Abstract
Context
To provide a systematic review and cost-effectiveness analysis on smoking interventions targeting smokers not ready to quit, a population that makes up approximately 32% of current smokers.
Evidence acquisition
Twenty-two studies on pharmacological, behavioral, and combination smoking cessation interventions targeting smokers not ready to quit (defined as those who reported they were not ready to quit at the time of the study) published between 2000 and 2017 were analyzed. The effectiveness (measured by the number needed to treat) and cost effectiveness (measured by costs per quit) of interventions were calculated. All data collection and analyses were performed in 2017.
Evidence synthesis
Smoking interventions targeting smokers not ready to quit can be as effective as similar interventions for smokers ready to quit; however, costs of intervening on this group may be higher for some intervention types. The most cost-effective interventions identified for this group were those using varenicline and those using behavioral interventions.
Conclusions
Updating clinical recommendations to provide cessation interventions for this group is recommended. Further research on development of cost-effective treatments and effective strategies for recruitment and outreach for this group are needed. Additional studies may allow for more nuanced comparisons of treatment types among this group.
CONTEXT
Despite declining smoking rates, smoking remains the single largest cause of preventable disease and death.1 Although the majority of smokers would like to quit sometime in the future, only 68% to 69% of smokers report willingness to quit within a year.1,2 Most smoking cessation literature focuses on those ready to set a quit date.2,3 In fact, the clinical practice guidelines devotes nearly 250 pages and provides scores of supporting tables for smokers ready to quit smoking whereas spending only three and a half pages and one supporting table on smokers not ready to quit (SNRTQ).4 The current clinical practice guidelines for SNRTQ recommend motivational interviewing (MI) to encourage these smokers to consider quitting, but do not make further recommendations for this group until they express a motivation to quit.1,5
Research examining differences between SNRTQ and those ready to make a quit attempt find that many of the behavioral differences between these groups, such as self-efficacy to cope with temptation to smoke, support of quit attempts by significant other, and use of nicotine replacement therapy (NRT), can be addressed directly through interventions.6 Currently, the most validated and widely studied method for treating SNRTQ is combined behavioral and pharmacological rate reduction, used as an approach to produce initial reductions in tobacco use and to enhance self-efficacy for ultimate smoking cessation.7–9 Combined behavioral/pharmacologic rate reduction has been shown in a meta-analysis to more than double the odds of cessation in SNRTQ.10 However, since the writing of this meta-analysis, the treatment field has taken considerable leaps forward, both in terms of pharmacologic interventions and combined pharmacologic and behavioral treatments for SNRTQ.11,12 Additionally, because the types and intensity of studies targeting SNRTQ may differ from smokers ready to quit, it is important to evaluate the cost effectiveness in conjunction with the efficacy of interventions in this difficult to treat group of smokers.
This study combines a systematic review to estimate the effectiveness of pharmacological, behavioral, and combination smoking cessation interventions targeted towards SNRTQ with a cost-effectiveness analysis to examine the most economical methods for intervening on this group. Effectiveness is expressed in the estimated number needed to treat (NNT) in order to produce one additional quit and cost effectiveness is expressed by the estimated cost per quit. Although many factors that promote smoking in those ready to quit and those not ready to quit are likely to be similar, it is hypothesized that the intensity, and thereby the cost of interventions (cost per quit) would be higher than typically found in the literature on smokers ready to quit.10
EVIDENCE ACQUISITION
Search Strategy
Studies of interventions targeting SNRTQ were reviewed. SNRTQ were defined as smokers who are either in the pre-contemplation (not thinking about quitting within the next 6 months) or contemplation (thinking about quitting but not ready to quit within the next 6 months) stage of the transtheoretical or stages of change model; these groups combined make up between 32% and 46% of current smokers.2 Ten papers reviewed in a previous study by Asfar et al.10 are summarized in Appendix Table 1.13–22 A search for additional clinical trials published after the Asfar et al. review was performed (between 2011 and 2017), using MEDLINE and Google Scholar and the search terms smoking, smoker, or smokers with the phrases not ready or unmotivated and quit.5
Selection Criteria
This search yielded 619 initial results; 12 of which met the criteria as RCTs that specifically recruited adult (aged ≥18 years) smokers who were not ready or willing to quit either immediately, within the next 30 days, or within the next 6 months. Ten of these studies are summarized in Appendix Table 2. Two were excluded and discussed below.
Including studies from Asfar et al. and the new literature search, 20 total studies were reviewed, 15 of which had abstinence outcomes which were biochemically verified and were therefore included in the meta-analysis (Figure 1). Studies examined pharmacological interventions, behavioral interventions, or combinations of these two and were grouped according to intervention type. Interventions using other types of tobacco, such as smokeless tobacco, snus, or e-cigarettes were not included. Each study had one comparison group, which received either placebo, usual care, or no treatment, noted in Appendix Tables 1 and 2. Studies with multiple treatment arms compared pooled treatments against the control group.
Figure 1.
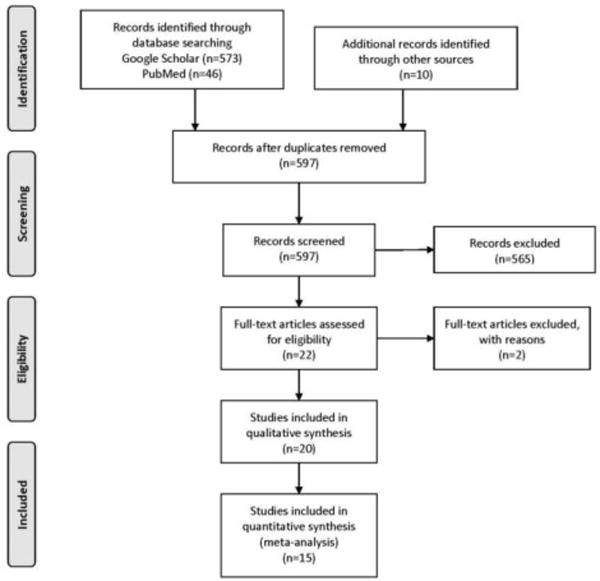
PRISMA flow diagram.
