 6MWD results; the Portuguese registry did not assess patient exercise tolerance and the ASPIRE registry used the incremental shuttle walking test, which was recently proven to be more accurate in assessing exercise tolerance in PAH patients than the 6MWD.25 However, methodology and distance covered differ between the two tests;26 therefore, we did not include these results in the current analysis. Despite similarities of studied populations, there were some notable differences. Age was one of the parameters that differed the most, youngest patients (possibly due to younger inclusion age) were reported in Portuguese and REHAP registries; mean age was 43 ± 16 and 45 ± 17 years, respectively. COMPERA and SPAHR patients were the oldest at 65 ± 15 and 67 ± 22 years, respectively.
Table 3.
Demographic, clinical, and hemodynamic characteristics of PAH patients of national and multinational PH registries in Europe.
| Registry | Age (years) | Female (%) | NYHA II/III/IV (%) | 6MWD (m) | RAP (mmHg) | mPAP (mmHg) | PVR (WU) | PAWP (mmHg) | CI (L·min–1·m–2) |
|---|---|---|---|---|---|---|---|---|---|
| Latvian | 59 ± 16 | 73 | 26/65/7 | 322 ± 122 | 11 ± 7 | 49 ± 18 | 8 ± 5 | 12 ± 4 | 2.5 ± 0.7 |
| Portuguese16 | 43 ± 16 | 65 | 27/51/20 | – | 8 ± 6 | 51 ± 18 | 11 ± 7 | 10 ± 4 | 2.7 ± 1.1 |
| REHAP6 | 45 ± 17 | 71 | 31*/58/11 | 363 ± 120 | 9 ± 5 | 54 ± 16 | 12 ± 6 | – | 2.6 ± 0.9 |
| Swedish7 | 67 ± 22 | 64 | 21/68/9 | 280 (224)† | 7 (6)† | 45 (16)† | 9 (6)† | 8 (5)† | 2.4 (1.0)† |
| Danish17 | 50 ± 21 | 58 | 30*/62/8 | 328 ± 131 | 10 ± 6 | 49 ± 15 | 10 ± 7 | 11 ± 5 | 2.4 ± 0.9 |
| Swiss18 | 57 ± 16 | 60 | 24/57/17 | 362 ± 137 | 9 ± 4 | 48 ± 15 | 9 ± 6 | 12 ± 7 | 2.5 ± 0.8 |
| Gi-PH-Reg19 | 51 ± 16 | 65 | 19/59/22 | 325 ± 126 | 8 ± 6 | 51 ± 16 | 11 (9)† | 8 ± 4 | 2.3 ± 0.8 |
| ASPIRE‡ 20 | 54 ± 18 | 70 | 22*/64/14 | – | 10 ± 6 | 48 ± 13 | 10 ± 6 | 9 ± 3 | 2.7 ± 0.9 |
| UK and Ireland§ 21 | 50 ± 17 | 70 | 16*/67/17 | 292 ± 123 | 10 ± 6 | 54 ± 14 | 13 ± 6 | 9 ± 4 | 2.1 ± 0.7 |
| French4 | 50 ± 15 | 65 | –/75 (III–IV) | 329 ± 109 | 8 ± 5 | 55 ± 15 | – | 8 ± 3 | 2.5 ± 0.8 |
| COMPERA**2 | 65 ± 15 | 60 | 9/75/16 | 293 ± 126 | 8 ± 5 | 44 ± 12 | 10 ± 6 | 10 ± 3 | 2.2 ± 0.7 |
NYHA class I–II.
Data presented as median (IQR).
Patients with pulmonary veno-occlusive disease (n = 2) were not included in the analysis.
IPAH, HPAH, and anorexigen-associated PAH.
IPAH only.
Abbreviations as in Table 1.
CTEPH patients
Nine registries included patients with CTEPH. Main patient characteristics of these nine registries are presented in Table 4. With the exception of the Latvian registry, CTEPH patients were generally older than PAH patients from corresponding registries. However, similar to PAH, most CTEPH patients were already diagnosed with impaired functional capacity and hemodynamic parameters were similar both compared to PAH patients and between CTEPH patients from different registries.
Table 4.
