Abstract
Background:
Multiple surgical approaches have been described for the management of anterior cruciate ligament (ACL) tears in skeletally immature patients.
Purpose:
To provide a detailed description of a modified all-epiphyseal ACL reconstruction and report early outcomes and complications with this new technique.
Study Design:
Case series; Level of evidence, 4.
Methods:
A retrospective review of all skeletally immature patients undergoing ACL reconstruction via a modified all-epiphyseal technique prior to July 2015 was performed. Skeletally immature male patients with a bone age of 8 to 15 years and female patients with a bone age of 8 to 12 years were selectively indicated for this procedure. The surgical technique involved an all-epiphyseal femoral tunnel drilled parallel and distal to the physis as well as an all-epiphyseal tibial tunnel. Both tunnels were placed in the anatomic footprint of the ACL. Tibial fixation was achieved first with a suspensory cortical fixation device followed by fixation on the femur with an interference screw.
Results:
During the study period, 30 patients with a mean bone age of 11.8 years underwent ACL reconstruction with this physeal-sparing technique; 26 patients (87%) achieved a minimum follow-up of 2 years. At final follow-up, the mean Lysholm score, Single Assessment Numeric Evaluation score, patient satisfaction, return-to-sport rate, and Tegner activity score were 93, 89, 9.2, 94%, and 7.6, respectively. Four graft failures (15%) and 3 (12%) contralateral ACL tears were identified. One patient was noted to have a 12-mm leg-length discrepancy at final follow-up, which required no additional treatment. No other leg-length discrepancies or angular deformities were identified.
Conclusion:
The modified all-epiphyseal ACL reconstruction technique achieved good functional outcomes, a high rate of return to sport, low failure rates, and low physeal injury rates at a mean follow-up of 3.2 years. Skeletally immature patients with an ACL tear requiring reconstruction pose a unique challenge for sports medicine clinicians. While several previous approaches have been described for this patient population, the proposed benefits of this new technique are that it is anatomic, it is physeal sparing, it uses osseous tunnels, and it provides good initial graft fixation strength.
Keywords: skeletal immaturity, ACL tear, open growth plates, all-epiphyseal ACL reconstruction
Anterior cruciate ligament (ACL) reconstructions in the skeletally immature population are being increasingly performed in the United States.4 Four primary approaches can be used in this patient population: an iliotibial band extra-articular intra-articular reconstruction, a transphyseal reconstruction, an all-epiphyseal reconstruction, and a hybrid approach using one of the aforementioned techniques on the femur and a separate technique on the tibia. The decision as to which approach to use remains controversial and is largely based on multiple factors such as patient age, height, and Tanner stage as well as the surgeon’s training and experience. To date, there are no head-to-head comparison studies to help guide treatment decisions.
An iliotibial band extra-articular intra-articular reconstruction is a modification of the previous MacIntosh procedure and has recently been popularized by Kocher et al.9 Several studies have shown that this reconstruction provides good functional outcomes, with low rates of complication.9,19 The primary advantage of this technique is that it does not require drilling osseous tunnels in either the distal femur or proximal tibia and can therefore be used in skeletally immature patients of all ages and all sizes. The primary drawback of this technique is that it is nonanatomic, and biomechanical studies have demonstrated that it may lead to overconstraint of the knee.8 At our institution, this technique is typically the preferred surgical option in patients with a bone age of less than 8 years.
A transphyseal approach has been advocated by several groups as a less challenging and technically demanding reconstruction that can be used in pediatric and adolescent patients.7,8 This technique uses osseous tunnels and a soft tissue graft that are placed across the physis (often in a more vertical fashion) to enable future growth of the distal femur and proximal tibia.10,15 The primary advantage of this approach is that it is similar to techniques currently used for reconstructions in skeletally mature patients—techniques with which most orthopaedic surgeons are comfortable and familiar. The major drawback with this technique is that the transphyseal tunnels result in an iatrogenic injury to the physis that may result in an angular deformity or leg-length discrepancy if a substantial portion of the cross-sectional area of the physis is affected.21 At our institution, this approach is used in patients who are approaching skeletal maturity, typically male patients with a bone age of greater than 15 years or females with a bone age of greater than 12 years.
