Abstract
Essentials.
The safety of apixaban in patients on dialysis is currently debated.
Bleeding events were retrospectively compared for dialysis patients on warfarin vs apixaban.
Overall bleeding events were significantly less frequent in apixaban group compared to warfarin group.
Apixaban appears to be a safe and effective choice for anticoagulation in patients on dialysis.
Background
The use of apixaban for stroke prophylaxis or for the treatment of venous thromboembolism in end stage renal disease (ESRD) patients maintained on dialysis is based on one single‐dose pharmacokinetic study. There is a deficiency of clinical evidence supporting safety in this population.
Objective
The purpose of this study was to determine the safety and efficacy of apixaban compared with warfarin in dialysis patients.
Patients/methods
This is a retrospective cohort study conducted at the University of Virginia Medical Center. A total of 124 ESRD patients maintained on dialysis who either received apixaban (n = 74) or warfarin (n = 50) between January 1, 2014 and October 31, 2016 were included in the study. We used multivariable logistic regression to compare the likelihood of patients experiencing a bleeding event based on anticoagulant therapy.
Results
The apixaban group experienced fewer overall bleeding events than the warfarin group (18.9% vs 42.0%; P = .01); this significant difference persisted in adjusted analysis (OR = 0.15; 95% CI = 0.05‐0.46; P = .001). Major bleeding events were less frequent in the apixaban group compared with patients on warfarin (5.4% vs 22.0%; P = .01). There were no recurrent ischemic strokes in either groups. A lower, non‐significant, incidence of recurrent VTE was found in patients on apixaban compared with warfarin (4.4% vs 28.6%; P = .99).
Conclusion
Compared to warfarin, our findings suggest that apixaban is a safe and effective alternative in patients with ESRD maintained on dialysis, with apixaban patients experiencing fewer bleeding events than warfarin patients.
Keywords: anticoagulation, apixaban, dialysis, hemorrhage, venous thromboembolism, warfarin
1. INTRODUCTION
Patients undergoing dialysis are at increased risk for developing atrial fibrillation or venous thromboembolism (VTE) compared with patients with normal renal function.1, 2, 3, 4, 5, 6 These medical indications pose the need for oral anticoagulation in this population, and for many years warfarin has been the only oral anticoagulation option for end stage renal disease (ESRD) patients.
Warfarin has been shown to have a narrow therapeutic window in the ESRD population.7 The described bleeding rates for warfarin use in hemodialysis patients range from 0.10 to 0.54 events/person‐year.8 In a recent meta‐analysis assessing warfarin use in patients with atrial fibrillation and chronic kidney disease (CKD), warfarin increased the risk of major bleeding in patients with ESRD on renal replacement therapy without decreasing risk of stroke or mortality.9 Available retrospective cohort data using warfarin for atrial fibrillation in patients with ESRD on hemodialysis suggests controversy in whether clinical benefits outweigh the bleeding risk.4, 5, 6, 10, 11, 12, 13, 14 Warfarin is also associated with increased vascular calcification in ESRD patients on dialysis leading to increased cardiovascular events, strokes, and mortality.15, 16, 17
A number of additional factors may complicate anticoagulant use in those with ESRD on hemodialysis. In addition to their known uremic platelet dysfunction, many patients have cardiovascular risk factors or indications for antiplatelet medications, both of which may increase their risk for bleeding with anticoagulation therapy.18, 19 International Normalized Ratio (INR) results may be fluctuant and unpredictable in ESRD on hemodialysis and time in therapeutic range is poor.20, 21 The aforementioned dilemmas underscore a need for additional anticoagulation options in ESRD patients on hemodialysis.
