Abstract
The timing of the circadian clock, circadian period and chronotype varies among individuals. To date, not much is known about how these parameters vary over time in an individual. We performed an analysis of the following five common circadian clock and chronotype measures: 1) the dim light melatonin onset (DLMO, a measure of circadian phase), 2) phase angle of entrainment (the phase the circadian clock assumes within the 24-h day, measured here as the interval between DLMO and bedtime/dark onset), 3) free-running circadian period (tau) from an ultradian forced desynchrony protocol; tau influences circadian phase and phase angle of entrainment, 4) mid-sleep on work-free days (MSF from the Munich ChronoType Questionnaire; MCTQ), and 5) the score from the Morningness-Eveningness Questionnaire (MEQ). The first three are objective physiological measures, and the last two are measures of chronotype obtained from questionnaires. These data were collected from 18 individuals (10 men, 8 women, ages 21 to 44) who participated in two studies with identical protocols for the first 10 days. We show how much these circadian rhythm and chronotype measures changed from the first to the second study. The time between the two studies ranged from 9 months to almost 3 years, depending on the individual. Since the full experiment required living in the laboratory for 14 days, participants were either unemployed, had part-time jobs or were free-lance workers with flexible hours. Thus, they did not have many constraints on their sleep schedules before the studies. The DLMO was measured on the first night in the lab, after free-sleeping at home, and also after sleeping in the lab on fixed 8-h sleep schedules (loosely tailored to their sleep times before entering the laboratory) for four nights. Graphs with lines of unity (when the value from the first study is identical to the value from the second study) showed how much each variable changed from the first to the second study. The DLMO did not change more than 2 h from the first to the second study, except for two participants whose sleep schedules changed the most between studies, a change in sleep times of 3 h. Phase angle did not change more than 2 h regardless of changes in the sleep schedule. Circadian period did not change more than 0.2 h, except for one participant. MSF did not change more than 1 h, except for 2 participants. MEQ did not change more than 10 points and the categories (e.g. M-type) did not change. Pearson’s correlations for the DLMO between the first and second study increased after participants slept in the lab on their individually timed fixed 8-h sleep schedules for four nights. A longer time between the two studies did not increase the difference between any of the variables from the first to the second study. This analysis shows that the circadian clock and chronotype measures were fairly reproducible, even after many months between the two studies.
Introduction
One of the most remarkable qualities of living organisms is the ability to time biological processes crucial to survival with respect to predictable environmental signals. The evolutionary ground for this biological quality in most organisms including humans is a circadian clock that is able to generate endogenous circadian rhythms, which, in turn, are entrainable to light/dark (LD) cycles (Pittendrigh & Daan, 1976; Roenneberg et al., 2013; Wever et al., 1983). Circadian misalignment (sleeping, working, eating, etc. at the wrong phases of the internal circadian clock) results in poor sleep and health and performance decrements (Akerstedt et al., 1984; Archer & Oster, 2015; Gold et al., 1992; Kantermann et al., 2012; Lunn et al., 2017; McHill et al., 2017; Smith & Eastman, 2012). Hence, maintaining the proper alignment between circadian rhythms and the sleep schedule is essential for health and safety.
During most of human history the natural alternation of day and night guaranteed predictable LD cycles. Today, light is provided by many mostly uncontrollable sources at all times of a day obscuring the natural alternation of day (light) and night (darkness) (Kantermann & Roenneberg, 2009; Kantermann, 2013; Kyba & Kantermann, 2016). Lifestyle choices and behaviors that use light during the time of natural darkness carry the risk of changing the phase of the circadian clock. In addition, light allows for activities like work or food consumption at the times for natural sleep, which is circadian misalignment and conflicts with a healthy lifestyle (Roenneberg et al., 2012; Wittmann et al., 2006). One prominent example thereof is shift work, which is associated with negative sleep and health outcomes (Akerstedt et al., 1984; Folkard et al., 2005; Gold et al., 1992; Kantermann et al., 2013; Kantermann et al., 2014; Knutsson, 2003; Lunn et al., 2017; Smith & Eastman, 2012).
