Abstract
Essentials.
Anti‐protein S autoantibodies (anti‐PS) may lead to a PS deficiency and recurrent pregnancy loss.
We performed epitope mapping of anti‐PS in patients with recurrent pregnancy loss.
Anti‐PS recognized epidermal growth factor (EGF)‐like domains in PS and recombinant human EGF.
Anti‐PS may be associated with not only thrombophilia but also the disruption of the EGF system.
Background
Protein S (PS) deficiency is a risk factor for adverse pregnancy outcomes including recurrent pregnancy loss. Several studies have shown that the presence of anti‐PS autoantibodies (anti‐PS) leads to an acquired PS deficiency. Hence, an epitope mapping study was conducted to know the pathogenesis of anti‐PS in patients with recurrent pregnancy loss.
Methods
PS was treated with thrombin to divide the protein into γ‐carboxyglutamic acid (Gla) domain and Gla‐domain free PS. For the preparation of fragments of epidermal growth factor (EGF)‐like domains (EGF1‐4), PS was subjected to proteolysis using lysyl endopeptidase. The epitopes were identified in immunoblot. Whether anti‐PS recognized EGF family proteins in anti‐PS‐positive patients was also examined.
Results
Anti‐PS recognized Gla‐domain free PS, especially the three fragments of EGF‐like domains, EGF1‐2, EGF3‐4, and EGF1‐4. Anti‐PS recognized recombinant human EGF. Anti‐PS and polyclonal antibodies to recombinant human EGF recognized PS in the absence of Ca2+ but not in the presence of Ca2+. In competitive inhibition studies, polyclonal antibodies to recombinant mouse EGF blocked anti‐PS binding to PS in a concentration‐dependent manner.
Conclusions
These results suggest that anti‐PS in patients with recurrent pregnancy loss recognize EGF‐like domains in PS. Interestingly, anti‐PS also recognized EGF family proteins. Anti‐PS in patients with recurrent pregnancy loss may be associated with not only thrombophilia but also the disruption of the EGF system.
Keywords: antibody binding sites, autoantibodies, epidermal growth factor, miscarriage, protein S
1. INTRODUCTION
Protein S (PS) is a vitamin K‐dependent glycoprotein that functions as a cofactor to an anticoagulant serine protease, activated protein C (APC), and is an important regulator of blood coagulation.1
Recently, many studies have suggested associations between adverse pregnancy outcome and PS deficiency.2, 3, 4, 5, 6 Rey et al. performed a meta‐analysis and reported that PS deficiency was associated with recurrent pregnancy loss and fetal loss after 22 weeks.2 Robertson et al. reported in a systematic review that PS deficiency was associated with late fetal loss.3
Some studies have suggested that the presence of anti‐PS autoantibodies (anti‐PS) leads to an acquired PS deficiency.7, 8 Reports revealed low free PS levels in numerous patients with human immunodeficiency virus infection and an overall 28.6% anti‐PS positivity with higher incidence in patients with PS levels of <50%.7
D'Angelo et al. described a type of acquired PS deficiency associated with a circulating antibody against PS in a child suffering from severe thromboembolic disease while recovering from chickenpox.8 They suggested that low PS antigen levels may have resulted from the rapid clearance of the circulating immune complex. In addition, they reported that the antibody did not show direct inhibitory effect on PS activity, which indicated that the interaction with a PS epitope(s) was not involved in the APC cofactor activity expression.
Consistent with the study, Morange et al. reported no evidence of PS activity inhibition by anti‐PS.9 In contrast, Sorice et al. demonstrated the possibility of PS activity inhibition by anti‐PS.10 Thus, existing literature fails to clarify whether anti‐PS affects PS activity or determines the sites recognized by anti‐PS.
Mature PS has a modular structure consisting of a γ‐carboxyglutamic acid (Gla) domain, a thrombin‐sensitive region (TSR), four epidermal growth factor (EGF)‐like domains (EGF1‐4), and a sex hormone‐binding globulin‐like domain containing two laminin G‐like‐domain‐like repeats (LGR).
The amino‐terminal Gla domain confers the high‐affinity interaction of PS with negatively charged phospholipid surfaces that is essential for APC‐dependent functions of PS.11, 12
TSR and EGF1 contribute to functionally crucial interactions of PS with APC.13, 14, 15 Indeed, a three‐dimensional structural model of the Gla, TSR, and EGF1 domains of PS indicates an interaction between the Gla domain and the membrane, which makes the other two domains available for interaction with the membrane‐bound APC.15
Additionally, other EGF‐like domains appear to be involved in the APC cofactor function of PS. A recent report has indicated that EGF2 plays a role in the APC cofactor function of PS because of the resultant loss of function on replacing or deleting it.16 Perhaps, EGF4 of PS is crucial for keeping EGF1 in optimal alignment for interacting with APC,17 even though an unaided recombinant EGF4 has been unsuccessful in exhibiting APC cofactor activity in a clotting assay.18
LGR is also involved in APC cofactor function, as indicated by the recent identification of the factor Va‐binding site in the second LGR.19
In the present study, we used PS treated with thrombin or lysyl endopeptidase, recombinant EGF, and polyclonal antibodies to EGF for inhibition and direct‐binding for identifying anti‐PS antigenic binding sites in patients with recurrent pregnancy loss.
2. MATERIALS AND METHODS
2.1. Patients
From October 2016 to December 2016, plasmas from 100 patients with recurrent pregnancy loss who were referred to Sugi Women's Clinic (Yokohama, Japan) were screened for anti‐PS by immunoblotting. Patients with recurrent pregnancy loss had two or more pregnancy losses before 10 weeks of gestation, not including ectopic pregnancy and/or elective abortion. Each patient was evaluated with three‐dimensional vaginal ultrasound, endocrine monitoring (eg, thyroid function and fasting blood sugar), and autoantibodies (eg, lupus anticoagulant, anticardiolipin, antiphosphatidylethanolamine, antinuclear antibodies, and anti‐PS antibodies). The mean age of patients was 38.5 (range: 31‐44) years; the mean number of pregnancy losses was 3.2 (range: 2‐4). No patient fulfilled criteria for definite antiphospholipid syndrome. No patient had thrombotic history or autoimmune diseases such as systemic lupus erythematosus. No patient was pregnant and taken any treatment when plasma was obtained. We identified 20 patients who were seropositive for anti‐PS. Zero, 12, and 5 of 100 patients each were positive for lupus anticoagulant, antiphosphatidylethanolamine and anticardiolipin antibodies, respectively. There was no significant association between anti‐PS and these antiphospholipid antibodies. In this epitope mapping study, we used blood samples from patients with recurrent pregnancy loss who had a high‐titer anti‐PS and whose sample volume was sufficient to do further examination. We also recruited 56 healthy non‐pregnant female volunteers who had no history of miscarriage. Among them, four women (7.1%) were seropositive for anti‐PS. We collected blood samples in non‐activating plastic tubes with 3.2% trisodium citrate (9:1 v/v). After centrifugation, plasma aliquots were instantly stored at −60°C until further use. All participants gave informed consent, and the study protocol was approved by the local ethics review committee.