Multiple variations of the all-epiphyseal ACL reconstruction have been described over the past 15 years, all with reported good outcomes.1,2,18 The main advantage of this strategy over the other strategies is that it does not place any fixation across the physis, theoretically reducing the risk of physeal disruption. The primary disadvantage is that the reported techniques are technically demanding. At our institution, we typically use a modification of this reported strategy for male patients with a bone age between 8 and 15 years and female patients with a bone age between 8 and 12 years. Our modification reverses the fixation proposed by Anderson1 by using suspensory fixation on the tibia and interference screw fixation on the femur. We believe that this modification has several advantages: no fixation or sutures cross the physis; the graft can traverse the entire length of the femoral and tibial tunnel, providing maximal surface area for ingrowth; and fixation is achieved with a suspensory button and interference screw, which may be superior to postfixation or suture fixation to the periosteum. The purpose of the current study was to provide a detailed description of our modified surgical technique and report the early outcomes since we began using this all-epiphyseal approach in 2009.
Methods
This institutional review board–approved study evaluated all skeletally immature patients who underwent an ACL reconstruction with a modified all-epiphyseal technique at a single pediatric hospital prior to July 2015. The surgical technique was a modification of the Anderson technique that was described in 2003.1
Patient Selection
Patient selection included skeletally immature patients with a bone age between 8 and 15 years for boys and a bone age between 8 and 12 years for girls. Bone age was assessed by use of preoperative magnetic resonance imaging (MRI) or a left-hand radiograph, as has been previously described.6,16 The preoperative MRI was also assessed to confirm the presence of adequate epiphyseal bone to safely accommodate a 6- to 8-mm osseous tunnel within the proximal tibia and distal femur (Figure 1). To assess the height of the femoral epiphysis, the T1-weighted coronal image corresponding to the native attachment of the ACL on the femur was identified. The ossified component was then measured to ensure that a 6- to 8-mm tunnel could be created without violating either the articular cartilage or the physis. We used a similar technique for the tibia, using the T1-weighted sagittal image corresponding to the tibial attachment of the ACL. It has been our experience that the tibia has less ossified epiphyseal bone compared with the femur. If a 6- to 8-mm tunnel cannot be safely placed within either bone, an iliotibial band reconstruction is then performed instead of an all-epiphyseal reconstruction. Unless the patient had clinical varus or valgus malalignment, routine standing long-leg alignment films were not obtained, given concerns about radiation exposure and cost.
Figure 1.
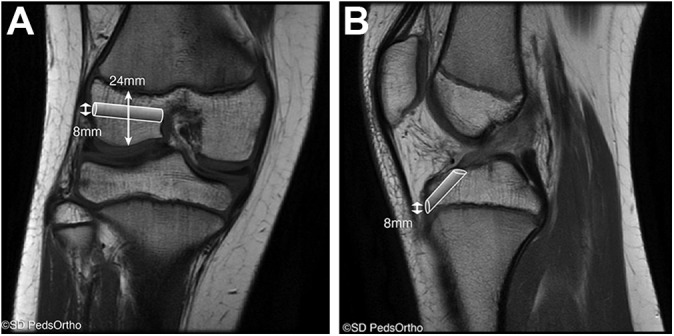
Preoperative magnetic resonance imaging of a 12-year-old boy with a complete anterior cruciate ligament tear. (A) Coronal T1-weighted image demonstrating an open physis with an ossified epiphyseal height of 24 mm and the proposed 8-mm femoral tunnel. (B) Sagittal T1-weighted image demonstrating the proposed 8-mm tibial tunnel proximal to the tibial physis. Images courtesy of SD PedsOrtho.