One potential solution to these problems is to proactively use the individuality of sleep and circadian timing, also known as chronotype (Lack et al., 2009; Roenneberg et al., 2007) or morningness-eveningness (Baehr et al., 2000; Mongrain et al., 2006). There is evidence that sleep time individuality moderates the impact of shift work on sleep. For example, individuals with a habitual late sleep behavior have been shown to be more suitable for night work compared to individuals with a habitual early sleep behavior, quantified by the difference in the amount of their social jetlag (Fischer et al., 2016; Kantermann et al., 2013; Vetter et al., 2015). Applying chronobiology to the practice can help reduce the sleep and health burden in many shift workers (Boivin & James, 2005; Eastman, 2016; Kantermann et al., 2012; Smith & Eastman, 2012).
Another example besides shift work is medicine. One branch of medicine in which an individual’s circadian phase is taken into account is using bright light and/or melatonin to treat patients who have various circadian rhythm sleep disorders (Auger et al., 2015; Emens & Eastman, 2017; Sack et al., 2007). Or, how about identifying an individual’s best time of day for a medical intervention or surgery? Imagine one could have surgeons work at their “best time” and most restored, with also the patient being under surgery when her/his body is most restored. It has successfully been shown that appropriately timed medication and chemotherapy enhance the effectiveness of a treatment both in experimental and clinical situations (Halberg et al., 1980; Hrushesky, 1990; Ortiz-Tudela et al., 2016; Truong et al., 2016). Of course, most hospital routines would need to be restructured to permit such flexibility. Furthermore, exceptions are emergency cases when urgent surgical help is needed. In addition, often there is a gap of several days or even weeks between the day of a medical diagnosis and the day of a treatment (e.g. the surgery). Similarly, health check-ups for shift workers can be weeks before the actual shift employment begins. Therefore, any assessment of a circadian clock parameter to be used for any individually timed action (e.g. light therapy, chemotherapy, surgery or shift work scheduling) requires reproducibility and stability of that same parameter. The reproducibility, ideally, is independent of the time that has passed by since the first assessment of that parameter.
To date, there is limited knowledge on the reproducibility of any chronotype and circadian clock measures, which, in turn, limits the applicability of these parameters. Hence, a better understanding of the variability of these parameters in healthy individuals is needed (Goel, 2016; Van Reen et al., 2013). This will also help to better understand phenomena like circadian misalignment and social jetlag, which have, for example, been associated with adverse lifestyle habits (Wittmann et al., 2006), cardiovascular problems in shift workers (Kantermann et al., 2013) and increased rates of obesity (Roenneberg et al., 2012).
To address these points, we present findings from a post-hoc analysis of data collected in two separate studies performed in the same individuals and in the same laboratory at Rush University Medical Center in Chicago. The purpose of this post-hoc analysis was to analyze the reproducibility of the following most commonly used circadian clock and chronotype measures: (1) the dim light melatonin onset (DLMO, the gold standard of objective circadian phase in humans) (Kantermann et al., 2015; Klerman et al., 2002; Lewy & Sack, 1989), (2) free-running circadian period (tau), (3) phase angle of entrainment (the phase position an individual’s circadian clock assumes relative to the environment, measured here by the interval between the DLMO and bedtime/dark onset), (4) mid-sleep on work-free days (MSF, a measure of chronotype derived from actual sleep time entries to the Munich ChronoType Questionnaire) (Roenneberg et al., 2003), and (5) the morningnesse-veningness (MEQ) score (a measure of an individual’s preferred times for sleep and other activities and ratings of feelings according to time of day) (Horne & Östberg, 1976).
Materials and Methods
Ethical approval
The Institutional Review Board of the Rush University Medical Center approved the study. Written informed consent was collected from all study participants. All participants were reimbursed for taking part in the study.