Apixaban is an oral direct factor Xa inhibitor approved in the United States for use in patients with non‐valvular atrial fibrillation (NVAF), VTE treatment or secondary/extended prophylaxis, or deep vein thrombosis (DVT) prophylaxis post total hip/total knee arthroplasty.22 While prospective studies demonstrated favorable safety and efficacy with apixaban compared with warfarin, they excluded dialysis patients.23, 24, 25, 26 Apixaban has a 27% renal excretion of the parent compound.22 Wang et al. administered a single dose of apixaban 5 mg to healthy patients and two doses of apixaban 5 mg to hemodialysis patients. Relative to non‐ESRD subjects, the authors found that ESRD patients experienced a 36% increase in apixaban area under the curve (AUC), no increase in maximum concentration levels and a negligible decrease in apixaban removal after a 4‐hour hemodialysis session.27 Based off of these results in ESRD, the FDA recommended dose adjustment for patients with atrial fibrillation with age >80 years or weight <60 kg as previously described in apixaban trials.22
Recent pharmacokinetic and retrospective study data describe further experience with apixaban use in ESRD patients on dialysis. A pharmacokinetic steady state dosing study demonstrated that apixaban 2.5 mg twice daily dosing in hemodialysis patients resulted in drug exposure comparable to apixaban 5 mg twice daily dosing in patients with preserved renal function; in contrast, apixaban 5 mg twice daily dosing in hemodialysis patients led to supra‐therapeutic drug exposure and trough levels.28 In a retrospective, matched‐cohort study in patients with severe renal disease, Stanton et al. found no statistically significant difference in the major or overall bleeding event rate of patients taking apixaban vs warfarin, including the subgroup of patients on dialysis.29 In addition, continuation of apixaban after inpatient admission, the daily dose of apixaban, and the number of hemodialysis sessions have been associated with an increased risk of bleeding.30
The University of Virginia Medical Center's Hematology Consult service has recommended apixaban use in some of its dialysis patients. The purpose of this study was to compare bleeding events between maintenance dialysis patients at our institution on apixaban vs warfarin.
2. METHODS
We performed a retrospective single institution review of clinical outcomes with the use of apixaban compared with warfarin in ESRD patients maintained on dialysis at a large academic hospital. The Institutional Review Board for Health Sciences Research (IRB‐HS) approved this study. Given the retrospective nature of the study, consent was waived. Data was collected from electronic medical records including inpatient admission notes, discharge summaries and outpatient documentation. Patients older than 18 years of age with ESRD requiring peritoneal or hemodialysis that received either at least two doses of apixaban or 5 days of warfarin for anticoagulation between January 1, 2014 and October 31, 2016 were included in the study. Patients with any indication for anticoagulation—including stroke prophylaxis in NVAF, VTE treatment, or VTE prophylaxis were included. Continuous renal replacement and acute kidney injury (AKI) patients were excluded. Further exclusions included lack of follow‐up documentation and switching anticoagulation during analysis period. Figure 1 shows further exclusion criteria and a schematic for analysis. Follow‐up time was 10 months for both groups. The primary outcome was overall bleeding event rate, which we defined as experiencing at least one bleeding event after at least two doses of starting apixaban and at least one bleeding event after 5 days of starting warfarin. We based censoring time off of the known pharmacokinetics of warfarin and apixaban. Secondary outcomes were major bleeding events, clinically relevant non‐major bleeding events, minor bleeding, recurrent VTE in patients treated for DVT or pulmonary embolism, and ischemic stroke in patients with NVAF.
Figure 1.
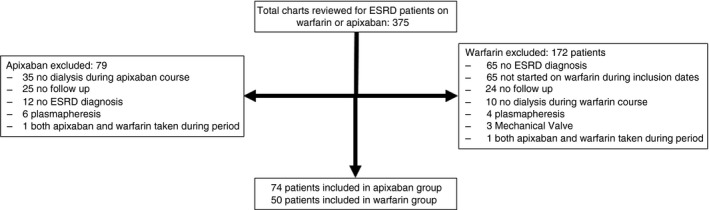
Analysis and inclusion schematic. Total of 375 charts reviewed with 74 patients ultimately analyzed in the apixaban group and 50 patients in the warfarin group. The most common reasons for exclusion were: not having end stage renal disease (ESRD) diagnosis, or not receiving dialysis during course of anticoagulation treatment
Major bleeding was defined using International Society of Thrombosis and Haemostasis (ISTH) criteria as clinically overt bleeding accompanied by at least one of the following: bleed leading to fatal outcome, a decrease in hemoglobin level of at least 2 g/dL, transfusion of at least two units of packed red blood cells, or involvement of a critical site (intracranial, spinal, ocular, pericardial, articular, retroperitoneal or intramuscular with compartment syndrome).31 Clinically relevant non‐major bleeding events were classified as clinically overt bleeding that did not satisfy the criteria for major bleeding and led to hospital admission for bleeding, physician‐guided medical or surgical treatment for bleeding, or a change in antithrombotic therapy due to bleeding. All other bleeding events were classified as minor. Ischemic stroke in the NVAF population was defined as a new neurologic deficit and corresponding imaging confirmation; rates of recurrent VTE were defined as new imaging confirmation during anticoagulation treatment in patients with VTE. In addition, we calculated HAS‐BLED scores for patients who were on warfarin or apixaban for NVAF to describe the risk of bleeding demonstrated in previous studies.32
2.1. Statistical analysis
We made unadjusted comparisons of apixaban and warfarin patients using student's t, chi‐square and Fisher's exact tests. We made adjusted comparisons of the likelihood of experiencing a bleeding event using a multivariable logistic model that included the following patient risk factors: anticoagulant, sex, race, age at start of anticoagulation, weight at start of anticoagulation, indication, type of concurrent anti‐platelet, and a history of alcohol use, cardiovascular disease, bleeding or stroke. We performed all analyses using SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. RESULTS
A total of 375 patients met the inclusion criteria for this review. Of the 153 patients in the apixaban group, 74 were ultimately included; of the 222 warfarin patients, 50 were ultimately included (Figure 1). Demographic and clinical baseline characteristics were similar between the groups (Table 1).