Demographic, clinical, and hemodynamic characteristics of CTEPH patients in national registries from different countries in Europe.
| Registry | Age (years) | Female (%) | NYHA II/III/IV (%) | 6MWD (m) | RAP (mmHg) | mPAP (mmHg) | PVR (WU) | PAWP (mmHg) | CI (L·min–1·m–2) | Patients undergoing PEA (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Latvian | All CTEPH pts | 59 ± 17 | 61 | 16/75/9 | 274 ± 111 | 13 ± 8 | 51 ± 15 | 10 ± 5 | 11 ± 4 | 1.9 ± 0.7 | 16 | |
| Portuguese16 | All CTEPH pts | 60 ± 13 | 70 | 21/46/32 | – | 11 ± 5 | 47 ± 10 | 11 ± 6 | 10 ± 3 | 2.5 ± 1.1 | 15 | |
| REHAP5 | PEA | 50 (24)* | 44 | 28†/68/4 | 400 (185)* | – | 48 ± 13 | 9 (7)* | – | – | 31 | |
| Non-PEA | 69 (20)* | 64 | 30†/62/9 | 320 (197)* | – | 45 ± 12 | 8 (7)* | – | – | |||
| Swedish7 | All CTEPH pts | 70 ± 14 | 50 | 20/73/8 | 345 (198)* | 7 (7)* | 46 (17)* | 9 (5)* | 10 (6)* | 2.2 (1.0)* | 33 | |
| Swiss18 | All CTEPH pts | 63 ± 14 | 52 | – | 365 ± 138 | 9 ± 6 | 45 ± 12 | 10 ± 6 | 12 ± 6 | 2.3 ± 0.6 | 14 | |
| Gi-PH-Reg19 | All CTEPH pts | 62 ± 13 | 56 | 15/60/25 | 308 ± 116 | 8 ± 5 | 44 ± 13 | 9 (7)* | 9 ± 4 | 2.2 ± 0.6 | 20 | |
| ASPIRE20 | All CTEPH pts | 61 ± 15 | 54 | 13†/70/17 | – | 11 ± 6 | 48 ± 11 | 9 ± 5 | 11 ± 5 | 2.5 ± 0.7 | 45 | |
| UK and Ireland22 | PEA | 57 ± 15 | 47 | 9/68/23 | 260 ± 126 | – | 47 ± 11 | 10 ± 5 | – | – | 100 | |
| International CTEPH3 | PEA | 60 | 45 | 19†/69/12 | 340* | 9* | 48* | 9* | 10* | 2.2* | 60 | |
| Non-PEA | 67 | 57 | 18†/69/13 | 315* | 8* | 45* | 8* | 10* | 2.3* | |||
Data presented as median (IQR), where available.
NYHA class I–II.
PEA, pulmonary endarterectomy; other abbreviations as in Table 1.
The proportion of patients with PEA differed among the registries; the lowest proportion (14% of CTEPH patients) was observed in the Swiss registry which could be explained by the fact that this registry started to include patients since 1998 when PEA was not widely available and an approved method of treatment. A similar explanation could be attributed to Gi-PH-Reg in which the proportion of CTEPH patients with PEA was 20%. However, the PEA proportion was quite low in more recently established Portuguese and Latvian registries (15% and 16%, respectively), possibly due to the small registry size and consequent lack of patient volume to safely perform PEA locally.27 The PEA proportion in other registries was from 31% in REHAP to 60% in the international CTEPH registry and even 100% in the UK and Ireland registry, confirming that PEA is a recognized treatment option for CTEPH patients. Both registries that compared PEA and non-PEA patients (REHAP and International CTEPH registry) reported that PEA patients were younger, predominantly male, had higher 6MWD, and higher mPAP.
Survival
Twelve registries reported at least one-year survival data (Table 5). Overall, the one-year survival of PAH and CTEPH patients was similar among registries and between patient groups, with best survival observed for PEA patients.
Table 5.