2.2. Materials
PS was purchased from Enzyme Research Laboratories (South Bend, IN, USA). Polyclonal antibodies to PS was purchased from Abnova (Taipei, Taiwan). Polyclonal antibodies to LGR in PS (residues 419 to 516) (anti‐ LGR) was purchased from Abnova (Taipei, Taiwan). Factor XII (FXII) was purchased from Enzyme Research Laboratories (South Bend, IN, USA). Polyclonal antibodies to recombinant human EGF (anti‐human EGF) was purchased from Abcam (Tokyo, Japan). Polyclonal antibodies to recombinant human heparin‐binding EGF‐like growth factor (anti‐HB‐EGF) was purchased from PeproTech (Rocky Hill, NJ, USA). Polyclonal antibodies to recombinant mouse EGF (anti‐mouse EGF) was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). Recombinant mouse EGF was purchased from ProSpec‐Tany TechnoGene Ltd (Ness Ziona, Israel). Recombinant human EGF was purchased from Higeta Shoyu Co., LTD (Ibaraki, Japan). Recombinant human HB‐EGF was purchased from PeproTech.
2.3. Assays for PS activity and total PS antigen
PS activity was measured using functional assays and prothrombin time method with a HemosIL PS clot test kit (reference range 56‐126%, Instrumentation Laboratory, Bedford, MA, USA). Total PS antigen was measured by sandwich enzyme immunoassay (EIA) using commercial kits (reference range 65‐135%, Instrumentation Laboratory, Bedford, MA, USA). Standard operating procedures were used.
2.4. Dose‐dependent binding of anti‐PS to PS in enzyme‐linked immunosorbent assay (ELISA)
MaxiSorp Nunc‐Immuno plates (Thermo Fisher Scientific, Roskilde, Denmark) were coated for 2 hour (h) at 25°C with increasing concentrations (50 μL of 0.16, 0.31, 0.63, 1.25, 2.5, 5.0, and 10 μg mL−1 solution) of PS in Tris‐buffered saline (TBS). Each well was blocked for 1 hour with 10% bovine serum albumin (BSA) in TBS followed by incubation with 50 μL of anti‐PS‐positive patient plasma (1:50) for 1 hour. We added alkaline phosphatase‐conjugated polyclonal antibodies to human IgG (Sigma, St. Louis, MO, USA), followed by the substrate solution. We washed the plates three times with TBS containing 0.03% Tween 20 after PS coating, blocking, serum, and conjugate incubations. Then, the optical density (OD) was assessed at 405 nm post color development produced by paranitrophenyl phosphate substrate. The color development was stopped with 75 μL of 3N NaOH when the OD of a positive control reached 1.0.
MaxiSorp Nunc‐Immuno plates were coated for 2 hour at 25°C with 50 μL of 5 μg mL−1 solution of PS in TBS. Each well was blocked for 1 hour with 10% BSA in TBS followed by incubation with several dilutions (1:3,200, 1:1600, 1:800, 1:400, 1:200, 1:100, and 1:50) of anti‐PS‐positive patient plasma for 1 hour. We added alkaline phosphatase‐conjugated polyclonal antibodies to human IgG, followed by the substrate solution. We washed the plates three times with TBS containing 0.03% Tween 20 after PS coating, blocking, serum, and conjugate incubations. OD was assessed as mentioned above.
2.5. IgG purification from plasma
IgGs were purified from plasma by protein G‐Agarose (Protein Ark Ltd, Sheffield, UK) chromatography following the manufacturer's recommendations.
2.6. Dose‐dependent binding of anti‐PS to PS in an immunoblot assay
We performed sodium dodecyl sulfate ‐polyacrylamide gel electrophoresis (SDS‐PAGE) using 10% polyacrylamide gel. PS was prepared in non‐reducing forms. Increasing concentrations (5 μL of 7.5, 15, 30, 60, 120 μg mL−1 solution) of PS were applied to each lane. Furthermore, it was transferred to polyvinylidene difluoride (PVDF) membrane for 20 min at 0.1 A; 1% BSA in TBS at pH 7.3. was used to block membranes for 1.5 hour. PS was incubated with purified IgG from anti‐PS‐positive patient plasma for 2 hour followed by three washes with 0.05% Tween 20/TBS. Additionally, we exposed the membrane to horseradish peroxidase‐conjugated polyclonal antibodies to human IgG for 1 hour followed by washing as mentioned above. Then, immunobands were developed using 3,3′,5,5′‐tetramethylbenzidine.
We performed SDS‐PAGE using 10% polyacrylamide gel. PS was prepared in non‐reducing forms. PS (5 μL of 15 μg mL−1 solution) was applied to each lane. We then transferred the slab gel contents to PVDF membranes and immunoblotted the contents with several concentrations (10, 20, 40, 80 μg mL−1) of purified IgG from anti‐PS‐positive patient plasma as described above.
2.7. Cleavage of PS by thrombin
For the preparation of Gla‐domain free PS, PS was incubated with thrombin at 37°C for 2 h. Then, we added dithiothreitol (DTT) to the thrombin‐treated PS and vortexed and boiled the mixture at 100°C for 5 minute. Remarkably, thrombin acts in cleaving PS at two sites, Arg49 and Arg70, which releases TSR and leaves the Gla domain of PS attached to the rest of the PS with the help of a disulfide linkage. DTT cleaves disulfide bonds, releasing Gla domain and Gla‐ domain free PS.
2.8. Proteolysis of PS
For the preparation of fragments of EGF‐like domains in PS, PS was incubated with high concentrations (30% or 60%) of lysyl endopeptidase (cleaves COOH‐terminal of lysines) at 37°C for 1 hour in the presence of Ca2+ (2 mM).
2.9. Effects of Ca2+ in immunoblot for anti‐PS
We performed SDS‐PAGE using 10% polyacrylamide gel. PS was prepared in non‐reducing forms. PS (5 μL of 10 μg mL−1 solution) was applied to each lane. Furthermore, it was transferred to PVDF membrane for 20 minute at 0.1 A; 1% BSA in TBS at pH 7.3. was used to block membranes for 1.5 hour. PS was incubated with patient plasma (1/100) in the presence of 2.0 mmol L−1 of Ca2+ or of increasing concentrations (0.125, 0.25, 0.5, 1.0, and 2.0 mmol L−1) of Ca2+ for 2 hour followed by three washes with 0.05% Tween 20/TBS. Additionally, we exposed the membrane to horseradish peroxidase‐conjugated polyclonal antibodies to human IgM for 1 h followed by washing as mentioned above. Then, immunobands were developed using 3,3',5,5'‐tetramethylbenzidine.
