Abstract
Essentials.
There remains clinical concern regarding the optimal management of direct oral (DOAC) anticoagulant effect for emergencies such as bleeding or urgent surgery/procedures.
Idarucizumab is the preferred agent for urgent reversal of dabigatran for severe bleeding or urgent surgeries/procedures.
There are currently no commercially available specific reversal agents for direct Xa inhibitors.
Evidence for prothrombin complex concentrate (PCC), activated PCC (aPCC), and recombinant VIIa (rVIIa) is limited primarily to animal bleeding models and studies in human volunteer subjects.
PCC, aPCC, or rVIIa may contribute to hemostasis for severe bleeding or urgent surgery/procedures in patients receiving factor Xa inhibitors, or dabigatran if idarucizumab is unavailable but benefits and harms are uncertain.
The direct oral anticoagulants (DOACs) are used for stroke prevention in atrial fibrillation (SPAF) and the prevention and treatment of venous thromboembolic disease (VTE). Although DOAC‐associated bleeding events are less frequent as compared to vitamin K antagonists, there is significant concern surrounding physicians’ ability to evaluate and manage DOAC‐associated bleeding when it does occur. Idarucizumab is a specific reversal agent for dabigatran and is the agent of choice for dabigatran reversal in the setting of major bleeding or urgent surgery/procedures. There are no commercially available specific reversal agents for the direct Xa inhibitors. Although they have not been rigorously studied in DOAC‐treated patients requiring urgent anticoagulant reversal, limited evidence from in vitro studies, animal bleeding models, human volunteer studies (in vivo and in vitro) and case series suggest that coagulation factor replacement with prothrombin complex concentrate (PCC) and activated PCC (FEIBA) may contribute to hemostasis. However, the safety and efficacy of these agents and the optimal dosing strategies remain uncertain.
Keywords: andexanet, ciraparantag, direct oral anticoaglants, FEIBA, idarucizumab, prothrombin complex concentrate, recombinant factor VIIa, reversal
1. INTRODUCTION
The direct oral anticoagulants (DOACs) are used for stroke prevention in atrial fibrillation (SPAF) and the prevention and treatment of venous thromboembolic disease (VTE).1, 2, 3, 4, 5, 6, 7, 8 DOACs have advantages over vitamin K antagonists (VKAs), such as rapid onset of action, short half‐lives, predictable pharmacokinetics enabling fixed dosing, wide therapeutic windows that obviate the need for routine laboratory monitoring of anticoagulant effect, and fewer drug‐drug and drug‐food interactions.9 DOACs are associated with fewer bleeding complications compared to VKAs, particularly intracranial hemorrhage (ICH).1, 2, 3, 10, 11 Although DOAC‐associated bleeding events may be less frequent, there remains significant concern regarding management of bleeding events when they occur.12 The anticoagulant effect of VKAs can be reversed with vitamin K, as well as coagulation factor replacement using prothrombin complex concentrates (PCCs) or plasma.13 The degree of VKA anticoagulation and its reversal can be monitored with the international normalized ratio (INR). While dabigatran can be reversed using idarucizumab, there are no specific reversal agents for factor Xa inhibitors. The objective of this narrative review is to provide a comprehensive summary of the evidence regarding pharmacological reversal of DOAC anticoagulant effect.
2. PREPARING FOR REVERSAL: ARE CLINICALLY SIGNIFICANT DOAC LEVELS PRESENT?
2.1. General considerations
Anticoagulant reversal agents and hemostatic products are generally reserved for emergency situations when rapid establishment of normal hemostasis is desired such as severe, refractory and life‐threatening bleeding, and urgent surgery. When considering whether DOAC reversal is required, determining the likely presence of clinically significant drug levels should begin by documenting the type of DOAC taken (including frequency and dosing) and the timing of the last dose. Although DOACs have short half‐lives typically ranging between 5 and 17 hours,14, 15, 16 metabolic derangements (such as renal or liver failure) can influence DOAC plasma concentration and the expected duration of clinically significant drug levels in a given patient. A medication review should identify drug interactions which may influence DOAC levels (eg, inducers or inhibitors of Pg‐P or CYP3A4) and/or contribute to bleeding (eg, antiplatelet therapy).
2.2. Coagulation testing
Although DOACs do not require routine laboratory monitoring of anticoagulant effect, laboratory assessment of hemostasis is useful for emergency situations where DOAC reversal is being contemplated. Unlike VKA anticoagulants for which the INR is used to determine the degree of anticoagulation, routine coagulation testing such as the INR, prothrombin time (PT), and activated partial thromboplastin time (aPTT) do not reliably reflect the presence or degree of DOAC anticoagulant effect.17, 18 Specialized assays which reliably measure DOAC levels are not widely available, particularly for emergency assessment.
Because of their limited sensitivity and reliability,17, 18 routine coagulation tests (PT/INR, aPTT) must be interpreted in light of the clinical context, timing of last dose, and renal function (particularly for dabigatran). These tests can provide qualitative information regarding the presence or absence of clinically significant drug levels (ie, typical on‐therapy or above‐therapy levels). For example, the thrombin time (TT) is very sensitive to the presence of any dabigatran and a normal TT likely excludes clinically significant levels of dabigatran.19, 20, 21 The sensitivity of aPTT for detecting dabigatran is variable, but a prolonged aPTT suggests clinically significant dabigatran levels (especially if using a sensitive assay).22 The most accurate and reliable tests for measuring dabigatran levels are the dilute thrombin time (dTT), ecarin clotting time (ECT), and ecarin chromogenic assay (ECA).18, 19, 21 The PT can be helpful for determining the presence of rivaroxaban and edoxaban, where a prolonged PT suggests clinically significant drug levels.23, 24 However, a normal PT may not exclude clinically significant levels of rivaroxaban or edoxaban.24 The PT and aPTT are relatively insensitive to apixaban and should not be used to exclude the presence of apixaban.17, 18 A prolonged PT suggests clinically significant apixaban levels.17 The most accurate and reliable tests for measuring factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) is a chromogenic anti‐Xa activity assay calibrated to the drug of interest.25 If a drug‐specific calibrated anti‐Xa assay is unavailable, calibration with a low molecular weight heparin standard can be used to exclude clinically significant drug levels (but not quantitation).18
Further, assessment of DOAC plasma levels is complicated by a lack of established therapeutic ranges.17, 18 Typical “on therapy” drug levels represent those measured in pharmacokinetic studies and clinical trials. Threshold DOAC levels below which hemostasis is considered “normal” for both bleeding complications and surgical intervention are uncertain. Current expert consensus guidelines endorsed by the International Society on Thrombosis and Haemostasis Scientific Subcommittee (ISTH SSC) recommend consideration of anticoagulation reversal in the setting of serious bleeding and DOAC concentrations in excess of 50 ng/mL,26 although there is little available evidence to support this recommendation. It is important to highlight that administration of prohemostatic therapy should not be delayed in patients with life‐threatening bleeding (eg, intracranial hemorrhage) while waiting for drug levels. Until rapid turnaround assays are available, administration of prohemostatic therapies should be guided by clinical assessment of the likelihood of clinically significant drug levels, including DOAC dose, frequency, timing of the last dose, and renal function.
2.3. Thrombin generation assays and thromboelastography
Global coagulation tests such as thrombin generation assays (TGAs), thromboelastrography (TEG) or ROTEM are under study for determining DOAC anticoagulant effect and the efficacy of reversal strategies.27, 28, 29 TGAs quantify the rate and amount of thrombin generation as a surrogate marker for fibrin clot formation. TEG/ROTEM measure the forces created by the formation of a clot, which provides information on the rate of clot formation, as well as clot strength and fibrinolysis. Both TGAs and TEG/ROTEM report kinetic/latency parameters (time to significant clot/thrombin endpoint) and quantitative parameters (amount of thrombin generation/strength of clot). With respect to TGAs, the kinetic parameters include the lag time (LT) and time‐to‐peak thrombin generation (TTP), whereas the quantitative parameters usually reported are the endogenous thrombin potential (ETP) and peak thrombin generation.30 Rivaroxaban has been shown to inhibit both kinetic and quantitative TGA parameters.31 Argatroban, a direct thrombin inhibitor similar to dabigatran, has been shown to also affect kinetic and quantitative TGA parameters.32 These assays are not widely available, nor validated for measuring DOAC plasma concentration, and the long turnaround time for TGAs is a limitation for application in emergency settings. Although TEG/ROTEM has a rapid turnaround time that may facilitate its future use as a point‐of‐care assay, its current clinical use is limited to specialized settings such as cardiac surgery.33
3. DABIGATRAN REVERSAL
3.1. Idarucizumab for dabigatran reversal
Idarucizumab is a humanized, monoclonal antibody fragment against dabigatran34 that binds to dabigatran with high affinity (approximately 350‐fold that of thrombin), thereby effectively preventing dabigatran from interacting with its therapeutic target.34 In phase I/II studies, idarucizumab rapidly and fully reversed dabigatran anticoagulation, as assessed by clotting assays such as the dTT and ECT, and reduction in unbound (active) dabigatran.35 Reversal of dabigatran‐anticoagulant effect by idarucizumab has been demonstrated in both young healthy adults,35 as well as older volunteer subjects with mild to moderate renal dysfunction based on correction of laboratory parameters and reduction of unbound dabigatran.36 The prospective cohort REVERSE‐AD study evaluated idarucizumab (5 g intravenously) in dabigatran‐treated patients with major bleeding (n = 301) or requiring emergent procedures or surgery (n = 202).37 Median correction of anticoagulant effect was 100% (95% CI 100‐100) based on correction of prolonged dTT and ECT. Although hemostatic efficacy was included as an outcome, the clinical assessment of hemostasis can be subjective, and cessation of bleeding is often difficult to ascertain, depending on the site of bleeding. Hemostatic efficacy could not be assessed in patients presenting with intracranial hemorrhage, as not all patients underwent scheduled follow‐up cranial imaging to assess for hematoma expansion. Among patients with non‐intracranial bleeding, cessation of bleeding was observed within 24 hours for 67.7% of patients, with a median time to hemostasis of 2.5 hours (95% CI 2.2‐3.9). Among patients receiving idarucizumab for preoperative reversal, normal hemostasis during the procedure was obtained for the vast majority of patients (93.4%).