Primary Outcome Definition
Included studies measured smoking abstinence, or quits, using either continuous abstinence or point prevalence (PP) measures which were either self-reported or biochemically verified by cotinine levels in saliva, urine, or carbon monoxide exhalation. If available, 7-day PP outcomes were reported.
One author performed the searches and abstracted data from included studies into a spreadsheet; a second author checked abstracted data for accuracy. The study design was registered as a systematic review in PROSPERO. All data collection and analyses were performed in 2017.
Calculation of Efficacy Estimates
For each study, an OR representing the odds of quitting for participants in the treatment group relative to the control group with a 95% CI was calculated using the reported number of quits and the reported sample size in both treatment and control groups. In this case, an OR >1 indicated that participants in the intervention group were more likely than participants in the control group to achieve quitting. Summary, or pooled effects across studies in each treatment type were calculated as the weighted mean of individual ORs.23 To minimize risk of bias, the summary measures were calculated using only studies with biochemically validated outcomes; funnel plots and statistical tests for small-study bias were estimated.
The measure I2 was used to assess whether heterogeneity of effect sizes was due to more than would be expected from sampling error alone.24 Moderator analysis to estimate the effect of study design factors on the estimated effect size was conducted and reported in the footnote of Table 2 and Appendix Table 3. Potential moderators included variations in study design features such as: sampling individuals willing to reduce smoking, months of treatment, duration and number of treatment sessions, inclusion of secondary treatments given to both treatment and control subjects, usual care versus no treatment in the control group, and inclusion of clinic visits as part of the study protocol.
Table 2.
Summary Effectiveness of Cessation Interventions for SNRTQ
| Intervention | Treatment | Control | Weight | OR | NNT | Cost per smoker | Cost per quit | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Quits | Total | Quits | Total | % | M-H fixed (95% CI) | ||||
| Pharmacological interventions | |||||||||
| Bollinger et al. 2000 | 21 | 200 | 17 | 200 | 14.20 | 1.26 (0.65, 2.47) | 50 | $5,166.43 | $258,321.69 |
| Wennike et al. 2003 | 19 | 205 | 7 | 206 | 5.90 | 2.90 (1.19, 7.07) | 17 | $1,046.16 | $17,784.72 |
| Hatsukami et al. 2004 | 20 | 295 | 16 | 299 | 13.80 | 1.29 (0.65, 2.53) | 70 | $1,436.62 | $100,563.46 |
| Batra et al. 2005 | 22 | 184 | 8 | 180 | 6.60 | 2.92 (1.26, 6.74) | 13 | $1,046.16 | $13,600.08 |
| Rennard et al. 2006 | 17 | 215 | 3 | 214 | 2.60 | 6.04 (1.74, 20.92) | 15 | $3,444.29 | $51,664.34 |
| Ebbert et al. 2015 | 205 | 760 | 74 | 750 | 50.60 | 3.37 (2.53, 4.50) | 6 | $1,650.05 | $9,900.32 |
| Hughes et al. 2011 | 15 | 107 | 8 | 111 | 6.30 | 2.10 (0.85, 5.18) | 15 | $550.02 | $8,250.26 |
| Etter et al. 2004a | 31 | 265 | 25 | 269 | 0.00 | 1.29 (0.74, 2.26) | 42 | $833.29 | $34,998.10 |
| Carpenter et al. 2011a | 68 | 426 | 60 | 423 | 0.00 | 1.15 (0.79, 1.67) | 56 | $64.56 | $3,615.36 |
| Jardin et al. 2014a | 8 | 53 | 3 | 51 | 0.00 | 2.84 (0.71, 11.40) | 11 | $85.78 | $943.58 |
| Pooled NRT inhalerb | 38 | 415 | 20 | 414 | – | 2.00 (1.14, 3.51) | 45 | $4,901.22 | $218,643.58 |
| Pooled NRT gumb | 41 | 389 | 15 | 386 | – | 2.91 (1.58, 5.36) | 15 | $1,046.16 | $15,571.05 |
| Pooled Vareniclineb | 220 | 867 | 82 | 861 | – | 3.23 (2.46, 4.25) | 7 | $1,529.05 | $10,688.05 |
| Pooled pharmacological | 319 | 1,966 | 133 | 1,960 | 100.00 | 2.72 (2.19, 3.37) | 10 | $2,021.79 | $19,510.24 |
| Heterogeneity: χ2 =13.78, df=6 (p=0.03); I2 =56% | |||||||||
| Test for overall effect: z=9.10 (p<0.00001) | |||||||||
| Behavioral interventions | |||||||||
| Glasgow et al. 2009 | 11 | 164 | 7 | 156 | 71.10 | 1.53 (0.58, 4.05) | 45 | $268.48 | $12,081.40 |
| Davis et al. 2011 | 0 | 109 | 1 | 109 | 15.90 | 0.33 (0.01, 8.20) | – | $86.98 | – |
| Catley et al. 2016c | 11 | 204 | 0 | 51 | 8.00 | 6.12 (0.35, 105.62) | 19 | $275.13 | $5,227.51 |
| Huang et al. 2016 | 2 | 72 | 0 | 76 | 5.00 | 5.43 [0.26, 114.97] | 36 | $275.13 | $9,904.75 |
| Danan et al. 2016a | 63 | 948 | 49 | 981 | 0.00 | 1.35 (0.92, 1.99) | 61 | $101.00 | $6,160.75 |
| Klemperer et al. 2016a,c | 35 | 371 | 10 | 189 | 0.00 | 1.86 (0.90, 3.85) | 24 | $254.46 | $6,107.03 |
| Pooled behavioral | 24 | 549 | 8 | 392 | 100.00 | 1.90 (0.86, 4.23) | 47 | $240.48 | $11,415.74 |
| Heterogeneity: χ2 =2.43, df=3 (p=0.49); I2 =0% | |||||||||
| Test for overall effect: z=1.58 (p<0.11) | |||||||||
| Combination interventions | |||||||||
| Carpenter et al. 2003 | 4 | 32 | 3 | 35 | 7.20 | 1.52 (0.31, 7.40) | 25 | $1,108.42 | $27,710.51 |
| Carpenter et al. 2004c | 83 | 409 | 9 | 207 | 27.20 | 5.60 (2.75, 11.39) | 6 | $462.78 | $2,776.69 |
| Chan et al. 2011c | 74 | 928 | 10 | 226 | 42.30 | 1.87 (0.95, 3.68) | 28 | $552.89 | $15,481.05 |
| Joseph et al. 2008 | 9 | 78 | 9 | 74 | 23.30 | 0.94 (0.35, 2.52) | – | $2,098.83 | – |
| Pooled combination | 170 | 1,447 | 31 | 542 | 100.00 | 2.64 (1.76, 3.97) | 16 | $928.59 | $14,662.36 |
| Heterogeneity: χ2 =9.99, df=3 (p=0.02); I2 =70% | |||||||||
| Test for overall effect: z=4.68 (p<0.00001) | |||||||||
Indicates study used self-reported cessation outcomes. Pooled calculations include only studies where cessation outcomes are bio-chemicaly verified through saliva, urine, or exhaled cotinine levels.