Participants
Data for this post-hoc analysis was collected in two studies performed at Rush University Medical Center. Details about the study protocols and the study populations are provided in the two primary publications (Eastman et al., 2015; Eastman et al., 2016). There were 36 participants in the first study (conducted between January 2013 and May 2014) (Eastman et al., 2015) and 45 participants in the second study (November 2014 to July 2016) (Eastman et al., 2016). Participants were run during all seasons except for summer. This analysis is on data from the 18 participants who participated in both studies. The order of the two studies was the same for all participants. The time between the two studies was between 9 and 33 months (mean ± SD = 16 ± 7) for these 18 people. The participants were free from medication, except for a few women taking oral contraceptives. Only those volunteers with a body mass index of < 35 kg/m2 and with no night work the preceding month were eligible for study participation.
Protocol
This analysis is on the data from the first 10 days of each 14-day study. These 2 × 10 days had identical protocols (Eastman et al., 2015; Eastman et al., 2016). The first 5 of these days were used to determine the free-running circadian period (tau). Day 1 consisted of a circadian phase assessment with saliva samples every 30 minutes to calculate the DLMO at study entry. The next three days consisted of an ultradian LD cycle (a forced desynchrony protocol, to produce free-running circadian rhythms), and the 5th day was another circadian phase assessment to calculate another DLMO. The phase shift between these two DLMOs yielded circadian period.
Then there were four days in which the participants slept on a fixed 8-hour sleep schedule similar to their usual sleep schedule (determined by sleep diaries prior to study start and discussions with each participant about when they felt they obtained the best sleep). The full protocols required living in the laboratory for 14 days, so participants were either unemployed, had part-time jobs or were free-lance workers with flexible hours. Thus, many of them had rather irregular sleep schedules before beginning the two studies. Bedtimes (lights off) and wake times (lights on) in the laboratory were on the hour, so the sleep/dark schedules were 11 pm to 7 am or midnight to 8 am or 1 am to 9 am, etc. Twelve of the 18 participants were assigned to the same sleep schedule during both studies; six had different sleep schedules (ranging from 1 to 3 h different).
Following the four days on a fixed sleep schedule, i.e. on day 10, there was another phase assessment to calculate the DLMO at study end. Participants lived in the lab under controlled lighting conditions (most importantly dark during the 8 h sleep opportunity) and slept in private bedrooms throughout the study except for an 8-h break after the first 8-h sleep/dark episode when they were permitted to leave the lab.
Phase angle was calculated as the interval between the DLMO at study end and bedtime (dark onset) during the preceding fixed 8-h sleep/dark episodes. The participants completed the Morningness-Eveningness Questionnaire (MEQ) (Horne & Östberg, 1976) and Munich ChronoType Questionnaire (MCTQ) (Roenneberg et al., 2003) during the second day in the lab in both studies.
Data analysis
Welch Two Sample t-tests and were used to compare the circadian clock and sleep measures (DLMO, circadian period, phase angle, MSF and MEQ score) collected in study one with those collected in study two. Graphs with lines of unity to compare the numbers from the two studies were used to see how reproducible each parameter was for each individual. Pearson’s productmoment correlations were also used to compare the circadian clock and chronotype measures collected in study one and study two. Statistical analyses and graphical representations of the findings were performed using statistical software R Version 1.0.143 and Graph Pad Prism.
Results
Demographics
Demographic information, circadian parameters and results from the chronotype questionnaires are provided in Table 1. Welch Two Sample t-tests showed that none of these variables were significantly different between the first and the second study (Table 1).
Table 1.
Demographics, circadian rhythm variables and chronotype measures, mean (SD), of 18 participants that took part in both study one and study two.