The 30‐day thromboembolic complication rate following anticoagulation reversal with idarucizumab was 4.8%. Although idarucizumab has not been shown to exert a prothrombotic effect based on laboratory markers,38, 39 a lack of a control group precludes firm conclusions regarding the contribution of idarucizumab to thrombotic risk in patients who have increased thrombotic risk at baseline and in whom anticoagulants have been withheld during acute medical illness. Idarucizumab has a relatively short half‐life of 45 minutes.38 The thrombotic complications occurring within 72 hours of idarucizumab administration all occurred in patients in whom therapeutic anticoagulation had not been reinitiated. Only 72.8% of patients who experienced a major bleeding event had their therapeutic anticoagulation reinitiated by day 90.
Some patients experienced a rise in unbound dabigatran levels around 24 hours following the dose of idarucizumab which may be related to redistribution of dabigatran from the extravascular compartment. There have been a few isolated reports of incomplete dabigatran reversal using the standard 5 g idarucizumab dosing in the setting of severe renal failure.40, 41 There may be a role for repeated doses idarucizumab in these situations, as well as hemodialysis for dabigatran reversal.40
4. NONSPECIFIC PROHEMOSTATIC AGENTS—PCC, APCC, AND RECOMBINANT FACTOR VIIA
Prothrombin complex concentrates (nonactivated and activated) are prepared from pooled human plasma and contain the vitamin K–dependent coagulation factors. Three‐factor PCC (3FPCC) contains factors II, IX, and X, whereas four‐factor PCC (4FPCC) contains relevant levels of factor VII as well. Factor levels are standardized based on factor IX content and can be quite variable across different PCC products.42 Newer formulations of PCC also contain variable amounts of the natural anticoagulants protein C, protein S, as well as small amounts of heparin as a stabilizing agent, so they are contraindicated in patients with heparin‐induced thrombocytopenia (HIT). Activated PCC (FEIBA) contains significant amounts of activated factor VII. Recombinant factor VIIa (rVIIa, NovoSeven, Niastase) was developed to treat bleeding complications in hemophilia patients with inhibitors to factors VIII and IX.
Thrombotic complications have been reported with use of PCC, aPCC, and rFVIIa.43, 44, 45, 46 It remains unclear whether PCC possesses a direct prothrombotic effect at doses used for VKA reversal, or whether thrombotic complications that occur following its use are due to the patients’ underlying thrombotic risk and withdrawal of anticoagulation in the setting of an acute bleeding event. There was no difference in the incidence of thrombotic events in randomized controlled trials of VKA reversal with PCC compared to plasma for major bleeding47 or urgent surgery.48 In a meta‐analysis of PCC for VKA reversal, the incidence of thromboembolic complications was 1.4%, although the reported thrombotic complication rates in individual studies vary widely.49 Activated PCCs were associated with an incidence of thrombotic events of approximately 4:100 000 FEIBA infusions when used in hemophiliacs.45 The thrombotic risk associated with the use of FEIBA in patients for VKA/DOAC reversal or PCC for DOAC reversal remains unclear, although a few small prospective cohort studies, as well as a small retrospective review revealed relatively low 30‐day thrombotic complication rates.50, 51, 52
A growing body of evidence reviewed herein suggests that PCCs and aPCC may be useful adjunctive therapies to promote hemostasis for DOAC‐associated life‐threatening bleeding. However, there is a lack of high‐quality evidence for hemostatic efficacy and safety of PCC, aPCC, and rVIIa in patients requiring urgent DOAC reversal for bleeding or urgent surgery. Thus far, the majority of this evidence is limited to studies in which PCC, aPCC, or rVIIa were added in vitro to plasma from healthy volunteers receiving DOACs, as well as animal models evaluating hemostatic efficacy using various bleeding models. In vivo use of these prohemostatic agents in bleeding patients has been limited to case reports/series, as well as two small prospective cohort studies.50, 51
When assessing the potential benefits and harms of these treatments, it is important to recognize that results from in vitro studies may not necessarily reflect hemostasis in vivo, and therefore, these studies should be interpreted with caution. Further, coagulation assays have variable sensitivity and reproducibility in the presence of DOACs as discussed above. The evidence presented herein should also be interpreted with the caveat that different assays, reagents, PCCs, PCC dosing and animal bleeding models were used in the various studies. This may partially account for some of the variation seen between the study outcomes. For the remainder of the review, we will refer to PCC, aPCC, and rFVIIa as “nonspecific prohemostatic agents”.
5. PCC, APCC, AND RECOMBINANT FACTOR VIIA FOR REVERSAL OF DABIGATRAN
A total of 14 studies evaluated dabigatran reversal with PCC, aPCC, or FVIIa, including eight animal model studies,53, 54, 55, 56, 57, 58, 59, 60 five in vitro studies,61, 62, 63, 64, 65 and one in vivo human volunteer study66 (Tables S1–S3).
5.1. Studies in animal models
5.1.1. PCC
PCC reversed dabigatran's effect on ETP and increased peak thrombin generation.57, 58, 59, 60 ETP overshoot was seen in all studies. Two studies showed complete reversal of dabigatran's effect on ROTEM/TEG clotting time,55, 60 while another two studies demonstrated a partial correction.56, 59 With respect to hemostasis, five of six studies demonstrated at least partial hemostatic efficacy as measured by hematoma expansion, blood loss, bleeding time, or time to hemostasis,53, 55, 57, 58, 60 whereas one study did not show a significant difference in blood loss following PCC administration.54
5.1.2. aPCC
The majority of studies (four of six) showed no effect of aPCC on coagulation assays, with the exception of two separate studies in which the aPTT60 and ECT57 were partially corrected. When combined with rVIIa, partial correction of the aPTT and TT was achieved.54 aPCC reversed dabigatran's effect on ETP57 and partially corrected ROTEM clotting time.56 The effect of aPCC on hemostasis was less certain with one study showing reduced bleeding time57 and another study which failed to show an effect on bleeding time and volume of blood loss.55
5.1.3. rVIIa
Overall, rVIIa had no effect on the aPTT, TT, dTT, and ECT with the exception of one study showing partial correction of aPTT. A total of four studies evaluated the impact of rFVIIa on hemostasis, with three studies demonstrating a significant benefit.54, 57, 58
5.2. Studies in human volunteer subjects
All studies that incorporated TGAs for dabigatran reversal demonstrated significant improvements in ETP and peak thrombin generation following PCC administration61, 62, 63, 64 (Table S3). ETP overshoot was seen in two studies.61, 63 PCC administration had a variable effect on the kinetic parameters of the TGAs, demonstrating a significant improvement in lag time in three out of five studies assessing this parameter.62, 63, 64 PCC had no effect on TTP in two studies.61, 63 All human studies evaluating aPCC demonstrated at least a partial significant correction in both kinetic and quantitative TGA parameters.62, 63, 64 Human studies evaluating rFVIIa demonstrated a significant impact on TGA kinetic parameters, but failed to show any benefit with respect to TGA quantitative parameters.61, 62, 63 The single in vivo human study reported that no thrombotic events were observed during the 24 hours following 4FPCC administration, although this study only had 12 patients in total, and is underpowered to detect thrombotic events.66
5.3. Dabigatran‐treated patients with bleeding
A small prospective cohort study evaluated the use of aPCC (FEIBA) in 14 patients with dabigatran‐associated bleeding.50 Hemostasis was assessed by the treating physician, and arterial/venous thrombotic events were evaluated up to 30 days post‐aPCC administration. The hemostatic efficacy of aPCC was rated as “good” in 64% of patients and “moderate” in 36% of patients. No thromboembolic events occurred during the 30‐day follow‐up. The median dose of aPCC in this study was 44 IU/kg (range, 24‐98 IU/kg). One death occurred 3 days after administration of aPCC in a patient with a large intracranial hemorrhage following withdrawal of life support.
