Abstract
Essentials.
Cognitive impairment is frequent after stroke and knowledge on predictors is limited.
We investigated hemostatic biomarkers as predictors of long‐term cognitive function after stroke.
Fibrinogen, von Willebrand factor and tissue‐type plasminogen activator correlated to cognitive outcome.
In young patients, fibrinogen was independently associated to worse cognitive outcome 7 years post‐stroke.
Background
Cognitive impairment is frequent after stroke, and young patients may live with this consequence for a long time. Predictors of cognitive outcomes after stroke represent a current gap of knowledge.
Objectives
To investigate levels of three hemostatic biomarkers as predictors of long‐term cognitive function after stroke.
Methods
This longitudinal study included consecutively recruited patients with ischemic stroke at 18‐69 years (n = 268). Blood was collected 3 months after index stroke and analyzed for plasma concentrations of fibrinogen, von Willebrand factor (VWF) and tissue‐type plasminogen activator (t‐PA) antigen. Cognitive function 7 years after index stroke was assessed by the Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS). Participants with stroke <50 years of age were also examined by the Trail Making Test A and B (n = 41). Associations between biomarker concentrations and cognitive scales were assessed in the whole group and in participants with stroke <50 years of age.
Results
The hemostatic biomarkers fibrinogen, VWF and t‐PA, were all correlated to total BNIS score, but these associations did not withstand adjustment for confounding factors in the whole group. However, in patients <50 years, we found an independent association between fibrinogen concentrations and total BNIS score (βstd = −.27, 95% confidence interval [CI], −0.47 to −0.07) and to performance on the Trail Making Test A (βstd = .31, 95% CI, 0.03–0.58). No such association was seen for the Trail Making Test B.
Conclusion
High convalescent fibrinogen concentrations were associated with worse long‐term cognitive outcomes in ischemic stroke <50 years of age. We propose further investigations of fibrinogen in relation to cognitive function in stroke in the young.
Keywords: cardiovascular diseases, cognition, hemostasis, prognosis, stroke
1. INTRODUCTION
Persisting cognitive impairment is a frequent symptom after stroke,1, 2, 3 a disability with great impact both on work and social life. Working‐aged patients frequently describe consequences of cognitive dysfunction despite a low residual neurological deficit. This group of patients is generally subject to higher demands on cognitive functioning and may live with the consequences of their stroke for many years. To date, most studies on post‐stroke cognitive outcome are focused on older stroke sufferers.4, 5 To our knowledge, there is only one stroke study including long‐term cognitive assessment of patients of occupational age. The FUTURE study prospectively followed ischemic stroke patients, 18‐50 years of age, for a mean follow‐up time of 11 years. They showed that long‐term cognitive outcome was worse in most cognitive domains compared to a non‐stroke population and a substantially higher proportion of ischemic stroke patients had cognitive impairment or a below average performance compared with controls.6 Since stroke incidence is increasing in younger ages,7 the long‐term effects of cognitive impairment constitute a growing problem both for society and affected individuals as well as their families.8 Biomarkers able to predict cognitive outcomes after stroke could contribute to a better understanding of the mechanisms behind cognitive decline or lack of recovery and might be of value for healthcare and rehabilitation planning.
Plasma concentrations of the hemostatic biomarkers fibrinogen, von Willebrand factor (VWF) and tissue‐type plasminogen activator (t‐PA) are associated to stroke risk, risk of recurrent events and functional outcomes.9, 10, 11, 12, 13 Moreover, both prospective and case‐control studies have suggested a relationship between these hemostatic biomarkers and vascular dementia.14, 15 Regarding high concentrations of fibrinogen, associations to incident vascular dementia have been demonstrated in prospective studies with mean follow‐up times of up to seventeen years.14, 16 Furthermore, in a population with atherosclerosis, but no cardiovascular disease at baseline, an association to cognitive decline during a five‐year follow‐up was demonstrated.17 Altogether, this makes hemostatic proteins, and especially fibrinogen, promising as candidate biomarkers of cognitive outcomes after stroke.
We hypothesized that hemostatic biomarkers predict long‐term cognitive outcome after ischemic stroke. To investigate this hypothesis the biomarkers fibrinogen, VWF, and t‐PA were selected for the present study. Young patients (<50 years) were investigated specifically.
2. MATERIALS AND METHODS
2.1. Study population
The study sample comprised of participants in the Sahlgrenska Academy Study on Ischemic Stroke (SAHLSIS), which consecutively recruits patients with ischemic stroke, aged 18‐69 years, at stroke units, and has been described in detail elsewhere.18 We studied patients recruited between 1998 and 2003 at the Sahlgrenska University Hospital who attended a follow‐up visit with blood sampling approximately 3 months after the event. To avoid influence of an acute vascular event on convalescent biomarker measures, patients suffering from a recurrent vascular event before the three‐month follow‐up were excluded from this study. These events were identified by review of the medical records, and this was performed by a stroke neurologist. To obtain data on recurrent strokes after 3 months, we used the National Hospital Discharge Registry and confirmed events by reviewing the corresponding medical record, according to criteria as previously described.19 Informed consent was obtained from all participants prior to enrollment. This study was approved by the Ethics Committee of the University of Gothenburg.