| Study 1 | Study 2 | p value$ | |
|---|---|---|---|
| Sex | 8 f & 10 m | 8 f & 10 m | − |
| Age (years) | 32.2 (6.7) | 33.6 (6.6) | 0.52 |
| BMI | 25.7 (4.3) | 25.6 (4.1) | 0.95 |
| DLMO study entry (h:min) | 21:58 (2.36) | 21:31 (1.54) | 0.56 |
| DLMO study end (h:min) | 22:14 (2.18) | 21:56 (2.18) | 0.69 |
| Phase angle (h) | −2.2 (1.5) | −2.0 (1.5) | 0.70 |
| Circadian period (h) | 24.22 (0.26) | 24.15 (0.25) | 0.42 |
| MSF (h) | 5.1 (1.5) | 4.8 (1.4) | 0.47 |
| MEQ score | 54.3 (9.4) | 54.8 (8.6) | 0.85 |
= Welch Two Sample t-test. BMI = body mass index. DLMO study entry = dim light melatonin onset on the first night in the lab in clock time. DLMO study end = DLMO after participants spent 4 days in the lab on a fixed, individually timed, 8-h sleep\dark schedule. Phase angle = time interval between DLMO end and bedtime/dark during the 4 nights on the fixed sleep schedule, with negative numbers meaning the time of the DLMO was earlier than bedtime. MSF = Mid-Sleep on work-Free days from the Munich ChronoType Questionnaire. MEQ score = score from the Morningness-Eveningness (Owl-Lark) Questionnaire.
Reproducibility of the circadian clock and chronotype measures
Figure 1 shows that the DLMO at entry to the study (upper left panel), after free sleeping at home, differed by 2 h or less between the two studies except for two participants (points below the lower dotted line). The DLMO at the end of the study (upper right panel), after the 4 days on the fixed 8-h sleep/dark schedule, differed by 2 h or less except for two participants. The two outliers in each of these graphs, showing earlier DLMOs during the second study, were the same two participants. Both of them had in-lab sleep schedules that were 3 h earlier during the second study. Recall that the in-lab sleep schedules were determined by the home sleep schedules before entering the lab. These two participants were the only ones whose sleep schedule changed by 3 h. Most of the others had the same sleep schedules in both studies (n=12), one had a sleep schedule that differed by 2 h, and 3 had schedules that differed by 1 h. When the data point for the greatest outlier was omitted, the correlation coefficient for the DLMO at entry to study increased greatly (r = .80, p = 0.0001) and the DLMO at the end of the study also increased (r = .92, p < 0.0001). We could not find any relation between the change in months (season) between the two studies and the change in the DLMOs in this small sample of 18 participants.
Figure 1.
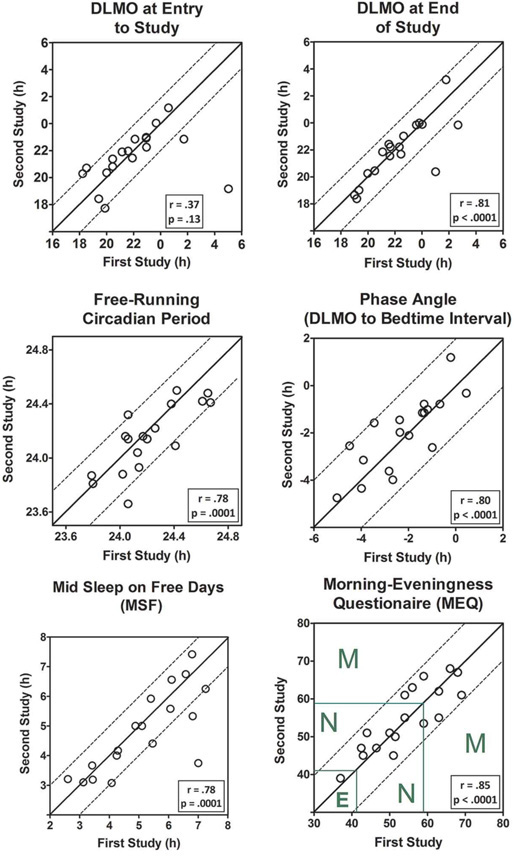
Comparison of circadian clock and chronotype measures collected during two different studies in the same 18 participants. The data are from the first 10 days of each study, days with identical protocols. The second study started 9 to 33 months after the first study depending on when each individual participated. The solid diagonal lines are the lines of unity. Data points on the line of unity mean that both studies produced the same number. Dotted lines parallel to the lines of unity help show how much each point differed between the two studies. Pearson’s product-moment correlations between the data from the first and second study are also presented. Upper left panel: DLMO at entry to study = dim light melatonin onset on the first day in the lab after free-sleeping at home. Upper right panel: DLMO at the end of the study = DLMO after 4 days in the lab on a fixed, individually timed, sleep\dark schedule. Middle left panel: Free-running circadian period was determined from an ultradian light-dark cycle forced desynchrony protocol. Middle right panel: Phase angle is the interval between the DLMO at the end of the study to the in-lab fixed bedtime/lights out. Negative numbers mean that the time of the DLMO was before bedtime. Bottom left panel: MSF is from the Munich ChronoType Questionnaire (MCTQ). Green lines on the MEQ graph (bottom right panel) show the cut offs between evening type (E), neither type (N), and morning type (M).