Table 1.
Baseline demographic and clinical characteristics of the study patients
| Characteristic | Apixaban (n = 74) | Warfarin (n = 50) | P‐value |
|---|---|---|---|
| Age (years) | 59.5 ± 14.7 | 62.0 ± 14.4 | .36 |
| Weight (kg) | 86.1 ± 27.0 | 79.0 ± 20.5 | .11 |
| BMI (kg/m2) | 27.9 ± 6.5 | 29.2 ± 10.1 | .38 |
| African American | 34 (45.9%) | 23 (46%) | .99 |
| Female | 36 (48.6%) | 19 (38%) | .24 |
| HASBLED score | 3.2 ± 1.4 | 3.4 ± 1.4 | .70 |
| CHAjDSj‐VASc score | 4.1 ± 1.2 | 4.0 ± 1.4 | .69 |
| Months on anticoagulation | 7.9 ± 7.3 | 10.8 ± 9.3 | .06 |
| Prior stroke | 4 (5.4%) | 3 (6%) | .71 |
| Liver Disease | 20 (27%) | 8 (16%) | .15 |
| Anticoagulation indication | |||
| Atrial fibrillation | 29 (39.2%) | 29 (58%) | .04 |
| VTE treatment/VTE PPX | 45 (60.8%) | 21 (42%) | |
| Antiplatelet | |||
| Aspirin | 21 (28.4%) | 18 (36%) | .06 |
| DAPT | 4 (5.4%) | 8 (16%) | |
| None | 49 (66.2%) | 24 (48%) | |
Comparisons were made via t test for age, weight, HASBLED score (Hypertension, Renal disease, Liver disease, Stroke history, Prior major bleeding, Labile INR, Age >65, Medication usage predisposing to bleeding, Alcohol use), CHA2DS2‐VASc score (Congestive heart failure, Hypertension, Age, Diabetes, Stroke/TIA/thromboembolism, Vascular disease, Sex), and months on anticoagulation therapy. Given the paucity of events, comparisons were made via Fisher's exact test for prior stroke. All other comparisons were made via chi‐square test. Values displayed are mean ± SD or (%) when appropriate.
VTE, venous thromboembolism; PPX, prophylaxis; DAPT, Dual antiplatelet therapy.
The sample patients experienced a total of 35 bleeding events during the analysis time period. Of these, 15 events were classified as major. During the analysis period, there were also eight recurrent VTE events (Table 2).
Table 2.
Incidence proportion of selected outcomes, by type of anticoagulation
| Characteristic | Apixaban | Warfarin | P‐value |
|---|---|---|---|
| Bleeding event | |||
| Any | 14 (18.9%) | 21 (42%) | .01 |
| Major (among all patients) | 4 (5.4%) | 11 (22%) | .01 |
| Recurrent venous thromboembolisma | 2 (4.4%) | 6 (28.6%) | .99 |
Numbers are presented with (%). Comparisons were made via chi‐square test for the bleeding event outcomes. Given the paucity of events, comparisons were made via Fisher's exact test for recurring VTE.
Among patients with a VTE or VTE prophylaxis indication; patients with an indication of atrial fibrillation are excluded.