3. RESULTS
3.1. Dose‐dependent binding of anti‐PS to PS in ELISA
PS was coated onto microtiter plate wells in increasing concentrations (50 μL of 0.16, 0.31, 0.63, 1.25, 2.5, 5.0, and 10 μg mL−1). We then applied anti‐PS‐positive patient plasma (X) or normal control plasma (1:50) followed by an alkaline phosphatase‐conjugated secondary antibody. The absorption was assessed at 405 nm. Figure 1A shows the concentration‐dependent binding of anti‐PS to PS.
Figure 1.
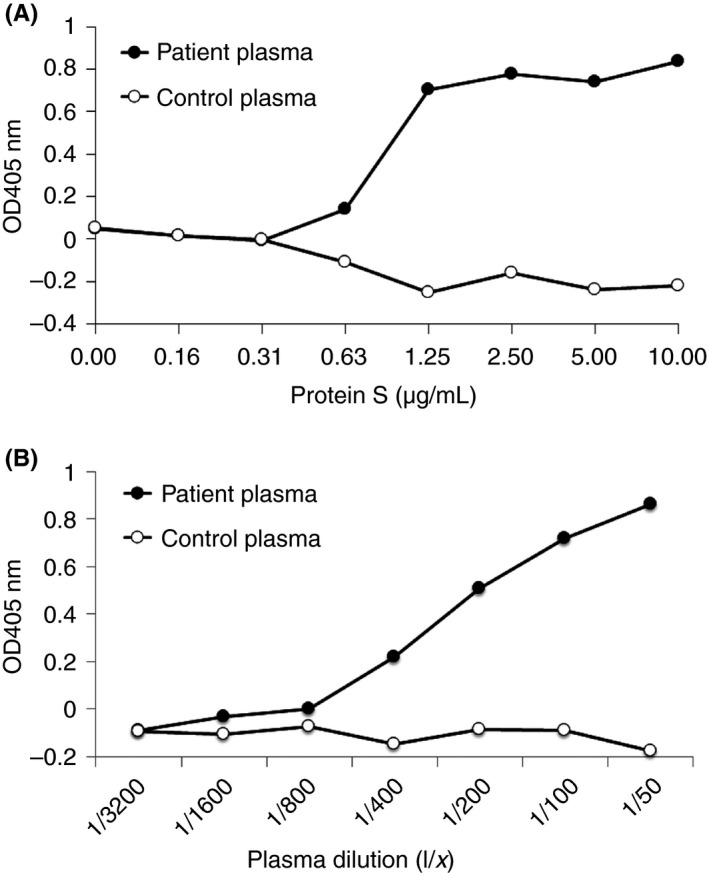
Dose‐dependent anti‐PS binding to PS in ELISA (A) Increasing concentrations of PS were coated on microtiter plate wells. Anti‐PS‐positive patient plasma (X) or normal control plasma (1:50) was applied followed by an alkaline phosphatase‐conjugated secondary antibody. The absorption at 405 nm was measured. (B) PS was coated on microtiter plate wells. Several dilutions of anti‐PS‐positive patient plasma (X) or normal control plasma were applied followed by an alkaline phosphatase‐conjugated secondary antibody. The absorption at 405 nm was measured. PS, protein S; ELISA, enzyme‐linked immunosorbent assay; OD, optical density
PS (50 μL of 5 μg mL−1 solution) was coated on microtiter plate wells. We then applied several dilutions (1:3,200, 1:1600, 1:800, 1:400, 1:200, 1:100, and 1:50) of anti–PS‐positive patient plasma (X) or normal control plasma followed by an alkaline phosphatase‐conjugated secondary antibody. The absorption was assessed at 405 nm. Figure 1B shows the concentration‐dependent binding of anti‐PS to PS.
3.2. Dose‐dependent binding of anti‐PS to PS in an immunoblot assay
Increasing concentrations (5 μL of 7.5, 15, 30, 60, 120 μg mL−1 solution) of PS were applied to each lane. After transferring to PVDF membranes, membranes were incubated with purified IgG from anti‐PS‐positive patient or normal control and immunoblotting was performed. Figure 2A shows the concentration‐dependent binding of anti‐PS to PS.
Figure 2.
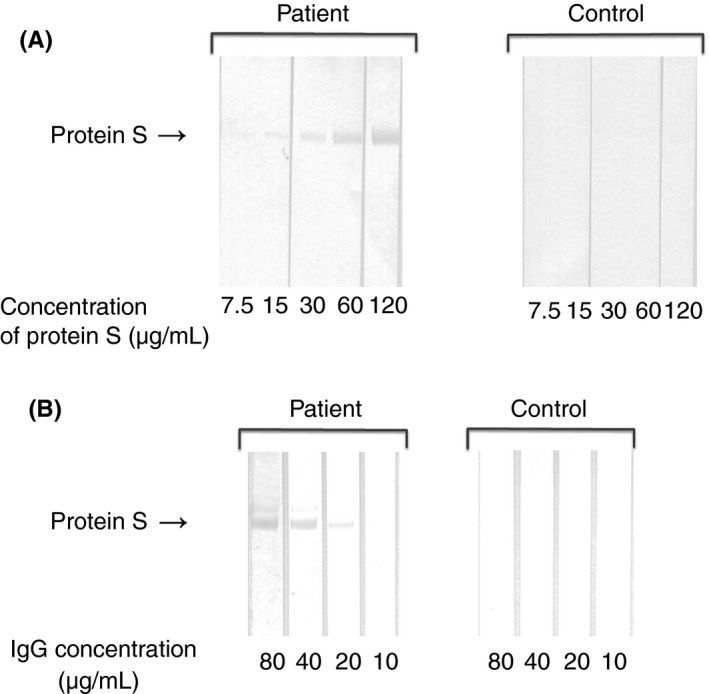
Dose‐dependent anti‐PS binding to PS in an immunoblot assay (A) Increasing concentrations of PS were subjected to SDS‐PAGE under non‐reducing conditions. Immunoblotting was performed with purified IgG from anti‐PS‐positive patient, or normal control (20 μg mL−1). (B) PS (15 μg mL−1) was subjected to SDS‐PAGE under non‐reducing conditions. Immunoblotting was performed with several dilutions of purified IgG from anti‐PS‐positive patient, or normal control. PS, protein S; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis
PS (5 μL of 15 μg mL−1 solution) was applied to each lane. After transferring to PVDF membranes, membranes were incubated with several concentrations (10, 20, 40, 80 μg mL−1) of purified IgG from anti‐PS‐positive patient or normal control and immunoblotting was performed. Figure 2B shows the concentration‐dependent binding of anti‐PS to PS.