5.4. Thrombotic indices
Transient elevations in D‐dimer values were reported following 4FPCC infusion in an animal model, but no evidence of post‐PCC thrombotic changes was found on histopathologic analysis.60
6. PCC, APCC AND RECOMBINANT FACTOR VIIA FOR REVERSAL OF FACTOR XA INHIBITORS
A total of 8 animal studies,67, 68, 69, 70, 71, 72, 73, 74 13 in vitro human volunteer studies,30, 61, 62, 63, 75, 76, 77, 78, 79, 80, 81, 82, 83 and 8 in vivo human volunteer studies66, 84, 85, 86, 87, 88, 89, 90 evaluated the use of PCC, aPCC, or rFVIIa to reverse the anticoagulant effect of direct factor Xa inhibitors (Tables 1, 2, 3, 4).
Table 1.
Xa inhibitor animal studies—PCC/aPCC/rFVIIa assay results
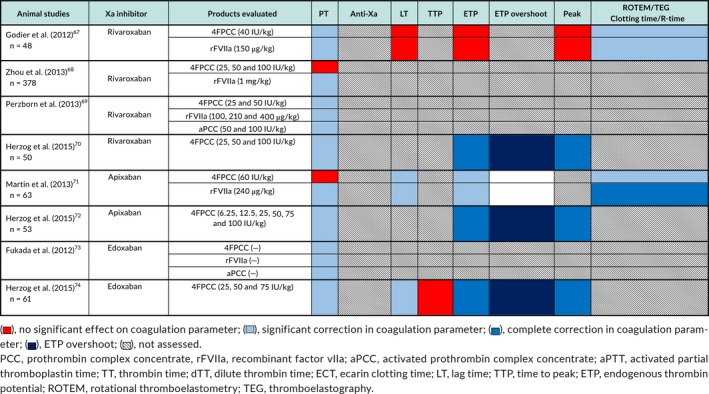
Table 2.
Xa inhibitor animal studies—PCC/aPCC/rFVIIa hemostasis/thrombosis results
| Animal studies | Xa inhibitor | Bleeding model | Products evaluated | Hemostatic efficacy | Thrombotic complications |
|---|---|---|---|---|---|
|
Godier et al. (2012)67
n = 48 |
Rivaroxaban | Rabbit Hepatosplenic Bleeding/Foltz Thrombosis Model | 4FPCC (40 IU/kg) |
Blood Loss: No effect of either 4FPCC or rFVIIa on blood loss Bleeding Time: rFVIIa significantly reduced bleeding time |
Foltz Thrombosis Model: No change in cyclic flow reduction in thrombosis model |
| rFVIIa (150 μg/kg) | |||||
|
Zhou et al. (2013)68
n = 378 |
Rivaroxaban | Murine ICH Bleeding Model | 4FPCC (25, 50 and 100 IU/kg) | Hematoma Expansion: Could be prevented by both PCC (50‐100 IU/kg) and rFVIIa | |
| rFVIIa (1 mg/kg) | |||||
| Perzborn et al. (2013)69 | Rivaroxaban | Murine Mesenteric Artery + Primate Incision Bleeding Model | 4FPCC (25 and 50 IU/kg) |
Murine Bleeding Time: PCC 50 IU/kg, aPCC 50/100 IU/kg and rFVII 400 μg/kg all significantly reduced bleeding time Primate Bleeding Time: aPCC 50 IU/kg normalized bleeding time. rFVIIa 210 μg/kg did not significantly reduce bleeding time |
|
| rFVIIa (100, 210 and 400 μg/kg) | |||||
| aPCC (50 and 100 IU/kg) | |||||
|
Herzog et al. (2015)70
n = 50 |
Rivaroxaban | Rabbit Renal Incision Bleeding Model | 4FPCC (25, 50 and 100 IU/kg) |
Blood Loss: 4FPCC 25‐100 IU/kg successfully reduced total blood loss Time to Hemostasis: 4FPCC 25‐100 IU/kg successfully reduced time to hemostasis |
|
| Martin et al. (2013)71n = 63 | Apixaban | Rabbit Ear Emersion Bleeding Time + Hepatosplenic Blood Loss/Foltz Thrombosis Model | 4FPCC (60 IU/kg) |
Bleeding Time: Only rFVIIa partly corrected bleeding time Blood Loss: Not corrected by either PCC or rFVIIa |
Foltz Thrombosis Model: No significant change in cyclic flow reduction in thrombosis model |
| rFVIIa (240 μg/kg) | |||||
|
Herzog et al. (2015)72
n = 53 |
Apixaban | Rabbit Renal Incision Bleeding Model | 4FPCC (6.25, 12.5, 25, 50, 75 and 100 IU/kg |
Blood Loss: 4FPCC significantly reduced blood loss at doses ≥12.5 IU/kg Time to Hemostasis: 4FPCC significantly reduced time to hemostasis at all doses |
|
| Fukada et al. (2012)73 | Edoxaban | Murine Plantar Bleeding Model/IVC Thrombosis Model | 4FPCC (—) | Bleeding Time: rFVIIa dose‐dependently reversed prolonged bleeding time. Higher dosing normalized bleeding time. FEIBA® 100 IU/kg significantly shortened bleeding time | Thrombus Formation: rFVIIa did not produce a significant difference in thrombus formation in the presence of edoxaban |
| rFVIIa (—) | |||||
| aPCC (—) | |||||
|
Herzog et al. (2015)74
n = 61 |
Edoxaban | Rabbit Renal Incision Bleeding Model | 4FPCC (50 IU/kg) |
Blood Loss: 4FPCC 50, 75 IU/kg dose‐dependently reduced blood loss Time to Hemostasis: 4FPCC 50, 75 IU/kg dose‐dependently reduced time to hemostasis |
PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa; aPCC, activated prothrombin complex concentrate; aPTT, activated partial thromboplastin time; TT, thrombin time; dTT, dilute thrombin time; ECT, ecarin clotting time; LT, lag time; TTP, time to peak; ETP, endogenous thrombin potential; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
Table 3.
Xa inhibitor human studies—PCC/aPCC/rFVIIa assay results
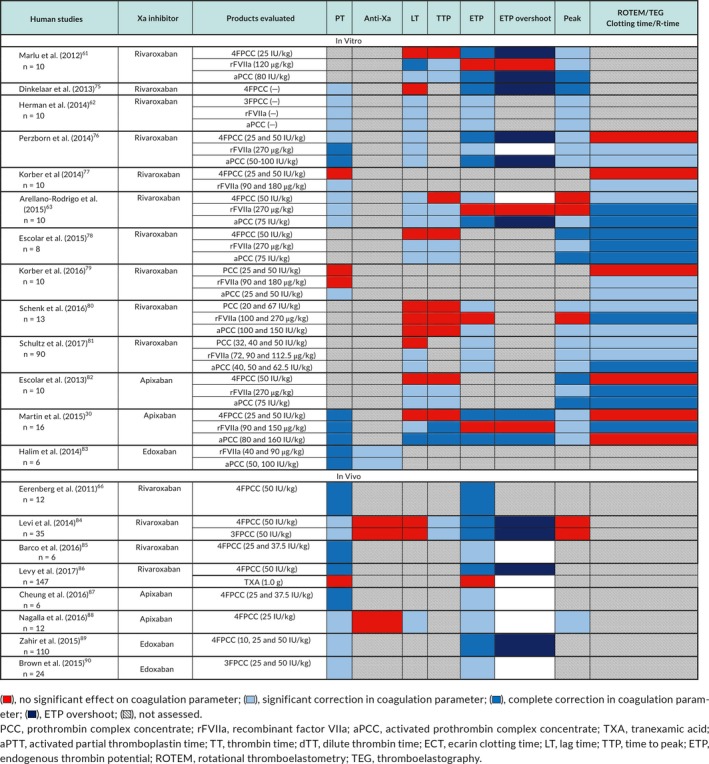
Table 4.