2.2. Stroke severity, vascular risk factors, and education
Maximum stroke severity within the first 7 days after the stroke was scored using the Scandinavian Stroke Scale (SSS), a scale that describes clinical deficit with a maximum score of 58 points (no deficit). Information on vascular risk factors including hypertension, diabetes, and smoking was collected at inclusion and at the three‐month follow‐up visit by examinations and structured questionnaires, as described.18 To define hypertension and diabetes, measurements at three‐month follow‐up were used, as described elsewhere.18, 20 In brief, hypertension was defined as pharmacological treatment for hypertension, systolic blood pressure ≥160 mm Hg, and/or diastolic blood pressure ≥90 mm Hg. Diabetes was defined as dietary or pharmacological treatment, fasting plasma glucose ≥7.0 mmol/L, and/or fasting blood glucose ≥6.1 mmol/L. Smoking was defined as current or cessation within the last year from inclusion. The educational level of the patients was categorized as low, medium, or high based on answers obtained through questionnaires. Low education corresponds to compulsory school (nine years or less), medium corresponds to secondary school, and high to post‐secondary education.
2.3. Cognitive outcomes
The surviving patients were invited to participate in a seven‐year follow‐up. This consisted of questionnaires and visits to a physician trained in stroke medicine and a research nurse. Home visits were offered to patients unable to visit our clinic. Cognitive functions were tested using the Barrow Neurological Institute Screen for Higher Cerebral Functions (BNIS), and all patients were tested by the same research nurse (IE). This nurse had been trained in administering the BNIS by a neuropsychologist, was supervised by this neuropsychologist throughout the study, and was blinded to biomarker results. The total score (maximum 50p) of the BNIS test reflects the overall cognitive function and consists of a pre‐screen (level of arousal 3p, basic communication 3p, and co‐operation 3p) to evaluate whether the patient is capable to take part in further testing, and the seven subscales: speech and language 15p, orientation 3p, attention/concentration 3p, visual and visuospatial problem solving 8p, memory 7p, affect 4p, and awareness 1p.21 When the BNIS is used as a screening tool for cognitive impairment after stroke, a commonly used cut‐off is a score of 47 or less.22
Patients below 50 years of age at index stroke (n = 67) were also invited to the outpatient clinic at the hospital for an additional more comprehensive cognitive testing including the Trail Making Test A and B.23 All patients were tested by one neuropsychologist (JV) or by our research nurse (IE) who had been trained in administering the tests by JV. The Trail Making Test is a timed task divided into parts A and B. For part A, individuals rapidly connect numbered circles sequentially. Part B is a more complex task that requires examinees to shift between number sequencing and alphabetic sequencing. Part A requires rapid visual scanning and processing speed, part B further requires executive functions such as working memory and attentional shifting.
2.4. Blood sampling and biomarker measurements
Blood sampling was performed at a follow‐up visit approximately three months after the event (median 101 days, range 85‐125 days). Blood was collected between 8.30 and 10.30 a.m. after an overnight fast. Venous blood was collected in tubes that contained 10% by volume of 0.13 mol L−1 sodium citrate. Plasma was isolated within 2 h by centrifugation 2000 × g at 4°C for 20 min and stored at −80°C before assay. The plasma concentrations of VWF and t‐PA antigen were analyzed by ELISAs, the plasma concentration of fibrinogen was measured with an automated clot rate assay, and the serum concentration of hsCRP was determined by a high‐sensitivity immunometric assay, all described in detail previously.9, 11, 24, 25
2.5. Missing data
The success rate for biomarker measurements was: fibrinogen 93%, VWF 96%, t‐PA 97%, and hsCRP 98%. Missing data were mainly due to technical reasons.
2.6. Statistical analyses
Normality of continuous variables was tested. The total score of the BNIS and the total time of Trail Making Test A and B were treated as continuous scales and were normally distributed. For the statistical analyses all biomarker concentrations were log transformed to reduce skewness.
Correlations between biomarker concentrations and clinical variables as well as cognitive scales were assessed by calculating Pearson correlation coefficients. A multivariable linear regression model was created to assess the associations between biomarker concentrations and cognitive scales. To be able to compare the relative importance between the biomarkers, standardized betas (βstd) were used. The standardized betas refer to how many standard deviations the dependent variable (BNIS) is estimated to change per standard deviation increase in the predictor variable. For the multivariable models, we selected variables based on their known or plausible confounding effect, with a priority of variables with the strongest correlations to total BNIS score. For BNIS, variables included in the final model were age, SSS, educational level and time from last stroke (ie, a shorter time than the total follow‐up time for those who experienced a recurrent stroke during the follow‐up period), diabetes and hypertension. Additionally, age <65 years at index stroke was included in the model. This variable was chosen since patients in working‐age are generally offered more intensive rehabilitation compared to patients in non‐working age. For inclusion in the linear regression model, the missing values of acute SSS (n = 7) and educational level (n = 5) were replaced by the median value. Since the influence of biomarker concentrations as well as consequences of cognitive impairment after stroke may differ by age, we additionally performed prespecified analyses in patients <50 years of age at index stroke. For the analyses of Trail Making Test A and B, variables included in this model were age, SSS, and time from last stroke. The standardized betas for the seven BNIS subscales were tested for homogeneity by Cochrans Q test.
