Abstract
Essentials.
There is a bidirectional relation between acute infections and immobilization
We studied the impact of infection and immobilization on risk of VTE in a case‐crossover design
Acute infection was a strong trigger for VTE independent of concomitant immobilization
Infection and immobilization had a synergistic effect on the VTE‐risk
Background
A bidirectional relation exists between acute infection and immobilization, and both are triggers for venous thromboembolism (VTE). To what extent the association between infection and VTE‐risk is explained by immobilization is unknown.
Aims
To investigate the impact of hospitalization with acute infection on the VTE‐risk in patients with and without concomitant immobilization, and to explore the differential impact of respiratory‐ (RTI) and urinary‐ (UTI) tract infections on the risk of deep vein thrombosis (DVT) and pulmonary embolism (PE).
Methods
We conducted a case‐crossover study of VTE‐patients (n = 707) recruited from a general population. Hospitalizations and VTE‐triggers were registered during the 90 days before a VTE (hazard period) and in four preceding 90‐day control periods. Conditional logistic regression was used to estimate odds ratios (ORs) for VTE according to triggers.
Results
Acute infection was registered in 267 (37.8%) of the hazard periods and in 107 (3.8%) of the control periods, corresponding to a high VTE‐risk after infection (OR 24.2, 95% CI 17.2‐34.0), that was attenuated to 15‐fold increased after adjustment for immobilization. The risk was 20‐fold increased after infection without concomitant immobilization, 73‐fold increased after immobilization without infection, and 141‐fold increased with the two combined. The risk of PE was apparently higher after RTIs (OR 48.3, 95% CI 19.4‐120.0) than UTIs (OR 12.6, 95% CI 6.4‐24.7), but diminished in sensitivity analyses excluding uncertain RTI diagnoses.
Conclusions
Our findings suggest that hospitalization with infection is a strong VTE‐trigger also in non‐immobilized patients. Infection and immobilization had a synergistic effect on the VTE‐risk.
Keywords: deep vein thrombosis, immobilization, infection, pulmonary embolism, venous thromboembolism
1. INTRODUCTION
Venous thromboembolism (VTE), which encompasses deep vein thrombosis (DVT) and pulmonary embolism (PE), is a frequent complication in hospitalized patients,1 and 40‐50% of all VTEs are hospital‐related.2, 3 Established risk factors like active cancer, major surgery, central venous catheter and acute medical conditions, including acute infections, all contribute to the increased risk of VTE in relation to hospitalization.4 Acute infections are associated with increased risk of VTE in both hospitalized and non‐hospitalized patients.5, 6, 7
In most observational study designs, confounding remains a methodological challenge. This is also the case when studying the relationship between infection and VTE, where for instance immobilization can act both as a confounder and as an intermediate in the causal pathway. Immobilization is an important risk factor for VTE,8 but it is also a risk factor for infectious disease. For example, the risk of pneumonia is increased in functionally impaired, and decreased in mobile subjects engaged in daily activity (walking >0.5‐1 h/d).9 Pneumonia is the most common clinical complication after stroke, and stroke severity was an independent risk factor for pneumonia in a retrospective cohort study.10 Moreover, pneumonia could be prevented in patients with acute stroke by adding a passive turning and mobilization program to usual care.11 The relationship between immobilization and infection is bidirectional, as infection often leads to bed‐rest and immobilization. In a study of patients hospitalized with an acute medical disease in the two‐month period before a VTE diagnosis, infection was the most common cause of immobilization.12
In approximately 50% of the cases, PE occurs secondary to embolization of thrombus material from a DVT.13 PEs can also originate from thrombi at the right side of the heart. Atrial fibrillation, pre‐disposing for intra‐cardiac thrombus formation, has been shown to be associated with VTE and PE in particular.14 Respiratory tract infection (RTI), including pneumonia, is associated with increased risk of VTE, and results from a case‐control study (the MEGA study) suggest that pneumonia has a stronger association with PE than DVT.6, 15 This suggests that local inflammation may trigger local activation of coagulation and thrombus formation. In a recent review, Violi and coworkers underscored the need for more knowledge on the relation between pneumonia and VTE.16
In this study, we aimed to investigate the impact of acute infections alone and in combination with immobilization on the risk of VTE. We also aimed to explore the differential impact of the most common infectious foci, namely respiratory tract infection (RTI) and urinary tract infection (UTI), on the location of the VTE (ie DVT and PE). To address these aims, we conducted a case‐crossover study with incident VTE cases recruited from the general population. In this design, each subject serves as its own control, and confounding by chronic conditions, comorbidities, anthropometric and genetic predisposition is therefore largely controlled for through the study design.17
2. METHODS
2.1. Study population
The source population comprised subjects participating in the fourth survey of the Tromsø Study, a single‐center, population‐based cohort study. In 1994/95, all inhabitants over 24 years living in the municipality of Tromsø were invited and 27 158 (77% of the eligible population) participated. The Tromsø Study cohort is described in detail elsewhere.18 Incident VTE events among the study participants were recorded from the date of enrollment (1994‐95) until December 31, 2012. For each potential VTE case the medical records were reviewed by trained personnel, and VTE events were adjudicated and recorded when clinical signs and symptoms of DVT or PE were combined with objective confirmation by radiological procedures, and resulted in a VTE diagnosis requiring treatment, as described in detail previously.19 The University Hospital of North Norway is the only hospital serving the Tromsø region, and all relevant diagnostics and hospital care are provided by this hospital. The study was approved by the regional ethics committee, and all participants provided informed written consent.