Survival data of national and multinational PH registries in Europe.
| PH registry | Study cohort | 1 year (%) |
3 years (%) |
5 years (%) |
|||
|---|---|---|---|---|---|---|---|
| PAH | CTEPH | PAH | CTEPH | PAH | CTEPH | ||
| Latvian | Inc | 88 | 84 | 73 | 76 | 58 | 44 |
| Portuguese16 | Inc | 94 | PEA 94 Non-PEA 93 | – | – | – | – |
| Spanish*,† 5,6 | Prev and Inc | 89 | PEA 97 Non-PEA 93 | 77 | PEA 91 Non-PEA 81 | 68 | PEA 86 Non-PEA 65 |
| Swedish7 | Inc | 85 | Ent 93 PEA 96 Non-PEA 91 | 71 | Ent 80 PEA 89 Non-PEA 75 | 59 | Ent 74 PEA 86 Non-PEA 69 |
| Danish17 | Inc | 86 | – | 73 | – | 65 | – |
| Swiss18 | Prev and Inc | 87 | Unop 91 | 69 | Unop 77 | – | – |
| German (Gi-PH-Reg)19 | Prev and Inc | 88 | PEA 96 Non-PEA 85 | 72 | PEA 87 Non-PEA 73 | 59 | PEA 77 Non-PEA 62 |
| ASPIRE20 | Inc | 88 | 89 | 68 | 71 | – | – |
| UK and Ireland21,22 | Inc | Ent 93 ≤50 years, 95 > 50 years, 90 | PEA 86 | Ent 73 ≤ 50 years, 87 > 50 years, 75 | PEA 84 | Ent 61 ≤ 50 years, 75 > 50 years, 44 | PEA 79 |
| French4 | Inc | 88 | – | – | – | – | – |
| COMPERA* 2 | Inc | Ent 92 ≤ 65 years, 96 > 65 years, 90 | – | Ent 74 ≤ 65 years, 83 > 65 years, 68 | – | – | – |
| International CTEPH3 | Inc | – | PEA 93 Non-PEA 88 | – | PEA 89 Non-PEA 70 | – | – |
Survival in PAH group analyzed for IPAH only.
Survival in CTEPH group reported from study with larger CTEPH cohort.7
Ent, entire study population; Inc, incident or newly diagnosed patients; Prev, prevalent or previously diagnosed patients; Unop, unoperated CTEPH patients; PEA, pulmonary endarterectomy; other abbreviations as in Table 1.
Interestingly, the one-year survival rate of 88% in Latvian PAH and 84% in CTEPH population is the fourth lowest and lowest in Europe, respectively. However, due to the relatively short follow-up period (especially in the Latvian CTEPH group) the three- and five- year survival data (which also are among the lowest in Europe) should be interpreted with caution.
Discussion
Comparing our study results with data from other PH registries in Europe, it is noticeable that most registries had common patient inclusion criteria, as they were based on international guidelines, even though guidelines have slightly changed over time.28–30
Despite the relatively small size of the Latvian PH registry, the observed incidence and prevalence of PAH and CTEPH in Latvia in 2016 was among the highest in Europe. This could be attributed to successful informative campaigning (lectures and scientific conferences for healthcare professionals, events organized by PH patient support group, etc.) which resulted in a steady increase of newly diagnosed patients each year up to 27 new PAH and ten CTEPH patients in 2016.
Age, gender, as well as distribution by PH diagnosis did not markedly differ between Latvian and European registries and similarly to Europe, PH patients in Latvia are diagnosed with more advanced disease and poor functional status (high NYHA functional class, low 6MWD) and hemodynamic parameters (high right atrial pressure (RAP), mPAP, pulmonary vascular resistance, low cardiac index). The survival of Latvian PAH and CTEPH patients is among the lowest in Europe.
Although V/Q scan remains the main first-line imaging modality for CTEPH, contrast-enhanced CT has only slightly lower diagnostic accuracy in expert centers (100% sensitivity, 93.7% specificity, and 96.5% accuracy for V/Q and 96.1%, 95.2%, and 95.6%, respectively, for CT pulmonary angiography). Dual-source CT may, in the future, replace V/Q scanning but has not been widely available in Latvia. Still, we believe that given the expertise of Latvian radiologists, the number of Latvian CTEPH cases misdiagnosed as IPAH is likely is to be low.
Only 16% of Latvian CTEPH patients underwent PEA, which is one of the lowest proportions in Europe, translating into only seven PEA procedures performed over nine years, despite PEA being the treatment of choice for CTEPH and demonstrating a positive effect on both patient quality of life and survival.30
Balloon pulmonary angioplasty (BPA) is an emerging treatment method for patients with inoperable CTEPH or patients who have recurrent or persistent PH following PEA.30,31 None of the analyzed registries reported patients with BPA; however, its role in the future will likely increase as there is accumulating evidence that BPA leads to hemodynamic and functional improvements. Several randomized controlled trials comparing BPA with targeted medical therapy (riociguat) are underway.31
Given the low CTEPH patient survival in our country, it is clear that Latvia has to make an increased effort in providing evidence-based care for patients with CTEPH who could benefit from PEA and/or BPA.