The warfarin group experienced more bleeding events than the apixaban group (42% and 18.9%, respectively; P = .01), with a lower prevalence of major bleeding in the apixaban group (5.4% vs 22% in the warfarin group; P = .01). The overall bleeding event rate in the apixaban and warfarin groups was 0.31 and 0.47 events per person year, respectively, with a lower rate of major bleeding events per person‐year in the apixaban group (0.07 vs 0.24). We also conducted adjusted analyses looking at the likelihood of experiencing a bleeding event. Using multivariable logistic regression adjusting for several demographic and clinical factors, we found that apixaban patients were significantly less likely to experience a bleeding event in the study period than were warfarin patients (OR = 0.15; 95% CI = 0.05‐0.46; P = .001). Results are shown in Table 3. As expected, patients who reported experiencing a bleed prior to the study were significantly more likely to experience a bleed during the study (OR = 4.78; 95% CI = 1.50‐15.21; P = .01). However, we were surprised to find that African‐American patients were less likely than their white counterparts to have a bleeding event (OR = 0.31; 95% CI = 0.1‐0.9; P = .03). No other factors were found to be significant predictors of a bleeding event.
Table 3.
Adjusted odds of experiencing a bleeding event
| Covariate | Adjusted odds ratio | 95% CI | P‐value |
|---|---|---|---|
| Anticoagulation | |||
| Warfarin (ref) | |||
| Apixaban | 0.15 | 0.05‐0.46 | .001 |
| Sex | |||
| Male (ref) | |||
| Female | 1.63 | 0.58‐4.56 | .35 |
| Age (y) | |||
| <50 (ref) | |||
| 50 to <70 | 1.40 | 0.37‐5.34 | .62 |
| 70+ | 3.73 | 0.89‐15.69 | .07 |
| Race | |||
| White (ref) | |||
| African American | 0.31 | 0.10‐0.90 | .03 |
| Weight (kg) | |||
| <60 (ref) | |||
| 60 to <120 | 1.85 | 0.38‐8.91 | .44 |
| 120+ | 8.62 | 0.81‐91.40 | .07 |
| Comorbidity history | |||
| Alcohol | 0.87 | 0.15‐5.21 | .88 |
| CVD | 2.04 | 0.63‐6.59 | .23 |
| Bleeding event | 4.78 | 1.50‐15.21 | .01 |
| Stroke | 0.99 | 0.15‐6.57 | .99 |
| Indication | |||
| Atrial fibrillation (ref) | |||
| VTE | 1.71 | 0.56‐5.21 | .34 |
| VTEPPX | 3.05 | 0.44‐21.05 | .26 |
| Concurrent antiplatelet | |||
| No (ref) | |||
| Aspirin | 1.13 | 0.39‐3.30 | .82 |
| DAPT | 0.99 | 0.19‐5.11 | .99 |
Numbers displayed are odds ratios with their corresponding 95% confidence intervals. These values are from a logistic model predicting the probability of a patient experiencing a bleeding event.
VTE, venous thromboembolism; PPX, prophylaxis; DAPT, Dual antiplatelet therapy.
Bold rows signify characteristics that were found to be statistically significant.
3.1. Apixaban group
In the apixaban group, most patients (46%; n = 34) required anticoagulation for the treatment of VTE, whereas 39.2% (n = 29) and 14.9% (n = 11) of patients required anticoagulation for NVAF and prophylaxis of VTE, respectively. Most patients were on therapeutic dosing of apixaban 5 mg every 12 hours (79.7%; n = 59). The remaining patients were on 2.5 mg twice daily (20.3%; n = 15) and met the criteria for either dose reduction (n = 4) or lifelong prophylactic anticoagulation dosing (n = 11). For patients receiving acute treatment of DVT, only three patients received the apixaban loading dose of 10 mg twice daily for 7 days prior to the maintenance dose, 5 mg every 12 hours. The average time patients were on anticoagulation in the study period was 7.9 months. There were 25 patients (33.8%) on concurrent antiplatelet medications, including 21 patients on aspirin and four patients on dual antiplatelet therapy (DAPT) with clopidogrel and aspirin. Most of the patients had been on warfarin prior to initiation of apixaban (n = 40; 54%), and one patient was on rivaroxaban prior to diagnosis of ESRD.