Figure 1 shows that the free-running circadian period (middle left panel) differed by 0.2 h or less except for one participant. This outlier had a period that was 24.06 h during the first study and 23.66 h during the second study. We could not find any relation between the difference in season (months) between the two studies and the change in circadian period in this small sample of 18 participants.
Phase angle differed by two hours or less (middle right panel). The MSF differed by one hour or less, except for two participants (bottom left panel). The MEQ scores (bottom right panel) differed by less than ten points between the two studies. The designation as morning-type (M), neither-type (N) and evening-type (E) did not change between the two studies for any of the participants.
Impact of the number of days between the two studies on the measures
The time between the two studies ranged from 260 days (0.7 years) to 976 days (2.7 years). The number of days between the two studies did not correlate with any difference in the data between the two studies (Pearson’s product-moment correlation for ‘days between the two studies’ and the difference in the study parameters was for DLMO at study entry r = .10 with p = 0.70 / for DLMO at study end r = .03 with p = 0.92 / for MSF r = −.26 with p = 0.30 / for MEQ r = −.09 with p = 0.71 / for phase angle r = .25 with p = 0.31 / for circadian period r = .26 with p = 0.29). Thus, there was no impact of the number of days between the two studies on these parameters.
Discussion
Circadian phase, phase angle, circadian period and chronotype showed good reproducibility from the first to the second study. This reproducibility was independent of the number of days between the two studies (which was up to 976 days or 2.7 years). In other words, the circadian and chronotype measures did not change more from the first to the second study as the days between the studies increased.
While living in the lab participants slept on fixed, individually-timed sleep schedules, based loosely on their habitual sleep times prior to each study. The reproducibility of the DLMO was diminished by the two participants who had the largest change in their sleep schedules between the two studies, which was an advance of 3 h, e.g., from 2–10 am to 11 pm–7 am. As expected, these two individuals had earlier DLMOs during the second study when they were on an earlier sleep schedule. When the most extreme outlier of the two was omitted from the calculations, the correlation coefficients for the DLMOs were similar and large (r = .80 for DLMO at entry to the study and r = .92 for DLMO at the end of the study). The correlations for the DLMOs collected at the end of the study, after sleeping in the lab for 4 nights on their fixed sleep schedules were larger than those after sleeping at home with no experimental constraints. This can be seen in the DLMO plots in Figure 1 by a tighter clustering of points around the line of unity.
It has long been common practice in circadian rhythm studies to keep participants on a relatively fixed sleep schedule at home for one to three weeks before an experiential manipulation or the measurement of circadian phase (Boivin et al., 1994; Dijk et al., 1999; Duffy et al., 1996; Eastman, 1992; Eastman et al., 2005). We show very good reproducibility of the DLMO after only four days on a fixed, individually-timed sleep schedule, especially when participants were on similar sleep schedules before each DLMO was measured. Most of those four days were spent in the laboratory, so there was relatively little variation in the pattern of light and dark between the two studies. Importantly, the participants were in complete darkness during the 8-h sleep opportunities. This stable LD cycle may have helped make the DLMOs more stable. Earlier reports suggest that under ecological conditions (outside the laboratory) the correlation between subjective sleep timing and objective circadian timing (DLMO) is rather noisy (Kantermann & Burgess, 2017; Kantermann, 2013; Kantermann et al., 2015; Lunn et al., 2017; Van Reen et al., 2013; Wright et al., 2013).