3.3. Binding of anti‐PS to Gla‐domain free PS
Thrombin‐treated PS or PS was subjected to SDS‐PAGE under reducing conditions. We then transferred the slab gel contents to PVDF membranes and immunoblotted the contents with anti‐PS‐positive patient plasmas. Figure 3 shows the binding of anti‐PS to Gla‐domain free PS. Intact PS should show a single band of 86 kDa. However, in this study, two bands were seen, one of 86 kDa corresponding to PS and that of approximately 80 kDa corresponding to Gla‐domain free PS. It was probably due to contamination of thrombin. Indeed, the same two bands were stained with Coomassie Brilliant Blue (data not shown).
Figure 3.
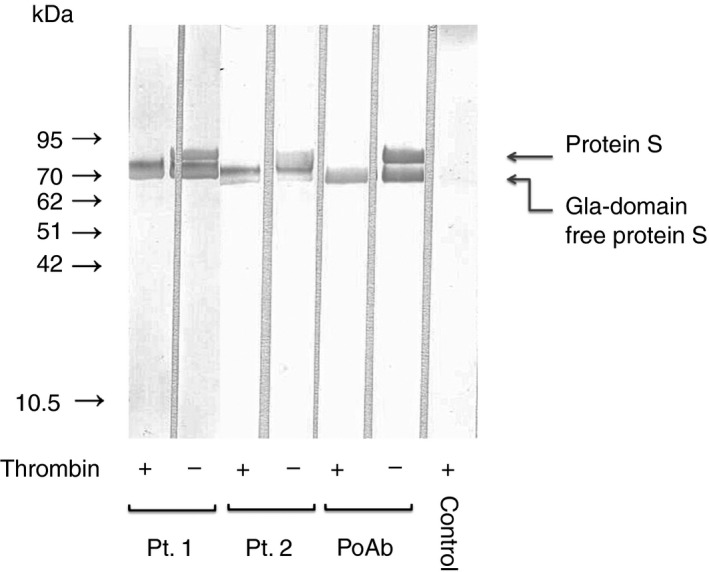
Representative experiment showing patient autoantibody binding to Gla‐domain free PS. SDS‐PAGE was performed using 10% polyacrylamide gel. Thrombin‐treated PS or PS under reducing conditions (5 μL of 10 μg mL−1 solution) was applied to each lane. Transfer to PVDF membrane was done for 20 min at 0.1 amps. Membranes were blocked for 2 h with 1% BSA in TBS at pH 7.3. Incubation with mouse polyclonal antibodies (PoAb) to PS, normal plasma (control), or anti‐PS‐positive patient plasmas (1/100) was done for 1.5 hour followed by three washes with 0.05% Tween 20/TBS. The membrane was exposed to horseradish peroxidase‐conjugated anti‐mouse immunoglobulins or polyclonal antibodies to human IgG or IgM for 1 hour. Gla, γ‐carboxyglutamic acid; PS, protein S; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis; PVDF, polyvinylidene difluoride; BSA, bovine serum albumin; TBS, Tris‐buffered saline
3.4. Binding of anti‐PS to EGF‐like domains
PS incubated with lysyl endopeptidase was subjected to SDS‐PAGE under non‐reducing conditions. We then transferred the slab gel contents to PVDF membranes and immunoblotted the contents with anti–PS‐positive patient plasmas.
Dahlbäck et al.13, 20 reported using lysyl endopeptidase digestion of PS and monoclonal antibodies to PS, and three bands were observed on Western blotting, that was, approximately 10‐15 kDa, 20‐24 kDa, and 30 kDa. From the results of the amino acid sequence analysis of these fragments, they revealed that 30 kDa fragment comprised EGF1‐4, whereas the 20‐24 kDa fragment contained only EGF3‐4, and the 10‐15 kDa fragment contained only EGF1‐2.
In the present study, as shown in Figure 4, three immunobands were observed, suggesting anti‐PS in the four patients bound to EGF1‐2 (10‐15 kDa fragment) and EGF3‐4 (20‐24 kDa fragment). PS activities in patients 1‐4 were 46%, 38%, 62%, and 49%, respectively. Patients 1, 2, and 4 were deficient for PS activity. In other words, patients 1‐4 were not deficient for total PS antigen (72%, 66%, 78%, and 70%, respectively). In control, PS activity and total PS antigen were 82% and 78%, respectively.
Figure 4.
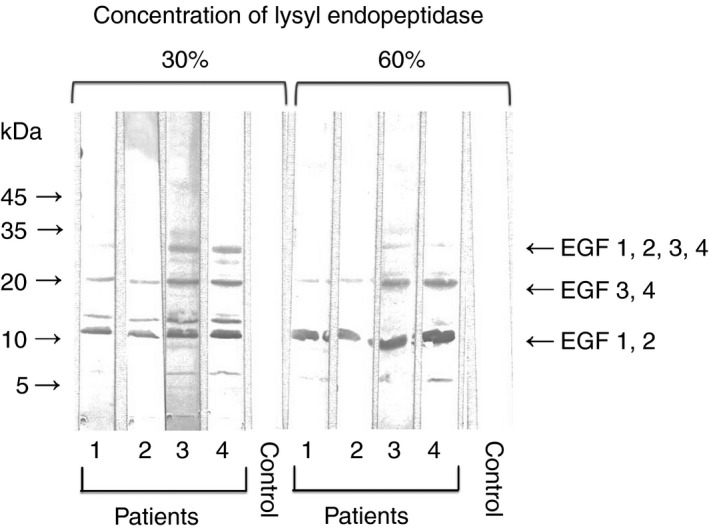
Representative experiment showing patient autoantibody binding to EGF‐like domains of PS. SDS‐PAGE was performed using a polyacrylamide gradient gel (5‐20%). For the preparation of fragments of EGF‐like domains in PS, PS was incubated with high concentrations (30% or 60%) of lysyl endopeptidase (cleaves COOH‐terminal of lysines) at 37°C for 1 h in the presence of Ca2+ (2 mmol L−1). PS (3 μL of 600 μg/mL solution) was applied to each lane. Transfer to PVDF membrane was done for 20 minute at 0.1 amps. Membranes were blocked for 1.5 hours with 1% BSA in TBS at pH 7.3. Incubation with normal plasma (control) or anti‐PS‐positive patient plasmas (1/100) was done for 2 hours followed by three washes with 0.05% Tween 20/TBS. The membrane was exposed to horseradish peroxidase‐conjugated polyclonal antibodies to human IgM for 1 hours. EGF, epidermal growth factor; PS, protein S; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis; PVDF, polyvinylidene difluoride; BSA, bovine serum albumin; TBS, Tris‐buffered saline
3.5. Effects of Ca2+ in immunoblot for anti‐PS
PS was subjected to SDS‐PAGE under reducing conditions. After we transferred the slab gel contents to PVDF membranes, the buffers were supplemented with 2.0 mmol L−1 of Ca2+ or increasing concentrations (0.125, 0.25, 0.5, 1.0, and 2.0 mmol L−1) of Ca2+ and then immunoblotted with anti‐PS‐positive patient plasmas. As shown in Figure 5A, in all patients, PS immunobands disappeared in the presence of 2.0 mmol L−1 Ca2+. As shown in Figure 5B, PS immunobands were reduced with >0.25 mmol L−1 Ca2+ in a concentration‐dependent manner.