2.2. Study design
We conducted a case‐crossover study including all incident VTE cases (n = 707) occurring among the participants of the Tromsø study during 1994‐2012. In the case‐crossover design, the participants serve as their own controls, implying that potential fixed confounders are controlled for through the study design. In this study, a hazard period of 90 days preceding the incident VTE was compared to four 90‐day control periods. We included a 90‐day washout period between the control and the hazard periods, to avoid carry‐over effects (Figure 1). For each VTE case, trained medical personnel searched the hospital medical records for relevant risk factors, diagnostic procedures, surgical and medical treatment, laboratory tests and diagnoses during hospital admissions, day care and outpatient clinic visits in any of the control or hazard periods. We did not have access to medical records from general practice.
Figure 1.
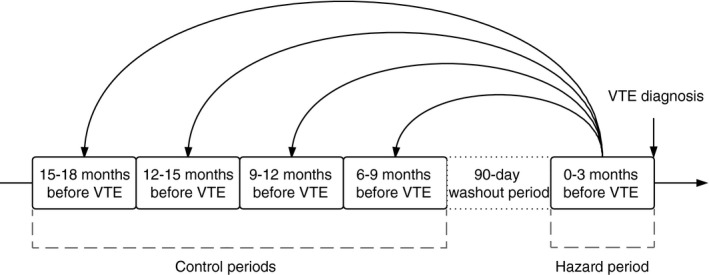
Case‐crossover study design. VTE, venous thromboembolism
2.3. Definition of transient risk factors
A transient risk factor, or trigger, was defined by its presence during the last 90 days before each admission. If an exposure occurred over several days, it was considered to have occurred if any of the days of the exposure fell within the specified 90‐day time period.
Immobilization was defined as the presence of one of the following: bedrest for three days or more, ECOG (Eastern Cooperative Oncology Group) score of four, or other immobilizing factors specified in the patient's medical record (eg, confinement to wheelchair, cast immobilization, etc.). Infection was recorded if an acute infection was noted by a physician in the patient's medical record, and this definition included both community‐acquired infections that required hospital admission and hospital‐acquired infections. Respiratory tract infection was defined as the presence of an upper or lower respiratory tract infection noted by a physician in the patient's medical record. As RTI and PE may have similar symptoms, some PEs could initially be diagnosed as a RTI. To address the possibility that a diagnosis of RTI preceding a PE was wrong, all cases with RTI and PE were re‐evaluated by a specialist in infectious diseases, and the diagnoses of RTI were classified as “most likely correct” (n = 28), “possible” (n = 37) or “most likely incorrect” (n = 8). The “most likely incorrect” RTI‐diagnoses were recoded as “no RTI”. Urinary tract infection was defined as upper or lower urinary tract infection noted by a physician in the patient's medical record, and/or if uro‐pathogen microbes (E. coli, Klebsiella species, and Enterococci) were found by urine culture. Patients with infection other than RTI and UTI were grouped together. Some patients had more than one infection during the hazard or control periods. Blood transfusion, central venous catheterization, trauma, and major surgery were recorded if noted in the medical record.