Data were analysed using SPSS 20.0, and two‐tailed P < .05 was considered significant.
3. RESULTS
Of 411 patients recruited at baseline, 395 attended the three‐month follow‐up visit and 375 of these had not experienced a recurrent vascular event. During the period between the three‐month and the seven‐year follow‐up, 43 individuals died. Thus, 332 individuals were eligible for cognitive testing 7 years after their index stroke. Of these, 268 were included in the final analyses of this study. Reasons for not participating are displayed in Figure 1. Among eligible study participants, the group that underwent cognitive testing by BNIS did not differ significantly with regards to age, sex, stroke severity, or plasma concentrations of fibrinogen or t‐PA compared to those who did not participate. However, the investigated group had significantly lower three‐month VWF concentrations compared to the group that did not participate (P < .01). Median time from index stroke to follow‐up was 7.4 years (IQR 7.3‐7.5). Forty‐five patients experienced a recurrent stroke during the follow‐up period from the three‐month to the seven‐year visit. Median time from this recurrent stroke to cognitive testing was 5.0 years (IQR 3.2‐7.2).
Figure 1.
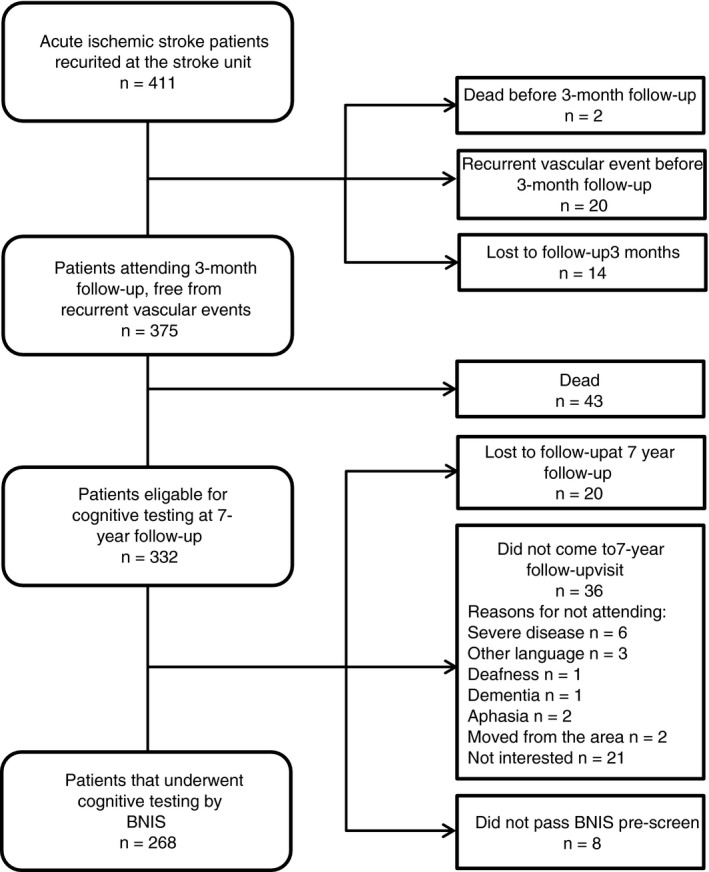
Flow chart of the study population. BNIS, Barrow Neurological Institute Screen for Higher Cerebral Functions
The median total BNIS score was 40 (interquartile range 35‐43) for the whole sample, and 43 (interquartile range 39‐47) for the group of participants with stroke <50 years of age. Clinical data, biomarker concentrations and correlations to total BNIS scores for both groups, are shown in Table 1. In the whole sample age, diabetes, educational level, SSS at baseline, and time since last stroke were significantly correlated to the total BNIS score at the seven‐year follow‐up. High three‐month concentrations of fibrinogen, VWF, and t‐PA were significantly correlated to a low BNIS score. In participants <50 years of age at index stroke, diabetes, low educational level, low SSS at baseline (ie, severe stroke), and high fibrinogen concentrations were significantly correlated to a low BNIS score. Plasma concentrations of fibrinogen in relation to the total BNIS score are shown in Figure 2.
Table 1.