There were 14 bleeding events recorded in patients anticoagulated with apixaban (Table 4), with four being major, seven being clinically relevant non‐major and three events classified as minor. The average HAS‐BLED score was 3.2. The average time to bleed in the patients who were on apixaban was 5.98 months. One patient had two major upper gastrointestinal (GI) bleeds, with each event occurring approximately 3 days after being initiated on apixaban after two separate occasions. The two other major bleeds were both secondary to a GI origin. None of the major bleeding events directly resulted in a patient death; however, after each case of major bleeding, the patients were discontinued from apixaban therapy. Other bleeding events are shown in Table 4. Interestingly, only three of these bleeding events occurred with patients taking 2.5 mg dose of apixaban and were all clinically relevant non‐major bleeding events. In all minor bleeding events, patients were continued on apixaban.
Table 4.
Bleeding events on anticoagulation
| Bleeding event | Apixaban (n = 14) | Warfarin (n = 21) |
|---|---|---|
| Gl etiology | 9 (64.3%) | 10 (47.6%) |
| Retroperitoneal hemorrhage | 0 | 2 (9.5%) |
| Hemoptysis | 1 (7.1%) | 0 |
| Hematoma | 3 (21.4%) | 4 (19%) |
| Subarachnoid hemorrhage | 0 | 1 (4.7%) |
| Hemopericardium | 0 | 2 (9.5%) |
| Hemothorax | 0 | 1 (4.7%) |
| Hematuria | 1 (7.1%) | 1 (4.7%) |
Bleeding events that occurred during the study period. Values are the number of events with (%) as appropriate.
3.2. Warfarin group
The indication for anticoagulation for the patients in the warfarin group comprised NVAF (58%; n = 29), treatment of VTE (36%; n = 18) and prophylaxis of VTE (6%; n = 3). The average amount of time patients were on warfarin during the study period was 10.8 months. Only one patient was on peritoneal dialysis. Most patients (52%; n = 26) were on concurrent antiplatelet medications, with 18 patients on aspirin and 8 patients on DAPT with clopidogrel and aspirin.
There were 21 patients who had a bleeding event while on warfarin. The average time to bleed in patients on warfarin was 7.2 months. Of those events, 11 patients (22%) had major bleeds, nine patients (18%) had clinically relevant non‐major bleeds and one patient (2%) had a minor bleed. Major bleeds included GI origin, hemopericardium, hemothorax, retroperitoneal bleeds, and a subarachnoid hemorrhage. Other types of bleeding events in the patients taking warfarin described in Table 4. The average HAS‐BLED score for patients on warfarin was 3.4. There was no difference in average INR at time of bleed between the major bleeding group (average INR = 3.85) and the minor bleeding group (average INR = 3.78).
3.3. Recurrent VTE/stroke
Given that maintenance dialysis patients are at increased risk for recurrent vascular complications, we also analyzed the rate of recurrent VTE in each group. Patients taking apixaban had a lower VTE recurrence than warfarin patients (4.4% vs 28.6%, respectively). No patients in either the apixaban group or the warfarin group suffered from an ischemic stroke. One patient in the warfarin group had a suspected transient ischemic attack (TIA); however, the patient did not demonstrate any evidence of stroke on imaging.
4. DISCUSSION
While there is no current consensus on the safety for apixaban use in ESRD patients, our study suggests that in maintenance dialysis patients, apixaban may be associated with a lower bleeding rate than warfarin. Given the high prevalence of atrial fibrillation and VTE in the ESRD population, it is important to find anticoagulation options that have a low risk of bleeding with favorable efficacy. To the authors’ knowledge, our study is the first retrospective cohort study to find a statistically significant lower bleeding event rate in patients with ESRD on apixaban vs warfarin.
The primary outcome of overall bleeding rate was significantly less frequent in patients on apixaban compared with patients on warfarin. The warfarin bleeding event rate of 0.47 events per person‐year was within the range published in a systematic review of warfarin anticoagulation in hemodialysis patients.8 The apixaban bleeding event rate was 0.30 events per person‐year with no established range in the literature but similar to rate Stanton et al. described with 0.26 event/1000 patient‐days. There was no evidence of thrombocytopenia at time of bleed in either group, with average platelet counts of 200 and 228 × 109/L for apixaban and warfarin, respectively.