Xa inhibitor human studies—PCC/aPCC/rFVIIa hemostasis/thrombosis results
| Human studies | Xa inhibitor | Bleeding model | Products evaluated | Hemostatic efficacy | Thrombotic complications |
|---|---|---|---|---|---|
| In Vitro | |||||
|
Marlu et al. (2012)61
n = 10 |
Rivaroxaban | 4FPCC (25 IU/kg) | |||
| rFVIIa (120 μg/kg) | |||||
| aPCC (80 IU/kg) | |||||
| Dinkelaar et al. (2013)75 | Rivaroxaban | 4FPCC (—) | |||
| Herman et al. (2014)62n = 10 | Rivaroxaban | 3FPCC (—) | |||
| rFVIIa (—) | |||||
| aPCC (—) | |||||
| Perzborn et al. (2014)76 | Rivaroxaban | 4FPCC (25 and 50 IU/kg) | |||
| rFVIIa (270 μg/kg) | |||||
| aPCC (50‐100 IU/kg) | |||||
|
Korber et al. (2014)77
n = 10 |
Rivaroxaban | 4FPCC (25 and 50 IU/kg | |||
| rFVIIa (90 and 180 μg/kg) | |||||
|
Arellano‐Rodrigo et al. (2015)63
n = 10 |
Rivaroxaban | 4FPCC (50 IU/kg) | |||
| rFVIIa (270 μg/kg) | |||||
| aPCC (75 IU/kg) | |||||
|
Escolar et al. (2015)78
n = 8 |
Rivaroxaban | 4FPCC (50 IU/kg) | |||
| rFVIIa (270 μg/kg) | |||||
| aPCC (75 IU/kg) | |||||
|
Korber et al. (2016)79
n = 10 |
Rivaroxaban | PCC (25 and 50 IU/kg) | |||
| rFVIIa (90 and 180 μg/kg) | |||||
| aPCC (25 and 50 IU/kg) | |||||
|
Schenk et al. (2016)80
n = 13 |
Rivaroxaban | PCC (20 and 67 IU/kg) | |||
| rFVIIa (100 and 270 μg/kg) | |||||
| aPCC (100 and 150 IU/kg) | |||||
|
Schultz et al. (2017)81
n = 90 |
Rivaroxaban | PCC (32, 40 and 50 IU/kg) | |||
| rFVIIa (72, 90 and 112.5 μg/kg) | |||||
| aPCC (40, 50 and 62.5 IU/kg) | |||||
|
Escolar et al. (2013)82
n = 10 |
Apixaban | 4FPCC (50 IU/kg) | |||
| rFVIIa (270 μg/kg) | |||||
| aPCC (75 IU/kg) | |||||
|
Martin et al. (2015)30
n = 16 |
Apixaban | 4FPCC (25 and 50 IU/kg) | |||
| rFVIIa (90 and 150 μg/kg) | |||||
| aPCC (80 and 160 IU/kg) | |||||
|
Halim et al. (2014)83
n = 6 |
Edoxaban | rFVIIa (40 and 90 μg/kg) | No increase in D‐Dimer was observed following administration of either rFVIIa or aPCC | ||
| aPCC (50 and 100 IU/kg) | |||||
| In Vivo | |||||
|
Eerenberg et al. (2011)66
n = 12 |
Rivaroxaban | 4FPCC (50 IU/kg) | No thrombotic events during 24 hour follow‐up | ||
|
Levi et al. (2014)84
n = 35 |
Rivaroxaban | 4FPCC (50 IU/kg) | No thrombotic events during 6 day follow‐up | ||
| 3FPCC (50 IU/kg) | |||||
|
Barco et al. (2016)85
n = 6 |
Rivaroxaban | 4FPCC (25 and 37.5 IU/kg) | No thrombotic events during 24 hour follow‐up | ||
|
Levy et al. (2017)86
n = 147 |
Rivaroxaban | Skin Punch Biopsy Bleeding Model | 4FPCC (50 IU/kg) |
Bleeding Duration: No impact of 4FPCC or TXA on bleeding duration Bleeding Volume: No impact of 4FPCC or TXA on bleeding volume |
Transient elevations in prothrombin fragment 1 + 2 following 4FPCC administration. Transient elevations in TAT following 4FPCC, TXA and saline administration. No change in d‐Dimer levels. No thromboembolic events during approximately 7 days of follow‐up |
| TXA (1.0 g) | |||||
|
Cheung et al. (2016)87
n = 6 |
Apixaban | 4FPCC (25 and 37.5 IU/kg) | No thrombotic events during 24 hour follow‐up | ||
|
Nagalla et al. (2016)88
n = 12 |
Apixaban | 4FPCC (25 IU/kg) | No thrombotic events during 72 hour follow‐up | ||
|
Zahir et al. (2015)89
n = 110 |
Edoxaban | Thigh Punch Biopsy Bleeding Model | 4FPCC (10, 25 and 50 IU/kg) | Bleeding Duration: 4FPCC 50 IU/kg produced complete reversal of edoxaban effect on bleeding duration. 4FPCC 25 IU/kg resulted in partial correction in bleeding duration | Transient elevations in prothrombin fragment 1 + 2 following 4FPCC infusion. No significant change in d‐dimer. No thromboembolic events |
|
Brown et al. (2015)90
n = 24 |
Edoxaban | 3FPCC (25 and 50 IU/kg) | 3FPCC infusion caused transient increase in prothrombin fragment 1 + 2 levels. 3FPCC infusion had no significant effect on d‐dimer. No thrombotic events | ||
PCC, prothrombin complex concentrate; rFVIIa, Recombinant Factor VIIa; aPCC, activated prothrombin complex concentrate; aPTT, activated partial thromboplastin time; TT, thrombin time; dTT, dilute thrombin time; ECT, ecarin clotting time; LT, lag time; TTP, time to peak; ETP, endogenous thrombin potential; ROTEM, rotational thromboelastometry; TEG, thromboelastography.
6.1. Studies in animal models
6.1.1. PCC
4FPCC administration resulted in at least partial correction of the PT in six of eight studies,67, 69, 70, 72, 73, 74 restoration of thrombin generation indices in three of four studies,70, 72, 74 and correction of ROTEM/TEG clotting time.67, 71 ETP overshoot (an increase in ETP above baseline values) was seen with 4FPCC administration in three studies.70, 72, 74 Improved hemostasis as measured by reduction in blood loss, bleeding time, time to hemostasis, or hematoma expansion was shown in five of eight studies.68, 69, 70, 72, 74
6.1.2. aPCC
aPCC administration corrected PT prolongation69, 73 and improved hemostasis as shown by reductions in bleeding time.69, 73
6.1.3. rVIIa
Administration of rFVIIa corrected the PT in all studies in which this was assessed.67, 68, 69, 71, 73 The effect on kinetic TGA parameters was variable with correction in only one of two studies.67, 71 Abnormal ROTEM/TEG clotting time was corrected in both studies that evaluated this measure.67, 71 There was at least partial improvement in hemostasis after rVIIa administration as assessed by bleeding time and hematoma expansion.67, 68, 69, 71, 73
6.2. Studies in human volunteer subjects
6.2.1. PCC
When added in vitro, 4FPCC and 3FPCC corrected factor Xa inhibitor‐induced PT prolongation in four of six studies.30, 62, 75, 76 In human volunteer subjects treated with factor Xa inhibitors, at least partial correction of prolonged PT was seen with in vivo administration of 4FPCC66, 84, 85, 87, 88, 89 and 3FPCC.84, 90 There was no correction of abnormal anti‐Xa activity seen with in vivo administration of 3FPCC or 4FPCC.84, 88 The effect of PCC on thrombin generation indices was variable, but most studies reported at least partial correction of ETP30, 61, 62, 63, 66, 75, 76, 80, 81, 84, 85, 86, 87, 88, 89, 90 and increased peak thrombin generation in 10 of 11 studies.30, 61, 62, 75, 76, 78, 80, 81, 82, 88 ETP overshoot was seen in six studies.61, 75, 76, 84, 86, 89 PCC had a variable effect on ROTEM/TEG clotting time, with four out of nine studies showing at least partial correction.63, 78, 80, 81 4FPCC (50 IU/kg) reduced bleeding duration after punch biopsy in edoxaban‐treated human volunteer subjects.89 However, administration of 4FPCC or TXA to healthy volunteers on rivaroxaban had no effect on bleeding duration or bleeding volume following skin punch biopsy.86
6.2.2. aPCC
When added in vitro, aPCC partially corrected PT prolongation30, 62, 63, 76, 79, 83 and abnormal anti‐Xa activity.83 aPCC restored thrombin generation indices in the majority of studies30, 61, 62, 63, 76, 78, 80, 81, 82 with only one study showing an effect on ETP but no effect on lag time and time to peak.80 ROTEM clotting time was corrected63, 76, 78, 79, 80, 81, 82 in all but one study.39 To our knowledge, aPCC has not been studied in vivo in humans.