Pooled NRT inhaler values come from Bolliger et al. 2000 and Rennard et al. 2006. Pooled NRT gum values come from Wennike et al. 2003 and Batra et al. 2005. Pooled varenicline values come from Ebbert et al. 2015 and Hughes et al. 2011.
Indicates studies with multiple treatment arms which have been pooled to estimate the overall effect of treatments versus control. Potential moderators to the effect size were identified using weighted least squares regression procedures; moderators included sample type, treatment type, secondary treatment availability, control group type. Only control group type was found to be a statistically significant moderator of effect size, at the p<0.05 level. Additional intervention details and calculations are described in Appendix Table 3. All cost calculations are taken from Table 1 and detailed cost assumptions are described in Appendix Table 5.
SNRTQ, smokers not ready to quit; NNT, number needed to treat; M-H, Mantel-Haenszel method to estimate pooled ORs, assuming a fixed effects model; NRT, nicotine replacement therapy
For each study, the number of observations from the full sample of smokers and number of quits in both treatment and control interventions were used to calculate the NNT.25 Summary NNT for each of the three intervention types was calculated, using only studies with biochemically validated outcomes. Efficiency estimates assumed that smokers lost to follow-up have resumed smoking and did not quit.
Calculation of Cost Estimates
Cost effectiveness was measured by cost per smoker and cost per quit. To make these calculations, costs were compiled from various sources for each of the types of pharmacological and behavioral interventions. Table 1 shows the estimated cost for a 1-month supply of pharmacological therapies and an average cost for each type of behavioral intervention. Assumptions used to determine costs are described in Table 1. These costs are used in Table 2 to estimate cost per smoker and cost per quit of each study. Appendix Table 5 shows additional details of cost calculations for behavioral treatments used to estimate average costs used in Table 1.
Table 1.
Summary of Costs of Smoking Cessation Interventions for General Smokers
| Interventions | Total cost/smoker (2016 $) | Cost assumptions |
|---|---|---|
| Pharmacological interventions | ||
| Varenicline (Chantix) | $275.01 | Starter pack (53 tablets) and continuing pack (56 tablets) provides 1 month supply, with recommended dosage at 0.5 mg once daily for 3 days, then 0.5 mg twice daily for 4 days, then 1 mg twice daily. |
| Bupropion (Zyban) | $221.02 | Each pack of 60 tablets provides 1 month supply, with recommended dosage at 150 mg once daily for 6 days, then 150 mg twice daily. |
| NRT lozenge (Nicorette) | $129.12 | Each 72 pack of lozenges will provide a 1 week supply if approximately 10 lozenges per day is assumed. Dosage in first 6 weeks is one lozenge every 1 to 2 hours, with a recommendation of at least nine lozenges per day. The estimate is 4 packs are needed per month. |
| NRT gum (Nicorette) | $87.18 | Three 100-count packs are needed per month if 10 pieces per day is assumed. Recommended dosage is 2 mg, up to 30 times per day (for smokers <25 cigarettes) or 4 mg, up to 20 times/day (for smokers ≥25 cigarettes). |
| NRT patch (Nicoderm CQ) | $42.44 | Each pack (Steps 1, 2, or 3) provides a 2-week supply containing 14 patches; the estimate is two packs are needed per month. |
| NRT inhaler (Nicotrol) | $287.02 | One inhaler (containing 168 cartridges) needed per month, if a mean of 6 cartridges per day is assumed. |
| Average cost of NRT | $138.88 | Average cost of NRT, excluding lozenges. |
| Behavioral interventions | ||
| Counselling or MI (In-person, about 4 sessions) | $275.13 | Average is calculated from costs reported for five 1-hour group sessions and four 30-minute individual sessions. |
| Counselling or MI (Telephone, 1 session) | $86.98 | Average is calculated from costs reported for one telephone session, duration 15–30 minutes. |
| Counselling or MI (Telephone, about 4 sessions) | $254.46 | Average is calculated from costs reported for two to six (average of four) 30-minute telephone sessions. |
| Mailings and followup calls only | $14.02 | Average is calculated 2 studies providing costs of mailing letters and other print materials. |
Notes: All cost estimates have been standardized to 2016 U.S. dollars. Pharmaceutical costs are calculated for a 1-month supply of each drug, using the assumptions shown and 121% of the Federal Supply Schedule costs in 2016. Costs of behavioral interventions are averaged by intervention type. Costs for in-person sessions from Barnett et al. 2013. Costs for telephone sessions from Hollis et al. 2007, McAlister et al. 2004, Richter et al. 2015 and Glasgow et al. 2009. Costs for Printed materials from McAlister et al. 2004 and Ruger et al. 2009.