Of the 26 patients on concurrent warfarin and antiplatelet therapy, 12 patients (41.4%) had a bleeding event prior to warfarin therapy. Of the patients on concurrent apixaban and antiplatelet therapy, 8 patients (32%) had a prior bleeding event. This suggests a higher baseline risk of bleeding in patients starting warfarin. The average HAS‐BLED score for patients on concurrent warfarin and antiplatelet therapy was 4.0 and 3.1 for those who bled and did not bleed respectively. This observation was also seen in patients on apixaban and concurrent antiplatelet therapy with an average HAS‐BLED score of 5.0 for patients who had a bleeding event and 3.25 for those who did not. Interestingly, of the patients who had a bleeding event, 11 and five were on warfarin or apixaban, respectively, with concurrent antiplatelet use.
We found no different levels of risk of recurrent stroke between the apixaban and warfarin groups. The mean CHA2DS2‐VASc was 4.1 and 4.0 for apixaban and warfarin, respectively. There was one TIA event in the warfarin group; however, no evidence of stroke was seen on imaging at the time of presentation. Also, we observed a lower risk of recurrent episodes of VTE while on anticoagulation with apixaban compared with warfarin. This is consistent with evidence from the AMPLIFY and AMPLIFY‐EXT trials.23, 24
Our study provides a depiction of safe use of apixaban treatment in a cohort of ESRD patients on maintenance dialysis, which we hope will expand the anticoagulation armamentarium for a population of patients in whom the safety of warfarin is in question. Available retrospective data using warfarin for atrial fibrillation in ESRD on hemodialysis patients evoke debate over whether clinical benefits outweigh the bleeding risk.4, 5, 6, 7, 11, 13, 33 Assessment is difficult due to heterogeneity in these trials, which differ in design, definitions of endpoints, and measurements of clinical efficacy or laboratory monitoring. Published trials question the benefit of warfarin use as there is disparity in the results characterizing stroke risk; some studies find no difference in stroke risk when warfarin is used,5, 6, 10, 11, 12 whereas others associate warfarin with increased stroke risk13, 14 and two studies found that warfarin use decreased stroke risk.33, 34
Our findings are similar to those reported by Stanton et al. in regard to decreased bleeding events in patients on apixaban with severe renal impairment; however, they analyzed patients with acute kidney injury (AKI), CKD and ESRD, while our study analyzed only patients with ESRD on dialysis.29 Most patients in their trial were on the reduced dose of apixaban at 2.5 mg every 12 hours and therefore may have been at a lower risk of bleeding. The question regarding safe dosing of apixaban in ESRD patients on dialysis has been asked in response to Stanton et al. analysis.35 The majority of patients on apixaban in our analysis were taking the recommended dose of 5 mg every 12 hours. In the Stanton et al. study, patients were cohort matched; there was no multivariable analysis of the bleeding events to assess for the contribution of other potential confounding variables.
In contrast to the above, a recent retrospective analysis of factors associated with apixaban related bleeding events in ESRD patients on hemodialysis, describe increased risk of bleeding with increased exposure to apixaban.30 This retrospective analysis did not include a warfarin comparator group. Many variables place patients with ESRD on dialysis at increased risk of bleeding; therefore, a warfarin comparator group assists in assessing the safety of apixaban use in these patients.
Our study has several limitations. First, it is a retrospective observational study on therapeutic effects, and therefore the results are likely to be confounded by factors that are not in the authors’ control, which introduces significant bias. As a retrospective review, the data collected are dependent on accurate documentation in the medical record and clinical interpretation. This can introduce misclassification, recall, and selection bias. We attempted to control for some of the confounding variables by performing a multivariable analysis. Bleeding events may not have been captured if patients did not present to our hospital. There may be factors that were not investigated that may have predisposed patients to bleeding events. Anticoagulation decisions were provider dependent and not standardized predisposing the study to some degree of sample bias. HAS‐BLED scores have not been validated in the direct oral anticoagulant population and efficacy to predict bleeding events in patients on apixaban is unknown. The study did not assess INR management or TTR for warfarin patients as it was a retrospective analysis of patients followed by electronic medical record in both inpatient and occasionally outpatient setting using different services for INR monitoring and therefore would not have been accurate. As discussed above, the larger number of warfarin patients compared with apixaban patients on concurrent antiplatelet agents may have confounded the bleeding rate in the warfarin group. Finally, the sample size is small and has low power to detect a meaningful difference. Therefore, caution is needed to interpret the external validity of the study. Our study did not have enough patients to analyze statistical significance of bleeding events on 2.5 mg every 12 hours dose of apixaban and therefore we cannot comment on the impact of dosing in this patient population. Ultimately, prospective randomized studies are needed to ensure safety and efficacy of apixaban use in the ESRD population.