Figure 5.
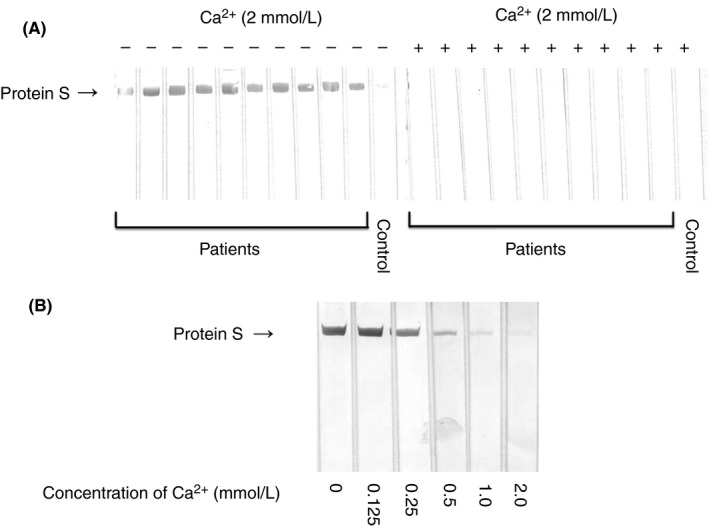
Effects of Ca2+ in immunoblot for anti‐PS. (A) PS was subjected to SDS‐PAGE under reducing conditions. Immunoblots with normal plasma (control) or anti‐PS‐positive patient plasmas in the absence or presence of 2.0 mmol L−1 of Ca2+. (B) PS was subjected to SDS‐PAGE under reducing conditions. Immunoblots with anti‐PS‐positive patient plasma in the presence of increasing concentrations of Ca2+. PS, protein S; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis
3.6. Binding of polyclonal antibodies to EGF to PS
PS or FXII was subjected to SDS‐PAGE under non‐reducing conditions. We then transferred the slab gel contents to PVDF membranes and immunoblotted the contents with anti‐human EGF or anti‐human HB‐EGF in the presence of Ca2+ (2 mmol L−1) or in the absence of Ca2+. As shown in Figure 6A, in the absence of Ca2+, anti‐human EGF recognized the whole PS and FXII molecule under non‐reducing conditions. In the presence of 2.0 mmol L−1 Ca2+, PS immunobands disappeared, but FXII immunobands did not. Anti‐human HB‐EGF did not recognize the whole PS or FXII molecule.
Figure 6.
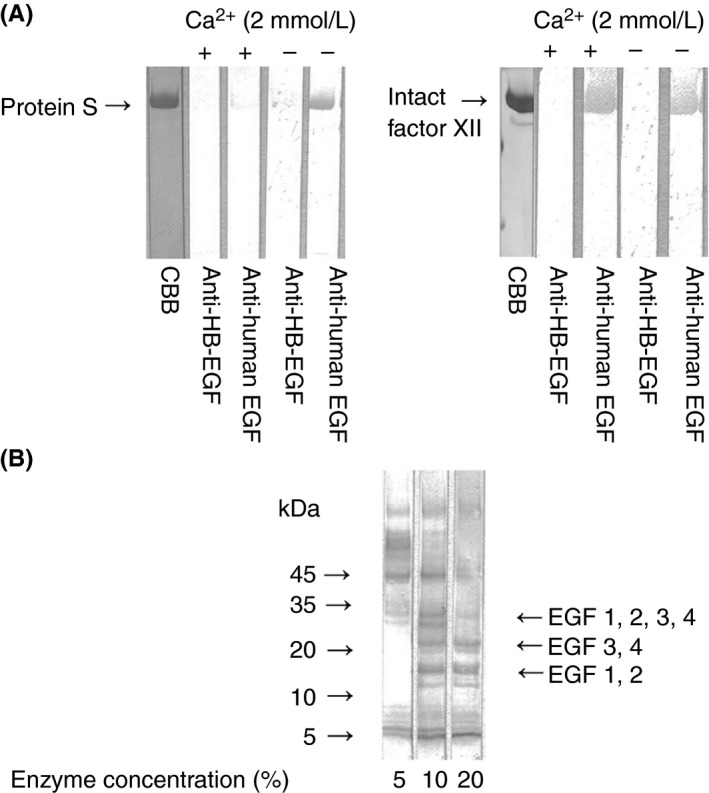
Binding of polyclonal antibodies to EGF to PS (A) SDS‐PAGE was performed using 10% polyacrylamide gel. PS and FXII were prepared in non‐reducing forms. PS (5 μL of 10 μg mL−1 solution) or FXII (5 μL of 10 μg mL−1 solution) was applied to each lane. Transfer to PVDF membrane was done for 20 minute at 0.1 amps. Membranes were blocked for 1.5 hours with 1% BSA in TBS at pH 7.3. Incubation with anti‐human EGF (2 μg mL−1) or anti‐HB‐EGF (2 μg mL−1) as a control was done in the presence of Ca2+ at 2 mmol L−1 or in the absence of Ca2+ for 2 hours followed by three washes with 0.05% Tween 20/TBS. The membrane was exposed to horseradish peroxidase‐conjugated polyclonal antibodies to human IgG for 1 hours. CBB: Coomassie Brilliant Blue. (B) SDS‐PAGE was performed using a polyacrylamide gradient gel (5‐20%). For the preparation of fragments of EGF‐like domains in PS, PS was incubated with several concentrations (5%, 10%, and 20%) of lysyl endopeptidase at 37°C for 1 hour in the presence of Ca2+ (2 mmol L−1). PS (3 μL of 600 μg mL−1 solution) was applied to each lane. Transfer to PVDF membrane was done for 20 minute at 0.1 amps. Membranes were blocked for 1.5 hour with 1% BSA in TBS at pH 7.3. Incubation with anti‐human EGF (2 μg mL−1) was done for 2 hour followed by three washes with 0.05% Tween 20/TBS. The membrane was exposed to horseradish peroxidase‐conjugated polyclonal antibodies to human IgG for 1 hour. EGF, epidermal growth factor; PS, protein S; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis; FXII, factor XII; PVDF, polyvinylidene difluoride; BSA, bovine serum albumin; TBS, Tris‐buffered saline; HB, heparin‐binding
PS incubated with lysyl endopeptidase was subjected to SDS‐PAGE under non‐reducing conditions. We then transferred the slab gel contents to PVDF membranes and immunoblotted the contents with anti‐human EGF. As shown in Figure 6B, the three immunobands (approximately 10‐15 kDa, 20‐24 kDa, and 30 kDa) reported by Dahlbäck et al.13, 20 were seen, suggesting that anti‐human EGF bound to EGF1‐2 (10‐15 kDa fragment) and EGF3‐4 (20‐24 kDa fragment).
