Abstract
Background:
Osteochondritis dissecans (OCD) is a disorder primarily affecting subchondral bone, with secondary effects on the overlying articular cartilage. Knee joint (75%) and radiocapitellar joint (6%) are the most common sites for OCD lesions. The presence of an open growth plate differentiates juvenile osteochondritis dissecans from adult form of osteochondritis. Early diagnosis and treatment produce best long term results. The objective of this study is to determine the best mode of management of a Grade I osteochondritis lesion in a young athlete.
Materials and Methods:
A PubMed search was made using the keywords “OCD” and “athlete”. Articles that were based on participants between the ages of 6–24 years (children, adolescent and young adult) and early stages of OCD were included in this study. A total of 25 articles were thus included for the review.
Results:
The healing potential is based on the age of the patient, status of physis, and stage of the lesion. Most authors have observed good to excellent results of drilling of early OCD in skeletally mature patients. Similarly, most authors also reported equally successful outcomes of nonoperative treatment for early OCD in skeletally immature patients.
Conclusions:
We recommend initial nonoperative line of management in patients with open physis. In case of progression of the lesion or failure of conservative treatment a reparative, restorative or palliative surgical intervention can be done. For Stage I OCD lesions in patients with closed physis, we advocate reparative surgery either by means of retro- or trans-articular drilling.
Keywords: Athlete, Grade I, osteochondritis dissecans
MeSH terms: Osteochondritis, cartilage, articular, knee joint
Introduction
The term osteochondritis dissecans (OCD) was coined by König in 1888 when1 OCD is a disorder primarily affecting subchondral bone, with secondary effects on the overlying articular cartilage. Knee joint (75%) and radiocapitellar joint (6%) are the most common sites for OCD lesions.2,3 The talus (4%) is the third most frequently affected anatomic site.4
Incidence is higher in boys than girls.5 Majority of the patients (55%–60%) are regularly involved in sports. Young athletes can be frustrated by the condition due to slow healing, extended period of sports restriction, and persistent pain or discomfort. The rationale behind conducting this review was to settle the debate on how to accurately predict which patients will heal with nonoperative treatment and which will require operative treatment.
Materials and Methods
A PubMed search was made using the keywords “OCD” and “athlete.” A total of 152 articles were found. Out of these, only studies in humans were included in this study, which narrowed the search down to 127 articles. We included articles for which only English full text was available that were 93 in number. We focused on articles that were based on subjects between the ages of 6–24 years (children, adolescent, and young adult), thus further narrowing our selection of studies to only 72 in number. We excluded articles based solely on advanced stages of OCD. We also excluded articles that were predominantly focused on injuries to ligaments, menisci, and other such structures, not directly pertaining to osteochondritis. Twenty five articles were shortlisted; out of which only those published in the last two decades were included for the review of the literature, giving us a final list of 18 articles [Figure 1]. All articles were reviewed for an in-depth study of the etiology, pathology, clinical presentation, evaluation, and management of early OCD in young athletes.
Figure 1.
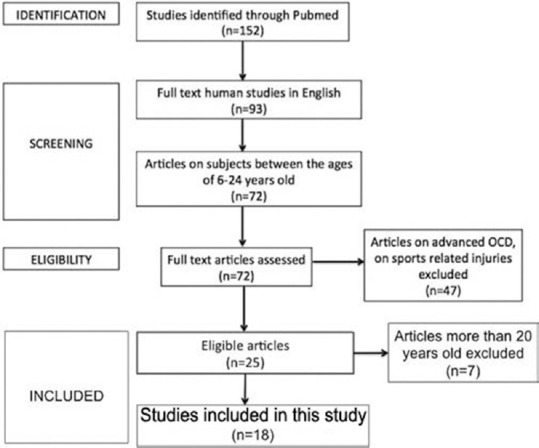
Flowchart showing the process of selecting studies for the review of literature
Etiology
The exact etiology of juvenile osteochondritis dissecans (JOCD) still remains unknown. The three most well-recognized etiologic factors are repetitive trauma, ischemia, and genetics.6 Limb malalignment, presence of lateral discoid meniscus, and inflammatory pathologies are other factors that are thought to contribute to the development of OCD.7,8
Repetitive stress, especially in young athletes, is one of the most accepted causes of JOCD. Repetitive cyclical stress through a joint may lead to chondral injury and possible vascular damage, leading to ischemia. It has also been postulated that JOCD lesions result from repetitive stress to the growth plate of the secondary ossification center that lies just between the articular cartilage and its supporting bone in the epiphysis.
OCD of the capitellum is a well-defined condition in adolescent javelin throwers, gymnasts, and weightlifters.9 During valgus loading, the radiocapitellar joint carries the majority of the force across the elbow and provides axial stability. During late cocking and acceleration phase of throwing, there is compressive force on the lateral aspect of elbow. During the follow through phase, there are shear forces across the joint.10 These motions are repetitive in athletes and are thought to underlie the pathologic process of OCD development. Microtrauma from compressive and shear forces sustained at the radiocapitellar joint during such activities may lead to microvascular injury and eventually to OCD.
Paget described loss of blood flow as an etiological factor.11 In skeletally immature patients, blood supply to the capitellum is provided by very few end vessels posteriorly, leaving the epiphysis vulnerable to vascular insufficiency.12 Most histological studies support “ischemic theory”due to the presence of osteonecrosis.13,14 However, few studies have found rich blood supply and high percentages of viable chondrocytes in detached fragments.15,16 Ischemia hypothesis forms the basis for some of the treatment options such as drilling and microfracture.
Mubarak found an autosomal dominant pattern of inheritance.17 However, Petrie refuted this, citing a lack of familial inheritance.18 As yet, there is no conclusive evidence backing specific genes or gene products in the development of OCD lesion.