Surgical Technique
The surgery was performed with the patient in the supine position on a radiolucent table that enabled intraoperative fluoroscopy. Once the ACL rupture was confirmed with an examination under anesthesia, the hamstring tendons were harvested via a 2-cm incision centered over the medial tibial crest at the pes anserinus. Both the semitendinosus and gracilis tendons were harvested and prepared on the back table with No. 2 nonabsorbable, high-strength sutures (FiberWire; Arthrex). Grafts 16 cm in length were typically obtained and were more than adequate for this reconstructive technique . If grafts less than 14 cm are obtained, an allograft or alternative graft source may be necessary. The grafts were then sized as a quadrupled graft and tensioned on a graft board. Graft diameter has been found to be dependent on patient age, sex, and size but is typically between 6 and 8 mm in this patient age group. To date, we have not routinely augmented these smaller grafts with soft tissue allograft, as the size of the graft seems to match the size of these younger patients. While various graft diameter thresholds have been suggested by previous studies in skeletally mature patients (7 mm and 8 mm), it is not clear how these thresholds apply to skeletally immature patients with smaller anatomic features and residual growth.13,14
A diagnostic arthroscopy was then performed by use of standard anteromedial and anterolateral portals. Any chondral or meniscal abnormality was addressed. The remnant native ACL was then debrided. A notchplasty was not routinely performed. A 10- to 20-mm, lateral incision was made just distal to the lateral epicondyle with the knee flexed 90°. A 2.4-mm guide wire was drilled into the joint either using a freehand technique or using a femoral guide typically set around 95° (Figure 2). The guide wire was inserted into the lateral femoral epiphysis just posterior to the popliteus tendon and fibular collateral ligament and parallel to the physis, exiting within the joint at the anatomic footprint of the ACL. The wire placement was confirmed fluoroscopically prior to the creation of the femoral tunnel, which was drilled with an outside-in technique using an acorn reamer. The arthroscope can be used to evaluate the femoral tunnel and ensure that the physis has not been violated.
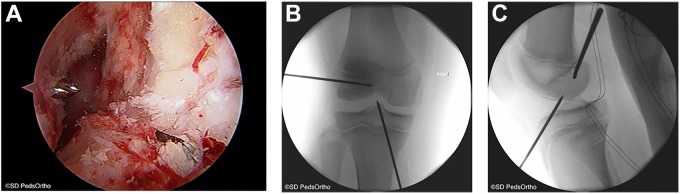
Figure 2.
(A) Arthroscopic view from the anterolateral portal demonstrating the guide wires entering the knee joint in the anatomic footprints of the anterior cruciate ligament. (B) Anteroposterior fluoroscopic image demonstrating the 2.4-mm guide wire placement in the femur and tibia. (C) Lateral fluoroscopic image showing the guide wire placement within the epiphysis of the femur and tibia. Images courtesy of SD PedsOrtho.
Attention was then turned to drilling the tibial tunnel. A separate 10- to 20-mm incision was made just medial and parallel to the patellar tendon and just distal to the anterior joint line. Another option entails extending the graft harvest incision 2 cm proximal toward the anterior joint line. A 2.4-mm guide wire was then drilled into the joint with either a freehand technique or using a tibial guide typically set as low as the device will allow (40°). The surgeon’s hand may be lifted toward the ceiling if necessary to flatten the trajectory of the wire to effectively reduce the drill angle. The guide wire was inserted proximal to the tibial physis within the ossified portion of the tubercle apophysis and was passed into the anatomic footprint of the ACL. Prior to drilling of the tibial tunnel, the placement of the guide wire was confirmed arthroscopically and fluoroscopically. The tibial tunnel was drilled from outside-in by use of an acorn reamer. The arthroscope can be used to evaluate the tibial tunnel and ensure that the physis has not been violated.
The hamstring grafts were then pulled antegrade through the femoral tunnel, across the joint, and through the tibial tunnel. We used a cortical suspensory fixation device that allows maximal graft length within the tibial tunnel: either an OrthoPediatrics ArmorLink (which was placed after shuttling the graft through the joint) or a Smith & Nephew Endobutton Direct (which was placed prior to shuttling the graft through the joint). The knee was then cycled and the graft was fixed on the femur with a 23 to 25 mm–long absorbable interference screw that was inserted from outside-in, with the screw placed eccentrically in the femoral tunnel as distal as possible from the physis. The screw diameter typically matched the femoral tunnel diameter size. The graft was secured with the knee flexed approximately 20° with a posterior drawer applied to the knee. Final fluoroscopic images were then taken to confirm the tunnel and implant positions. After skin closure, a range-of-motion brace was applied (Figure 3).
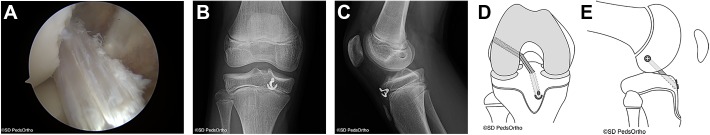
Figure 3.
(A) Arthroscopic view of the anterior cruciate ligament (ACL) graft viewed from the anterolateral portal. (B) Anteroposterior view demonstrating the final ACL construct. (C) Lateral view. (D) Schematic diagram demonstrating the final ACL construct in the coronal plane. (E) Schematic diagram showing the final ACL construct in the sagittal plane. Images courtesy of SD PedsOrtho.