6.2.3. rVIIa
The majority of in vitro studies showed at least partial correction of prolonged PT.30, 62, 63, 76, 77, 83 One in vitro study, showed partial reduction of anti‐Xa activity with rFVIIa.83 rFVIIa corrected kinetic thrombin generation parameters (lag time, time to peak) in all30, 61, 62, 63, 76, 78, 81 but one study,80 whereas it had a variable effect on quantitative thrombin generation parameters (ETP, peak thrombin). rFVIIa corrected ROTEM/TEG clotting time in all studies that evaluated this component.30, 63, 76, 78, 79, 80, 81, 82
6.3. Factor Xa‐Inhibitor Treated Patients with Bleeding
A prospective cohort study evaluated 4FPCC for the management of rivaroxaban or apixaban‐associated bleeding.51 This study included a total of 84 patients, 45 of whom were on rivaroxaban and 39 on apixaban. 4FPCC was given at a median dose of 26.7 IU/kg (IQR, 21.4‐29.9 IU/kg). Hemostatic efficacy was rated as “effective” in 58 patients (69.1%) and “ineffective” in 26 patients (30.9%) based on International Society on Thrombosis and Haemostasis criteria.51 Two patients developed ischemic strokes at days 5 and 10 following administration of 4FPCC. A total of 15 patients (18%) out of the 84 evaluated in this study died within 30 days of 4FPCC administration. A majority of these patients (13 patients, 86.7%) had experienced an intracranial hemorrhage as the initial bleeding event. Out of these 15 deaths, 14 were attributed to major bleeding, whereas a single fatal event was attributed to stroke 5 days post‐4FPCC.
6.4. Thrombotic indices
Three animal model studies evaluated the impact of prohemostatic agents on thrombogenesis, none of which demonstrated a significant thrombotic signal.67, 71, 73 Prothrombotic laboratory markers were evaluated in one in vitro83 and seven in vivo human volunteer studies studies.66, 84, 85, 87, 88, 89, 90 Transient elevations in prothrombin fragment 1 + 2 were seen following 4FPCC infusion in two studies,89, 90 with no significant impact on D‐dimer values and no clinical thrombotic events in either study.
7. PCC, APCC, AND RECOMBINANT FACTOR VIIA—SUMMARY
Overall, nonspecific prohemostatic agents such as PCC, aPCC, and rFVIIa may have some effect on reversal of DOAC anticoagulant effect as shown by an effect on laboratory parameters. However, these effects are overall modest and inconsistent between studies. This may be due to differences in study design, assays, reagents, as well as prohemostatic agents and dosing. This variation could also be because assays have not yet been optimized to adequately assess the effects of DOAC reversal using these nonspecific agents.
Idarucizumab is the agent of choice for dabigatran reversal for severe bleeding or urgent surgery in dabigatran‐treated patients. However, there are currently no approved specific reversal agents for the management of direct Xa inhibitor‐associated bleeding. Based on the evidence reviewed, PCC, aPCC, and rVIIa may have a role in promoting hemostasis in patients receiving factor Xa inhibitors, or in patients receiving dabigatran when idarucizumab is not readily available. Only PCC has been studied in vivo for factor Xa inhibitor reversal in humans. This, in addition to its wider availability and reduced cost compared to aPCC make it a reasonable first‐line option for emergency reversal of factor Xa inhibitors. However, there remains significant uncertainty regarding efficacy and potential harms associated with these agents which should be used cautiously and in conjunction with maximal supportive measures, mechanical compression and definitive procedural/surgical intervention as required. Patients should be monitored for thrombotic complications following the administration of these agents, and consideration should be given to resuming anticoagulation once clinically appropriate.
The effect of nonspecific prohemostatic agents is particularly uncertain for DOAC‐treated patients requiring urgent surgery. Elective surgeries should be delayed, if possible, until adequate drug clearance can be achieved or assessment of DOAC levels can be assessed using reliable tests. This issue is further complicated by uncertainty regarding the DOAC levels that would provide adequate hemostasis to undertake surgery safely. Hemostasis may still be impaired following administration of one of these nonspecific agents, and high‐bleed risk procedures (eg, spinal anesthesia) should be avoided.
There is a lack of evidence regarding the dosing strategy for nonspecific hemostatic strategies. Initial dosing of PCC and aPCC at 50 IU/kg (maximum dose as per product monograph) has been suggested because some studies show incomplete correction of laboratory tests with lower doses.53, 54, 67, 69, 89 The use of aPCC (FEIBA) at doses of 75‐100 IU/kg has been studied exclusively in animal models or in vitro human studies, making dosing recommendations challenging and uncertain.79, 81, 83 Dosing of rFVIIa in the animal studies highlighted above is highly variable, and in vitro addition to plasma was done using concentrations equivalent to 90‐270 μg/kg.
8. INVESTIGATIONAL DOAC REVERSAL AGENTS
8.1. Andexanet Alfa for factor Xa inhibitor reversal
Andexanet alfa is a recombinant modified human factor Xa protein, currently undergoing clinical development. Andexanet alfa can bind to both direct (rivaroxaban, apixaban, edoxaban, betrixaban) and indirect (low molecular weight heparin, unfractionated heparin) factor Xa inhibitors.91 Because it lacks catalytic activity and a membrane binding domain, it acts as a decoy protein to sequester circulating factor Xa inhibitors, thereby preventing them from inhibiting endogenous factor Xa. Andexanet alfa has been shown to rapidly reverse the anticoagulant effects of rivaroxaban (ANNEXA‐R) and apixaban (ANNEXA‐A) in older healthy non‐bleeding volunteers based on correction of anti‐Xa activity.92 The half‐life of andexanet alfa is approximately 1 hour,92 and as a result, it is given as a bolus followed by an infusion to produce sustained anticoagulation reversal.
ANNEXA‐4 is an ongoing multicenter, open‐label, prospective study evaluating andexanet alfa for reversal of Xa inhibitor anticoagulant effect in patients with major bleeding.93 Apixaban‐treated patients, or rivaroxaban‐treated patients who presented more than 7 hours after their last dose receive andexanet as a 400 mg intravenous (IV) bolus followed by a 480 mg 2‐hour infusion. Rivaroxaban‐treated patients with recent ingestion of rivaroxaban within 7 hours of presentation receive andexanet as an 800 mg bolus followed by a 960 mg 2‐hour infusion. Higher doses of andexanet alfa are required for rivaroxaban due to higher initial plasma concentrations, as well as a larger volume of distribution.92, 94, 95, 96, 97
In an interim analysis of ANNEXA‐4, 67 patients received andexanet alfa for factor Xa inhibitor reversal (32 receiving rivaroxaban, 31 receiving apixaban, and 4 receiving enoxaparin). The relative reduction in anti‐Xa activity at the end of the andexanet alfa bolus was 89% (95% CI 58‐94) for rivaroxaban and 93% (95% CI 87‐94) for apixaban and this reduction was sustained for the duration of the 2‐hour infusion for both drugs. A rebound increase in anti‐Xa activity was observed at the 4‐hour measurement for both rivaroxaban and apixaban, after the 2‐hour infusion was completed. Clinical hemostasis was assessed in this study, based on predefined criteria.93 Among the 47 patients included in the efficacy analysis, hemostasis was rated as excellent or good in 79% of cases (95% CI, 64‐89). Several patients had persistently elevated anti‐Xa levels at the end of the andexanet infusion. Among patients with the 10% highest anti‐Xa activity levels at the end of the infusion, the median values for anti‐Xa activity was 327.4 ng/mL (IQR = 283.9‐330.1). Although precise therapeutic ranges for DOACs have not been established, these levels are consistent with typical “on‐therapy” DOAC plasma levels.17
All 67 patients in this study were included in the safety analysis. Of note, 18% of patients (12 patients) experienced a thromboembolic event during the 30‐day follow‐up. Four of these 12 patients experienced the thrombotic event within 3 days of the andexanet alfa infusion. Only 1 out of these 12 patients had their therapeutic anticoagulation reinitiated at the time of the thrombotic event. Although direct comparisons between andexanet alfa and idarucizumab cannot be made, transient elevations in prothrombotic laboratory markers (D‐dimer, prothrombin fragment 1 + 2) contribute to concerns about the rate of thrombotic events in the ANNEXA‐4 study. Again, it is unclear whether thrombotic events are associated with a prothrombic effect from the andexanet alfa, or due to underlying thrombotic risk and withdrawal of therapeutic anticoagulation following major bleeding in the absence of a control group in which these markers were measures. Transient elevations in D‐dimer and prothrombin fragment 1 + 2 levels were also noted following andexanet alfa administration in healthy individuals.92 This is thought to be mediated by binding of andexanet alfa to tissue factor pathway inhibitor (TFPI).98 Further study into a potential prothrombotic effect of andexanet alfa is warranted.