Malalignment of the lower limb leading to mechanical axis deviation would increase strain on specific aspects of the joint. Valgus alignment has been associated with lateral condyle lesions, whereas varus alignment has been associated with medial condyle lesions.7
Clinical assessment
Patients usually present with activity-related pain and stiffness, which gradually worsens. Dominant extremity is affected. Occasionally, clicking, locking, and catching might be present. These symptoms point toward the presence of loose bodies.
Radiocapitellar OCD patients have tenderness over radiocapitellar joint, with associated crepitus during pronation and supination. Terminal 15°–30° of extension might become restricted. Radiocapitellar compression test can become positive, where active pronation and supination with elbow in extension produces pain.19
Knee OCD presents with nonspecific knee pain, effusion, catching, locking, and muscle atrophy. Tenderness is usually present at medial femoral condyle, owing to the fact that the lateral surface of the medial femoral condyle is the most common site for OCD of knee. Wilson's sign, pain on extension and internal rotation, is no longer considered specific to OCD.20
Imaging and investigations
Initial imaging for OCD elbow includes three-view plain films of the elbow: extension anterior-posterior (AP), 45°-flexion AP, and lateral. Initial radiographs may not show any abnormality. However, later findings include lucencies, flattening, sclerosis, and fragmentation along with intraarticular loose bodies.19
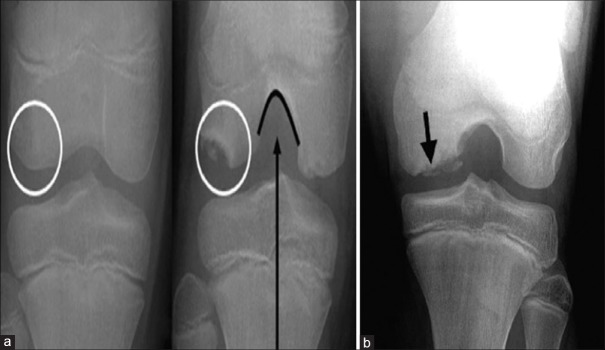
For the knee, since most lesions are located posteriorly, tunnel or notch view radiographs are required [Figure 2]. The lesions appear as dark bone deficient areas just beneath articular surface. However, radiographs have low sensitivity for early OCD lesions.21
Figure 2.
(a) Anterior-posterior and notch views of the same patient. The lesion (white circle) cannot be seen on the anterior-posterior radiograph. The lesion is well visualized on the notch radiograph. (b) Radiograph of an osteochondritis dissecans lesion showing lucency in subchondral region
Technetium-99 m diphosphonate quantitative bone scans were also tried in the past for osteochondritis but was demonstrated to have only limited prognostic significance.22 In addition, the tracer from the bone scan persists in the lesion for a long time even after healing of the lesion and hence limits the ability of bone scan to determine resolution of the lesion.23
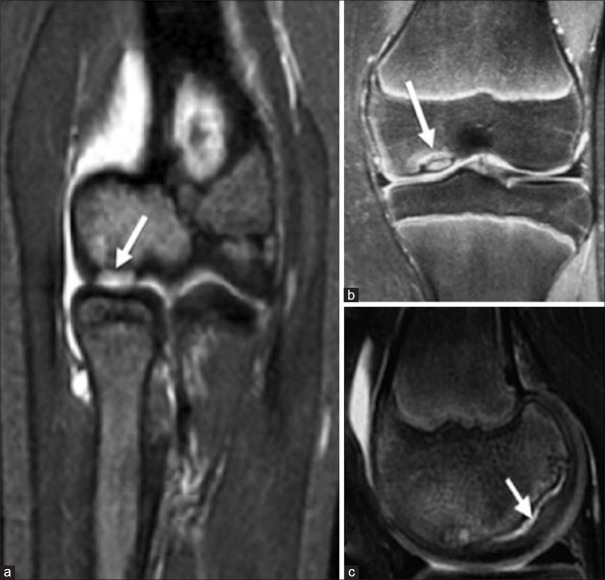
Magnetic resonance imaging (MRI) is the best imaging modality and diagnostic method of choice for evaluating OCD [Figure 3]. The articular cartilage can also be visualized and assessed on MRI. It can demonstrate early stage lesions when radiographs may appear normal. The earliest findings on MRI are low-signal-intensity changes on T1-weighted imaging with normal T2 imaging. Changes are seen on both T1 and T2 imaging with progression of the lesion.24 Gadolinium contrast enhancement of the lesion suggests vascularity of the fragment and good viability. MRI also helps in identifying poor prognostic factors such as bone cysts, subarticular high-signal lines, and articular fissures.10
Figure 3.
(a) T2-weighted coronal magnetic resonance imaging elbow, showing an osteochondritis dissecans lesion of the capitellum. (b) Coronal magnetic resonance imaging of juvenile osteochondritis dissecans lesion clearly demarcated from underlying subchondral bone and an intact articular mantle. (c) Magnetic resonance imaging showing a high-signal line at the interface between the osteochondritis dissecans lesion and its bed indicating an early deep separation at the bone-cartilage junction, which would not be seen on arthroscopy
Lately, ultrasound has garnered attention as a screening tool. It can show loss of smooth articular surface, which is a good indicator of osteochondral lesion, with reportedly 100% positive predictive value.25 In the hands of an experienced operator, ultrasound can not only identify lesions but also provide information on stability of the lesion.26
Arthroscopy is widely considered to be the gold standard for the assessment of articular cartilage and detachment of subchondral bone.27 However, for early lesions, where articular cartilage may not be defective, arthroscopy findings may be falsely negative. MRI is more sensitive for early stage lesions.