NRT, nicotine replacement therapy; MI, motivational interviewing
To calculate the cost per smoker in pharmacological interventions in Table 2, the cost for a 1-month supply of the selected drug from Table 1 was multiplied by the number of months of treatment, which was noted in Appendix Tables 1 and 2. Drug costs were calculated using 121% of prices listed under the Federal Supply Schedule in 2016; this is in line with current cost-effectiveness guidelines on estimating drug costs.26,27 Although U.S. Preventive Services Task Force guidelines recommend 90 days for NRT treatments, in many cases, pharmacological therapies were offered to enrollees for an extended period of time, with no requirements for tapering usage (e.g., 12 to18 months); as a result, cost estimates assumed enrollees used therapies as long as they were offered. This provides a conservative estimate of cost effectiveness; typically, costs would be lower if individuals stopped treatment early or gradually tapered during the treatment period. In some studies, treatment options varied either by amount of time treatments were offered or by amount of treatment available, or both. To account for variations, Appendix Table 4 presents a sensitivity analysis showing estimates of minimum and maximum cost per smoker and minimum and maximum cost per quit. These estimates were based on American Cancer Society guidelines for recommended usage and on reported actual use in each study.28 For example, in a study that provided nicotine gum for a up to 12 months, the minimum use estimate assumed six and a half pieces per day, which was the mean reported use in the study, for 12 months.17 The maximum use estimate assumed ten pieces per day for 12 months the treatment was offered. Similarly, in studies where treatment groups were offered a choice of NRT therapy, minimum cost was calculated based on minimum use of the lowest-cost type of therapy; and maximum cost was based on maximum use of the highest-cost type of therapy. In studies of non-NRT pharmacological therapies, such as buproprion or varenicline, dosage is standardized, so minimum and maximum cost assumptions were equivalent unless variation in treatment time was reported. Additional details about variations in treatment protocols, actual use, and assumptions about cost estimates were described in detail in Appendix Table 4.
To calculate cost per smoker in behavioral interventions, the estimated mean cost by intervention type was taken from Table 1. These costs were calculated based on a selection of studies with reported treatment costs and are described in Appendix Table 5. These studies focused on a more typical smoking population, and did not target SNRTQ, with the exception of one study by Glasgow and colleagues19 Costs of in-person counseling or MI sessions came from a cost-effectiveness study of cessation treatment in older smokers.29 Costs of telephone counseling or MI were averaged across four different cost-effectiveness studies of telephone counseling for smoking cessation.19,30–32 Costs for printed materials and mailings were taken from two studies that report costs of printed and mailed materials for smoking cessation.31,33 All costs were adjusted to 2016 U.S. dollars.
In cases where a study includes secondary therapies given to both treatment and control group alike, costs were only calculated for the therapies that were given to the treatment group alone. This allows for identification of the marginal effect of the treatment on the treated group.
In Table 2, cost per quit for each of the interventions was calculated by multiplying the estimated cost per smoker by the estimated NNT.
Fixed effects meta-analysis was conducted for studies using biochemically verified cessation outcomes. All analyses were conducted using Stata, version 14.2 and were cross-checked against results from the same analyses using RevMan, version 5.3 software.35,36
EVIDENCE SYNTHESIS
Summary of Recent Studies
The ten studies originally identified in the review by Asfar et al. have been summarized in Appendix Table 1. In Appendix Table 2, ten of the 12 new studies on smoking cessation interventions targeting SNRTQ were summarized. Two studies were not included and are described in detail below. Studies were categorized by intervention type: pharmacological, behavioral, or a combination of both. To remain consistent with published recommendations, the primary outcome of interest was the 7-day PP of smoking abstinence at 6 months. Where this was unavailable, the longest follow-up period reported was used.34 Cases where studies used other measures of smoking abstinence, such as continuous abstinence or 1-day PP, or where outcomes were not biochemically validated were noted.
One study, not included in Appendix Table 2, by Cook and colleagues,37 had a 2 × 2 × 2 × 2 factorial design, which resulted in 16 separate experimental conditions and were not easily comparable with results of other studies summarized in Table 2. The authors reported no significant main effects but found a significant four-way interaction.37 The other excluded study, Meyer et al.,46 had a cessation arm and a reduction arm, where participants received tailored letters and self-help guides focusing on cessation or reduction, respectively. The reported treatment and control effects and ORs were not replicable using the information provided and therefore have not been included in the summary.
Four studies of pharmacological interventions for smoking cessation against a placebo were identified.11,38,39 Two of these studies, by Ebbert and colleagues11 and Hughes et al.,39 examined use of varenicline, over a 6-month and 3-month period respectively, for increasing smoking abstinence rates through smoking reduction among individuals with no intention to quit smoking. The study by Jardin and colleagues38 examined the effect of providing access to free 2-week NRT samples on self-reported quit rates among smokers with varying degrees of motivation. Another study by Carpenter et al.40 examined the effect of providing NRT lozenges within the context of a practice quit attempt among a group of smokers not motivated to quit within 30 days.