8.2. CIRAPARANTAG
Ciraparantag (PER977, aripazine) is a synthetic, rationally designed, small cationic molecule.99 It was designed via in silico modelling to specifically bind directly to unfractionated heparin, low molecular weight heparin, dabigatran, rivaroxaban, apixaban, and edoxaban through noncovalent hydrogen bonding and charge–charge interactions without binding to human coagulation proteins or albumin.99 Ciraparantag reversed rivaroxaban and apixaban‐induced increases in anti‐Xa activity in an in vitro human plasma study and reduced bleeding by >90% after tail transection in rats receiving dabigatran, rivaroxaban, and apixaban.100 In this study, authors report that no procoagulant effects were observed following administration of ciraparantag in both rats and human plasma, although it is unclear how this was assessed.100
Ciraparantag reduced blood loss in a rat‐tail transection model evaluating dabigatran, rivaroxaban, apixaban, and edoxaban, while also restoring PT, aPTT, and TEG parameters to baseline.101 There were no procoagulant effects observed in either rats or humans to date.101 In a rabbit liver laceration model, both andexanet alfa and ciraparantag induced comparable and statistically significant reductions in blood loss at the highest doses studied.102 Ciraparantag, however, did not show reductions in rivaroxaban‐induced prolongation of PT, aPTT, and it did not affect anti‐Xa activity. No change in total plasma rivaroxaban concentrations was seen after ciraparantag administration. A higher molar ratio of ciraparantag to rivaroxaban (30:1) was required to reduce blood loss as compared to andexanet alfa (1:1).102
Ciraparantag has been assessed in healthy volunteers following administration of a single 60‐mg dose of edoxaban.103, 104 In a placebo‐controlled, double blind, escalating dose study involving 80 healthy subjects, whole‐blood clotting time (WBCT) was used to assess the effects of edoxaban on coagulation and reversal with ciraparantag which reduced the WBCT to within 10% of baseline at 10 minutes post‐administration and persisted for 24 hours. Clot structure (as assessed by scanning electron microscopy) was restored to baseline following a single 100 mg IV dose of ciraparantag. In 40 heathy volunteer subjects, ciraparantag (100‐300 mg) reversed the anticoagulant effects of enoxaparin as measured by WBCT.105 Scanning electron micrographs also demonstrated a dose‐dependent increase in fibrin structure formation following ciraparantag administration. There was no evidence of procoagulant effects following administration of ciraparantag as assessed by D‐dimer, prothrombin fragment 1 + 2 and tissue factor pathway inhibitor (TFPI) levels.103, 104, 105 Ciraparantag is currently under further investigation in phase 2 clinical trials (NCT03288454, NCT03172910).
9. CONCLUSIONS
DOAC reversal is indicated when rapid normalization of hemostasis is required for severe bleeding or urgent surgeries/procedures. Idarucizumab is the recommended agent for dabigatran reversal for major bleeding or urgent surgery. PCC, aPCC, or rFVIIa may have a role in the setting of Xa‐inhibitor associated bleeding, or when idarucizumab is not readily available for dabigatran‐associated bleeding, but the efficacy of these agents and their optimal dosing is uncertain. Based on limited data, administration of PCC or aPCC at doses of 25‐50 IU/kg is reasonable for DOAC‐associated life‐threatening bleeding. Andexanet alfa is currently under clinical development as a factor Xa inhibitor reversal agent, whereas ciraparantag is under development as a universal reversal agent.
RELATIONSHIP DISCLOSURE
JS has previously received unrestricted grant funding from Portola Pharmaceuticals. DS reports personal fees and nonfinancial support from Bayer, personal fees from Servier, Portola, and Pfizer.
AUTHOR CONTRIBUTIONS
JS and DS conceived of and designed the review. JS conducted the literature search, collected and summarized data, and wrote the manuscript. DS provided critical revisions to the manuscript content and organization.
Supporting information
ACKNOWLEDGMENTS
DS is the recipient of a Research Early Career Award from the Hamilton Health Sciences Foundation and an ERLI Grant from the Heart and Stroke Foundation of Canada.
Shaw JR, Siegal DM. Pharmacological reversal of the direct oral anticoagulants—A comprehensive review of the literature. Res Pract Thromb Haemost. 2018;2:251–265. 10.1002/rth2.12089
Contributor Information
Joseph R. Shaw, https://twitter.com/JRand083.
Deborah M. Siegal, Email: siegald@mcmaster.ca, https://twitter.com/DebSiegal.
REFERENCES
- 1. Connolly SJ, Ezekowitz MD, Yusuf S, et al.; RELY Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Mahaffey KW, Garg J, et al.; ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 3. Granger CB, Alexander JH, McMurray JJ, et al.; ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 4. Giugliano RP, Ruff CT, Braunwald E, et al.; ENGAGE AF‐TIMI 48 Investigators . Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 5. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 6. Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 7. Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 8. Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 9. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy. Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e326S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chai‐Adisaksopha C, Hillis C, Isayama T, Lim W, Iorio A, Crowther M. Mortality outcomes in patients receiving direct oral anticoagulants: a systematic review and meta‐analysis of randomized controlled trials. J Thromb Haemost. 2015;13:2012–20. [DOI] [PubMed] [Google Scholar]
- 11. Castellucci LA, Cameron C, Le Gal G, et al. Clinical and safety outcomes associated with treatment of acute venous thromboembolism: a systematic review and meta‐analysis. JAMA. 2014;312:1122–35. [DOI] [PubMed] [Google Scholar]
- 12. Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med. 2013;368:1272–4. [DOI] [PubMed] [Google Scholar]
- 13. Ansell J, Hirsh J, Hylek E, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2008;133(Suppl 6):160–98. [DOI] [PubMed] [Google Scholar]
- 14. Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59‐7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–21. [DOI] [PubMed] [Google Scholar]
- 16. Raghavan N, Frost CE, Xu Z, et al. Apixaban metabolism and pharmacokinetics after oral administration in humans. Drug Metab Dispos. 2009;37:74–81. [DOI] [PubMed] [Google Scholar]
- 17. Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151:127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209–19. [DOI] [PubMed] [Google Scholar]
- 19. Douxfils J, Lessire S, Dincq AS, et al. Estimation of dabigatran plasma concentrations in the perioperative setting. An ex vivo study using dedicated coagulation assays. Thromb Haemost. 2015;113:862–9. [DOI] [PubMed] [Google Scholar]
- 20. Lessire S, Douxfils J, Baudar J, et al. Is thrombin time useful for the assessment of dabigatran concentrations? An in vitro and ex vivo study. Thromb Res. 2015;136:693–6. [DOI] [PubMed] [Google Scholar]
- 21. Jabet A, Stepanian A, Golmard JL, et al. CA27: Can PT, aPTT and TT reliably be used to dtect DOAC at clinically relevant concentrations in emergency situations? Results from a 660‐patient cohort study. J Thromb Haemost. 2016;14:20. [Google Scholar]
- 22. Douxfils J, Dogne JM, Mullier F, et al. Comparison of calibrated dilute thrombin time and aPTT tests with LC‐MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate. Thromb Haemost. 2013;110:543–9. [DOI] [PubMed] [Google Scholar]
- 23. Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti‐Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol. 2016;38:505–13. [DOI] [PubMed] [Google Scholar]
- 24. Douxfils J, Chatelain B, Chatelain C, Dogne JM, Mullier F. Edoxaban: impact on routine and specific coagulation assays. A practical laboratory guide. Thromb Haemost. 2016;115:368–81. [DOI] [PubMed] [Google Scholar]
- 25. Douxfils J, Gosselin RC. Laboratory assessment of direct oral anticoagulants. Semin Thromb Hemost. 2017;43:277–90. [DOI] [PubMed] [Google Scholar]
- 26. Levy JH, Ageno W, Chan NC, Crowther M, Verhamme P, Weitz JI. Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2016;14:623–7. [DOI] [PubMed] [Google Scholar]
- 27. Keeling D, Cotter F. Management of bleeding in patients taking FXa and FIIa inhibitors. Br J Haematol. 2013;160:1–2. [DOI] [PubMed] [Google Scholar]
- 28. Dinkelaar J, Patiwael S, Harenberg J, Leyte A, Brinkman HJ. Global coagulation tests: their applicability for measuring direct factor Xa‐ and thrombin inhibition and reversal of anticoagulation by prothrombin complex concentrate. Clin Chem Lab Med. 2014;52:1615–23. [DOI] [PubMed] [Google Scholar]