An individual’s circadian period is important because it helps determine circadian phase and circadian phase angle (Duffy et al., 2001., Eastman et al., 2015; Eastman et al., 2016; Gronfier et al., 2007; Pittendrigh & Daan, 1976; Sharma & Chandrashekaran, 1998; Wright et al., 2005). Circadian period also determines the direction and magnitude of the phase shift of the circadian clock after a large phase shift of the sleep schedule as in shift work and jet travel (Eastman et al., 2015; Eastman et al., 2016). Furthermore, circadian period is shorter in African-Americans compared to European-Americans (Eastman et al., 2015; Eastman et al., 2016; Eastman et al., 2017) and thus has practical implications for the ailments produced by modern society. A shorter circadian period is better for early morning shifts, early school start times and most types of social jet lag, whereas a longer circadian period is better for night shift work and flying west.
Our results have several implications, such as (1) Medical treatments: if personalized medical treatments are to be designed based on circadian phase (e.g. DLMO) then these parameters should be stable and predictable. Based on our findings here, to support stable entrainment of the circadian clock, patients should keep a stable sleep and light schedule prior to an intervention, for at least four days. (2) Shift work: since our results show that the reproducibility of the DLMO improves with stable sleep and light schedules, which are virtually impossible for shift workers, any single assessments of sleep and circadian clock measures appear risky. Instead, for shift work, repeated assessments of sleep and circadian clock measures are advised to allow for possibly necessary adjustments of the chronobiology informed shift work schedule once in practice.
In conclusion, we show that the most widely used circadian clock and chronotype measures are reproducible and stable at the individual level, even after many months. In addition, the reproducibility of the DLMO (circadian phase) improved when individuals had similar habitual sleep schedules before the DLMO was measured, and was further improved when they were kept on fixed sleep schedules, based on their habitual sleep schedules, for only four days before the DLMO was assessed.
Acknowledgements
We thank Victoria A. Tomaka for performing preliminary analyses, such as calculating the DLMOs, circadian periods and MEQ scores, and for preparing preliminary figures. Supported by National Institutes of Health (NIH) grant R01NR007677 from the National Institute of Nursing Research (NINR) to C.I.E. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINR or the NIH. The NINR and NIH had no involvement in designing the study, data collection, data analysis and interpretation, writing of the manuscript, or in the decision to submit the manuscript for publication.
Footnotes
Declaration of Interest statement:
The authors declare that they have no competing interests.
Author Contributions
CIE designed and coordinated the running of the studies that produced these data. TK performed statistical analyses, prepared Table 1, and wrote the manuscript. CIE edited and approved the manuscript.
References
- Akerstedt T, Knutsson A, Alfredsson L, Theorell T. (1984). Shift work and cardiovascular disease. Scand. J. Work Environ. Health 10:409–414. [DOI] [PubMed] [Google Scholar]
- Archer SN, Oster H. (2015). How sleep and wakefulness influence circadian rhythmicity: effects of insufficient and mistimed sleep on the animal and human transcriptome. J. Sleep Res 24:476–493. [DOI] [PubMed] [Google Scholar]
- Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. (2015). Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An update for 2015: an American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med 11:1199–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr EK, Revelle W, Eastman CI. (2000). Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res 9:117–127. [DOI] [PubMed] [Google Scholar]
- Boivin DB, James FO. (2005). Light treatment and circadian adaptation to shift work. Ind Health 43:34–48. [DOI] [PubMed] [Google Scholar]
- Boivin DB, Duffy JF, Kronauer RE, Czeisler CA. (1994). Sensitivity of the human circadian pacemaker to moderately bright light. J. Biol. Rhythms 9:315–331. [DOI] [PubMed] [Google Scholar]