3.7. Inhibition of anti‐PS binding by anti‐mouse EGF
PS was subjected to SDS‐PAGE under non‐reducing conditions. We then transferred the slab gel contents to PVDF membranes and incubated the contents with increasing concentrations (0, 0.5, and 1.0 μg mL−1) of anti‐mouse EGF or polyclonal antibodies to LGR in PS (residues 419 to 516) as a control. Then, immunoblotting was performed with anti‐PS‐positive patient plasma. As shown in Figure 7, anti‐mouse EGF blocked anti‐PS binding to PS in a concentration‐dependent manner. Although anti‐human EGF recognized PS, the reactivity was not strong enough for competitive inhibition studies. Therefore, we used anti‐mouse EGF.
Figure 7.
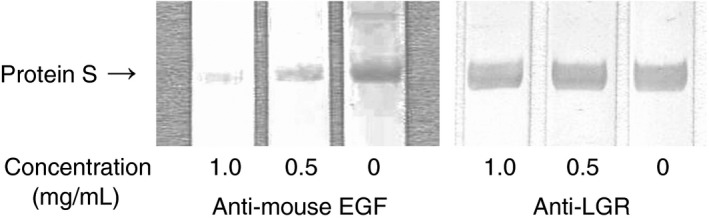
Inhibition of anti‐PS binding by anti‐mouse EGF antibodies. SDS‐PAGE was performed using 10% polyacrylamide gel. PS was prepared in non‐reducing forms. PS (5 μL of 15 μg mL−1 solution) was applied to each lane. Transfer to PVDF membrane was done for 20 minute at 0.1 amps. Membranes were blocked for 1.5 hour with 1% BSA in TBS at pH 7.3. Incubation with increasing concentrations (0, 0.5, 1.0 μg mL−1) of anti‐mouse EGF or polyclonal antibodies to LGR in PS (residues 419 to 516) (anti‐ LGR) as a control was done for 1 hour. Thereafter, incubation with anti‐PS‐positive patient plasma (1/100) was done for 2 hour followed by three washes with 0.05% Tween 20/TBS. The membrane was exposed to horseradish peroxidase‐conjugated polyclonal antibodies to human IgM for 1 hour. PS, protein S; EGF, epidermal growth factor; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis; PVDF, polyvinylidene difluoride; BSA, bovine serum albumin; TBS, Tris‐buffered saline; LGR, a sex hormone‐binding globulin‐like domain containing two laminin G‐like‐domain‐like repeats
3.8. Binding of anti‐PS to EGF
Recombinant mouse EGF, recombinant human EGF or human HB‐EGF was subjected to SDS‐PAGE. We then transferred the slab gel contents to PVDF membranes and immunoblotted the contents with anti‐PS‐positive patient plasmas. Figure 8 shows the binding of anti‐PS to mouse EGF and human EGF, but not human HB‐EGF. Among plasmas from 20 patients with recurrent pregnancy loss who were seropositive for anti‐PS, 14 patients (70.0%) recognized mouse EGF.
Figure 8.
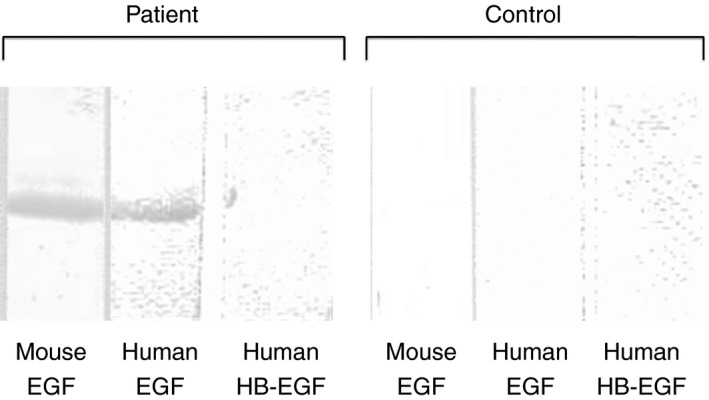
Representative experiment showing patient autoantibody binding to EGF. SDS‐PAGE was performed using a polyacrylamide gradient gel (5‐20%). Recombinant mouse EGF (6 μL of 30 μmol L−1 solution), recombinant human EGF (7 μL of 25 μmol L−1 solution) or recombinant human HB‐EGF (7 μL of 10 μmol L−1 solution) was applied to each lane. Subsequent transfer to PVDF membranes was performed for 20 minute at 0.1 amps. Membranes were blocked for 1.5 hour with 1% BSA in TBS at pH 7.3. Incubation with anti‐PS‐positive patient plasma (1/100) was performed for 2 hours followed by three washes with 0.05% Tween 20/TBS. The membranes were exposed to horseradish peroxidase‐conjugated polyclonal antibodies to human IgG for 1 hour. EGF, epidermal growth factor; SDS‐PAGE, sodium dodecyl sulfate‐polyacrylamide gel electrophoresis; HB, heparin‐binding; PVDF, polyvinylidene difluoride; BSA, bovine serum albumin; TBS, Tris‐buffered saline; PS, protein S
4. DISCUSSION
In the present study, we used thrombin to examine if anti‐PS identified the Gla domain or Gla domain‐free PS. In the immunoblot analysis by means of PS treated with thrombin under reducing conditions, anti‐PS in patients with recurrent pregnancy loss recognized Gla‐domain free PS.
Furthermore, we used high concentrations of lysyl endopeptidase (cleaves COOH‐terminal of lysines) to examine whether anti‐PS in patients with recurrent pregnancy loss bound to EGF‐like domains or LGR in Gla‐domain free PS. Anti‐PS recognized the 10‐15 kDa, 20‐24 kDa, and 30 kDa fragments containing EGF‐like domains.
It has been suggested that PS has an important role in multiple biological processes in addition to coagulation, including apoptosis, angiogenesis/vasculogenesis, and cancer progression.21 PS accumulated around damaged placental trophoblastic cells in early and late pregnancy, indicating that there was a possibility that PS could protect or restore damaged villi and had physiological effects on the placenta.22 Autoantibodies which recognize EGF‐like domains in PS might be related to inhibition of angiogenesis and clearance of apoptotic cells during normal pregnancy.