Arthroscopic evaluation of osteochondritis lesion is prone to interobserver and intraobserver variations. Ultrasound arthroscopy (UA) is generating interest for objective intraoperative assessment of cartilage and subchondral bone. Quantitative ultrasonography detects an increase in articular cartilage surface roughness, degradation of superficial collagen, changes in subchondral bone mineralization, and cartilage healing after surgical repair.28 Ultrasound catheter is inserted into the joint through conventional portals. US and arthroscopy videos were synchronously recorded. US parameters for cartilage and subchondral bone characteristics are measured and combined to perform cartilage grading. UA has the advantage of visualization of an OCD lesion not detected by conventional arthroscopy. US-guidance can also aid retrograde drilling and decrease the need for fluoroscopy.29
Classification
Plain radiography is not optimal for staging of lesions due to inadequate assessment of articular cartilage; it is used extensively to assess healing progression. MRI is more accurate for classifying OCD lesions. Dipaola et al. classified OCD by correlating radiographs, MRI, and arthroscopic findings [Table 1].30 MRI and arthroscopy together can help differentiate between stable and unstable lesions. De Smet et al. described four signs of instability [Table 2].31
Table 1.
Classification of osteochondritis dissecans

Table 2.
Signs of instability of lesion on magnetic resonance imaging
Management
Treatment of OCD has come a long way from the era of open surgical exploration and debridement to arthroscopic stem cell implantation. Stage I OCD lesions are stable lesions with minimal cartilage changes, and hence, the aim of treatment is healing of the lesion with early return to sports in professional athletes. The main debate on the management of Stage I OCD in athletes is about which lesions will nonoperatively and which ones will require surgical intervention. This decision mainly rests on the age of the patient. Healing potential of the lesion is good in patients with open physis. Success rate of nonoperative treatment in patients with open physis and stable lesions is reported to be between 50% and 100%.27,32,33,34,35,36,37
Apart from age and lesion staging, the size and site of the lesion has also been shown to influence healing potential. Small longitudinal diameter and surface area of the lesion as measured on MRI are more likely to heal by nonoperative means.34,35 Lesions at atypical sites also show poor healing potential.38
The treatment for a stable JOCD lesion remains controversial because the condition is rare, the success rate of various treatments is unknown, the progression or healing of the condition is slow, and there are no uniform criteria to determine success. In addition, symptoms often resolve before radiographic signs of resolution, which leads to poor treatment compliance and loss to followup.34
Nonoperative management
Most authors agree that small stable lesions, in young patients with open growth plates show greatest potential for healing by nonoperative methods. Nonoperative treatment involves immediate cessation of aggravating activities.39 Alternate forms of exercise can be encouraged such as swimming, stationary bikes, deep-water running, and other nonimpact activities.40
Controversy exists regarding the duration of immobilization and speed of rehabilitation. Authors who focus on subchondral bone argue that the limb should be protected in a cast or immobilizer and treated as a fracture. Conversely, authors focused on articular cartilage cite the role of continuous motion. The use of continuous passive motion device or repetitive active-assisted range of motion may help to nourish the articular cartilage. Ahmad et al. suggested the use of a hinge elbow brace for 1–6 weeks to allow intermittent range of motion exercises and hence prevent stiffness.41 Greiwe et al. recommended only rest, without immobilization.19
For OCD knee, immobilization may be required for 4–16 weeks by means of cylindrical cast, hinged brace, unloader-type brace, or ambulatory aids. Immobilization of more than 16 weeks can lead to detrimental effects.42 There are still no established guidelines for the duration of rest, immobilization, and allowable range of motion. The American Academy of Orthopaedic Surgeons clinical practice guideline was also unable to recommend any one particular form on nonoperative treatment.43
Some authors suggest a 3-phase nonoperative management for OCD [Table 3].23 The first phase involves knee immobilization for 4–6 weeks, with crutch-protected partial weight-bearing gait. At the end of this period, the patient should be pain free. In phase 2 (weeks 6–12), weight bearing as tolerated is permitted without immobilization. A rehabilitation program is initiated emphasizing knee range of motion, quadriceps, and hamstring strengthening exercises. Electrical stimulation can facilitate muscle strengthening. Phase 3 can begin, if there are radiographic and clinical signs of healing at 3–4 months after the initial diagnosis. This includes supervised initiation of running, jumping, and sports readiness activities. A gradual return to sports with increasing intensity is allowed in the absence of knee symptoms.
Table 3.
Rehabilitation protocol for nonoperative management of osteochondritis dissecans
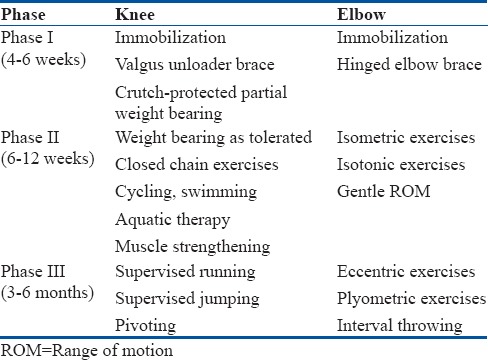
Most authors report that athletes that respond to conservative treatment may start gentle sports at 3–4 months and return to competitive sports at 6 months.32
Younger patients are likely to be less compliant, and hence, immobilization may be more beneficial in them. Patients who experience symptoms even in daily activities may require a slower and stricter rehabilitation protocol.34
Healing should be assessed at 6–8-week interval. Clinical assessment can be made on the basis of decrease in symptoms. Early radiographic and MRI healing can be seen as early as 4 weeks.44 Progressive reossification is a sign of radiographic healing.34 Reduction in size and reduction in high-signal intensity around the lesion are signs of healing seen on MRI.33
Operative management
Operative treatment in Stage I OCD should be considered in the following situations:45
Skeletally mature patients
Patients approaching physeal closure
Failure of nonoperative management.