2.4. Statistical analysis
Statistical analysis was carried out using STATA version 14.0 (Stata Corporation, College Station, TX, USA). We performed a post‐hoc power analysis (asymptomatic z‐test, 1:4 matched design) using the incidence of infection based on our data. If 35% had an infection in the hazard period, and 5% had an infection in a control period, 45 cases would be sufficient to reject the null hypothesis (OR = 1) with 99% power and an alpha level of 5%. We used conditional logistic regression to obtain odds ratios (ORs) with 95% confidence intervals (CI) to estimate the relative risk of VTE according to the presence of infection and immobilization. For infection, we included analyses adjusted for immobilization alone, and in another model, we additionally adjusted for cancer, major surgery, red blood cell transfusion, trauma, and central venous catheter. These adjustment variables were chosen since they are potential triggers of VTE that often co‐exist with both infection and immobilization and thereby could serve as confounders. In a third model, we adjusted for the number of hospitalizations in the hazard and control periods. Hospital‐admissions occurring less than 2 days before the VTE‐diagnosis were excluded, to avoid adjusting for hospitalizations that were due to the VTE. The same models were used in analyses of immobilization; however, these analyses were adjusted for infection instead of immobilization. Conditional logistic regression was used to calculate ORs with 95% CI for the presence of combinations of infection and immobilization, using the combination of no infection and no immobilization as the reference group. The synergy index with corresponding 95% CIs were calculated according to Andersson et al.20 using an Excel sheet (epinet.se/res/xls/epinetcalculation.xls).
Finally, we calculated ORs with 95% CI for infection, RTI, UTI, and other infections through conditional logistic regression in subjects with VTE, DVT, and PE respectively. For sensitivity purposes, we also conducted analyses where those with a possible RTI (n = 37) were counted as no infection.
3. RESULTS
Among the 707 VTE cases, there were 408 DVTs, 254 PEs, and 45 cases of PE with concomitant DVT (Table 1). A total of 1868 hospital contacts, including 441 outpatient or day care visits, were registered during the hazard period and the four control periods among the 707 VTE cases. The number of hospital contacts was higher in the periods closest to the VTE, increasing from 170, 173, 187, and 201 respectively in the control periods, to 1137 hospital contacts in the hazard period. Characteristics of study participants at the time of VTE, and the distribution of VTE‐triggers in the hazard and control periods are shown in Table 1. The median age at VTE was 71 years, and 53.6% were women. Among the 707 cases, 172 (24.3%) had active cancer at the time of VTE‐diagnosis. Prophylactic treatment with low‐molecular weight heparin was prescribed in 138 of the 707 (19.5%) hazard periods, and in 78 of the 2828 (2.8%) control periods.
Table 1.
Characteristics of study participants
| At time of VTE‐diagnosis | |
|---|---|
| Median age, years ± SD | 71 ± 14 |
| Female sex (n, %) | 379 (53.6) |
| DVT only (n, %) | 408 (57.7) |
| DVT + PE (n, %) | 45 (6.4) |
| PE only (n, %) | 254 (35.9) |
| Community‐acquired VTE (n, %) | |
| Outpatient care (n, %) | 154 (21.8) |
| Hospitalized with VTE (n, %) | 418 (59.1) |
| VTE during hospitalization (n, %) | 135 (19.1) |
| Triggers/risk factors | Hazard period (n = 707) | Control periods (n = 2828)b |
|---|---|---|
| Infection (n, %) | 267 (37.8) | 107 (3.8) |
| Immobilizationa (n, %) | 222 (31.4) | 57 (2.0) |
| Cancer (n, %) | 172 (24.3) | 375 (13.2)c |
| Surgery (n, %) | 118 (16.7) | 88 (3.1) |
| Red blood cell transfusion | 82 (11.6) | 28 (1.0) |
| Trauma (n, %) | 71 (10.0) | 25 (0.9) |
| Central venous catheter (n, %) | 56 (7.9) | 17 (0.6) |
DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Bedrest >3 days, ECOG 4, other immobilizing factor (wheelchair use etc.)
707 cases, four control periods for each case.
Based on 106 unique individuals with cancer in one or more of the control periods.
Table 2 shows the frequencies of acute infection and immobilization in the hazard and control periods, with corresponding ORs as estimates of the relative risk of VTE by these triggers. Acute infection occurred in 267 (37.8%) of the hazard periods and 107 (3.8%) of the control periods, and the estimated risk of VTE was high (OR 24.2, 95% CI 17.2‐34.0) after acute infection. The VTE‐risk associated with infection decreased, but remained considerably elevated, after adjusting for immobilization (OR 14.6, 95% CI 10.1‐21.2), and was further slightly attenuated after adding cancer, major surgery, trauma, red blood cell transfusion, and central venous catheter to the adjusted model (OR 10.8, 95% CI 7.2‐16.0). Adjustment for prior hospitalizations yielded similar results (OR 11.6, 95% CI 8.0‐16.7). Immobilization was present in 222 (31.4%) of the hazard periods and 57 (2.0%) of the control periods, yielding a high risk of VTE (OR 66.7, 95% CI 37.3‐119.4). The risk of VTE associated with immobilization decreased after adjusting for infection (OR 37.9, 95% CI 20.6‐70.0) and further after multivariable adjustment (OR 26.3, 95% CI 14.1‐49.2). Sensitivity analysis in which a possible diagnosis of RTI were counted as no infection, yielded high risk estimates for VTE after infection (OR 20.1, 95% CI 14.4‐28.2), and the risk was still considerable after multivariable adjustment (OR 8.1, 95% CI 5.4‐12.1) (Table S1).