Several studies focus on the effects of anti‐PS on PS activity. Two studies reported no evidence of PS activity inhibition by anti‐PS.8, 9 In contrast, Sorice et al. demonstrated the possibility of PS activity inhibition by anti‐PS.10 Whether anti‐PS inhibits PS activity depends on the antigenic binding sites of anti‐PS. In the present study, anti‐PS recognized both the 10‐15 kDa fragment comprising only EGF1‐2 and the 20‐24 kDa fragment comprising only EGF3‐4 in the four patients. EGF1‐2 are known to be directly involved in APC cofactor function.13, 14, 15, 16 Three of the four patients whose anti‐PS recognized EGF1‐2 were in the low levels of PS activity. From these findings, anti‐PS recognizing EGF1‐2 may inhibit PS activity. Given that EGF3‐4 in PS are not directly involved in APC cofactor function,18 we speculate that autoantibodies which recognize these EGF‐like domains might be related to inhibition of angiogenesis other than coagulation.
In addition, anti‐PS reactivities against PS under several Ca2+ concentrations were investigated in the immunoblot. PS immunobands were reduced with >0.25 mmol L−1 Ca2+ in a concentration‐dependent manner. It has been suggested that regions crucial for Ca2+ binding in PS are EGF2‐4 in PS.23 The study using recombinant human EGF‐like domains in PS demonstrated that Ca2+ induced conformational changes in these domains.13, 23 These conformational changes may make anti‐PS unable or able to recognize the epitopes. EGF‐like domains in PS contain Ca2+ binding sites, whereas those in FXII do not. Anti‐human EGF recognized the whole PS and FXII molecule. In the presence of Ca2+, PS immunobands disappeared; however, FXII immunobands did not. It was probably due to whether Ca2+ binding induced conformational changes. For anti‐PS, immunobands also disappeared in the presence of Ca2+. These results are consistent with the hypothesis that anti‐PS recognized EGF‐like domains. We hypothesize that anti‐PS recognize PS under physiological conditions in the presence of Ca2+. Indeed, three of the four patients whose anti‐PS recognized EGF1‐2 in immunoblot analysis were in the low levels of PS activity. Anti‐PS can't recognize PS coated on the membrane in the presence of Ca2+ probably due to the conformational changes induced by the membrane. On the other hand, in the epitope mapping study using lysyl endopeptidase, anti‐PS in the four patients recognized EGF‐like domains in the presence of Ca2+.
Interestingly, in the present study, a possibility that autoantibodies affect patients with recurrent pregnancy loss on EGF family proteins arose.
Anti‐human EGF recognized the whole PS molecule and anti‐mouse EGF blocked anti‐PS in patients with recurrent pregnancy loss binding to PS in a concentration‐dependent manner. In this competitive inhibition studies, we used anti‐mouse EGF instead of anti‐human EGF because anti‐mouse EGF recognized PS strongly (data not shown), whereas the reactivity of anti‐human EGF was not strong enough. These findings indicate that anti‐PS in patients with recurrent pregnancy loss and antibodies to EGF probably recognize the same sites of EGF‐like domain in PS and that these are similar antibodies. Indeed, autoantibodies in anti‐PS‐positive patients also recognized human EGF and mouse EGF.
EGF family proteins play an important role in pregnancy. Several studies suggested that human trophoblast survival and invasive ability were associated with intercellular signaling downstream of EGF family proteins.24, 25, 26, 27 EGF is able to protect from apoptosis induced during in vitro culture of human term cytotrophoblast cells,24, 25 which indicates the ability of EGF family proteins to function as survival factors. Autoantibodies to EGF family proteins may inhibit uterine and placental angiogenesis crucial for the survival of embryo and fetus, leading to pregnancy loss.
Recently, our preliminary data suggested that anti‐FXII autoantibodies (anti‐FXII) in patients with recurrent pregnancy loss recognized EGF‐like domains in FXII. Many of these anti‐FXII also recognized PS.28 FXII functions as a growth factor, which mediates cell signaling that leads to proliferation and stimulates angiogenesis.29, 30, 31 Anti‐FXII may inhibit these FXII functions.
In summary, our present study suggests that anti‐PS in patients with recurrent pregnancy loss recognize EGF‐like domains in PS. Interestingly, anti‐PS also recognized EGF family proteins. To date, thrombophilia has been especially focused on recurrent pregnancy loss; however, autoantibody‐associated disruption of the EGF system can be one of the causes of recurrent pregnancy loss. There are limitations of this epitope mapping study. It is unclear whether anti‐PS in patients with recurrent pregnancy loss are pathological in physiological conditions, because studies of antigen‐antibody interactions were only performed. Further studies using in vivo animal models and in vitro culture system are needed to clarify this possibility.
AUTHOR CONTRIBUTIONS
Study concept and design, Y. Sato and T. Sugi; acquisition of data, Y. Sato, T. Sugi, and R. Sakai; analysis and interpretation of data, Y. Sato and T. Sugi; patient inclusion and management, Y. Sato and T. Sugi; drafting of the manuscript, Y. Sato and T. Sugi; study supervisor, T. Sugi.
RELATIONSHIP DISCLOSURE
This research was supported in part by the Project for Baby and Infant Research of Health and Development to Adolescent and Young Adult from Japan Agency for Medical Research and Development, AMED.