Baseline clinical data and biomarker concentrations in ischemic stroke patients and their correlations to total BNIS score at 7‐year follow‐up
| Whole sample, n = 268 | Stroke <50 years, n = 67 | |||||
|---|---|---|---|---|---|---|
| Correlation coefficient (r) | 95% CI for r | Correlation coefficient (r) | 95% CI for r | |||
| Age at index stroke, years, mean (SD) | 55 (11) | −.28 | −0.39, −0.17 | 40 (8) | .04 | −0.20, 0.28 |
| Age <65 years at index stroke, n (%) | 223 (83%) | −.30 | −0.41, −0.19 | NA | NA | |
| Male sex, n (%) | 169 (63%) | −.05 | −0.17, 0.07 | 32 (48%) | −.10 | −0.33, 0.14 |
| Education | ||||||
| 1 Low level, n (%) | 83 (31%) | .38 | 0.27, 0.48 | 13 (20%) | .37 | 0.14, 0.56 |
| 2 Medium level, n (%) | 103 (38%) | 28 (42%) | ||||
| 3 High level, n (%) | 77 (29%) | 25 (37%) | ||||
| Hypertension, n (%) | 142 (53%) | −.14 | −0.26, −0.02 | 17 (25%) | −.04 | −0.28, 0.20 |
| Smoking, n (%) | 95 (35%) | −.07 | −0.19, 0.05 | 25 (37%) | −.17 | −0.39, 0.07 |
| Diabetes mellitus, n (%) | 45 (17%) | −.20 | −0.31, −0.08 | 11 (16%) | −.40 | −0.58, −0.18 |
| Hyperlipedemia, n (%) | 190 (71%) | −.09 | −0.21, 0.03 | 34 (51%) | −.07 | −0.31, 0.18 |
| SSS score, median (IQR) | 54 (47‐57) | .36 | 0.25, 0.46 | 54 (47‐58) | .67 | 0.51, 0.78 |
| Years from last stroke, median (IQR) | 7.3 (7.2‐7.5) | .25 | 0.14, 0.36 | 7.3 (7.2‐7.5) | .13 | −0.11, 0.36 |
| Fibrinogen, g/L, median (IQR) | 3.2 (2.8‐3.7) | −.22 | −0.33, −0.10 | 2.9 (2.5‐3.4) | −.35 | −0.55, −0.11 |
| VWF antigen, IU/dL, median (IQR) | 208.6 (164.6‐274.3) | −.18 | −0.30, −0.06 | 185.3 (150.5‐226.3) | −.17 | −0.40, 0.08 |
| t‐PA antigen, μg/L, median (IQR) | 11.2 (8.6‐14.4) | −.18 | −0.29, ‐0.06 | 9.6 (6.8‐11.9) | −.23 | −0.45, 0.02 |
BNIS, Barrow Neurological Institute Screen for Higher Cerebral Functions; SSS, Scandinavian Stroke Scale; VWF, von Willebrand factor; t‐PA, tissue‐type plasminogen activator; CI, confidence interval. Pearson correlation was used to calculate correlation to total BNIS score. Data are shown as median and interquartile range (IQR), mean and standard deviation (SD), or number (n) and percentage.
Figure 2.
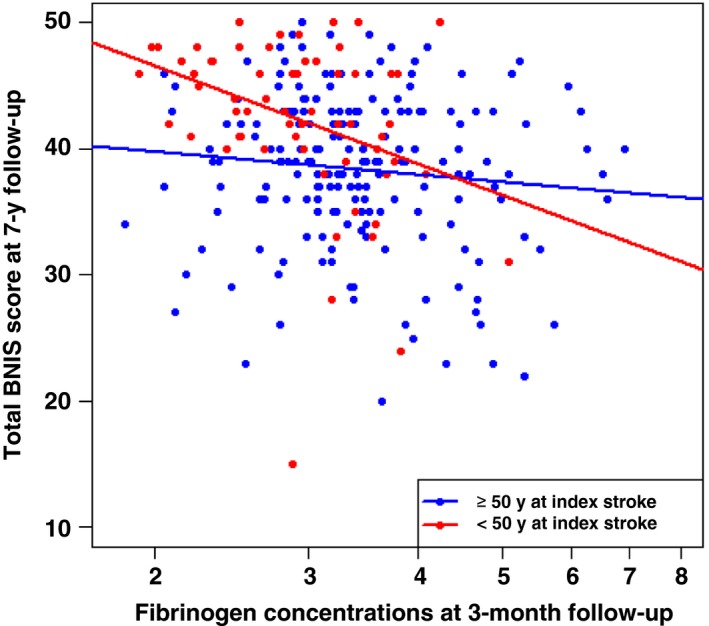
Scatterplot with regression lines for plasma concentrations of fibrinogen at baseline in relation to total BNIS score at 7‐year follow‐up. BNIS, Barrow Neurological Institute Screen for Higher Cerebral Functions
The results from the multivariable linear regression models are shown in Table 2. Age, educational level, SSS at baseline, time since last stroke, and diabetes were all independent predictors of the total BNIS score at follow‐up in the whole group, whereas the associations for the biomarker concentrations were not retained.
Table 2.