- 29. Martin AC, Gouin‐Thibault I, Siguret V, et al. Multimodal assessment of non‐specific hemostatic agents for apixaban reversal. J Thromb Haemost. 2015;13:426–36. [DOI] [PubMed] [Google Scholar]
- 30. van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142:889–903. [DOI] [PubMed] [Google Scholar]
- 31. Gerotziafas GT, Elalamy I, Depasse F, Perzborn E, Samama MM. In vitro inhibition of thrombin generation, after tissue factor pathway activation, by the oral, direct factor Xa inhibitor rivaroxaban. J Thromb Haemost. 2007;5:886–8. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka KA, Szlam F, Katori N, Sato N, Vega JD, Levy JH. The effects of argatroban on thrombin generation and hemostatic activation in vitro. Anesth Analg. 2004;99:1283–9. [DOI] [PubMed] [Google Scholar]
- 33. Shore‐Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela‐Cantos F, Ergin MA. Thromboelastography‐guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg 1999;88:312. [DOI] [PubMed] [Google Scholar]
- 34. Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554–62. [DOI] [PubMed] [Google Scholar]
- 35. Glund S, Stangier J, Schmohl M, et al. Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo‐controlled, double‐blind phase I trial. Lancet. 2015;386:680–90. [DOI] [PubMed] [Google Scholar]
- 36. Glund S, Stangier J, Schmohl M, et al. Idarucizumab, a specific antidote for dabigatran: immediate, complete and sustained reversal of dabigatran induced anticoagulation in elderly and renally impaired subjects. Blood. 2014;124:344.24914142 [Google Scholar]
- 37. Pollack CV Jr, Reilly PA, van Ryn J, et al. Idarucizumab for dabigatran reversal—Full cohort analysis. N Engl J Med. 2017;377:431–41. [DOI] [PubMed] [Google Scholar]
- 38. Glund S, Stangier J, van Ryn J, et al. Effect of age and renal function on idarucizumab pharmacokinetics and idarucizumab‐mediated reversal of dabigatran anticoagulant activity in a randomized, double‐blind, crossover phase Ib study. Clin Pharmacokinet. 2017;56:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glund S, Moschetti V, Norris S, et al. A randomised study in healthy volunteers to investigate the safety, tolerability and pharmacokinetics of idarucizumab, a specific antidote to dabigatran. Thromb Haemost. 2015;113:943–51. [DOI] [PubMed] [Google Scholar]
- 40. Simon A, Domanovits H, Ay C, Sengoelge G, Levy JH, Spiel AO. The recommended dose of idarucizumab may not always be sufficient for sustained reversal of dabigatran. J Thromb Haemost. 2017;15:1317–21. [DOI] [PubMed] [Google Scholar]
- 41. Steele AP, Lee JA, Dager WA. Incomplete dabigatran reversal with dabigatran. Clin Toxicol (Phila). 2018;56:216–8. [DOI] [PubMed] [Google Scholar]
- 42. Key NS, Negrier C. Coagulation factor concentrates: past, present, and future. Lancet. 2007;370:439–48. [DOI] [PubMed] [Google Scholar]
- 43. Chavin SI, Siegel DM, Rocco TA Jr, Olson JP. Acute myocardial infarction during treatment with an activated prothrombin complex concentrate in a patient with factor VIII deficiency and a factor VIII inhibitor. Am J Med. 1988;85:245–9. [DOI] [PubMed] [Google Scholar]
- 44. Lusher JM. Thrombogenicity associated with factor IX complex concentrates. Semin Hematol. 1991;28(3 Suppl 6):3–5. [PubMed] [Google Scholar]
- 45. Luu H, Ewenstein B. FEIBA® safety profile in mutliple modes of clinical and home‐therapy application. Haemophilia. 2004;10(suppl. 2):10–6. [DOI] [PubMed] [Google Scholar]
- 46. O'Connoll KA, Wood JJ, Wise RP, Lozier JN, Braun MM. Thromboembolic adverse events after use of a recombinant human coagulation factor VIIa. JAMA. 2006;295:293–8. [DOI] [PubMed] [Google Scholar]
- 47. Sarode R, Milling TJ Jr, Refaai MA, et al. Efficacy and safety of a 4‐factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma‐controlled, phase IIIB study. Circulation. 2013;128:1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goldstein JN, Refaai MA, Milling TJ Jr, et al. Four‐factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open‐label, non‐inferiority, randomised trial. Lancet. 2015;385:2077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dentali F, Marchesi C, Pierfranceschi MG, et al. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. Thromb Haemost. 2011;106:429–38. [DOI] [PubMed] [Google Scholar]
- 50. Schulman S, Ritchie B, Nahirniak S, et al.; Study Investigators . Reversal of dabigatran‐associated major bleeding with activated prothrombin concentrate: a prospective cohort study. Thromb Res. 2017;152:44–8. [DOI] [PubMed] [Google Scholar]
- 51. Majeed A, Agren A, Holmstrom M, et al. Management of rivaroxaban or apixaban associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706–12. [DOI] [PubMed] [Google Scholar]
- 52. Shaw J, Buyting R, Latrous M, et al. Thrombotic complication rates following anticoagulation reversal: a retrospective evaluation of prothrombin complex concentrates. Res Pract Thromb Haemost. 2017;1(Suppl 1):902 (PB 282). [Google Scholar]
- 53. Zhou W, Schwarting S, Illanes S, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–9. [DOI] [PubMed] [Google Scholar]
- 54. Lambourne MD, Eltringham‐Smith LJ, Gataiance S, Arnorld DM, Crowther MA, Sheffield WP. Prothrombin complex concentrates reduce blood loss in murine coagulopathy induced by warfarin, but not in that induced by dabigatran etexilate. J Thromb Haemost. 2012;10:1830–40. [DOI] [PubMed] [Google Scholar]
- 55. Pragst J, Zeitler SH, Doerr B, et al. Reversal of dabigatran anticoagulation by prothrombin complex concentrate (Beriplex P/N) in a rabbit model. J Thromb Haemost. 2012;10:1841–8. [DOI] [PubMed] [Google Scholar]
- 56. Grottke O, van Ryn J, Spronk HMH, Rossaint R. Prothrombin complex concentrate and a specific antidote to dabigatran are effective ex‐vivo in reversing the effects of dabigatran in an anticoagulation/liver trauma experimental model. Crit Care. 2014;18:R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Van Ryn J, Schurer J, Kink‐Eiband M, Clemens A. Reversal of dabigatran‐induced bleeding by coagulation factor concentrates in a rat‐tail bleeding model and lack of effect on assays of coagulation. Anesthesiology. 2014;120:1429–40. [DOI] [PubMed] [Google Scholar]
- 58. Hoffman M, Volovyk Z, Monroe DM. Reversal of dabigatran effects in models of thrombin generation and hemostasis by factor VIIa and prothrombin complex concentrate. Anesthesiology. 2015;122:353–62. [DOI] [PubMed] [Google Scholar]
- 59. Honickel M, Treutler S, van Ryn J, Tillmann S, Rossaint R, Grottke O. Reversal of dabigatran anticoagulation ex vivo: porcine study comparing prothrombin complex concentrates and idarucizumab. Thromb Haemost. 2015;113:728–40. [DOI] [PubMed] [Google Scholar]
- 60. Honickel M, Braunschweig T, van Ryn J, et al. Prothrombin complex concentrate is effective in treating the anticoagulant effects of dabigatran in a porcine polytrauma model. Anesthesiology. 2015;123:1350–61. [DOI] [PubMed] [Google Scholar]
- 61. Marlu R, Hodaj E, Paris A, Albaladejo P, Crackowski JL, Pernod G. Effect of non‐specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban. Thromb Haemost. 2012;108:217–24. [DOI] [PubMed] [Google Scholar]
- 62. Hermann R, Thom J, Wood A, Phillips M, Muhammad S, Baker R. Thrombin generation using the calibrated automated thrombinoscope to assess reversibility of dabigatran and rivaroxaban. Thromb Haemost. 2014;111:989–95. [DOI] [PubMed] [Google Scholar]
- 63. Arellano‐Rodrigo E, Lopez‐Vilchez I, Galan AM, et al. Coagulation factor concentrates fail to restore alterations in fibrin formation caused by rivaroxaban or dabigatran in studies with flowing blood from treated healthy volunteers. Transfus Med Rev. 2015;29:242–9. [DOI] [PubMed] [Google Scholar]
- 64. Khoo TL, Weatherburn C, Kershaw G, Reddel CJ, Crunow J, Dunkley S. The use of FEIBA® in the correction of coagulation abnormalities induced by dabigatran. Int J Lab Hematol. 2013;35:222–4. [DOI] [PubMed] [Google Scholar]
- 65. Solbeck S, Meyer MAS, Johansson PI, et al. Monitoring of dabigatran anticoagulation and its reversal in vitro by thromboelastrography. Int J Cardiol. 2014;176:794–9. [DOI] [PubMed] [Google Scholar]
- 66. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo‐controlled, cross study in healthy subjects. Circulation. 2011;124:1573–9. [DOI] [PubMed] [Google Scholar]
- 67. Godier A, Miclot A, Bonniec BL, et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology 2012;116:94–102. [DOI] [PubMed] [Google Scholar]
- 68. Zhou W, Zorn M, Nawroth P, et al. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke. 2013;44:771–8. [DOI] [PubMed] [Google Scholar]