- Dijk DJ, Duffy JF, Riel E, Shanahan TL, Czeisler CA. (1999). Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J. Physiol 516 ( Pt 2):611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Rimmer DW, Czeisler CA. (2001). Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav. Neurosci 115:895–899. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Kronauer RE, Czeisler CA. (1996). Phase-shifting human circadian rhythms: influence of sleep timing, social contact and light exposure. J. Physiol 495 (Pt 1):289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI. (2016). How to reduce circadian misalignment in rotating shift workers. ChronoPhysiology and Therapy 6:41–46. [Google Scholar]
- Eastman CI. (1992). High-intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol 263:R428–36. [DOI] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA, Crowley SJ. (2017). Sex and ancestry determine the free-running circadian period. J. Sleep Res 26(5):547–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Tomaka VA, Crowley SJ. (2016). Circadian rhythms of European and African-Americans after a large delay of sleep as in jet lag and night work. Sci. Rep 6:36716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Suh C, Tomaka VA, Crowley SJ. (2015). Circadian rhythm phase shifts and endogenous free-running circadian period differ between African-Americans and European-Americans. Sci. Rep 5:8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastman CI, Gazda CJ, Burgess HJ, Crowley SJ, Fogg LF. (2005). Advancing circadian rhythms before eastward flight: a strategy to prevent or reduce jet lag. Sleep 28:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens JS, Eastman CI. (2017). Diagnosis and treatment of non-24-h sleepwake disorder in the blind. Drugs 77:637–650. [DOI] [PubMed] [Google Scholar]
- Fischer D, Vetter C, Oberlinner C, Wegener S, Roenneberg T. (2016). A unique, fast-forwards rotating schedule with 12-h long shifts prevents chronic sleep debt. Chronobiol. Int 33:98–107. [DOI] [PubMed] [Google Scholar]
- Folkard S, Lombardi DA, Tucker PT. (2005). Shiftwork: safety, sleepiness and sleep. Ind Health 43:20–23. [DOI] [PubMed] [Google Scholar]
- Goel N. (2016). Probing personalized genetic platforms for novel molecular clues for circadian chronotype. Ann. Transl. Med 4:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Rogacz S, Bock N, Tosteson TD, Baum TM, Speizer FE, Czeisler CA. (1992). Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am. J. Public Health 82:1011–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronfier C, Wright KP Jr, Kronauer RE, Czeisler CA. (2007). Entrainment of the human circadian pacemaker to longer-than-24-h days. Proc. Natl. Acad. Sci. U. S. A 104:9081–9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F, Kabat HF, Klein P (1980). Chronopharmacology: a therapeutic frontier. Am. J. Hosp. Pharm 37:101–106. [PubMed] [Google Scholar]
- Horne JA, Östberg O. (1976). A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int. J. Chronobiol 4:97–110. [PubMed] [Google Scholar]
- Hrushesky WJ. (1990). Cancer chronotherapy: a drug delivery challenge. Prog. Clin. Biol. Res 341A:1–10. [PubMed] [Google Scholar]
- Kantermann T, Burgess HJ. (2017). Average mid-sleep time as a proxy for circadian phase. PsyCh Journal [Epub ahead of print] [DOI] [PubMed]
- Kantermann T. (2013). Circadian biology: sleep-styles shaped by light-styles. Curr Biol 23:R689–90. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Roenneberg T. (2009). Is light-at-night a health risk factor or a health risk predictor? Chronobiol Int 26:1069–1074. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Sung H, Burgess HJ. (2015). Comparing the MorningnessEveningness Questionnaire and Munich ChronoType Questionnaire to the Dim Light Melatonin Onset. J. Biol. Rhythms 30:449–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantermann T, Wehrens SM, Ulhoa MA, Moreno C, Skene DJ. (2012). Noisy and individual, but doable: shift-work research in humans. Prog Brain Res 199:399–411. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Duboutay F, Haubruge D, Kerkhofs M, Schmidt-Trucksass A, Skene DJ. (2013). Atherosclerotic risk and social jetlag in rotating shift-workers: first evidence from a pilot study. Work 46:273–282. [DOI] [PubMed] [Google Scholar]
- Kantermann T, Duboutay F, Haubruge D, Hampton S, Darling AL, Berry JL, Kerkhofs M, Boudjeltia KZ, Skene DJ. (2014). The direction of shiftwork rotation impacts metabolic risk independent of chronotype and social jetlag - An exploratory pilot study. Chronobiol. Int 31:1139–1145. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. (2002). Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms 17:181–193. [DOI] [PubMed] [Google Scholar]