Surgical techniques for OCD of the knee can be classified as reparative, restorative, and palliative. Reparative techniques include transarticular and retroarticular drilling, microfracture, open reduction or arthroscopic-assisted reduction, and fixation. Restorative techniques include autologous chondrocyte implantation and auto or allograft transplantation into the osteochondral lesion. Palliative techniques including loose body removal or debridement of the defect.46 Restorative and palliative techniques are reserved for advanced stage lesions and failed cases of reparative techniques.
For Grade I OCD, the aim of surgery is to stimulate healing of the lesion. This is achieved by drilling of subchondral bone either in a retrograde manner without articular penetration (transepiphyseal/retroarticular) or through the articular surface (transarticular).
Drilling through the epiphysis avoids articular surface violation but is associated with technical challenges of maintaining drill depth and placement accuracy. Posterior condylar lesions are more readily accessible through retroarticular technique.47 In comparison, transarticular drilling is technically straightforward; however, it involves the penetration of the articular cartilage. These drill holes may not heal even a year later.48
For retroarticular drilling, patient is placed supine on a radiolucent operating table, with a tourniquet on the proximal thigh. The lower extremity is placed in about 20° of hip flexion, to facilitate fluoroscopic imaging. Diagnostic arthroscopy is performed to inspect the OCD lesion. The C-arm is positioned in such a way that the lesion can be visualized in AP, notch, and lateral views. Under fluoroscopic guidance, a smooth Kirschner-wire (K-wire) of 1.6 mm (0.062 inch) diameter is inserted through a 1 cm skin incision. The K-wire is inserted just anterior to the midcondyle on the lateral view and in skeletally immature patients; it must begin just distal to the femoral growth plate. It is directed into the OCD lesion up to the bone-cartilage junction, avoiding penetration into the articular cartilage. Using this first K-wire as a guide, multiple K-wires can be inserted about 3 mm–8 mm away from each other. The K-wires can be over-drilled using a 3.2-mm-cannulated drill bit. The initial pass of the drill is stopped 10 mm short of the tip because the drill often pushes the wire into the joint. The drill is then backed out, and K-wire is removed. The blunt end of the wire is then inserted into the bone tunnel, and the drill is advanced over the blunt-tipped K-wire to penetrate into the OCD lesion, but not into any of the articular cartilage. The cannulated drill bit is disconnected from the drill and is removed with twisting by manually.49 To accelerate healing, these tunnels can be filled with stem cells from iliac crest. Lately, electromagnetic navigation system assisted retroarticular drilling has shown promise and is being explored to eliminate the need for fluoroscopy.50,51 For transarticular drilling, the patient is placed supine on the operating table, with a tourniquet applied on the proximal thigh. Diagnostic arthroscopy is performed through the anteromedial and anterolateral portals. The fibrillations of articular cartilage and softening of cartilage observed on probing identify the OCD lesion. Multiple drill holes are then made into the lesion using 0.45 mm–0.62 mm K-wires. Drilling is done from periphery to center and up to a depth of 2 cm. Oozing of blood and fat droplets from the drill holes indicates that adequate depth of drilling has been achieved.
A meta-analysis by Gunton et al. compared short-term outcomes after retroarticular and transarticular drilling.48 They reported comparable healing rates (86% in retroarticular and 91% in transarticular at an average of 5.6 months and 4.5 months, respectively) after both types of procedures. No major complication of either procedure was reported.
Rehabilitation following drilling procedures usually involves 4–12 weeks of nonweight-bearing ambulation. Full range of motion is allowed immediately following drilling procedures. Unloader braces are recommended to help resume weight bearing and mobility while protecting the knee from overload. After 4–6 months, the patient may transition to a staged running program and after 6 months, to sport-specific activities such as pivoting, cutting, and jumping.
Bradley and Dandyperformed transarticular drilling and noted healing on radiographs and pain relief in 9 of 11 knees within a 1-year period.52 Anderson et al. performed transarticular drilling in 20 knees with open physis and in four patients with closed physis.53 In the skeletally immature group, 90% lesions healed. In the skeletally mature group, 50% lesions healed at a followup of 5 years. At the Children's Hospital of Philadelphia, transarticular drilling was performed on 51 knees up to 18 years of age. It was curative in 83% of skeletally immature patients, in contrast to 75% in skeletally mature.54 Lesions in atypical locations, multiple lesions, and underlying medical conditions are associated with inadequate healing. Yonetani revealed a healing rate of 79% following transarticular drilling for stable JOCD lesions of the knee.55
Edmonds et al. reported on 59 JOCD patients treated with retroarticular drilling.56 The average time to return to sports was 3 months. Nearly 98 lesions had healed completely at 36 months. Similarly, Boughanem showed 94% healing rates after retroarticular drilling in stable JOCD.57 Adachi et al. also reported 95% healing rate at an average of 4.4 months following retroarticular drilling for stable JOCD.58
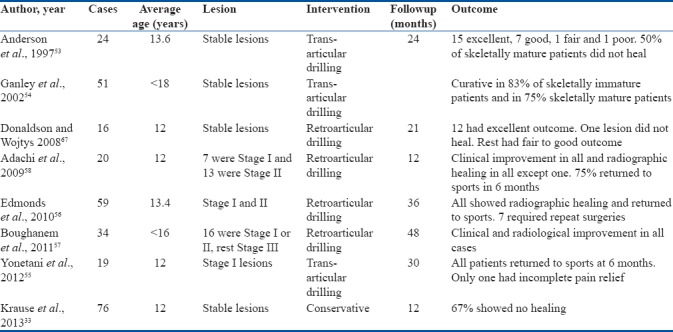
Although most of these studies report excellent outcomes of drilling procedures. A closer look at the profile of patients that improved reveals that a high percentage of patients had open physis; hence, better healing potential and might have fared well on conservative treatment alone. This critical point perhaps exaggerates the benefit of drilling procedures. However, there is no denying that in patients with closed physis nonoperative treatment is unlikely to benefit. A summary of recent results of operative management of early OCD in young athletes is provided in Tables 4 and 5.