- 69. Perzborn E, Gruber A, Tinel H, et al. Reversal of rivaroxaban anticoagulation by haemostatic agents in rats and primates. Thromb Haemost. 2013;110:162–72. [DOI] [PubMed] [Google Scholar]
- 70. Herzog E, Kaspereit F, Krege W, et al. Correlation of coagulation markers and 4F‐PCC‐mediated reversal of rivaroxaban in a rabbit model of acute bleeding. Thromb Res. 2015;135:554–60. [DOI] [PubMed] [Google Scholar]
- 71. Martin AC, Le Bonniec B, Fischer AF, et al. Evaluation of recombinant factor VII, prothrombin complex concentrate, and fibrinogen concentrate to reverse apixaban in a rabbit model of bleeding and thrombosis. Int J Cardiol. 2013;168:4228–33. [DOI] [PubMed] [Google Scholar]
- 72. Herzog E, Kaspereit F, Krege J, et al. Four‐factor prothrombin complex concentrate reverses apixaban‐associated bleeding in a rabbit model of acute hemorrhage. J Thromb Haemost. 2015;13:2220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fukada T, Honda Y, Kamisato C, Morishima Y, Shibano T. Reversal of anticoagulant effects of edoxaban, an oral, direct factor Xa inhibitor, with haemostatic agents. Thromb Haemost. 2012;107:253–9. [DOI] [PubMed] [Google Scholar]
- 74. Herzog E, Kaspereit F, Krege W, et al. Effective reversal of edoxaban‐associated bleeding with four‐factor prothrombin complex concentrate in a rabbit model of acute hemorrhage. Anesthesiology. 2015;122:387–98. [DOI] [PubMed] [Google Scholar]
- 75. Dinkelaar J, Molenaar PJ, Ninivaggi M, De Laat B, Brinkman HJM, Leyte A. In vitro assessment, using thrombin generation, of the applicability of prothrombin complex concentrate as an antidote for rivaroxaban. J Thromb Haemost. 2013;11:1111–8. [DOI] [PubMed] [Google Scholar]
- 76. Perzborn E, Heitmeier S, Laux V, Buchmuller A. Reversal of rivaroxaban‐induced anticoagulation with prothrombin complex concentrate, activated prothrombin complex concentrate and recombinant activated factor VII in vitro. Thromb Res. 2014;133:671–81. [DOI] [PubMed] [Google Scholar]
- 77. Korber MK, Langer E, Ziemer S, Perzborn E, Gericke C, von Heymann C. Measurement and reversal of prophylactic and therapeutic peak levels of rivaroxaban: an in vitro study. Clin Appl Thromb Hemost. 2014;20:735–40. [DOI] [PubMed] [Google Scholar]
- 78. Escolar G, Arellno‐Rodrigo E, Lopez‐Vilchez I, et al. Reversal of rivaroxaban‐induced alterations on hemostasis by different coagulation factor concentrates: in vitro studies with steady and circulating human blood. Circ J. 2015;79:331–8. [DOI] [PubMed] [Google Scholar]
- 79. Korber MK, Langer E, Kaufner L, Sander M, von Heymann CV. In vitro reversal of supratherapeutic rivaroxaban levels with coagulation factor concentrates. Blood Transfus. 2016;14:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Schenk B, Wurtinger P, Streif W, Sturm W, Fries D, Bachler M. Ex vivo reversal of effects of rivaroxaban evaluated using thrombolastometry and thrombin generation assay. Br J Anaesth. 2016;117:583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Schultz NH, Tran HTT, Bjornsen S, Henriksson CE, Sandset PM, Holme PA. The reversal effect of prothrombin complex concentrate (PCC), activated PCC and recombinant activated factor VII against anticoagulation of Xa inhibitor. Thromb J. 2017;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Escolar G, Fernandez‐Gallego V, Arellano‐Rodrigo E, et al. Reversal of apixaban induced alterations in hemostasis by different coagulation factor concentrates: significant of studies in vitro with circulating human blood. PLoS ONE. 2013;8:e78696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Halim AB, Samama MM, Mendell J. Ex vivo reversal of the anticoagulant effects of edoxaban. Thromb Res. 2014;134:909–13. [DOI] [PubMed] [Google Scholar]
- 84. Levi M, Moore KT, Castillejos CF, et al. Comparison of three‐factor and four‐factor prothrombin complex concentrates regarding reversal of the anticoagulant effects of rivaroxaban in healthy volunteers. J Thromb Haemost. 2014;12:1428–36. [DOI] [PubMed] [Google Scholar]
- 85. Barco S, Cheung YW, Coppens M, Hutten BA, Meijers JC, Middeldorp S. In vivo reversal of the anticoagulant effect of rivaroxaban with four‐factor prothrombin complex concentrate. Br J Haematol. 2016;172:255–61. [DOI] [PubMed] [Google Scholar]
- 86. Levy JH, Moore KT, Neal MD, et al. Rivaroxaban reversal with prothrombin complex concentrate or tranexamic acid in healthy volunteers. J Thromb Haemost. 2018;16:54–64. [DOI] [PubMed] [Google Scholar]
- 87. Cheung YW, Barco S, Hutten BA, Meijers CM, Middeldorp S, Coppens M. In vivo increase in thrombin generation by four‐factor prothrombin complex concentrate in apixaban‐treated healthy volunteers. J Thromb Haemost. 2015;13:1799–805. [DOI] [PubMed] [Google Scholar]
- 88. Nagalla S, Thomson L, Oppong Y, Bachman B, Chernoveva I, Kraft WK. Reversibility of apixaban anticoagulation with a four‐factor protrhrombin complex concentrate in healthy volunteers. Clin Transl Sci. 2016;9:176–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zahir H, Brown KS, Vandell AG, et al. Edoxaban effects on bleeding following punch biopsy and reversal by a 4‐factor prothrombin complex concentrate. Circulation. 2015;131:82–90. [DOI] [PubMed] [Google Scholar]
- 90. Brown KS, Wickremasingha P, Parasrampuria DA, et al. The impact of a three‐factor prothrombin complex concentrate on the anticoagulatory effects of the factor Xa inhibitor edoxaban. Thromb Res. 2015;136:825–31. [DOI] [PubMed] [Google Scholar]
- 91. Lu G, DeGuzman FR, Hollenbach SJ, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med. 2013;19:446–51. [DOI] [PubMed] [Google Scholar]
- 92. Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–24. [DOI] [PubMed] [Google Scholar]
- 93. Connolly SJ, Truman JM Jr, Eikelboom JW, et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375:1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Crowther M, Lu G, Conley PB, et al. Reversal of factor Xa inhibitors‐induced anticoagulation in healthy subjects by andexanet alfa. Crit Care Med. 2014;42:A1469. [Google Scholar]
- 95. Crowther M, Mathur V, Kitt M, et al. A phase 2 randomized, double‐blind, pacebo‐controlled trial demonstrating reversal of rivaroxaban‐induced anticoagulation in healty subjects by andexanet alfa (PRT064445), an antidote for FXa inhibitors. Blood. 2013;122:3636 (abstract). [Google Scholar]
- 96. Crowther M, Gold A, Lu G, et al. ANNEXA‐A PART 2: a phase 3 randomized, double‐blind, placebo‐controlled trial demonstrating sustained reversal of apixaban‐induced anticoagulation in older subjects by andexanet alfa (PRT064445), a universal antidote for factor Xa (FXA) inhibitors. J Thromb Haemost. 2013;11(Suppl 2):84 (abstract LB004).23809113 [Google Scholar]
- 97. Crowther MA, Levy G, Lu G, et al. A phase 2 randomized, double‐blind, placebo‐controlled trial demonstrating reversal of edoxaban‐induced anticoagulation in healthy subjects by andexanet alfa (PRT064445), a universal antidote for factor XA (fXa) inhibitors. Blood. 2014;124:4269 (abstract). [Google Scholar]
- 98. Lu G, Lin J, Coffey G, Curnutte JT, Conley PB. Interaction of andexanet alfa, a universal antidote to fXA inhibitors, with tissue factor pathway inhibitor enhances reversal of fXA inhibitor‐induced anticoagulation. J Thromb Haemost. 2015;13:635 (abstract PO351). [Google Scholar]
- 99. Laulicht B, Bakhru S, Jiang X, et al. Antidote for new oral anticoagulants: mechanism of action and binding specificity of PER977. J Thromb Haemost. 2013;11:75 (abstract).23809112 [Google Scholar]
- 100. Laulicht B, Bakhru S, Lee C, et al. Small molecular antidote for anticoagulants. Circulation. 2012;126:A11395 (abstract). [Google Scholar]
- 101. Bakhru S, Laulicht B, Jiang X, et al. PER977: a synthetic small molecular which reverses over‐dosage and bleeding by the new oral anticoagulants. Circulation. 2013;128:A18809 (abstract). [Google Scholar]
- 102. Hollenbach S, Lu G, DeGuzman F, et al. Andexanet alfa and PER977 (aripazine) correct blood loss in a rabbit liver laceration model—only andexanet reverses markers of fXa‐mediated anticoagulation. Circulation. 2014;130:A14657 (abstract). [Google Scholar]
- 103. Ansell JE, Bakhru SH, Grosso M, Noveck RJ, Costin JC. Use of PER977 to reverse the anticoagulant effect of edoxaban. N Engl J Med. 2014;371:2141–2. [DOI] [PubMed] [Google Scholar]
- 104. Ansell JE, Bakhru SH, Laulicht BE, et al. Single‐dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemost. 2017;117:238–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ansell JE, Laulicht BE, Bakhru SH, Hoffman M, Steiner SS, Costin JC. Ciraparantag safely and completely reverses the anticoagulant effects of low molecular weight heparin. Thromb Res. 2016;146:113–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