Postoperative Protocol
Range of motion was started immediately, and patients were allowed toe-touch weightbearing with crutches for approximately 1 week. Formal physical therapy was initiated 1 week after surgery. Running was postponed for 3 months and cutting sports were avoided for 6 to 12 months until the patient passed a return-to-sports test, which at our institution is based on several factors including isokinetic strength testing and the performance of several functional movements (Appendix). Radiographic evaluation consisting of a long-leg alignment film as well as a lateral radiograph of the surgical extremity was typically performed at 6 weeks, 6 months, 12 months, and annually until the physis had closed (Figures 4 and 5).
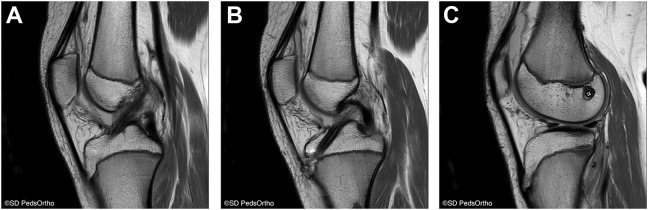
Figure 4.
Postoperative magnetic resonance imaging taken 22 months after the anterior cruciate ligament reconstruction as a result of a new knee injury. (A) Sagittal T1-weighted image demonstrating an intact graft. (B) Tibial tunnel safely positioned within the tibial epiphysis. (C) Femoral tunnel positioned safely within the femoral epiphysis. Images courtesy of SD PedsOrtho.
Figure 5.
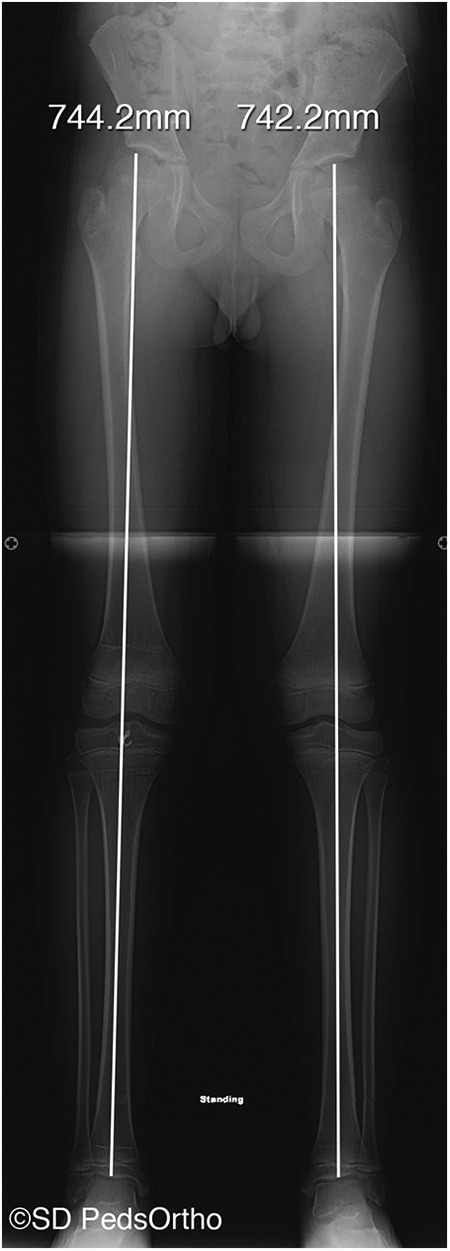
Postoperative long-leg alignment film taken 24 months after the surgery documenting no significant leg-length discrepancy or angular deformity. Image courtesy of SD PedsOrtho.
Results
Between 2009 and 2015, a total of 30 consecutive ACL reconstructions were performed at our institution with this modified all-epiphyseal technique. The mean chronologic and bone age of the male patients was 12.2 years (range, 8.7-15.8 years) and 12.2 years (range, 8-15 years), respectively, and for the female patients it was 11.0 years (range, 7.4-13.3 years) and 10.7 years (range, 8-12 years), respectively. Two patients were lost to follow-up prior to 1 year, and another 2 patients were lost to follow-up prior to 2 years. These patients were excluded from further analysis, yielding a minimum 2-year follow up rate of 87% and a study cohort of 26 patients.