- Knutsson A. (2003). Health disorders of shift workers. Occupational medicine (Oxford, England) 53:103–108. [DOI] [PubMed] [Google Scholar]
- Kyba CC, Kantermann T. (2016). Does ambient light at night reduce total melatonin production? Hormones (Athens) 15:142–143. [DOI] [PubMed] [Google Scholar]
- Lack L, Bailey M, Lovato N, Wright H. (2009). Chronotype differences in circadian rhythms of temperature, melatonin, and sleepiness as measured in a modified constant routine protocol. Nat. Sci. Sleep 1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. (1989). The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int 6:93–102. [DOI] [PubMed] [Google Scholar]
- Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, Hansen J, Nelson RJ, Panda S, Smolensky MH, Stevens RG, Turek FW, Vermeulen R, Carreon T, Caruso CC, Lawson CC, Thayer KA, Twery MJ, Ewens AD, Garner SC, Schwingl PJ, Boyd WA. (2017). Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ 607–608:1073–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, Garaulet M, Scheer FA, Klerman EB. (2017). Later circadian timing of food intake is associated with increased body fat. Am. J. Clin. Nutr [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Mongrain V, Carrier J, Dumont M (2006). Circadian and homeostatic sleep regulation in morningness-eveningness. J. Sleep Res 15:162–166. [DOI] [PubMed] [Google Scholar]
- Ortiz-Tudela E, Innominato PF, Rol MA, Levi F, Madrid JA. (2016). Relevance of internal time and circadian robustness for cancer patients. BMC Cancer 16:285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S (1976). A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol 106:291–331. [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, Vetter C. (2012). Social jetlag and obesity. Curr Biol 22:939–943. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. (2013). Light and the human circadian clock. Handb. Exp. Pharmacol 217:311–331. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, Merrow M. (2007). Epidemiology of the human circadian clock. Sleep Med Rev 11:429–438. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. (2003). Life between clocks - daily temporal patterns of human chronotypes. J. Biol. Rhythms 18:80–90. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP Jr, Vitiello MV, Zhdanova IV, American Academy of Sleep Medicine. (2007). Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 30:1484–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Chandrashekaran MK. (1998). Relationship between period and phase angle differences in mus booduga under abrupt versus gradual light-dark transitions. Naturwissenschaften 85:183–186. [DOI] [PubMed] [Google Scholar]
- Smith MR, Eastman CI. (2012). Shift work: health, performance and safety problems, traditional countermeasures, and innovative management strategies to reduce circadian misalignment. Nat. Sci. Sleep 4:111–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong KK, Lam MT, Grandner MA, Sassoon CS, Malhotra A. (2016). Timing matters: circadian rhythm in sepsis, obstructive lung disease, obstructive sleep apnea, and cancer. Ann. Am. Thorac. Soc 13:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reen E, Sharkey KM, Roane BM, Barker D, Seifer R, Raffray T, Bond TL, Carskadon MA. (2013). Sex of college students moderates associations among bedtime, time in bed, and circadian phase angle. J. Biol. Rhythms 28:425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter C, Fischer D, Matera JL, Roenneberg T. (2015). Aligning work and circadian time in shift workers improves sleep and reduces circadian disruption. Curr. Biol 25:907–911. [DOI] [PubMed] [Google Scholar]
- Wever RA, Polasek J, Wildgruber CM. (1983). Bright light affects human circadian rhythms. Pflugers Arch 396:85–87. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Dinich J, Merrow M, Roenneberg T. (2006). Social jetlag: misalignment of biological and social time. Chronobiol Int 23:497–509. [DOI] [PubMed] [Google Scholar]
- Wright KP Jr, Gronfier C, Duffy JF, Czeisler CA. (2005). Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J. Biol. Rhythms 20:168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP Jr, McHill AW, Birks BR, Griffin BR Rusterholz T, Chinoy ED. (2013). Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol 23:1554–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]