Table 2.
Odds ratios for infection and immobilization as triggers for venous thromboembolism
| Hazard period (N = 707) n, (%) | Control periods (N = 2828)a n, (%) | OR (95% CI) | Adjustedd OR (95% CI) | Adjustede OR (95% CI) | Adjustedf OR (95% CI) | |
|---|---|---|---|---|---|---|
| Infectionb | 267 (37.8) | 107 (3.8) | 24.2 (17.2‐34.0) | 14.6 (10.1‐21.2) | 10.8 (7.2‐16.0) | 11.6 (8.0‐16.7) |
| Immobilizationc | 222 (31.4) | 57 (2.0) | 66.7 (37.3‐119.4) | 37.9 (20.6‐70.0) | 26.3 (14.1‐49.2) | 37.6 (20.3‐69.6) |
CI, confidence interval; OR, odds ratio.
707 cases, four control periods for each case.
Reference: no infection.
Reference: no immobilization.
Infection adjusted for immobilization, immobilization adjusted for infection.
Adjusted as in model 1 with addition of cancer, major surgery, trauma, red blood cell transfusion, central venous catheter.
Adjusted for number of hospital admissions except for admission for VTE.
The frequencies of immobilization and acute infection separately and in combination, and the corresponding estimated risks of VTE are shown in Table 3. The estimated risks of VTE were high after acute infection without concomitant immobilization (OR 20.3, 95% CI 13.4‐30.8), and after immobilization without concomitant acute infection (OR 72.5, 95% CI 35.5‐148.0). The combination of acute infection and immobilization had an even greater impact on the estimated risk of VTE (OR 140.7, 95% CI 66.4‐297.9), yielding a synergy index of 1.5 (95% CI 0.7‐3.2), which suggested a positive interaction on an additive scale. Table 4 shows frequencies of infection and different infectious foci for all subjects with VTE and for subjects with DVT and PE separately, as well as the ORs for VTE, DVT, and PE by various infectious foci. The estimated risk of VTE was highest after RTI (OR 21.8, 95% CI 13.0‐36.5), followed by UTI (OR 14.6, 95% CI 9.4‐22.6), and other infections (OR 12.5, 95% CI 8.0‐19.6). Acute infection had a higher impact on the estimated risk of PE (OR 32.4, 95% CI 18.2‐57.5) than DVT (OR 19.9, 95% CI 13.0‐30.6). UTI was more prevalent than RTI in the hazard period preceding a DVT, and had a slightly greater impact on DVT risk (OR 16.1, 95% CI 9.0‐28.8 vs. 10.7, 95% CI 5.5‐20.8). RTI, however, displayed a higher estimated risk of PE than UTI (OR 48.3, 95% CI 19.4‐120.0 vs. 12.6, 95% CI 6.4‐24.7). In sensitivity analysis, where possible cases of RTI were counted as no infection, the estimated risk of PE did not differ significantly across various infectious foci (Table S2). Further, we performed sensitivity analysis including only lower UTI, to address variation in infection severity. These results were similar to those for all UTIs (VTE: OR 13.3, 95% CI 8.6‐20.5, DVT: OR 14.0, 95% CI 7.9‐24.8, PE: OR 12.3, 95% CI 6.2‐24.1).
Table 3.
Odds ratios for combinations of infection and immobilization as triggers for venous thromboembolism
| Hazard period (N = 707) n, (%) | Control periods (N = 2828)a n, (%) | OR (95% CI) | |
|---|---|---|---|
| Infection, no immobilizationb | 140 (19.8) | 84 (3.0) | 20.3 (13.4‐30.8) |
| Immobilization, no infectionb | 95 (13.4) | 34 (1.2) | 72.5 (35.5‐148.0) |
| Infection and immobilizationb | 127 (18.0) | 23 (0.8) | 140.7 (66.4‐297.9) |
CI, confidence interval; OR, odds ratio.
707 cases, four control periods for each case.
Reference: no infection, no immobilization.
Table 4.