At a mean follow-up of 3.2 years (range, 2-5 years), the mean (±SD) Lysholm score, Single Assessment Numeric Evaluation score, patient satisfaction score, and Tegner activity score were 93 ± 6, 89 ± 14, 9.2 ± 1.1, and 7.6 ± 1.0, respectively. Twenty-five out of 26 patients (94%) were able to return to sport, but 1 family chose not to return their son to football. Four patients (15%) sustained a graft tear at a mean of 1.8 ± 0.8 years from their index procedure that necessitated a revision surgery. Three patients (12%) sustained a contralateral ACL tear also requiring surgical intervention. No other patient underwent a second surgery as a result of arthrofibrosis, infection, symptomatic implants, or meniscal or chondral injury. One patient (4%) had a postoperative leg-length discrepancy of 12 mm that was identified radiographically 2 years postoperatively (with the surgical leg being longer); this discrepancy did not require further surgical intervention. No other clinically significant leg-length discrepancy (>1 cm) or angular deformity was identified.
Discussion
Multiple all-epiphyseal reconstruction techniques have evolved over the past decade as modifications of previous hybrid techniques that were described by Anderson,1 Henry et al,7 Gebhard et al,5 and others. The primary advantages of an all-epiphyseal technique are that it is physeal sparing and anatomic and uses osseous tunnels that are placed solely in the epiphyses that enter the joint within the footprint of the ACL. Several reports in the literature have shown good clinical outcomes, with high Lysholm scores, satisfaction scores, and rates of return to sport.2,3,11,18 The results of our proposed technique are similar to these previously published results demonstrating good patient outcomes, high rates of return to sport, and a low rate of complication.
Graft failure was noted to occur in 15% of the patients; this is similar to rates reported in the literature, which have ranged from 10% to 15% with other physeal-sparing techniques.3,12,17,20 In addition to these promising early outcomes, several advantages of our modified all-epiphyseal approach make it potentially superior to other all-epiphyseal techniques or other surgical approaches that may be used in the skeletally immature patient. First, similar to an iliotibial band technique, our all-epiphyseal approach theoretically avoids any damage to the physis and provides a significant advantage over the transphyseal technique. Our approach also provides a theoretical advantage to the all-epiphyseal technique described by Cruz et al,3 which requires a reverse cutting drill across the tibial physis. Although the cross-sectional area of injury of this reverse cutting drill is small, it still creates an iatrogenic injury to the physis that puts the tibial physis at theoretical risk.
A second advantage is that unlike an iliotibial band technique, an all-epiphyseal technique uses a single femoral tunnel and a single tibial osseous tunnel, which potentially allows better graft incorporation into a circumferential tunnel surrounded by cancellous bone. Additionally, the use of a single tibial tunnel simplifies our proposed technique compared with the technique described by Wall et al,18 which requires 2 tibial tunnels. The modification of the all-epiphyseal approach used at our institution has the further advantage of maximizing graft length in both the femoral and tibial tunnels, with soft tissue, which may be beneficial for graft incorporation, traversing the entire length of the tunnels. This differs from the technique proposed by Cordasco et al,2 which uses an all-inside approach with suspensory fixation on both the femur and tibia. Additionally, our modification places not only the tunnels but also the fixation devices strictly within the epiphysis, minimizing the chance of any suture or graft “tethering” the physis. Nearly all other techniques that have been described to treat skeletally immature patients—including iliotibial band reconstruction, various transphyseal approaches, and several of the described all-epiphyseal techniques—have at least one side of the ACL construct fixed on the metaphysis, which can lead to physeal tethering and a resultant angular deformity.