In conclusion, our study suggests apixaban may be a safe and effective anticoagulation option in patients with ESRD on dialysis.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper.
AUTHOR CONTRIBUTIONS
D. Reed contributed to the study design, acquisition and analysis and interpretation of the data, writing of the manuscript, and decision to submit manuscript for publication. S. Palkimas contributed to the study design, acquisition and analysis of the data, writing and reviewing of the manuscript and decision to submit manuscript for publication. R. Hockman contributed to the study design, acquisition and analysis of the data, writing and reviewing of the manuscript, and decision to submit manuscript for publication. S. Abraham contributed to the acquisition and analysis of the data, writing of the manuscript, and decision to submit manuscript for publication. T. Le contributed to the study design, reviewing of the manuscript, and decision to submit manuscript for publication. H. Maitland contributed to the acquisition and analysis of the data, critical review of the manuscript, and decision to submit the manuscript for publication.
ACKNOWLEDGMENTS
We thank E.E. Browning for help with data acquisition and B.G. Macik for assistance with study design. We also thank D. Chyn for her help with statistical modeling and analysis.
Reed D, Palkimas S, Hockman R, Abraham S, Le T, Maitland H. Safety and effectiveness of apixaban compared to warfarin in dialysis patients. Res Pract Thromb Haemost. 2018;2:291–298. 10.1002/rth2.12083
REFERENCES
- 1. Ocak G, Vossen CY, Rotmans JI, et al. Venous and arterial thrombosis in dialysis patients. Thromb Haemost. 2011;106:1046–52. [DOI] [PubMed] [Google Scholar]
- 2. Tveit DP, Hypolite IO, Hshieh P, et al. Chronic dialysis patients have high risk for pulmonary embolism. Am J Kidney Dis. 2002;39:1011–7. [DOI] [PubMed] [Google Scholar]
- 3. Wattanakit K, Cushman M, Stehman‐Breen C, Heckbert SR, Folsom AR. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008;19:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Friberg L, Benson L, Lip GYH. Balancing stroke and bleeding risks in patients with atrial fibrillation and renal failure: the swedish atrial fibrillation cohort study. Eur Heart J. 2015;36:297–306. [DOI] [PubMed] [Google Scholar]
- 5. Mitsuma W, Matsubara T, Hatada K, et al. Clinical characteristics of hemodialysis patients with atrial fibrillation: the RAKUEN (registry of atrial fibrillation in chronic kidney disease under hemodialysis from niigata) study. J Cardiol. 2016;68:148–55. [DOI] [PubMed] [Google Scholar]
- 6. Shah M, Avgil Tsadok M, Jackevicius CA, et al. Warfarin use and the risk for stroke and bleeding in patients with atrial fibrillation undergoing dialysis. Circulation. 2014;129:1196–203. [DOI] [PubMed] [Google Scholar]
- 7. Harel Z, Sood MM, Perl J. Comparison of novel oral anticoagulants versus vitamin K antagonists in patients with chronic kidney disease. Curr Opin Nephrol Hypertens. 2015;24:183–92. [DOI] [PubMed] [Google Scholar]
- 8. Elliott MJ, Zimmerman D, Holden RM. Warfarin anticoagulation in hemodialysis patients: a systematic review of bleeding rates. Am J Kidney Dis. 2007;50:433–40. [DOI] [PubMed] [Google Scholar]
- 9. Dahal K, Kunwar S, Rijal J, Schulman P, Lee J. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta‐analysis of observational studies. Chest. 2016;149:951–9. [DOI] [PubMed] [Google Scholar]
- 10. Wang TKM, Sathananthan J, Marshall M, Kerr A, Hood C. Relationships between anticoagulation, risk scores and adverse outcomes in dialysis patients with atrial fibrillation. Heart Lung Circ. 2016;25:243–9. [DOI] [PubMed] [Google Scholar]
- 11. Winkelmayer WC, Liu J, Setoguchi S, Choudhry NK. Effectiveness and safety of warfarin initiation in older hemodialysis patients with incident atrial fibrillation. Clin J Am Soc Nephrol. 2011;6:2662–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li J, Wang L, Hu J, Xu G. Warfarin use and the risks of stroke and bleeding in hemodialysis patients with atrial fibrillation: a systematic review and a meta‐analysis. Nutr Metab Cardiovasc Dis. 2015;25:706–13. [DOI] [PubMed] [Google Scholar]