Multivariable linear regression analyses showing the associations for baseline characteristics and biomarkers to cognitive function (ie, total BNIS score) 7 years after index ischemic stroke
| Baseline characteristics | Whole sample, n = 268 | Stroke <50 years, n = 67 | ||
|---|---|---|---|---|
| βstd | 95% CI for βstd | βstd | 95% CI for βstd | |
| Age | −0.12 | −0.24, 0.00 | 0.04 | −0.15, 0.23 |
| <65 years | −0.16 | −0.28, −0.05 | NA | NA |
| SSS | 0.35 | 0.25, 0.44 | 0.58 | 0.39, 0.76 |
| Education | 0.31 | 0.21, 0.40 | 0.26 | 0.09, 0.44 |
| Years from last stroke | 0.17 | 0.07, 0.27 | 0.08 | −0.10, 0.25 |
| Diabetes | −0.10 | −0.20, 0.00 | −0.16 | −0.35, 0.03 |
| Hypertension | −0.02 | −0.12, 0.08 | 0.01 | −0.18, 0.19 |
| Biomarkers | ||||
| Fibrinogen | −0.09 | −0.20, 0.01 | −0.27 | −0.47, −0.07 |
| VWF antigen | −0.07 | −0.17, 0.04 | −0.03 | −0.23, 0.18 |
| t‐PA antigen | −0.03 | −0.14, 0.07 | −0.05 | −0.26, 0.17 |
BNIS, Barrow Neurological Institute Screen for Higher Cerebral Functions; SSS, Scandinavian Stroke Scale; VWF, von Willebrand factor; t‐PA, tissue‐type plasminogen activator; βstd, standardized beta; CI, confidence interval. Multivariable linear regression models were used for calculation of βstd for total BNIS score. The betas represent an estimate of how many standard deviations the BNIS score will change per standard deviation increase in the predictor variable. Variables included were baseline characteristics and, for the biomarkers, each biomarker at a time. Units for the predictor variables are given in Table 1. The analyses were based on log transformed biomarker concentrations. For fibrinogen one standard deviation increase represents a biomarker concentration increase of approximately 30% and for t‐PA and VWF the corresponding figure is approximately 50%. Please note that a high SSS score represents a low stroke severity.
In the subgroup of participants who were <50 years of age at index stroke, a similar pattern of correlations between biomarker concentrations and the total BNIS score were observed as in the whole group (Table 1), and here the association for fibrinogen was independent of the clinical variables (βstd = −.27, Table 2) and significantly stronger than in the participants with stroke ≥50 years of age. With regards to the different BNIS subscales, high plasma concentrations of fibrinogen were associated to a low score for all subscales, and this association was not significantly different between subscales.
Forty‐four participants completed the Trail Making Test A, and 41 completed Trail Making Test B. The main reason for not participating was the unwillingness to undergo further cognitive testing. There was no significant difference in total BNIS score, fibrinogen levels or main vascular risk factors between the group who did and did not perform the Trail Making Test A. However, the group who did not perform the test was younger (P < .01) and had a lower SSS score (P = .05). The mean total time for Trail Making Test A was 34 (SD 12) seconds, and for Trail Making Test B 75 (SD 30) seconds. High fibrinogen concentrations were significantly correlated to worse performance (measured as total time) on Trail Making Test A, r = .37 (95% CI, 0.07–0.61). In the linear regression model this association was retained, βstd = .31 (95% CI, 0.03–0.58). No significant as association was detected for fibrinogen and Trail Making Test B, βstd = .09 (95% CI, −0.17 to 0.35).
Considering the significant results for fibrinogen, but not for the other investigated biomarkers, the question arose that, in contrast to our hypothesis, it might be inflammation rather than prothrombotic factors that influence cognitive function. Therefore, in the subgroup with stroke <50 years of age, we additionally investigated hsCRP in relation to total BNIS score. Replacing fibrinogen with hsCRP in the multivariable linear regression model yielded a non‐significant result for hsCRP βstd = −.13 (95% CI, −.32 to 0.07). Including both fibrinogen and hsCRP in the same model resulted in a significant association for fibrinogen βstd = −.27 (95% CI, −0.50 to −0.05), but not for hsCRP βstd = .003 (95% CI,−0.23 to 0.23).
4. DISCUSSION
In this study of young and middle‐aged ischemic stroke patients we found that in participants <50 years of age at index stroke, fibrinogen concentrations measured 3 months post‐stroke were independently associated to total BNIS score 7 years later. No significant difference regarding the association to fibrinogen concentrations was observed between the BNIS subscales. The results indicate an association to overall rather than domain‐specific cognitive function. In line with this, in the same subgroup of patients, fibrinogen concentrations were also independently associated to processing speed, measured as performance on the Trail Making Test A. However, no association was seen for Trail Making Test B, which is a more complex test, also requiring working memory and attentional shifting. The same pattern, with processing speed being affected, has been previously observed in patients with vascular disease and cognitive impairment.26, 27, 28 Our results indicate that fibrinogen concentrations predict long‐term cognitive function in the young, but not all, ischemic stroke patients. Older individuals may have multiple contributing factors that lead to reduced cognitive performance, hence the relative importance of fibrinogen would be less.