Odds ratios for all infections, respiratory and urinary tract infections as triggers for DVT, PE, and VTEa
| VTE | Hazard period (N = 707) n, (%) | Control periods (N = 2828)b n, (%) | OR (95% CI) |
|---|---|---|---|
| All infections | 267 (37.8) | 107 (3.8) | 24.2 (17.2‐34.0) |
| Respiratory tract infection | 98 (13.9) | 29 (1.0) | 21.8 (13.0‐36.5) |
| Urinary tract infection | 103 (14.6) | 47 (1.7) | 14.6 (9.4‐22.6) |
| Other infections | 84 (11.9) | 35 (1.2) | 12.5 (8.0‐19.6) |
| DVT | Hazard period (N = 408) n, (%) | Control periods (N = 1632)c n, (%) | OR (95% CI) |
|---|---|---|---|
| All infections | 143 (35.0) | 60 (3.7) | 19.9 (13.0‐30.6) |
| Respiratory tract infection | 33 (8.1) | 15 (0.9) | 10.7 (5.5‐20.8) |
| Urinary tract infection | 62 (15.2) | 24 (1.5) | 16.1 (9.0‐28.8) |
| Other infections | 59 (14.5) | 21 (1.3) | 14.0 (8.1‐24.4) |
| PE | Hazard period (N = 299) n, (%) | Control periods (N = 1196)d n, (%) | OR (95% CI) |
|---|---|---|---|
| All infections | 124 (41.5) | 47 (3.9) | 32.4 (18.2‐57.5) |
| Respiratory tract infection | 65 (21.7) | 14 (1.2) | 48.3 (19.4‐120.0) |
| Urinary tract infection | 41 (13.7) | 23 (1.9) | 12.6 (6.4‐24.7) |
| Other infections | 25 (8.4) | 14 (1.2) | 9.9 (4.6‐21.3) |
Reference: absence of the specified infection.
707 VTE‐cases, four control periods for each case.
408 DVT‐cases, four control periods for each case.
299 PE‐cases, four control periods for each caseCI, confidence interval; DVT, deep vein thrombosis; PE, pulmonary embolism; OR, odds ratio; VTE, venous thromboembolism.
Among the patients with PE, 65 had a RTI in the hazard period. Information on the location of both the RTI and the PE (from either chest X‐ray, CT‐scan, lung scintigraphy, or autopsy) was available in 43 of those 65 patients. In 22 of the 43 cases with available information, radiological signs of infection were described at the ipsilateral side of the PE, four had bilateral signs of both infection and PE, and 10 had unilateral signs of infection and bilateral PE.
4. DISCUSSION
In this case‐crossover study including 707 VTE patients, we found that acute infection was an important trigger for VTE independent of immobilization. In analysis of infection and immobilization separately, we found that each of them was associated with a high risk of VTE, and that the combination of these triggers had a synergistic effect on VTE‐risk. When investigating the impact of common infectious foci on VTE‐risk, we found that RTIs had a higher impact on the risk of VTE, and PE in particular, than UTIs. Our findings suggest that hospitalization with acute infection is a strong trigger for VTE independent of immobilization, and that RTI appear to be an especially important trigger for PE.
Although results from several studies confirm that acute infection is a trigger for VTE, only a few studies have addressed this question in hospitalized patients. In a population‐based case‐control study, infection was associated with a 4.2‐fold increased VTE‐risk regardless of health‐care setting, and the risk increased to 12.5‐fold for hospital‐related infections.5 Further adjustment for some comorbidities and risk factors for VTE decreased the VTE‐risk to 3.3‐fold for hospital‐diagnosed infections. Immobilization was not a specified variable in the study, but immobilizing conditions such as surgery, trauma, and recent hospitalization were included in the adjusted model.5 In a study of patients included in the MEDENOX‐study, originally investigating the impact of enoxaparin as thromboprophylaxis in hospitalized medical patients, acute infection was associated with a higher VTE‐risk (RR 1.47) compared to patients hospitalized with other pre‐defined medical conditions such heart failure and chronic obstructive pulmonary disease.21 However, the latter study did not address the impact of infection on risk of symptomatic VTE in hospitalized patients since the reference population also were at increased risk of VTE and most VTE events were asymptomatic due to bilateral examination by venography of the lower extremities of all participants.
A case‐crossover study investigating the impact of hospitalization with infection on the risk of VTE has recently been published.22 In this study, exposure was categorized as no hospitalization, hospitalization without infection and hospitalization with infection, and control periods one and two years before the VTE event were used. Using hospitalization without infection as reference, they found non‐significantly higher OR for hospitalization with infection. Information was not presented regarding other concomitant triggers, or relationship between infectious foci and VTE entity.