Five studies that examined the effect of behavioral interventions for smoking cessation against usual care were identified.41–46 Of these, two had three-arm designs; in order to provide comparisons between treatment and control, the separate treatment arms were pooled together.47 Research performed by Catley and colleagues42 had a MI arm and a health education arm, both consisted of four 20-minute individual counseling sessions (two in-person and two over the phone); the control group received brief advice. Individuals in either group who set a quit date were offered a choice of varenicline, or NRT lozenges or patches. Whereas the study by Klemperer et al.44 had a motivational intervention and a reduction intervention, both of which consisted of three 10- to 15-minute phone calls. The control group received a single 5-minute phone call with brief advice to quit; no additional treatment components were offered. The remaining three behavioral studies had single-arm designs. The study by Davis and colleagues41 provided one 15-minute MI; the control group received 15 minutes of prescriptive counseling. Research by Huang and colleagues45 examined the effectiveness of four 20-minute sessions of individual, tailored MI among a population who also reported having a family member willing to participate as a supporter. Treatment and control groups both received a self-help manual and a 25-minute group counseling session. Alternatively, a study by Danan and colleagues43 provided proactive mailings and telephone outreach as well as an offer of either in-person or telephone counseling as treatment for SNRTQ who were eligible for Veterans’ Affairs smoking cessation services. Both treatment and control groups had access to the Veterans’ Administration and state quitline smoking cessation services.
One new study which examined combining behavioral and pharmacological interventions for smoking cessation was identified.48 The study by Chan and colleagues48 had a three-arm design and reported combined results of two treatment arms against the control. One treatment group received three sessions of in-person reduction counseling and three sessions of NRT adherence counseling, the other treatment group received only reduction counseling. Both groups received 2 months of NRT therapy. The control group received only 10 minutes of cessation advice.
Effect Sizes of Treatment
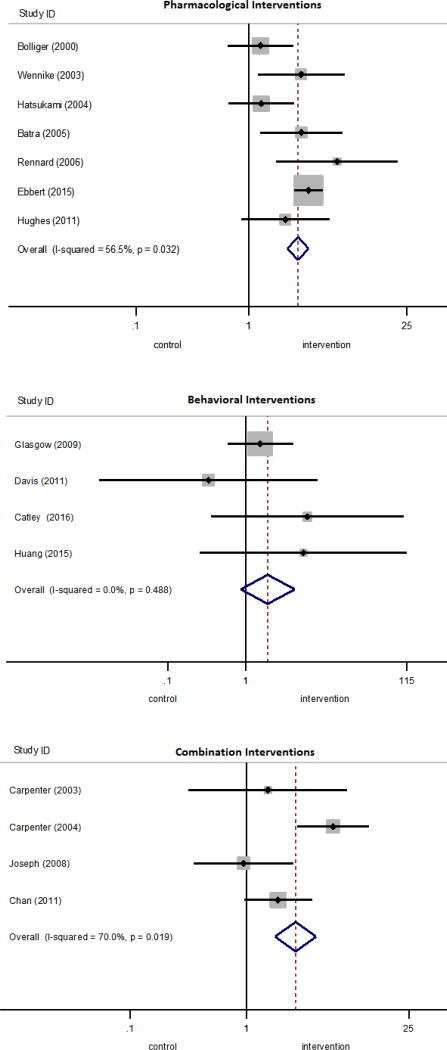
Estimates of the effect of smoking interventions on cessation among SNRTQ were pooled for 15 studies which used biochemically verified cessation outcomes in Table 2; forest plots are shown in Figure 2. Studies were pooled by intervention type: pharmacological, behavioral, and combination.
Figure 2.
Forest plots for pharmacologic, behavioral, and combination interventions.
The pooled OR for pharmacological interventions was 2.72 (95% CI=2.19, 3.37). Because there were multiple studies using varenicline, NRT inhalers, and NRT gum, pooled ORs for these pharmacological interventions are presented separately in Table 2 and Appendix Table 7. For behavioral interventions the OR was 1.90 (95% CI=0.86, 4.23), and for combination interventions the OR was 2.64 (95% CI=1.76, 3.97). These ORs were not statistically significantly different from each other. The measure I2 was used to assess whether effect sizes were more heterogeneous than would be expected due to sampling error alone.24 Pooled pharmacological studies and combination studies were found to have moderate heterogeneity (I2=56% and (I2=70%) whereas pooled behavioral studies had no heterogeneity. Providing no treatment to the control group (versus usual care) was found to be a statistically significant moderator of the effect size for combination studies, at the p<0.05 level.
Number Needed to Treat
Pooled NNT for studies with biochemically verified outcomes were presented in Table 2. For comparison, a summary of Cochrane review estimates of NNT for interventions targeting smokers ready to quit is presented in Appendix Table 6. The lowest estimated NNT for SNRTQ was six, for varenicline versus placebo interventions.11,39 This compares with an estimated pooled NNT of seven from Cochrane reviews for varenicline versus placebo interventions for smokers ready to quit.49 For interventions using NRT inhalers and gum, the pooled NNT were 37 and 15, respectively. This is similar to the range of NNT values of NRT interventions for smokers ready to quit found in a recent Cochrane review, which were between 11 and 42.50 One study using bupropion had an NNT of 70.16 The pooled NNT for all types of pharmacological interventions was ten.
For studies with behavioral interventions with biochemically verified outcomes, the estimated NNT ranged from 19 to 45.19,42 The pooled NNT for behavioral interventions was 47, which was somewhat higher than pooled NNT values for behavioral interventions for smokers ready to quit, which ranged from 19 to 38.19,41,42,51,52
The pooled NNT for combination interventions was 16.20–22,48 This was slightly higher than the pooled NNT for combination interventions for smokers ready to quit, which was 14.53
Cost Per Quit
Among pharmacological interventions, interventions using varenicline had the lowest cost per quit, at $9,822.58.11,39 The pooled cost per quit for pharmacological interventions was $19,510.24.