Table 4.
Summary of recent studies on early capitellum osteochondritis dissecans in young athletes
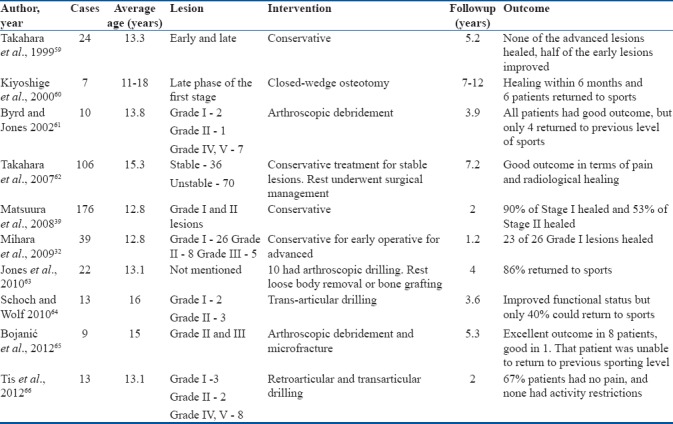
Table 5.
Summary of recent studies on early knee osteochondritis dissecans in young patients
Conclusions
The management of OCD in a young professional athlete is based on early diagnosis and assessment of healing potential of the lesion. The healing potential is based on the age of the patient, status of physis, and stage of the lesion. Size and site of lesion also have a bearing on the healing potential. The ultimate aim for an athlete is early return to the previous level of competitive sports. For Stage I OCD (stable) lesions, we recommend initial nonoperative line of management in patients with open physis [Figure 4]. Case of progression of the lesion or failure of conservative treatment a reparative, restorative, or palliative surgical intervention can be done. For Stage I OCD lesions in patients with closed physis, we advocate reparative surgery either by means of retro or transarticular drilling. The technique of drilling can be chosen as per surgeons’ preference and experience since both have comparative results.67
Figure 4.
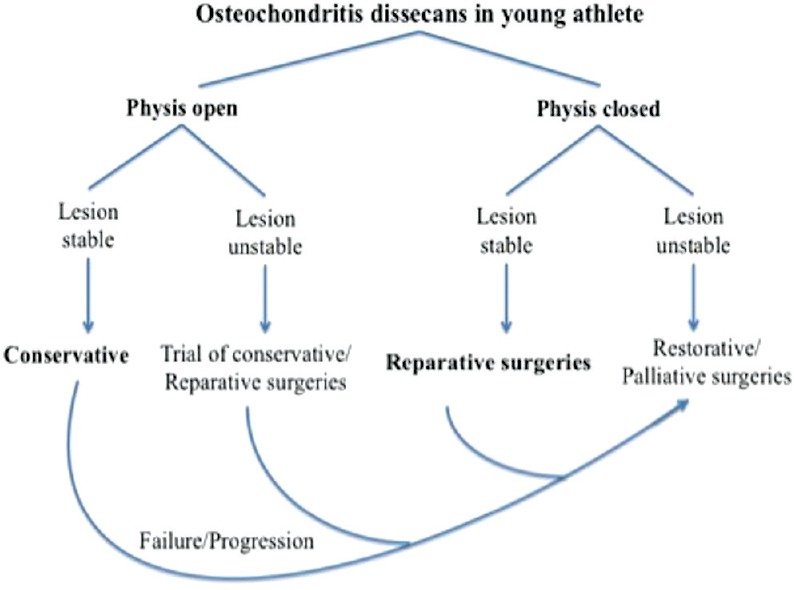
Protocol of authors preferred the treatment of osteochondritis dissecans
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
References
- 1.König F. The classic: On loose bodies in the joint 1887. Clin Orthop Relat Res. 2013;471:1107–15. doi: 10.1007/s11999-013-2824-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeLee JC, Drez D, Jr, Miller MD. 2nd ed. Philadelphia: Elsevier Science; 2003. DeLee & Drez's Orthopaedic Sports Medicine Principles and Practice. [Google Scholar]
- 3.Griffin LY, editor. Essentials of Musculoskeletal Care. 3rd ed. Rosemont: American Academy of Orthopedic Surgeons; 2005. [Google Scholar]
- 4.Steinhagen J, Niggemeyer O, Bruns J. Etiology and pathogenesis of osteochondrosis dissecans tali. Orthopade. 2001;30:20–7. doi: 10.1007/s001320050569. [DOI] [PubMed] [Google Scholar]
- 5.Kessler JI, Nikizad H, Shea KG, Jacobs JC, Jr, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42:320–6. doi: 10.1177/0363546513510390. [DOI] [PubMed] [Google Scholar]
- 6.Edmonds EW, Polousky J. A review of knowledge in osteochondritis dissecans: 123 years of minimal evolution from König to the ROCK study group. Clin Orthop Relat Res. 2013;471:1118–26. doi: 10.1007/s11999-012-2290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobi M, Wahl P, Bouaicha S, Jakob RP, Gautier E. Association between mechanical axis of the leg and osteochondritis dissecans of the knee: Radiographic study on 103 knees. Am J Sports Med. 2010;38:1425–8. doi: 10.1177/0363546509359070. [DOI] [PubMed] [Google Scholar]
- 8.Mitsuoka T, Shino K, Hamada M, Horibe S. Osteochondritis dissecans of the lateral femoral condyle of the knee joint. Arthroscopy. 1999;15:20–6. doi: 10.1053/ar.1999.v15.015002. [DOI] [PubMed] [Google Scholar]
- 9.Nissen CW. Osteochondritis dissecans of the elbow. Clin Sports Med. 2014;33:251–65. doi: 10.1016/j.csm.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Baker CL, 3rd, Romeo AA, Baker CL., Jr Osteochondritis dissecans of the capitellum. Am J Sports Med. 2010;38:1917–28. doi: 10.1177/0363546509354969. [DOI] [PubMed] [Google Scholar]
- 11.Paget J. On the production of some of the loose bodies in joints. St Barth Hosp Rep. 1870;6:1–4. [Google Scholar]
- 12.Haraldsson S. On osteochondrosis deformas juvenilis capituli humeri including investigation of intra-osseous vasculature in distal humerus. Acta Orthop Scand Suppl. 1959;38:1–232. [PubMed] [Google Scholar]
- 13.Yonetani Y, Matsuo T, Nakamura N, Natsuume T, Tanaka Y, Shiozaki Y, et al. Fixation of detached osteochondritis dissecans lesions with bioabsorbable pins: Clinical and histologic evaluation. Arthroscopy. 2010;26:782–9. doi: 10.1016/j.arthro.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Uozumi H, Sugita T, Aizawa T, Takahashi A, Ohnuma M, Itoi E, et al. Histologic findings and possible causes of osteochondritis dissecans of the knee. Am J Sports Med. 2009;37:2003–8. doi: 10.1177/0363546509346542. [DOI] [PubMed] [Google Scholar]