A third advantage to our technique is that it is truly anatomic, and both tunnels can be placed into the center of the ACL footprint. This is in contradistinction to an iliotibial band technique, where the graft is placed in the over-the-top position on the femur and an anterior position just below the intermeniscal ligament on the tibia. A vertical transphyseal approach may be nonanatomic; the graft is frequently placed higher in the notch to minimize iatrogenic damage to the physis, which may place the tunnel aperture outside the anatomic footprint on the femur. The fourth advantage of our proposed technique is that it can be modified if alternative graft choices need to be considered, such as a soft tissue quadriceps tendon where the graft sutures are tied over a suspensory button on the tibia. The fifth advantage of this modified technique is that it potentially has superior time-zero fixation strength compared with constructs that rely on sutures like the iliotibial band technique or other proposed all-epiphyseal techniques such as that proposed by Cordasco et al.2
As with any surgical procedure, our modified all-epiphyseal ACL reconstruction has several potential drawbacks. First, this technique requires intraoperative fluoroscopy. The radiation exposure during these procedures is quite low, however, and averaged 0.55 minutes (range, 0.1-2.1 minutes) at our institution. A second drawback is that a minor learning curve is required, especially with final graft fixation that is achieved on the femur as opposed to the tibia. The third drawback is that this technique potentially cannot be used in very young patients with a bone age of less than 8 years because a large portion of the femoral and tibial epiphyses is unossified, making it challenging to safely drill a 5- to 6-mm tunnel. However, ACL surgery is rare in patients younger than this age unless the patient has a congenital absence of the cruciate ligament. The fourth drawback, although not encountered in this series, is the potential for significant physeal damage if the guide wire is misplaced. The parallel trajectory of the guide wire and drill can theoretically damage a large cross-sectional area of the physis if the guide wire is placed within the physis or too close to the physis. Thus, it is imperative to perform this surgery with intraoperative fluoroscopy and to confirm that there is no physeal damage with the arthroscope after the tunnel is drilled. Additionally, the use of fluoroscopy can confirm that the implants (screw and button) are appropriately placed and not crossing the physis. A fifth potential drawback of this technique is that the relatively small tibial bone bridge could fracture, but this has not been observed intraoperatively or postoperatively at our institution. The sixth drawback is the theoretical chance that the popliteus tendon or fibular collateral ligament could be damaged during drilling of the femoral tunnel. To date, we have not had any problems postoperatively with varus or posterolateral instability.
This study has several limitations, including its retrospective design. Additionally, greater patient numbers, more objective clinical data (KT-1000 arthrometer, pivot shift, etc), longer term follow-up, and external validation of this surgical technique will be necessary to confirm the durability of these results. Finally, and potentially most important, this study had no comparison group. Future studies looking at the various reconstruction options for the skeletally immature population will be necessary to determine which surgery is optimal for this unique patient population.
In conclusion, ACL surgery using a modified all-epiphyseal technique in skeletally immature patients with substantial remaining growth provides an anatomic reconstructive option that is physeal sparing using osseous tunnels. Further, the technology needed to perform this modification is no different than the instruments used in the transphyseal strategy, making it potentially easier to master for a greater number of surgeons. This initial series reports good short-term functional outcomes, with high patient satisfaction, high rates of return to sports, low failure rates, and minimal risk of future leg-length discrepancy or angular deformity. This technique has therefore become our institution’s preferred surgical approach in this skeletally immature patient population.
Acknowledgment
The authors thank James Bomar, MPH, for preparing the figures for this manuscript.
Appendix
Footnotes
One or more of the authors has declared the following conflict of interest or source of funding: A.T.P. has received educational support from Arthrex, Smith & Nephew, and Sportstek Medical and is a paid speaker/presenter for Arthrex. H.G.C. is a consultant for OrthoPediatrics and Roche Molecular Systems. E.W.E. is a consultant for OrthoPediatrics and is a paid speaker/presenter for Arthrex.
Ethical approval for this study was obtained from the institutional review board at the University of California, San Diego.