To the best of our knowledge this is the first study to show an association between fibrinogen concentrations and long‐term cognitive outcomes in a population of young ischemic stroke patients. Several previous studies support a role for fibrinogen in cognitive functioning. In a population‐based prospective study on older subjects (mean age 73 years at first cognitive assessment and randomly selected from general practitioners), Rafnsson et al. found an association between five‐year cognitive decline and fibrinogen levels measured eleven years prior to the first cognitive assessment.29 Another study found an association between vascular dementia and fibrinogen levels measured seventeen years earlier in a cohort of men aged 65–84 years who were free of vascular disease at baseline.14 In line with this, fibrinogen levels were associated to both vascular dementia and Alzheimer disease in a prospective study following a cohort of men and women, ≥55 years and free of dementia at baseline, for a mean follow‐up time of 5.7 years.16 The number of studies on biomarkers and long‐term cognitive outcomes after stroke is very limited. Interestingly though, a study using a proteomic discovery approach to analyze microvesicle‐enriched fractions of plasma pools from patients with lacunar infarctions, found that fibrinogen alpha‐, beta‐, and gamma chains as well as VWF were among the proteins that were upregulated in patients with cognitive decline at five‐year follow‐up, and fibrinogen alpha was the protein that was most upregulated in this group.30
Several mechanisms could explain the role of hemostatic proteins in cognitive impairment. Generally the correlation between hemostatic biomarkers and vascular dementia is stronger compared to all dementia supporting the explanation of a prothrombotic state and progressive cerebral micro‐infraction.15, 16 In line with this reasoning, a study on young hypertensive patients with subcortical ischemic changes, but no manifest cerebrovascular disease, found that these patients had a reduced performance in cognitive testing as well as higher plasma concentrations of VWF compared to healthy controls.31 In the case of fibrinogen, another possible mechanism is through inflammation. Markers of inflammation have recently been demonstrated to be associated to cognitive function after ischemic stroke32 and infectious burden has also been shown to associate with cognitive decline.33 However, in our study, hsCRP was not significantly associated to cognitive function. Interestingly at last, although we do not know if peripheral fibrinogen concentrations correlate to those in the central nervous system, a previous in vitro study demonstrated that an interaction between amyloid beta and fibrinogen modifies fibrinogen's structure which may lead to abnormal fibrin clot formation and vascular abnormalities in Alzheimer’s disease.34
In our cohort, the correlations between BNIS and VWF as well as t‐PA did not withstand adjustment for confounding factors in the linear regression model. As far as we know, no previous study investigated those biomarkers in a long‐term follow‐up of cognitive function after ischemic stroke.
Our study has the advantage of a longitudinal design with long follow‐up‐time of a study sample including consecutive and well‐characterized ischemic stroke patients. The thorough nature of the available data enabled correction for several possible confounders. Blood sampling was strictly standardized and performed during the chronic, rather than acute, phase, avoiding influence from the acute event itself. Cognitive testing was performed in a standardized way, and the BNIS test was administered by the same study nurse to all participants. There are several limitations that should be mentioned. First, we did not have knowledge on cognitive function before or in the subacute phase of the index stroke and could therefore not investigate changes in relation to biomarker levels. However, most patients were in work at the time of index stroke, making cognitive impairment before the index stroke unlikely in a substantial proportion of patients. Moreover, the effects we observed were most pronounced in younger ages, making the likelihood that pre‐stroke cognitive decline influenced the results even less plausible. Second, although eligible participants who underwent cognitive testing by BNIS did not differ significantly with regards to age, sex, stroke severity, or plasma concentrations of fibrinogen or t‐PA compared to those who did not participate, selection bias could influence our results. Third, the independent associations that we found between fibrinogen concentrations and cognitive function were in a subgroup analysis. Although the analysis was prespecified, the findings need to be replicated. Moreover, the number of patients available for subgroup analyses was limited leading to low power. Fourth, we cannot exclude our results being explained by uncontrolled confounding. Finally, our study design does not allow any conclusion on causation. The authors of a previous study have suggested a reverse causation such that cognitive ability in early life may predict later change in hemostasis, possibly mediated through lifestyle.35
In this study on young and middle‐aged ischemic stroke patients we found that convalescent concentrations of the hemostatic biomarkers fibrinogen, VWF, and t‐PA were correlated to cognitive function 7 years post‐stroke, but these associations were not independent of confounding factors. However, in participants younger than 50 years at index stroke, fibrinogen concentrations were independently associated to cognitive outcomes as assessed by BNIS and Trail Making Test A. Although highly speculative, our results raise the idea that fibrinogen's association to cognitive outcome might not solely go through hemostasis or inflammation, but through a mechanism more specific for fibrinogen. Further, our findings indicate that future studies on hemostatic biomarkers and long‐term cognitive outcomes after stroke should focus on stroke in the young. In the future it would be advantageous to complement these data with vascular imaging studies, to elucidate the underlying mechanisms.