For behavioral interventions, the pooled cost per quit was $11,415.74. These estimates were considerably higher than estimates of cost effectiveness for similar interventions targeting smokers ready to quit. The literature on smokers ready to quit estimates cost per quit for behavioral interventions ranging from $1,807.00 to $3,326.40, in 2016 U.S. dollars.29–33,54
For studies that examined interventions which were a combination of pharmacological and behavioral treatments, the pooled cost per quit was $14,662.36. This is higher than cost per quit estimates for combination interventions for smokers ready to quit from one study, which ranged from $2,654.82 to $3,108.42.30
DISCUSSION
This work represents the largest and most comprehensive systematic review to date that focuses on interventions for SNRTQ. This is an important population that is often excluded from smoking intervention studies. Twenty-two studies that included this population were identified. Comparing cost effectiveness of all types of treatments for SNRTQ, behavioral interventions were the most cost effective and pharmacological interventions, the least. However, pharmacological interventions were the most effective whereas behavioral interventions were the least effective. Further, the average cost of pharmacological interventions was driven up by high costs of NRT inhaler and bupropion interventions. Among pharmacological interventions, varenicline was the most cost effective and was slightly more cost effective than the pooled behavioral intervention estimate.
The effectiveness of interventions targeting SNRTQ, measured by NNT, was similar to the effectiveness of interventions targeting smokers ready to quit. For pharmacological interventions, NRT and varenicline have similar effectiveness in both groups of smokers, although bupropion seems to be much less effective in the SNRTQ group. Behavioral and combination interventions were slightly less effective among SNRTQ than for smokers ready to quit.
In terms of cost effectiveness, comparisons of pharmacological and combination interventions for SNRTQ against the same types of interventions for smokers ready to quit could not be made because of unavailability of cost estimates of studies on smokers ready to quit. Behavioral interventions for SNRTQ were found to be less cost effective than similar behavioral interventions for smokers ready to quit. Part of this may be because of differences in intensity of treatment. For example, many studies offered counseling up to a certain number of minutes and sessions—it is possible that SNRTQ are using more of the available counseling than smokers ready to quit, resulting in increased cost and therefore decreased cost effectiveness of these studies.22,29,31,43 Previous work established that participant identification and recruitment was particularly intensive for SNRTQ, which also may contribute to their higher cost.19 Future work should explore differences in treatment intensity for these two groups and ways to more efficiently tailor recruitment and interventions for SNRTQ.
Much of this recent research shows that regardless of smokers’ readiness to quit, pharmacological, behavioral, and combination interventions can increase motivation and intention to quit. This suggests clinicians can offer or provide support and treatment rather than waiting for smokers to seek cessation treatment.
Limitations
The primary limitation of this study is the small number of studies that meet the selection criteria. Meta-analyses of interventions for the general population of smokers are able to include a large number of studies. This is addressed by following the example of Asfar et al. to pool all pharmacological interventions, all behavioral interventions, and all combination interventions together. This makes it somewhat difficult to disentangle effects of variations in treatment type, such as to compare MI with reduction counseling. Where multiple studies for a particular type of treatment, such as NRT gum, NRT inhaler, and varenicline, were available, pooled comparisons are presented in Table 2. Nevertheless, additional studies targeting SNRTQ may provide a better picture of how this group responds to variations in treatment. To assess small-study effects such as publication bias, the authors present funnel plots in Appendix Figure 1. Results from Egger’s test and Begg and Mazumdar’s rank correlation test suggested little evidence of small-study effects; coefficients were noted in the footnote of Appendix Figure 1.55
Among the studies identified, there were variations in intervention type, treatment intensity, sample selection criteria, follow-up times, outcome measures, and definitions of not ready to quit. To address this issue, a heterogeneity test and moderator analysis were conducted and variation in protocols for control groups were found to have statistically significant effect on the estimated effect size. However, there were not enough studies within each type of pharmacological intervention to perform a moderator analysis within each type. For example, two studies that provided 1 mg of varenicline twice daily, for 2 months and 6 months respectively, reported very different results.11,39 In this case, it was not possible to ascertain whether the larger OR of the second study was due to the increased length of treatment time in that study, to possible differences in study populations, to differences in the methods used to measure the outcome, or whether it was due to other treatment factors, such as the fact that the first study provided brief counseling sessions to treatment and control group alike whereas the second study did not provide any sort of counseling.
To address the issue of variations affecting cost estimates, information on the assumptions that were made for developing these estimates are shown in Appendix Table 5 and minimum and maximum cost ranges for each study are shown in Appendix Table 4.
An additional limitation of this work is that among studies identified, no studies incorporated newer technology-based interventions for SNRTQ, such as e-health, Skype-based counseling, or other app-based interventions. Because this group is typically younger, it is possible they may be more receptive to app-based interventions.6
CONCLUSIONS
Smoking cessation interventions and reduce to quit interventions targeting SNRTQ were found to be comparable in effectiveness to similar interventions for smokers who are ready to set a quit date. Existing clinical guidelines should be updated to encourage healthcare providers to offer smoking reduction and cessation assistance to this potentially large and under-targeted group of smokers.
Because these interventions appeared to be more expensive than similar interventions targeting smokers ready to quit, further research should seek to understand differences in treatment intensity, which may vary between these different groups of smokers. As suggested in previous work, standardization of outcome measures, follow-up times, treatment intensity, intervention costs, and definitions of not ready to quit using the stages of change definitions are all recommended for future clinical trials.10 Currently, few studies identify effective recruitment strategies for SNRTQ; further research on effectiveness of recruitment for individuals not ready to quit is also needed.19,56
Supplementary Material
Acknowledgments
This study was funded by a grant from the National Cancer Institute, Strategies to Promote Cessation in Smokers Who Are Not Ready To Quit (1R01CA193245-01A1), Klesges (PI), 2016–2021.