- 15.Chiroff RT, Cooke CP., 3rd Osteochondritis dissecans: A histologic and microradiographic analysis of surgically excised lesions. J Trauma. 1975;15:689–96. [PubMed] [Google Scholar]
- 16.Yonetani Y, Nakamura N, Natsuume T, Shiozaki Y, Tanaka Y, Horibe S, et al. Histological evaluation of juvenile osteochondritis dissecans of the knee: A case series. Knee Surg Sports Traumatol Arthrosc. 2010;18:723–30. doi: 10.1007/s00167-009-0898-6. [DOI] [PubMed] [Google Scholar]
- 17.Mubarak SJ, Carroll NC. Familial osteochondritis dissecans of the knee. Clin Orthop Relat Res. 1979;140:131–6. [PubMed] [Google Scholar]
- 18.Petrie PW. Aetiology of osteochondritis dissecans. Failure to establish a familial background. J Bone Joint Surg Br. 1977;59:366–7. doi: 10.1302/0301-620X.59B3.893517. [DOI] [PubMed] [Google Scholar]
- 19.Greiwe RM, Saifi C, Ahmad CS. Pediatric sports elbow injuries. Clin Sports Med. 2010;29:677–703. doi: 10.1016/j.csm.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Conrad JM, Stanitski CL. Osteochondritis dissecans: Wilson's sign revisited. Am J Sports Med. 2003;31:777–8. doi: 10.1177/03635465030310052301. [DOI] [PubMed] [Google Scholar]
- 21.Cahill BR, Berg BC. 99m-technetium phosphate compound joint scintigraphy in the manage- ment of juvenile osteochondritis dissecans of the femoral condyles. Am J Sports Med. 1983;11:329–5. doi: 10.1177/036354658301100509. [DOI] [PubMed] [Google Scholar]
- 22.Paletta GA, Jr, Bednarz PA, Stanitski CL, Sandman GA, Stanitski DF, Kottamasu S. The prognostic value of quantitative bone scan in knee osteochondritis dissecans A preliminary experience. Am J Sports Med. 1998;26:7–14. doi: 10.1177/03635465980260012901. [DOI] [PubMed] [Google Scholar]
- 23.Kocher MS, Tucker R, Ganley TJ, Flynn JM. Management of osteochondritis dissecans of the knee: Current concepts review. Am J Sports Med. 2006;34:1181–91. doi: 10.1177/0363546506290127. [DOI] [PubMed] [Google Scholar]
- 24.Zbojniewicz AM, Laor T. Imaging of osteochondritis dissecans. Clin Sports Med. 2014;33:221–50. doi: 10.1016/j.csm.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Kida Y, Morihara T, Kotoura Y, Hojo T, Tachiiri H, Sukenari T, et al. Prevalence and clinical characteristics of osteochondritis dissecans of the humeral capitellum among adolescent baseball players. Am J Sports Med. 2014;42:1963–71. doi: 10.1177/0363546514536843. [DOI] [PubMed] [Google Scholar]
- 26.Takahara M, Ogino T, Tsuchida H, Takagi M, Kashiwa H, Nambu T, et al. Sonographic assessment of osteochondritis dissecans of the humeral capitellum. AJR Am J Roentgenol. 2000;174:411–5. doi: 10.2214/ajr.174.2.1740411. [DOI] [PubMed] [Google Scholar]
- 27.Eismann EA, Pettit RJ, Wall EJ, Myer GD. Management strategies for osteochondritis dissecans of the knee in the skeletally immature athlete. J Orthop Sports Phys Ther. 2014;44:665–79. doi: 10.2519/jospt.2014.5140. [DOI] [PubMed] [Google Scholar]
- 28.Nieminen HJ, Zheng YP, Saarakkala S. Quantitative assessment of articular cartilage using high-frequency ultrasound: Research findings and diagnostic prospects. Crit Rev Biomed Eng. 2009;37:461–94. doi: 10.1615/critrevbiomedeng.v37.i6.20. [DOI] [PubMed] [Google Scholar]
- 29.Penttilä P, Liukkonen J, Joukainen A, Virén T, Jurvelin JS, Töyräs J, et al. Diagnosis of knee osteochondral lesions with ultrasound imaging. Arthrosc Tech. 2015;4:e429–33. doi: 10.1016/j.eats.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dipaola JD, Nelson DW, Colville MR. Characterizing osteochondral lesions by magnetic resonance imaging. Arthroscopy. 1991;7:101–4. doi: 10.1016/0749-8063(91)90087-e. [DOI] [PubMed] [Google Scholar]
- 31.De Smet AA, Fisher DR, Graf BK, Lange RH. Osteochondritis dissecans of the knee: Value of MR imaging in determining lesion stability and the presence of articular cartilage defects. AJR Am J Roentgenol. 1990;155:549–53. doi: 10.2214/ajr.155.3.2117355. [DOI] [PubMed] [Google Scholar]
- 32.Mihara K, Tsutsui H, Nishinaka N, Yamaguchi K. Nonoperative treatment for osteochondritis dissecans of the capitellum. Am J Sports Med. 2009;37:298–304. doi: 10.1177/0363546508324970. [DOI] [PubMed] [Google Scholar]