Our study included a high number of validated, symptomatic VTE cases, with information available from hospital contacts for 18 months before the VTE event. This made it possible to investigate the impact of acute infection on risk of VTE and to deal with possible confounders through the study design (chronic conditions) and adjusted models (other transient risk factors). This is especially valuable when investigating risk factors for VTE in a hospital setting, as VTE is a multifactorial disease, and hospitalized VTE patients often experience the presence of more than one trigger for VTE. As immobilization and infection are related in a bidirectional manner, and both are triggers for VTE, their individual impact on risk of VTE can be difficult to investigate.
Triggers of hospitalization for VTE have been investigated in 399 patients using a case‐crossover design.23 In this study, infection was the most common trigger and increased the risk of VTE 2.9‐fold after multivariable adjustment. Immobilization, defined as any non‐surgical hospitalization or skilled nursing facility stay, was included in the adjustment model. As infection is a common cause of hospitalization, adjusting for immobilization by this definition might have led to adjustment for infection, and thereby lowered risk estimates.
In our study, immobilization was defined as bedrest over three days, ECOG score of four (completely disabled/100% bedrest) or other immobilizing factors specified in the patient's medical notes (ie, wheelchair use). When we adjusted for immobilization, OR for VTE by infection was reduced, but remained substantially elevated. When studying the impact of different combinations of infection and immobilization on risk of VTE, we found that even though acute infection was a more frequent trigger of VTE in our study, immobilization alone had a higher impact on the risk of VTE. Furthermore, the presence of both infection and immobilization had an even stronger impact on risk of VTE. These findings could be explained in the context of the thrombosis threshold model emphasizing that VTE is a multifactorial disease.24 The presence of one strong trigger, either infection or immobilization, will on top of other risk factors (eg, advanced age, obesity, pro‐thrombotic genotypes) for some individuals be enough to reach the thrombosis threshold. However, the presence of both triggers at the same time increased the likelihood to reach the thrombosis threshold further.
In addition, we wanted to investigate whether various infectious foci had differential impact on the location of the VTE event (eg, DVT or PE). RTI displayed a strong association with PE. Our findings support previous results from the MEGA‐study.15 In this case‐control study, they found a 5‐fold increased risk of VTE after pneumonia, and after adjustment for immobilization and “healthy lifestyle,” the risk remained 3.8‐fold increased. The risk was higher for PE than DVT. In the MEGA‐study, all information on risk factors for VTE, lifestyle, and pneumonia was obtained through self‐administered questionnaires, and the participants were younger (median age for cases 50 years, controls 48 years). This implies a generally healthier study population, and less validated information on risk factors, which can partly explain lower risk estimates in their study compared to our results. In agreement with our findings, Rogers et al. found higher risk estimates for infection preceding PE than DVT, and RTI had a greater impact on VTE than non‐respiratory infections.23 They did not present data for other infectious foci on the risk of PE and DVT, separately. In a study using the self‐controlled case‐series method, Smeeth and co‐authors found that the risk of DVT and PE were 2‐fold increased after community‐acquired UTI, as well as the risk of DVT after RTI.6 They reported an 11‐fold increased risk of PE after RTI, but decided not to include analyses of the risk of PE after RTI, since the increase could be due to misdiagnosis of PE as respiratory infection. These results, however, are in line with the 48‐fold higher odds of having a RTI in the hazard period than in the control periods observed in our study.
Due to better diagnostic possibilities in hospital compared to general practice, we expect lower probability for misclassification of diagnoses in our study. According to guidelines, chest X‐ray is the first choice of radiological investigation when suspecting pneumonia in a hospital setting, followed by computerized tomography (CT) scan if doubt about diagnosis or suspected complications.25 As we defined RTI to be present only if a physician noted a diagnosis of RTI in the medical record, patients admitted to the hospital with a suspected RTI that later proved to be a misdiagnosed PE would not be registered as having a RTI. Our data source was hospital medical records. A strength of this data source is that it represents actual clinical practice, where the choice of diagnostic approaches and treatments are made at each clinician's decision and preference. Such data are, however, limited by various degrees of diagnostic precision, and a less likely diagnosis (for example of RTI) might be kept even if an alternative explanation for symptoms are made (for example PE), when the clinician cannot rule out the occurrence of both. To further address this problem, the medical records of cases with RTI in the hazard period before a PE were re‐evaluated as described, and those cases with a less likely diagnosis of RTI were re‐coded as “no RTI.” In sensitivity analyses, where possible RTI‐cases were counted as no infection, the risk of PE was essentially similar after RTI and UTI. The most common reason for a RTI to be categorized as “possible” was limited information about symptoms, treatment response, or time course.