- 13. Chan KE, Lazarus JM, Thadhani R, Hakim RM. Warfarin use associates with increased risk for stroke in hemodialysis patients with atrial fibrillation. J Am Soc Nephrol. 2009;20:2223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77:1098–106. [DOI] [PubMed] [Google Scholar]
- 15. Danziger J. Vitamin K‐dependent proteins, warfarin, and vascular calcification. Clin J Am Soc Nephrol. 2008;3:1504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fusaro M, Tripepi G, Noale M, et al. Vertebral Fractures and Vascular Calcifications Study Group . Prevalence of vertebral fractures, vascular calcifications, and mortality in warfarin treated hemodialysis patients. Curr Vasc Pharmacol. 2015;13:248–58. [DOI] [PubMed] [Google Scholar]
- 17. Poterucha TJ, Goldhaber SZ. Warfarin and vascular calcification. Am J Med. 2016;129:635.e1–4. [DOI] [PubMed] [Google Scholar]
- 18. Sohal AS, Gangji AS, Crowther MA, Treleaven D. Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res. 2006;118:417–22. [DOI] [PubMed] [Google Scholar]
- 19. Schwartzenberg S, Lev EI, Sagie A, Korzets A, Kornowski R. The quandary of oral anticoagulation in patients with atrial fibrillation and chronic kidney disease. Am J Cardiol. 2016;117:477–82. [DOI] [PubMed] [Google Scholar]
- 20. Delate T, Witt DM, Jones JR, Bhardwaja B, Senser M. Falsely elevated international normalized ratio values in patients undergoing anticoagulation therapy: a descriptive evaluation. Chest. 2007;131:816–22. [DOI] [PubMed] [Google Scholar]
- 21. Yang F, Hellyer JA, Than C, et al. Warfarin utilisation and anticoagulation control in patients with atrial fibrillation and chronic kidney disease. Heart. 2017;103:818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eliquis . (apixaban) [package insert]. Princeton: Bristol‐Myers Squibb Company; 2016.
- 23. Agnelli G, Buller HR, Cohen A, et al. AMPLIFY‐EXT Investigators . Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. [DOI] [PubMed] [Google Scholar]
- 24. Agnelli G, Buller HR, Cohen A, et al. AMPLIFY Investigators . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 25. Granger CB, Alexander JH, McMurray JJ, et al. ARISTOTLE Committees and Investigators . Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 26. Connolly SJ, Eikelboom J, Joyner C, et al. AVERROES Steering Committee and Investigators . Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–17. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Tirucherai G, Marbury TC, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end‐stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628–36. [DOI] [PubMed] [Google Scholar]
- 28. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacotherapy. 2017;37:412–9. [DOI] [PubMed] [Google Scholar]
- 30. Steuber TD, Shiltz DL, Cairns AC, Ding Q, Binger KJ, Courtney JR. A multicenter analysis of factors associated with apixaban‐related bleeding in hospitalized patients with end‐stage renal disease on hemodialysis. Ann Pharmacother 2017;51:954–60. [DOI] [PubMed] [Google Scholar]
- 31. Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 32. Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user‐friendly score (HAS‐BLED) to assess 1‐year risk of major bleeding in patients with atrial fibrillation: the euro heart survey. Chest. 2010;138:1093–100. [DOI] [PubMed] [Google Scholar]
- 33. Olesen JB, Lip GY, Kamper AL, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. 2012;367:625–35. [DOI] [PubMed] [Google Scholar]
- 34. Shen JI, Montez‐Rath ME, Lenihan CR, Turakhia MP, Chang TI, Winkelmayer WC. Outcomes after warfarin initiation in a cohort of hemodialysis patients with newly diagnosed atrial fibrillation. Am J Kidney Dis. 2015;66:677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts MZ, Farley TM, Owens RE. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment: an alternative viewpoint. Pharmacotherapy. 2017;37:e107–8. [DOI] [PubMed] [Google Scholar]