- 33.Krause M, Hapfelmeier A, Möller M, Amling M, Bohndorf K, Meenen NM, et al. Healing predictors of stable juvenile osteochondritis dissecans knee lesions after 6 and 12 months of nonoperative treatment. Am J Sports Med. 2013;41:2384–91. doi: 10.1177/0363546513496049. [DOI] [PubMed] [Google Scholar]
- 34.Wall EJ, Vourazeris J, Myer GD, Emery KH, Divine JG, Nick TG, et al. The healing potential of stable juvenile osteochondritis dissecans knee lesions. J Bone Joint Surg Am. 2008;90:2655–64. doi: 10.2106/JBJS.G.01103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellelli A, Avitto A, David V. Spontaneous remission of osteochondritis dissecans in 8 pediatric patients undergoing conservative treatment. Radiol Med. 2001;102:148–53. [PubMed] [Google Scholar]
- 36.Hughes JA, Cook JV, Churchill MA, Warren ME. Juvenile osteochondritis dissecans: A 5-year review of the natural history using clinical and MRI evaluation. Pediatr Radiol. 2003;33:410–7. doi: 10.1007/s00247-003-0876-y. [DOI] [PubMed] [Google Scholar]
- 37.Sales de Gauzy J, Mansat C, Darodes PH, Cahuzac JP. Natural course of osteochondritis dissecans in children. J Pediatr Orthop B. 1999;8:26–8. [PubMed] [Google Scholar]
- 38.Samora WP, Chevillet J, Adler B, Young GS, Klingele KE. Juvenile osteochondritis dissecans of the knee: Predictors of lesion stability. J Pediatr Orthop. 2012;32:1–4. doi: 10.1097/BPO.0b013e31823d8312. [DOI] [PubMed] [Google Scholar]
- 39.Matsuura T, Kashiwaguchi S, Iwase T, Takeda Y, Yasui N. Conservative treatment for osteochondrosis of the humeral capitellum. Am J Sports Med. 2008;36:868–72. doi: 10.1177/0363546507312168. [DOI] [PubMed] [Google Scholar]
- 40.Hurst JM, Steadman JR, O’Brien L, Rodkey WG, Briggs KK. Rehabilitation following microfracture for chondral injury in the knee. Clin Sports Med. 2010;29:257–65. doi: 10.1016/j.csm.2009.12.009. viii. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad CS, Vitale MA, ElAttrache NS. Elbow arthroscopy: Capitellar osteochondritis dissecans and radiocapitellar plica. Instr Course Lect. 2011;60:181–90. [PubMed] [Google Scholar]
- 42.Smillie I. Treatment of osteochondritis dissecans. J Bone Joint Surg Br. 1957;39:248–60. doi: 10.1302/0301-620X.39B2.248. [DOI] [PubMed] [Google Scholar]
- 43.Chambers HG, Shea KG, Carey JL. AAOS clinical practice guideline: Diagnosis and treatment of osteochondritis dissecans. J Am Acad Orthop Surg. 2011;19:307–9. doi: 10.5435/00124635-201105000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Detterline AJ, Goldstein JL, Rue JP, Bach BR., Jr Evaluation and treatment of osteochondritis dissecans lesions of the knee. J Knee Surg. 2008;21:106–15. doi: 10.1055/s-0030-1247804. [DOI] [PubMed] [Google Scholar]
- 45.Lewine EB, Miller PE, Micheli LJ, Waters PM, Bae DS. Early results of drilling and/or microfracture for grade IV osteochondritis dissecans of the capitellum. J Pediatr Orthop. 2016;36:803–9. doi: 10.1097/BPO.0000000000000575. [DOI] [PubMed] [Google Scholar]
- 46.Erickson BJ, Chalmers PN, Yanke AB, Cole BJ. Surgical management of osteochondritis dissecans of the knee. Curr Rev Musculoskelet Med. 2013;6:102–14. doi: 10.1007/s12178-013-9156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pennock AT, Bomar JD, Chambers HG. Extra-articular, intraepiphyseal drilling for osteochondritis dissecans of the knee. Arthrosc Tech. 2013;2:e231–5. doi: 10.1016/j.eats.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunton MJ, Carey JL, Shaw CR, Murnaghan ML. Drilling juvenile osteochondritis dissecans: Retro- or transarticular? Clin Orthop Relat Res. 2013;471:1144–51. doi: 10.1007/s11999-011-2237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lykissas MG, Wall EJ, Nathan S. Retro-articular drilling and bone grafting of juvenile knee osteochondritis dissecans: A technical description. Knee Surg Sports Traumatol Arthrosc. 2014;22:274–8. doi: 10.1007/s00167-013-2375-5. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmann M, Petersen JP, Schröder M, Hartel M, Kammal M, Rueger JM, et al. Accuracy analysis of a novel electromagnetic navigation procedure versus a standard fluoroscopic method for retrograde drilling of osteochondritis dissecans lesions of the knee. Am J Sports Med. 2012;40:920–6. doi: 10.1177/0363546511434407. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann M, Schröder M, Petersen JP, Spiro AS, Kammal M, Lehmann W, et al. Arthroscopically assisted retrograde drilling for osteochondritis dissecans (OCD) lesions of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:2257–62. doi: 10.1007/s00167-012-1886-9. [DOI] [PubMed] [Google Scholar]