Our center is the only PH reference center in Latvia and all diagnostic workups and treatment of PH patients is done in-house. Although this ensures that all patients diagnosed with PAH or CTEPH are included in the Latvian PH registry, patients with suspicion of PH may never be referred from local hospitals, therefore influencing reported incidence and prevalence of PAH and CTEPH as well as survival estimates in our study.
Current results are limited by the small study population and the observational study design, which creates unmeasured confounders and potential selection bias. Therefore, comparison between registries must be made with caution.
More efforts should be made towards a wider collaboration between countries/regions as new global clinical data on rare diseases, would permit to gain new insights on a broader range of PH types (prevalence of which differs between developed and developing countries) and would also allow to design more representative and purposeful clinical trials, therefore advancing the knowledge on PH and ultimately improving the quality of care.1,32,33
As numerous PH registries in Europe are small and patient inclusion criteria are practically the same, it would be reasonable to combine efforts and combine PH patient populations from multiple registries, therefore gaining more high-quality data and results.
Various approaches are possible to move towards such globalization of PH registries, including a recent creation of web-based Global Rare Diseases Patient Registry Data Repository (GDRD) under the NIH/NCATS GRDR program and Pulmonary Hypertension Association Registry (PHAR), a multi-center prospective registry across > 30 PH centers in the USA.33–35 A viable alternative could be networking (e.g. recently created European Reference Networks or ERNs) with future unification of national or regional PH registries using a common or overlapping case report forms and comparable enrollment principles while keeping their own funding sources and management.33 From a technical standpoint, a latter model would either require: (1) to develop a web-based software for management of PH registry data, which could then become as a standard tool for collaboration (e.g. iPHnet project in Italy);36 and (2) to join a group of PH centers/national registries (Portugal, France, Netherlands, etc.), and acquire a commercial, standardized web-based PH database (PAHtool, Inovultus, Portugal).16,37,38
Acknowledgments
The authors thank Professor Ugis Gruntmanis from University of Texas Southwestern Medical Center for proofreading the revised version of the article.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes, Rockville, MD: Agency for Healthcare Research and Quality, 2014. [PubMed] [Google Scholar]
- 2.Hoeper MM, Huscher D, Ghofrani HA, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: Results from the COMPERA registry. Int J Cardiol 2013; 168: 871–880. [DOI] [PubMed] [Google Scholar]
- 3.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. Circulation 2016; 133: 859–871. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: Results from a national registry. Am J Respir Crit Care Med 2006; 173: 1023–1030. [DOI] [PubMed] [Google Scholar]
- 5.Escribano-Subías P, Del Pozo R, Román-Broto A, et al. Management and outcomes in chronic thromboembolic pulmonary hypertension: From expert centers to a nationwide perspective. Int J Cardiol 2016; 203: 938–944. [DOI] [PubMed] [Google Scholar]
- 6.Escribano-Subias P, Blanco I, López-Meseguer M, et al. Survival in pulmonary hypertension in Spain: Insights from the Spanish registry. Eur Respir J 2012; 40: 596–603. [DOI] [PubMed] [Google Scholar]
- 7.Radegran G, Kjellstrom B, Ekmehag B, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000-2014. Scand Cardiovasc J 2016; 50: 243–250. [DOI] [PubMed] [Google Scholar]
- 8.El Moustaine D (ed.) Rare Disease Registries in Europe. Orphanet Report Series Rare Disease Collection. Paris: Orphanet, 2017.
- 9.Skride A, Sablinskis K, Lejnieks A, et al. Pulmonary hypertension in adults with congenital heart disease: First data from Latvian PAH registry. Eur J Intern Med 2016; 36: e20–21. [DOI] [PubMed] [Google Scholar]
- 10.Skride A, Sablinskis K, Rudzitis A, et al. First data from Latvian chronic thromboembolic pulmonary hypertension registry. Eur J Intern Med 2016; 32: e23–24. [DOI] [PubMed] [Google Scholar]
- 11.Skride A, Sablinskis K, Avidan Y, et al. Pulmonary arterial hypertension associated with connective tissue disease: Insights from Latvian PAH registry. Eur J Intern Med 2017; 40: e13–14. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J 2004; 25: 2243–2278. [DOI] [PubMed] [Google Scholar]
- 13.He J, Fang W, Lv B, et al. Diagnosis of chronic thromboembolic pulmonary hypertension. Nucl Med Commun 2012; 33: 459–463. [DOI] [PubMed] [Google Scholar]
- 14.Central Statistical Bureau of Latvia. Resident population at the beginning of the year 2016. Available at: http://data.csb.gov.lv/pxweb/en/Sociala/Sociala__ikgad__iedz__iedzskaits/IS0020.px/table/tableViewLayout2/?rxid=c0c89723-38f1-49ef-87ee-d7c42809edd2.