Sato Y, Sugi T, Sakai R. Antigenic binding sites of anti‐protein S autoantibodies in patients with recurrent pregnancy loss. Res Pract Thromb Haemost. 2018;2:357–365. 10.1002/rth2.12081
REFERENCES
- 1. Rezende SM, Simmonds RE, Lane DA. Coagulation, inflammation, and apoptosis: different roles for protein S and the protein S–C4b binding protein complex. Blood. 2004;103:1192–201. [DOI] [PubMed] [Google Scholar]
- 2. Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta‐analysis. Lancet. 2003;361:901–8. [DOI] [PubMed] [Google Scholar]
- 3. Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2006;132:171–96. [DOI] [PubMed] [Google Scholar]
- 4. Paidas MJ, Ku DH, Lee MJ, et al. Protein Z, protein S levels are lower in patients with thrombophilia and subsequent pregnancy complications. J Thromb Haemost. 2005;3:497–501. [DOI] [PubMed] [Google Scholar]
- 5. Hojo S, Tsukimori K, Kinukawa N, et al. Decreased maternal proteinS activity is associated with fetal growth restriction. Thromb Res. 2008;123:55–9. [DOI] [PubMed] [Google Scholar]
- 6. Ebina Y, Ieko M, Naito S, et al. Low levels of plasma protein S, protein C and coagulation factor XII during early pregnancy and adverse pregnancy outcome. Thromb Haemost. 2015;114:65–9. [DOI] [PubMed] [Google Scholar]
- 7. Sorice M, Griggi T, Arcieri P, et al. Protein S and HIV infection: the role of anticardiolipin and anti‐protein S antibodies. Thromb Res. 1994;73:165–75. [DOI] [PubMed] [Google Scholar]
- 8. D'Angelo A, Valle PD, Crippa L, Pattarini E, Grimaldi L, D'Angelo SV. Autoimmune protein S deficiency in a boy with severe thromboembolic disease. N Engl J Med. 1993;328:1753–7. [DOI] [PubMed] [Google Scholar]
- 9. Morange PE, Alessi MC, Barthet MC, et al. Acquired protein S deficiency, likely due to anti‐PS autoantibodies, following a thrombotic event in a patient with a systemic lupus erythematosus. Thromb Haemost 1997;78:1416–7. [PubMed] [Google Scholar]
- 10. Sorice M, Arcieri P, Griggi T, et al. Inhibition of protein S by autoantibodies in patients with acquired protein S deficiency. Thromb Haemost. 1996;75:555–9. [PubMed] [Google Scholar]
- 11. Furie B, Bouchard BA, Furie BC. Vitamin K‐dependent biosynthesis of gamma‐carboxyglutamic acid. Blood. 1999;93:1798–808. [PubMed] [Google Scholar]
- 12. Stenflo J. Contributions of Gla and EGF‐like domains to the function of vitamin K‐dependent coagulation factors. Crit Rev Eukaryot Gene Expr. 1999;9:59–88. [PubMed] [Google Scholar]
- 13. Dahlba¨ck B, Hildebrand B, Malm J. Characterization of functionally important domains in human vitamin K‐dependent protein S using monoclonal antibodies. J Biol Chem 1990;265:8127–35. [PubMed] [Google Scholar]
- 14. He X, Shen L, Villoutreix BO, Dahlba¨ck B. Amino acid residues in thrombin‐sensitive region and first epidermal growth factor domain of vitamin K‐dependent protein S determining specificity of the activated protein C cofactor function. J Biol Chem. 1998;273:27449–58. [DOI] [PubMed] [Google Scholar]
- 15. Giri TK, Villoutreix BO, Wallqvist A, Dahlba¨ck B, Garcia de Frutos P. Topological studies of the amino terminal modules of vitamin K‐dependent protein S using monoclonal antibody epitope mapping and molecular modeling. Thromb Haemost. 1998;80:798–804. [PubMed] [Google Scholar]
- 16. Mille‐Baker B, Rezende SM, Simmonds RE, Lane DA, Mason P, Laffan MA. Deletion or replacement of the second EGF‐like domain of protein S results in loss of APC cofactor activity. Blood. 2003;101:1416–8. [DOI] [PubMed] [Google Scholar]
- 17. Stenberg Y, Drakenberg T, Dahlba¨ck B, Stenflo J. Characterization of recombinant epidermal growth factor (EGF)‐like modules from vitamin‐Kdependent protein S expressed in Spodoptera cells—the cofactor activity depends on the N‐terminal EGF module in human protein S. Eur J Biochem. 1998;251:558–64. [DOI] [PubMed] [Google Scholar]
- 18. Saposnik B, Borgel D, Aiach M, Gandrille S. Functional properties of the sex‐hormone‐binding globulin (SHBG)‐like domain of the anticoagulant protein S. Eur J Biochem. 2003;270:545–55. [DOI] [PubMed] [Google Scholar]
- 19. Heeb MJ, Kojima Y, Rosing J, Tans G, Griffin J. C‐terminal residues 621‐635 of protein S are essential for binding to factor Va. J Biol Chem. 1999;274:36187–92. [DOI] [PubMed] [Google Scholar]
- 20. Dahlbäck B, Hildebrand B, Linse S. Novel type of very high affinity calcium‐binding sites in β‐ hydroxy‐ asparagine‐containing epidermal growth factor‐like domains in vitamin K‐dependent protein S. J Biol Chem. 1999;265:18481–9. [PubMed] [Google Scholar]
- 21. Suleiman L, Négrier C, Boukerche H. Protein S: a multifunctional anticoagulant vitamin K‐dependent protein at the crossroads of coagulation, inflammation, angiogenesis, and cancer. Crit Rev Oncol Hematol. 2013;88:637–54. [DOI] [PubMed] [Google Scholar]
- 22. Matsumoto M, Tachibana D, Nobeyama H, et al. Protein S deposition at placenta: a possible role of protein S other than anticoagulation. Blood Coagul Fibrinolysis. 2008;19:653–6. [DOI] [PubMed] [Google Scholar]
- 23. Stenberg Y, Linse S, Drakenberg T, Stenflo J. The high affinity calcium‐binding sites in the epidermal growth factor module region of vitamin K‐dependent protein S. J Biol Chem. 1997;272:23255–60. [DOI] [PubMed] [Google Scholar]
- 24. Payne SG, Brindley DN, Guilbert LJ. Epidermal growth factor inhibits ceramide‐induced apoptosis and lowers ceramide levels in primary placental trophoblasts. J Cell Physiol. 1999;180:263–70. [DOI] [PubMed] [Google Scholar]
- 25. Smith S, Francis R, Guilbert L, Baker PN. Growth factor rescue of cytokine mediated trophoblast apoptosis. Placenta. 2002;23:322–30. [DOI] [PubMed] [Google Scholar]
- 26. Bass KE, Morrish D, Roth I, et al. Human cytotrophoblast invasion is up‐regulated by epidermal growth factor: evidence that paracrine factors modify this process. Dev Biol. 1994;164:550–61. [DOI] [PubMed] [Google Scholar]
- 27. Leach RE, Kilburn B, Wang J, Liu Z, Romero R, Armant DR. Heparin‐binding EGF‐like growth factor regulates human extravillous cytotrophoblast development during conversion to the invasive phenotype. Dev Biol. 2004;266:223–37. [DOI] [PubMed] [Google Scholar]
- 28. Sato Y, Sugi T. A basic study to demonstrate that autoantibodies to factor XII in patients with recurrent pregnancy loss recognize EGF2 domain. J Reprod Immunol. 2016;118:111. [Google Scholar]
- 29. Schmeidler‐Sapiro KT, Ratnoff OD, Gordon EM. Mitogenic effects of coagulation factor XII and factor XIIa on HepG2 cells. Proc Natl Acad Sci U S A. 1991;88:4382–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gordon EM, Venkatesan N, Salazar R, et al. Factor XII‐induced mitogenesis is mediated via a distinct signal transduction pathway that activates a mitogen‐activated protein kinase. Proc Natl Acad Sci U S A. 1996;93:2174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. LaRusch GA, Mahdi F, Shariat‐Madar Z, et al. Factor XII stimulates ERK1/2 and Akt through uPAR, integrins, and the EGFR to initiate angiogenesis. Blood. 2010;115:5111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