Possible mechanisms for the increased risk of PE after RTI might be local inflammation leading to local activation of coagulation and to local vasoconstriction induced by the hypoxic lung environment in the infected area.26, 27 If this actually were the case, we would expect the PE to occur on the ipsilateral side as the pneumonia. To our knowledge, no studies are available addressing this question. We found radiological signs of infection and PE on the same side in more than half of the patients with radiological signs of pneumonia. This supports that local inflammation and stasis in the pulmonary circulation play a role for thrombus formation in pneumonia. Other studies have found increased risk of PE, but not DVT, in patients with severe asthma, which further supports local inflammation as an important mechanism for thrombosis in the lungs.28
Our findings emphasize that acute infection needs to be taken into account when considering thromboprophylaxis in hospitalized patients, and that awareness of symptoms of PE is especially important in those with an incident RTI during hospitalization.
The strengths of our study include the high attendance rate in the population‐based cohort where the cases come from, the complete and validated registry of VTE events and the study design enabling us to focus on transient risk factors. The case‐crossover design is well suited to investigate transient risk factors.17 In this design, risk of selection bias and possible confounding by chronic conditions and anthropometric measures are reduced since each subject serves as its own control. Our study has some limitations. First, we only had access to medical records from hospital, and therefore less severe infections diagnosed and treated solely in general practice are not taken into account. As previous studies have found increased risk of VTE after infections treated in the community,5, 6, 29 our results might therefore be diluted. Second, even if fixed confounders are controlled for through the study design, other (unknown) transient risk factors might have influenced the results. Thus, although we adjusted for several other VTE triggers, the presence of residual confounding cannot be completely ruled out. Third, we did not have information on severity of infections, and could therefore not stratify for infection severity. As we included all infections, clinical presentation will range from uncomplicated lower UTIs, with symptoms actually encouraging mobilization, to severe septic patients in need of intensive care. We did sensitivity analysis where only patients with lower UTI were included, and OR were essentially similar as for all UTIs. Fourth, surveillance bias might be present, as doctors could be more aware of VTE‐risk factors when VTE is suspected than during admissions for other conditions in the control periods. For example, as immobilization is a well‐known risk factor for VTE, clinicians might have been more prone to specify immobilization in the medical record when VTE was suspected. If so, this would lead to overestimation of the impact of immobilization on risk of VTE.
In conclusion, hospitalization with acute infection was a frequent and strong trigger for VTE independent of immobilization. Immobilization and infection had a synergetic effect on the VTE‐risk.
AUTHOR CONTRIBUTIONS
G. Grimnes contributed to data collection, data analysis and interpretation, and writing of the manuscript. T. Isaksen contributed to data collection and revision of the manuscript. V. Tichelaar contributed to data interpretation and revision of the manuscript. S.K. Brækkan contributed to data collection, statistical support, and revision of the manuscript. J.‐B. Hansen contributed to the conception and design of the study, and interpretation and revision of the manuscript.
RELATIONSHIP DISCLOSURES
K. G. Jebsen TREC is supported by an independent grant from Stiftelsen Kristian Gerhard Jebsen. G. Grimnes is in receipt of a grant from the Northern Norway Regional Health Authority.