This study was registered as a PROSPERO systematic review (Registration: CRD42017060372). AA performed the original search, summarized the articles, performed the meta-analysis, and wrote the first draft. CMK reviewed articles and assisted with the meta-analysis. KD also assisted with the meta-analysis. All authors contributed to the writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
References
- 1.U.S. DHHS. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: DHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. [Google Scholar]
- 2.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting smoking among adults — United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. doi: 10.15585/mmwr.mm6552a1. [DOI] [PubMed] [Google Scholar]
- 3.Velicer WF, Fava JL, Prochaska JO, Abrams DB, Emmons KM, Pierce JP. Distribution of smokers by stage in three representative samples. Prev Med. 1995;24(4):401–411. doi: 10.1006/pmed.1995.1065. [DOI] [PubMed] [Google Scholar]
- 4.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update: Clinical Practice Guideline. DIANE Publishing; 2008. https://bphc.hrsa.gov/buckets/treatingtobacco.pdf. Accessed March 23, 2018. [Google Scholar]
- 5.Tobacco Use and Dependence Guideline Panel. Treating Tobacco Use and Dependence: 2008 Update. U.S. DHHS; 2008. https://www.ncbi.nlm.nih.gov/books/NBK63952/. Accessed March 23, 2018. [Google Scholar]
- 6.Burris JL, Wahlquist AE, Carpenter MJ. Characteristics of cigarette smokers who want to quit now versus quit later. Addict Behav. 2013;38(6):2257–2260. doi: 10.1016/j.addbeh.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fjalldal SB, Janson C, Benediktsdóttir B, et al. Smoking, stages of change and decisional balance in Iceland and Sweden. Clin Respir J. 2011;5(2):76–83. doi: 10.1111/j.1752-699X.2010.00201.x. [DOI] [PubMed] [Google Scholar]
- 8.Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current population survey results. Prev Med. 2003;36(6):710–720. doi: 10.1016/S0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 9.Lim KH, Ibrahim N, Ghazali SM, et al. Stages of smoking cessation among Malaysian adults – findings from National Health Morbidity Survey 2006. Asian Pac J Cancer Prev. 2013;14(2):805–810. doi: 10.7314/APJCP.2013.14.2.805. [DOI] [PubMed] [Google Scholar]
- 10.Asfar T, Ebbert JO, Klesges RC, Relyea GE. Do smoking reduction interventions promote cessation in smokers not ready to quit? Addict Behav. 2011;36(7):764–768. doi: 10.1016/j.addbeh.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ebbert JO, Hughes JR, West RJ, et al. Effect of Varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687–694. doi: 10.1001/jama.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigotti NA. Strategies to help a smoker who is struggling to quit. JAMA. 2012;308(15):1573–1580. doi: 10.1001/jama.2012.13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolliger CT, Zellweger JP, Danielsson T, et al. Smoking reduction with oral nicotine inhalers: double blind, randomised clinical trial of efficacy and safety. BMJ. 2000;321(7257):329–333. doi: 10.1136/bmj.321.7257.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wennike P, Danielsson T, Landfeldt B, Westin Å, Tønnesen P. Smoking reduction promotes smoking cessation: results from a double blind, randomized, placebo-controlled trial of nicotine gum with 2-year follow-up. Addiction. 2003;98(10):1395–1402. doi: 10.1046/j.1360-0443.2003.00489.x. [DOI] [PubMed] [Google Scholar]
- 15.Etter JF, Laszlo E, Perneger TV. Postintervention effect of nicotine replacement therapy on smoking reduction in smokers who are unwilling to quit: randomized trial. J Clin Psychopharmacol. 2004;24(2):174–179. doi: 10.1097/01.jcp.0000115666.45074.d6. [DOI] [PubMed] [Google Scholar]
- 16.Hatsukami DK, Rennard S, Patel MK, et al. Effects of sustained-release bupropion among persons interested in reducing but not quitting smoking. Am J Med. 2004;116(3):151–157. doi: 10.1016/j.amjmed.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Batra A, Klingler K, Landfeldt B, Friederich Hm, Westin A, Danielsson T. Smoking reduction treatment with 4-mg nicotine gum: a double-blind, randomized, placebo-controlled study. Clin Pharmacol Ther. 2005;78(6):689–696. doi: 10.1016/j.clpt.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Rennard SI, Glover ED, Leischow S, et al. Efficacy of the nicotine inhaler in smoking reduction: a double-blind, randomized trial. Nicotine Tob Res. 2006;8(4):555–564. doi: 10.1080/14622200600789916. [DOI] [PubMed] [Google Scholar]
- 19.Glasgow RE, Gaglio B, Estabrooks PA, et al. Long-term results of a smoking reduction program. Med Care. 2009;47(1):115–120. doi: 10.1097/MLR.0b013e31817e18d1. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter MJ, Hughes JR, Keely JP. Effect of smoking reduction on later cessation: A pilot experimental study. Nicotine Tob Res. 2003;5(2):155–162. doi: 10.1080/146222003100007385. [DOI] [PubMed] [Google Scholar]
- 21.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72(3):371–381. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 22.Joseph AM, Hecht SS, Murphy SE, et al. Smoking reduction fails to improve clinical and biological markers of cardiac disease: a randomized controlled trial. Nicotine Tob Res. 2008;10(3):471–481. doi: 10.1080/14622200801901948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M, Davey-Smith G, Altman D. Systematic Reviews in Health Care: Meta-Analysis in Context. John Wiley & Sons; 2008. [Google Scholar]
- 24.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454. doi: 10.1136/bmj.310.6977.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders Gillian D, Neumann Peter J, Basu Anirban. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 27.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, editors. Cost-Effectiveness in Health and Medicine. Second. New York, NY: Oxford University Press; 2016. https://global.oup.com/academic/product/cost-effectiveness-in-health-and-medicine-9780190492939?cc=us&lang=en&. Accessed December 11, 2017. [Google Scholar]
- 28.American Cancer Society. Nicotine Replacement Therapy for Quitting Tobacco. www.cancer.org/healthy/stay-away-from-tobacco/guide-quitting-smoking/nicotine-replacement-therapy.html. Published 2017. Accessed December 14, 2017.