AUTHOR CONTRIBUTIONS
A Pedersen carried out the analyses, interpreted the data, and wrote the manuscript. TM Stanne contributed to interpretation of the data and writing of the manuscript. P Redfors contributed to data acquisition, and critically reviewed the manuscript. J Viken contributed to data acquisition, and critically reviewed the manuscript. H Samuelsson conceived of and designed the study, and critically reviewed the manuscript. S Nilsson carried out part of the statistical analyses, interpreted the data, and critically revised the manuscript. K Jood conceived of and designed the study, and critically reviewed the manuscript. C Jern: conceived of and designed the study, interpreted the data, and critically revised the manuscript. All authors read and approved the manuscript in its final version.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper.
ACKNOWLEDGMENTS
The authors thank research nurse Ingrid Eriksson (IE) for her excellent work and assistance with the study patients. This study was supported by the Swedish Research Council (2013‐3595), the Swedish Heart and Lung Foundation (20160316), the Swedish State under the ALF agreement (ALFGBG‐429981), the Swedish Stroke Association, the Gothenburg Foundation for Neurological Research.
Pedersen A, Stanne TM, Redfors P, et al. Fibrinogen concentrations predict long‐term cognitive outcome in young ischemic stroke patients. Res Pract Thromb Haemost. 2018;00:1–8. 10.1002/rth2.12078
REFERENCES
- 1. Douiri A, Rudd AG, Wolfe CD. Prevalence of poststroke cognitive impairment: South London Stroke Register 1995‐2010. Stroke. 2013;44:138–45. [DOI] [PubMed] [Google Scholar]
- 2. Tatemichi TK, Desmond DW, Stern Y, Paik M, Sano M, Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Planton M, Peiffer S, Albucher JF, et al. Neuropsychological outcome after a first symptomatic ischaemic stroke with ‘good recovery’. Eur J Neurol. 2012;19:212–9. [DOI] [PubMed] [Google Scholar]
- 4. Ben Assayag E, Korczyn AD, Giladi N, et al. Predictors for poststroke outcomes: the Tel Aviv Brain Acute Stroke Cohort (TABASCO) study protocol. Int J Stroke. 2012;7:341–7. [DOI] [PubMed] [Google Scholar]
- 5. Pendlebury ST, Cuthbertson FC, Welch SJ, Mehta Z, Rothwell PM. Underestimation of cognitive impairment by Mini‐Mental State Examination versus the Montreal Cognitive Assessment in patients with transient ischemic attack and stroke: a population‐based study. Stroke. 2010;41:1290–3. [DOI] [PubMed] [Google Scholar]
- 6. Schaapsmeerders P, Maaijwee NA, van Dijk EJ, et al. Long‐term cognitive impairment after first‐ever ischemic stroke in young adults. Stroke. 2013;44:1621–8. [DOI] [PubMed] [Google Scholar]
- 7. Rosengren A, Giang KW, Lappas G, Jern C, Toren K, Bjorck L. Twenty‐four‐year trends in the incidence of ischemic stroke in Sweden from 1987 to 2010. Stroke. 2013;44:2388–93. [DOI] [PubMed] [Google Scholar]
- 8. Persson J, Holmegaard L, Karlberg I, et al. Spouses of stroke survivors report reduced health‐related quality of life even in long‐term follow‐up: results from Sahlgrenska Academy Study on Ischemic Stroke. Stroke. 2015;46:2584–90. [DOI] [PubMed] [Google Scholar]
- 9. Jood K, Danielson J, Ladenvall C, Blomstrand C, Jern C. Fibrinogen gene variation and ischemic stroke. J Thromb Haemost. 2008;6:897–904. [DOI] [PubMed] [Google Scholar]
- 10. Rothwell PM, Howard SC, Power DA, et al. Fibrinogen concentration and risk of ischemic stroke and acute coronary events in 5113 patients with transient ischemic attack and minor ischemic stroke. Stroke. 2004;35:2300–5. [DOI] [PubMed] [Google Scholar]
- 11. Hanson E, Jood K, Karlsson S, Nilsson S, Blomstrand C, Jern C. Plasma levels of von Willebrand factor in the etiologic subtypes of ischemic stroke. J Thromb Haemost. 2011;9:275–81. [DOI] [PubMed] [Google Scholar]
- 12. Ridker PM, Hennekens CH, Stampfer MJ, Manson JE, Vaughan DE. Prospective study of endogenous tissue plasminogen activator and risk of stroke. Lancet. 1994;343:940–3. [DOI] [PubMed] [Google Scholar]
- 13. Whiteley W, Wardlaw J, Dennis M, et al. The use of blood biomarkers to predict poor outcome after acute transient ischemic attack or ischemic stroke. Stroke. 2012;43:86–91. [DOI] [PubMed] [Google Scholar]
- 14. Gallacher J, Bayer A, Lowe G, et al. Is sticky blood bad for the brain?: hemostatic and inflammatory systems and dementia in the Caerphilly Prospective Study. Arterioscler Thromb Vasc Biol. 2010;30:599–604. [DOI] [PubMed] [Google Scholar]