Supporting information
Grimnes G, Isaksen T, Tichelaar YIGV, Brækkan SK, Hansen J‐B. Acute infection as a trigger for incident venous thromboembolism: Results from a population‐based case‐crossover study. Res Pract Thromb Haemost. 2018;2:85–92. 10.1002/rth2.12065
REFERENCES
- 1. Heit JA, Melton LJ 3rd, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc. 2001;76:1102–10. [DOI] [PubMed] [Google Scholar]
- 2. Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population‐based study. Arch Intern Med. 2002;162:1245–8. [DOI] [PubMed] [Google Scholar]
- 3. Feland N, Wendelboe AM, McCumber MD, et al. Hospital associated venous thromboembolism in Oklahoma County. Blood. 2016;128:416. [Google Scholar]
- 4. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. 2015;12:464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schmidt M, Horvath‐Puho E, Thomsen RW, Smeeth L, Sorensen HT. Acute infections and venous thromboembolism. J Intern Med. 2012;271:608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–9. [DOI] [PubMed] [Google Scholar]
- 7. Dalager‐Pedersen M, Sogaard M, Schonheyder HC, Thomsen RW, Baron JA, Nielsen H. Venous thromboembolism after community‐acquired bacteraemia: a 20‐year danish cohort study. PLoS ONE. 2014;9:e86094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samama MM. An epidemiologic study of risk factors for deep vein thrombosis in medical outpatients: the Sirius study. Arch Intern Med. 2000;160:3415–20. [DOI] [PubMed] [Google Scholar]
- 9. Dang TT, Majumdar SR, Marrie TJ, Eurich DT. Recurrent pneumonia: a review with focus on clinical epidemiology and modifiable risk factors in elderly patients. Drugs Aging. 2015;32:13–9. [DOI] [PubMed] [Google Scholar]
- 10. Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–45. [DOI] [PubMed] [Google Scholar]
- 11. Cuesy PG, Sotomayor PL, Pina JO. Reduction in the incidence of poststroke nosocomial pneumonia by using the “turn‐mob” program. J Stroke Cerebrovasc Dis. 2010;19:23–8. [DOI] [PubMed] [Google Scholar]
- 12. Merah A, Bertoletti L, Ginzarly M, et al. Prior thromboprophylaxis and outcome in patients experiencing acute venous thromboembolism after an acute medical illness. Eur J Intern Med. 2016;30:72–6. [DOI] [PubMed] [Google Scholar]
- 13. Van Langevelde K, Šrámek A, Vincken PWJ, van Rooden J‐K, Rosendaal FR, Cannegieter SC. Finding the origin of pulmonary emboli with a total‐body magnetic resonance direct thrombus imaging technique. Haematologica. 2013;98:309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enga KF, Rye‐Holmboe I, Hald EM, et al. Atrial fibrillation and future risk of venous thromboembolism:the Tromso study. J Thromb Haemost. 2015;13:10–6. [DOI] [PubMed] [Google Scholar]
- 15. Ribeiro DD, Lijfering WM, Van Hylckama Vlieg A, Rosendaal FR, Cannegieter SC. Pneumonia and risk of venous thrombosis: results from the MEGA study. J Thromb Haemost. 2012;10:1179–82. [DOI] [PubMed] [Google Scholar]
- 16. Violi F, Cangemi R, Calvieri C. Pneumonia, thrombosis and vascular disease. J Thromb Haemost. 2014;12:1391–400. [DOI] [PubMed] [Google Scholar]
- 17. Maclure M. The case‐crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–53. [DOI] [PubMed] [Google Scholar]
- 18. Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njolstad I. Cohort profile: the Tromso Study. Int J Epidemiol. 2012;41:961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Mean platelet volume is a risk factor for venous thromboembolism: the Tromso Study, Tromso, Norway. J Thromb Haemost. 2010;8:157–62. [DOI] [PubMed] [Google Scholar]
- 20. Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–9. [DOI] [PubMed] [Google Scholar]
- 21. Alikhan R, Cohen AT, Combe S, et al. Risk factors for venous thromboembolism in hospitalized patients with acute medical illness: analysis of the MEDENOX Study. Arch Intern Med. 2004;164:963–8. [DOI] [PubMed] [Google Scholar]
- 22. Cowan LT, Lutsey PL, Pankow JS, Cushman M, Folsom AR. Hospitalization with infection and incident venous thromboembolism: The ARIC study. Thromb Res. 2017;151:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rogers MA, Levine DA, Blumberg N, Flanders SA, Chopra V, Langa KM. Triggers of hospitalization for venous thromboembolism. Circulation. 2012;125:2092–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosendaal FR. Venous thrombosis: a multicausal disease. Lancet. 1999;353:1167–73. [DOI] [PubMed] [Google Scholar]
- 25. Lim WS, Smith DL, Wise MP, Welham SA. British Thoracic Society community acquired pneumonia guideline and the NICE pneumonia guideline: how they fit together. Thorax. 2015;70:698–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schultz MJ, Haitsma JJ, Zhang H, Slutsky AS. Pulmonary coagulopathy as a new target in therapeutic studies of acute lung injury or pneumonia–a review. Crit Care Med. 2006;34:871–7. [PubMed] [Google Scholar]
- 27. Marshall BE, Hanson CW, Frasch F, Marshall C. Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. 2. Pathophysiology. Intensive Care Med. 1994;20:379–89. [DOI] [PubMed] [Google Scholar]
- 28. Majoor CJ, Kamphuisen PW, Zwinderman AH, et al. Risk of deep vein thrombosis and pulmonary embolism in asthma. Eur Respir J. 2013;42:655–61. [DOI] [PubMed] [Google Scholar]
- 29. Timp JF, Cannegieter SC, Tichelaar V, et al. Antibiotic use as a marker of acute infection and risk of first and recurrent venous thrombosis. Br J Haematol. 2017;176:961–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