- 29.Barnett PG, Wong W, Jeffers A, Munoz R, Humfleet G, Hall S. Cost-effectiveness of extended cessation treatment for older smokers. Addiction. 2014;109(2):314–322. doi: 10.1111/add.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16(suppl 1):i53–i59. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAlister A, Rabius V, Geiger A, Glynn T, Huang P, Todd R. Telephone assistance for smoking cessation: one year cost effectiveness estimations. Tob Control. 2004;13(1):85–86. doi: 10.1136/tc.2003.004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113. doi: 10.2196/jmir.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruger JP, Weinstein MC, Hammond SK, Kearney MH, Emmons KM. Cost-effectiveness of motivational interviewing for smoking cessation and relapse prevention among low-income pregnant women: a randomized controlled trial. Value Health. 2008;11(2):191–198. doi: 10.1111/j.1524-4733.2007.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce JP, Gilpin EA. A minimum 6-month prolonged abstinence should be required for evaluating smoking cessation trials. Nicotine Tob Res. 2003;5(2):151–153. doi: 10.1080/0955300031000083427. [DOI] [PubMed] [Google Scholar]
- 35.Sterne JAC, Bradburn MJ, Egger M. Meta-analysis in StataTM. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care. BMJ Publishing Group; 2001. pp. 347–369. [DOI] [Google Scholar]
- 36.Harbord RM, Harris RJ, Sterne JAC. Updated tests for small-study effects in meta-analyses. Stata J. 2009;9(2):197–210. [Google Scholar]
- 37.Cook JW, Collins LM, Fiore MC, et al. Comparative effectiveness of motivation phase intervention components for use with smokers unwilling to quit: a factorial screening experiment. Addiction. 2016;111(1):117–128. doi: 10.1111/add.13161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jardin BF, Cropsey KL, Wahlquist AE, et al. Evaluating the effect of access to free medication to quit smoking: a clinical trial testing the role of motivation. Nicotine Tob Res. 2014;16(7):992–999. doi: 10.1093/ntr/ntu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes JR, Rennard SI, Fingar JR, Talbot SK, Callas PW, Fagerstrom KO. Efficacy of Varenicline to prompt quit attempts in smokers not currently trying to quit: a randomized placebo-controlled trial. Nicotine Tob Res. 2011;13(10):955–964. doi: 10.1093/ntr/ntr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Arch Intern Med. 2011;171(21):1901–1907. doi: 10.1001/archinternmed.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis MF, Shapiro D, Windsor R, et al. Motivational interviewing versus prescriptive advice for smokers who are not ready to quit. Patient Educ Couns. 2011;83(1):129–133. doi: 10.1016/j.pec.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 42.Catley D, Goggin K, Harris KJ, et al. A randomized trial of motivational interviewing: cessation induction among smokers with low desire to quit. Am J Prev Med. 2016;50(5):573–583. doi: 10.1016/j.amepre.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danan ER, Joseph AM, Sherman SE, et al. Does motivation matter? Analysis of a randomized trial of proactive outreach to VA smokers. J Gen Intern Med. 2016;31(8):878–887. doi: 10.1007/s11606-016-3687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klemperer EM, Hughes JR, Solomon LJ, Callas PW, Fingar JR. Motivational, reduction and usual care interventions for smokers who are not ready to quit: a randomized controlled trial. Addiction. 2017;112(1):146–155. doi: 10.1111/add.13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang FF, Jiao NN, Zhang LY, Lei Y, Zhang JP. Effects of a family-assisted smoking cessation intervention based on motivational interviewing among low-motivated smokers in China. Patient Educ Couns. 2015;98(8):984–990. doi: 10.1016/j.pec.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 46.Meyer C, Ulbricht S, Haug S, et al. Motivating smokers to quit using computer-generated letters that target either reduction or cessation: A population-based randomized controlled trial among smokers who do not intend to quit. Drug Alcohol Depend. 2016;166(suppl C):177–186. doi: 10.1016/j.drugalcdep.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Scammacca N, Roberts G, Stuebing KK. Meta-analysis with complex research designs: dealing with dependence from multiple measures and multiple group comparisons. Rev Educ Res. 2014;84(3):328–364. doi: 10.3102/0034654313500826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan SSC, Leung DYP, Abdullah ASM, Wong VT, Hedley AJ, Lam T-H. A randomized controlled trial of a smoking reduction plus nicotine replacement therapy intervention for smokers not willing to quit smoking. Addiction. 2011;106(6):1155–1163. doi: 10.1111/j.1360-0443.2011.03363.x. [DOI] [PubMed] [Google Scholar]
- 49.Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016;5:CD006103. doi: 10.1002/14651858.CD006103.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 51.Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev. 2005;2:CD001007. doi: 10.1002/14651858.CD001007.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2013;8:CD002850. doi: 10.1002/14651858.CD002850.pub3. [DOI] [PubMed] [Google Scholar]
- 53.Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev. 2016;3:CD008286. doi: 10.1002/14651858.CD008286.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cummings KM, Fix B, Celestino P, Carlin-Menter S, O’Connor R, Hyland A. Reach, efficacy, and cost-effectiveness of free nicotine medication giveaway programs. J Public Health Manag Pract. 2006;12(1):37–43. doi: 10.1097/00124784-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 55.Sterne JA, Egger M, Moher D. Chapter 10: Addressing Reporting Biases. In: Higgins P, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons, Ltd; 2008. [DOI] [Google Scholar]
- 56.Harris KJ, Bradley-Ewing A, Goggin K, et al. Recruiting unmotivated smokers into a smoking induction trial. Health Educ Res. 2016;31(3):363–374. doi: 10.1093/her/cyw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.