- 15. Quinn TJ, Gallacher J, Deary IJ, Lowe GD, Fenton C, Stott DJ. Association between circulating hemostatic measures and dementia or cognitive impairment: systematic review and meta‐analyzes. J Thromb Haemost. 2011;9:1475–82. [DOI] [PubMed] [Google Scholar]
- 16. van Oijen M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Fibrinogen is associated with an increased risk of Alzheimer disease and vascular dementia. Stroke. 2005;36:2637–41. [DOI] [PubMed] [Google Scholar]
- 17. Marioni RE, Stewart MC, Murray GD, et al. Peripheral levels of fibrinogen, C‐reactive protein, and plasma viscosity predict future cognitive decline in individuals without dementia. Psychosom Med. 2009;71:901–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jood K, Ladenvall C, Rosengren A, Blomstrand C, Jern C. Family history in ischemic stroke before 70 years of age: the Sahlgrenska Academy Study on Ischemic Stroke. Stroke. 2005;36:1383–7. [DOI] [PubMed] [Google Scholar]
- 19. Redfors P, Jood K, Holmegaard L, Rosengren A, Blomstrand C, Jern C. Stroke subtype predicts outcome in young and middle‐aged stroke sufferers. Acta Neurol Scand. 2012;126:329–35. [DOI] [PubMed] [Google Scholar]
- 20. Hanson E, Kanse SM, Joshi A, et al. Plasma factor VII‐activating protease antigen levels and activity are increased in ischemic stroke. J Thromb Haemost. 2012;10:848–56. [DOI] [PubMed] [Google Scholar]
- 21. Prigatano GP. BNI screen for higher cerebral functions: rationale and initial validation. BNI Quart. 1991;7:2–9. [Google Scholar]
- 22. Redfors P, Hofgren C, Eriksson I, Holmegaard L, Samuelsson H, Jood K. The Barrow Neurological Institute screen for higher cerebral functions in cognitive screening after stroke. J Stroke Cerebrovasc Dis. 2014;23:349–55. [DOI] [PubMed] [Google Scholar]
- 23. Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol. 1955;19:393–4. [DOI] [PubMed] [Google Scholar]
- 24. Jern C, Blomstrand C, Westerlind A. Evidence of a net release of tissue‐type plasminogen activator across the human cerebral vasculature. Thromb Haemost. 2004;91:1019–25. [DOI] [PubMed] [Google Scholar]
- 25. Ladenvall C, Jood K, Blomstrand C, Nilsson S, Jern C, Ladenvall P. Serum C‐reactive protein concentration and genotype in relation to ischemic stroke subtype. Stroke. 2006;37:2018–23. [DOI] [PubMed] [Google Scholar]
- 26. Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke‐Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. [DOI] [PubMed] [Google Scholar]
- 27. Gorelick PB, Nyenhuis D, Materson BJ, et al. Blood pressure and treatment of persons with hypertension as it relates to cognitive outcomes including executive function. J Am Soc Hypertens. 2012;6:309–15. [DOI] [PubMed] [Google Scholar]
- 28. Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8:38–45. [DOI] [PubMed] [Google Scholar]
- 29. Rafnsson SB, Deary IJ, Smith FB, et al. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J Am Geriatr Soc. 2007;55:700–7. [DOI] [PubMed] [Google Scholar]
- 30. Datta A, Chen CP, Sze SK. Discovery of prognostic biomarker candidates of lacunar infarction by quantitative proteomics of microvesicles enriched plasma. PLoS ONE. 2014;9:e94663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Consoli D, Di Carlo A, Inzitari D, et al. Subcortical ischaemic changes in young hypertensive patients: frequency, effect on cognitive performance and relationship with markers of endothelial and haemostatic activation. Euro J Neurol. 2007;14:1222–9. [DOI] [PubMed] [Google Scholar]
- 32. Narasimhalu K, Lee J, Leong YL, et al. Inflammatory markers and their association with post stroke cognitive decline. Int J Stroke. 2015;10:513–8. [DOI] [PubMed] [Google Scholar]
- 33. Wright CB, Gardener H, Dong C, et al. Infectious burden and cognitive decline in the Northern Manhattan Study. J Am Geriatr Soc. 2015;63:1540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahn HJ, Zamolodchikov D, Cortes‐Canteli M, Norris EH, Glickman JF, Strickland S. Alzheimer's disease peptide beta‐amyloid interacts with fibrinogen and induces its oligomerization. Proc Nat Acad Sci U S A. 2010;107:21812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luciano M, Marioni RE, Gow AJ, Starr JM, Deary IJ. Reverse causation in the association between C‐reactive protein and fibrinogen levels and cognitive abilities in an aging sample. Psychosom Med. 2009;71:404–9. [DOI] [PubMed] [Google Scholar]