- 15.Olsson KM, Nickel NP, Tongers J, et al. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol 2013; 167: 2300–2305. [DOI] [PubMed] [Google Scholar]
- 16.Baptista R, Meireles J, Agapito A, et al. Pulmonary hypertension in Portugal: first data from a nationwide registry. Biomed Res Int 2013; 2013: 489574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korsholm K, Andersen A, Kirkfeldt RE, et al. Survival in an incident cohort of patients with pulmonary arterial hypertension in Denmark. Pulm Circ 2015; 5: 364–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mueller-Mottet S, Stricker H, Domeninghetti G, et al. Long-term data from the swiss pulmonary hypertension registry. Respiration 2015; 89: 127–140. [DOI] [PubMed] [Google Scholar]
- 19.Gall H, Felix JE, Schneck FK, et al. The Giessen Pulmonary Hypertension Registry: Survival in Pulmonary Hypertension Subgroups. J Hear Lung Transplant 2017; 36: 957–967. [DOI] [PubMed] [Google Scholar]
- 20.Hurdman J, Condliffe R, Elliot CA, et al. ASPIRE registry: Assessing the Spectrum of Pulmonary hypertension Identified at a REferral centre. Eur Respir J 2012; 39: 945–955. [DOI] [PubMed] [Google Scholar]
- 21.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: Results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med 2012; 186: 790–796. [DOI] [PubMed] [Google Scholar]
- 22.Cannon JE, Su L, Kiely DG, et al. Dynamic risk stratification of patient long-term outcome after pulmonary Endarterectomy: Results from the United Kingdom national cohort. Circulation 2016; 133: 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–41. [DOI] [PubMed] [Google Scholar]
- 24.Badesch DB, Champion HC, Gomez Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S55–66. [DOI] [PubMed] [Google Scholar]
- 25.Irisawa H, Takeuchi K, Inui N, et al. Incremental shuttle walk test as a valuable assessment of exercise performance in patients with pulmonary arterial hypertension. Circ J 2014; 78: 215–221. [DOI] [PubMed] [Google Scholar]
- 26.Pulz C, Diniz RV, Alves ANF, et al. Incremental shuttle and six-minute walking tests in the assessment of functional capacity in chronic heart failure. Can J Cardiol 2008; 24: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2015; 24: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barst RJ, McGoon M, Torbicki A, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43: S40–47. [DOI] [PubMed] [Google Scholar]
- 29.Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: The Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 30.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 31.Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomberg-Maitland M, Michelakis ED. A global pulmonary arterial hypertension registry: is it needed? Is it feasible? Chest 2010; 137: 95S–101S. [DOI] [PubMed] [Google Scholar]
- 33.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013; 62: D51–59. [DOI] [PubMed] [Google Scholar]
- 34.Rubinstein YR, McInnes P. NIH/NCATS/GRDR® Common Data Elements: A leading force for standardized data collection. Contemp Clin Trials 2015; 42: 78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pulmonary Hypertension Association Registry (PHAR). Available at: https://phccregistry.org/ (accessed 4 March 2018).
- 36.Poscia R, Ghio S, D’Alto M, et al. “Real-life” information on pulmonary arterial hypertension: the iPHnet Project. Curr Med Res Opin 2014; 30: 2409–2414. [DOI] [PubMed] [Google Scholar]
- 37.Reis A. PAHTool: A dedicated software for PAH National and international experience. Available at: http://www.pahtool.net/news/PAHToolProject2016.pdf (accessed 6 July 2017).
- 38.Huis in ’t Veld A, Vonk Noordegraaf A, Boonstra A, et al. The OPTIEK1 study: Design and preliminary results of a Dutch national registry. Eur Respir J 2016; 48: PA1881. [Google Scholar]