References
- 1. Anderson AF. Transepiphyseal replacement of the anterior cruciate ligament in skeletally immature patients: a preliminary report. J Bone Joint Surg Am. 2003;85-A(7):1255–1263. [DOI] [PubMed] [Google Scholar]
- 2. Cordasco FA, Mayer SW, Green DW. All-inside, all-epiphyseal anterior cruciate ligament reconstruction in skeletally immature athletes: return to sport, incidence of second surgery, and 2-year clinical outcomes. Am J Sports Med. 2017;45(4):856–863. [DOI] [PubMed] [Google Scholar]
- 3. Cruz AI, Jr, Fabricant PD, McGraw M, et al. All-epiphyseal ACL reconstruction in children: review of safety and early complications. J Pediatr Orthop. 2017;37(3):204–209. [DOI] [PubMed] [Google Scholar]
- 4. Dodwell ER, Lamont LE, Green DW, et al. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am J Sports Med. 2014;42(3):675–680. [DOI] [PubMed] [Google Scholar]
- 5. Gebhard F, Ellermann A, Hoffmann F, et al. Multicenter-study of operative treatment of intraligamentous tears of the anterior cruciate ligament in children and adolescents: comparison of four different techniques. Knee Surg Sports Traumatol Arthrosc. 2006;14(9):797–803. [DOI] [PubMed] [Google Scholar]
- 6. Greulich WW, Pyle SI. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd ed Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 7. Henry J, Chotel F, Chouteau J, et al. Rupture of the anterior cruciate ligament in children: early reconstruction with open physes or delayed reconstruction to skeletal maturity? Knee Surg Sports Traumatol Arthrosc. 2009;17(7):748–755. [DOI] [PubMed] [Google Scholar]
- 8. Kennedy A, Coughlin DG, Metzger MF, et al. Biomechanical evaluation of pediatric anterior cruciate ligament reconstruction techniques. Am J Sports Med. 2011;39(5):964–971. [DOI] [PubMed] [Google Scholar]
- 9. Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents. J Bone Joint Surg Am. 2005;87(11):2371–2379. [DOI] [PubMed] [Google Scholar]
- 10. Kocher MS, Smith JT, Zoric BJ, et al. Transphyseal anterior cruciate ligament reconstruction in skeletally immature pubescent adolescents. J Bone Joint Surg Am. 2007;89(12):2632–2639. [DOI] [PubMed] [Google Scholar]
- 11. Lawrence JT, Bowers AL, Belding J, et al. All-epiphyseal anterior cruciate ligament reconstruction in skeletally immature patients. Clin Orthop Relat Res. 2010;468(7):1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Longo UG, Ciuffreda M, Casciaro C, et al. Anterior cruciate ligament reconstruction in skeletally immature patients: a systematic review. Bone Joint J. 2017;99-B(8):1053–1060. [DOI] [PubMed] [Google Scholar]
- 13. Magnussen RA, Lawrence JT, West RL, et al. Graft size and patient age are predictors of early revision after anterior cruciate ligament reconstruction with hamstring autograft. Arthroscopy. 2012;28(4):526–531. [DOI] [PubMed] [Google Scholar]
- 14. Mariscalco MW, Flanigan DC, Mitchell J, et al. The influence of hamstring autograft size on patient-reported outcomes and risk of revision after anterior cruciate ligament reconstruction: a Multicenter Orthopaedic Outcomes Network (MOON) cohort study. Arthroscopy. 2013;29(12):1948–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Paletta GA., Jr Complete transphyseal reconstruction of the anterior cruciate ligament in the skeletally immature. Clin Sports Med. 2011;30(4):779–788. [DOI] [PubMed] [Google Scholar]
- 16. Pennock AT, Bomar JD, Manning JD. The Creation and Validation of a Knee Bone Age Atlas Utilizing MRI [published online February 21, 2018]. J Bone Joint Surg Am. doi: 10.2106/JBJS.17.00693. [DOI] [PubMed] [Google Scholar]
- 17. Pierce TP, Issa K, Festa A, et al. Pediatric anterior cruciate ligament reconstruction: a systematic review of transphyseal versus physeal-sparing techniques. Am J Sports Med. 2017;45(2):488–494. [DOI] [PubMed] [Google Scholar]
- 18. Wall EJ, Ghattas PJ, Eismann EA, et al. Outcomes and complications after all-epiphyseal anterior cruciate ligament reconstruction in skeletally immature patients. Orthop J Sports Med. 2017;5(3):2325967117693604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willimon SC, Jones CR, Herzog MM, et al. Anterior cruciate ligament reconstruction in skeletally immature youths: a retrospective case series with a mean 3-year follow-up. Am J Sports Med. 2015;43(12):2974–2981. [DOI] [PubMed] [Google Scholar]
- 20. Wong SE, Feeley BT, Pandya NK. Complications after pediatric ACL reconstruction: a meta-analysis [published online September 22, 2017]. J Pediatr Orthop. doi:10.1097/BPO.0000000000001075 [DOI] [PubMed] [Google Scholar]
- 21. Yoo WJ, Kocher MS, Micheli LJ. Growth plate disturbance after transphyseal reconstruction of the anterior cruciate ligament in skeletally immature adolescent patients: an MR imaging study. J Pediatr Orthop. 2011;31(6):691–696. [DOI] [PubMed] [Google Scholar]