- 52.Bradley J, Dandy D. Results of drilling osteochondritis dissecans before skeletal maturity. J Bone Joint Surg Br. 1989;71:642–4. doi: 10.1302/0301-620X.71B4.2768313. [DOI] [PubMed] [Google Scholar]
- 53.Anderson AF, Richards DB, Pagnani MJ, Hovis WD. Antegrade drilling for osteochondritis dissecans of the knee. Arthroscopy. 1997;13:319–24. doi: 10.1016/s0749-8063(97)90028-1. [DOI] [PubMed] [Google Scholar]
- 54.Ganley TJ, Amro RR, Gregg JR, Halpern KV. Salt Lake City, Utah: 2002. Antegrade drilling for osteo-chondritis dissecans of the knee Paper Presented at: Pediatric Orthopaedic Society of North America 2002 Annual Meeting; May 3-5, 2002. [Google Scholar]
- 55.Yonetani Y, Tanaka Y, Shiozaki Y, Kanamoto T, Kusano M, Tsujii A, et al. Transarticular drilling for stable juvenile osteochondritis dissecans of the medial femoral condyle. Knee Surg Sports Traumatol Arthrosc. 2012;20:1528–32. doi: 10.1007/s00167-011-1736-1. [DOI] [PubMed] [Google Scholar]
- 56.Edmonds EW, Albright J, Bastrom T, Chambers HG. Outcomes of extra-articular, intra-epiphyseal drilling for osteochondritis dissecans of the knee. J Pediatr Orthop. 2010;30:870–8. doi: 10.1097/BPO.0b013e3181f5a216. [DOI] [PubMed] [Google Scholar]
- 57.Boughanem J, Riaz R, Patel RM, Sarwark JF. Functional and radiographic outcomes of juvenile osteochondritis dissecans of the knee treated with extra-articular retrograde drilling. Am J Sports Med. 2011;39:2212–7. doi: 10.1177/0363546511416594. [DOI] [PubMed] [Google Scholar]
- 58.Adachi N, Deie M, Nakamae A, Ishikawa M, Motoyama M, Ochi M, et al. Functional and radiographic outcome of stable juvenile osteochondritis dissecans of the knee treated with retroarticular drilling without bone grafting. Arthroscopy. 2009;25:145–52. doi: 10.1016/j.arthro.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27:728–32. doi: 10.1177/03635465990270060701. [DOI] [PubMed] [Google Scholar]
- 60.Kiyoshige Y, Takagi M, Yuasa K, Hamasaki M. Closed-wedge osteotomy for osteochondritis dissecans of the capitellum. A 7- to 12-year followup. Am J Sports Med. 2000;28:534–7. doi: 10.1177/03635465000280041401. [DOI] [PubMed] [Google Scholar]
- 61.Byrd JW, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: Minimum three-year followup. Am J Sports Med. 2002;30:474–8. doi: 10.1177/03635465020300040401. [DOI] [PubMed] [Google Scholar]
- 62.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89:1205–14. doi: 10.2106/JBJS.F.00622. [DOI] [PubMed] [Google Scholar]
- 63.Jones KJ, Wiesel BB, Sankar WN, Ganley TJ. Arthroscopic management of osteochondritis dissecans of the capitellum: Mid-term results in adolescent athletes. J Pediatr Orthop. 2010;30:8–13. doi: 10.1097/BPO.0b013e3181c3be83. [DOI] [PubMed] [Google Scholar]
- 64.Schoch B, Wolf BR. Osteochondritis dissecans of the capitellum: Minimum 1-year followup after arthroscopic debridement. Arthroscopy. 2010;26:1469–73. doi: 10.1016/j.arthro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Bojanić I, Smoljanović T, Dokuzović S. Osteochondritis dissecans of the elbow: Excellent results in teenage athletes treated by arthroscopic debridement and microfracture. Croat Med J. 2012;53:40–7. doi: 10.3325/cmj.2012.53.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tis JE, Edmonds EW, Bastrom T, Chambers HG. Short-term results of arthroscopic treatment of osteochondritis dissecans in skeletally immature patients. J Pediatr Orthop. 2012;32:226–31. doi: 10.1097/BPO.0b013e31824afeb8. [DOI] [PubMed] [Google Scholar]
- 67.Donaldson LD, Wojtys EM. Extraarticular drilling for stable osteochondritis dissecans in the skeletally immature knee. J Pediatr Orthop. 2008;28:831–5. doi: 10.1097/BPO.0b013e31818ee248. [DOI] [PubMed] [Google Scholar]