Abstract
Background
Finding the optimal duration of anticoagulant treatment following an acute event of deep vein thrombosis (DVT) is challenging. Residual venous obstruction (RVO) has been identified as a risk factor for recurrence, but data on management strategies incorporating the presence of RVO and associated recurrence rates in defined clinical care pathways (CCP) are lacking.
Objectives
We aimed to investigate the long‐term clinical outcomes and predictors of venous thromboembolism (VTE) recurrence in a contemporary cohort of patients with proximal DVT and managed in a CCP incorporating the presence of RVO.
Patients
All patients treated at the Maastricht University Medical Center within an established clinical care pathway from June 2003 through June 2013 were prospectively followed for up to 11 years in a prospective management study.
Results
Of 479 patients diagnosed with proximal DVT, 474 completed the two‐year CCP (99%), and 457 (94.7%) the extended follow‐up (2231.2 patient‐years; median follow‐up 4.6 years). Overall VTE recurrence was 2.9 per 100 patient‐years, 1.3 if provoked by surgery, 2.1 if a non‐surgical transient risk factor was present and 4.0 if unprovoked. Predictors of recurrent events were unprovoked VTE (adjusted hazard ratio [HR] 4.6; 95% CI 1.7, 11.9), elevated D‐dimer one month after treatment was stopped (HR 3.3; 1.8, 6.1), male sex (HR 2.8; 1.5, 5.1), high factor VIII (HR 2.2; 1.2, 4.0) and use of contraceptives (HR 0.1; 0.0, 0.9).
Conclusions
Patients with DVT managed within an established clinical care pathway incorporating the presence of RVO had relatively low incidences of VTE recurrence.
Keywords: clinical decision making, epidemiology, health services research, mortality, risk factors, therapy, venous thromboembolism
1. INTRODUCTION
The optimal management strategy for the prevention of recurrent venous thromboembolism (VTE) is still uncertain. Venous thromboembolism contributes significantly to global disease burden.1 Within the European Union, it is estimated that the annual incidence of deep‐vein thrombosis (DVT) and pulmonary embolism (PE) cases is 684 000 and 435 000, respectively, and VTE‐related deaths exceed 543 000.2 While prevention of recurrent VTE is important to reduce the burden of disease,1 anticoagulation treatment, the mainstay of VTE prevention, is accompanied by a significant risk of bleeding complications.3 Efficient prevention thus critically depends on optimal assessment of recurrence risk in individual patients. Even though a number of clinical criteria, laboratory assays, and even imaging tests have been proposed as risk factors for recurrent VTE,4, 5 their clinical value is limited.4 Recent guidelines comment on previous study results and suggest that the demonstration of residual vein obstruction (RVO) at the end of the regular period of anticoagulation treatment might improve risk assessment and management of recurrent VTE.3, 6 Indeed, observational data has established RVO as a risk factor for recurrent VTE with a relative risk of about 1.5.7, 8, 9, 10, 11 These data were confirmed in a randomized controlled trial that compared RVO‐guided anticoagulation therapy vs stopping anticoagulation treatment after 3 to 6 months.10 However, it is not known if RVO is useful for risk assessment in clinical practice, although it is used in combination with other diagnostic and prognostic tools in a management strategy. In particular, the effects of a management strategy comprising the presence of RVO in clinical practice are unknown.
Applying evidence‐based health care is a difficult task, particularly in VTE patients. Clinical care pathways (CCPs) have been introduced to guide diagnostic and therapeutic decisions for patients with defined clinical problems in complex organisations.12 CCPs aim to translate evidence‐based medicine into clinical practice, improve collaboration among multiple specialized care providers and standardize health care procedures.13, 14 CCPs have been introduced for patients with a variety of clinical problems including venous thromboembolism.15, 16, 17, 18, 19 However, knowledge on the long‐term effects of CCPs on clinical outcomes of patients with VTE is lacking. The present investigation aimed to investigate both the long‐term clinical outcomes in patients with DVT managed in a defined CCP incorporating the presence of RVO and also to determine risk factors for VTE recurrence in a contemporary cohort of patients with proximal DVT managed within a defined CCP.
Essentials.
Outcomes of clinical care pathways (CCP) for treatment of deep vein thrombosis (DVT) are unknown.
We followed 479 DVT patients treated within a CCP incorporating RVO for a median of five years.
Patients had relatively low incidences of VTE recurrences and deaths.
Unprovoked DVT, D‐dimer, male sex, factor VIII and contraceptive use predicted recurrent events.
2. METHODS
2.1. Study design and population
The present study represents a observational health care management study and no control group was assessed. Clinical outcomes of all patients that were treated within a clinical care pathway (CCP) of the Maastricht University Medical Center (MUMC) between 2003 and 2013 were investigated. MUMC is the only tertiary hospital in the Dutch province of Limburg, the Netherlands. Consecutive adult patients diagnosed with an acute, objectively confirmed first proximal DVT (popliteal vein, femoral vein, common femoral vein, or iliac vein) between 2003 and 2013 were followed for two years within the CCP, and additional outcomes data was collected for an extended period. No exclusion criteria were applied. However, certain patient groups were usually not treated within the CCP: distal DVT, DVT complicated by PE, patients who follow further treatment in other institutions than MUMC, and patients with cancer. The study was carried out in accordance with the Declaration of Helsinki, and the study protocol and collection of data was approved by the local MUMC ethical committee (METC 15‐4‐256).
2.2. Clinical care pathway
In 2003, a CCP was implemented at the MUMC to guide management of patients diagnosed with proximal DVT. All patients objectively diagnosed with proximal DVT (popliteal vein, femoral vein, common femoral vein, or iliac vein) at the MUMC are managed in a specialized outpatient clinic according to a strict protocol. Regular visits are scheduled 0.5, 3, 6, 12, and 24 months after diagnosis. Structured history and physical examination as well as an assessment of clinical risk factors are performed at the first visit. The Villalta score is performed at every visit.20 Laboratory tests are performed 1 month after cessation of anticoagulation treatment, and 12 and 24 months after diagnosis (levels of D‐dimer, factor VIII, and C‐reactive protein [CRP]). Thrombophilia markers are not ordered routinely. RVO is assessed by ultrasonography 1 week before the intended cessation of anticoagulant treatment (after 3 or 6 months, respectively).
2.3. Risk assessment and treatment decisions
The criteria by which the risk of recurrent VTE is assessed are delineated by a strict protocol and instructions are in line with current guidelines. The major principles are illustrated in Figure 1. Patients are assigned to three different categories: (i) patients with a provoked DVT in the course of a reversible risk factor such as recent surgery are assigned to three months of anticoagulation treatment; (ii) patients with an unprovoked DVT are assigned to 6 months of anticoagulation therapy and extensive risk assessment; (iii) high‐risk patients are assigned to an indefinite anticoagulant treatment regimen. “Provoked DVT” was defined as DVT with the presence of a reversible risk factor (surgery within 2 months, contraceptive use, pregnancy, long‐distance travel of more than 10 hours, and immobilization). “Unprovoked DVT” was defined as DVT without the presence of a reversible risk factor (see above). “High‐risk patients” were defined as unprovoked DVT in the presence of recurrent VTE, elevated D‐dimer, high factor VIII, known high‐risk thrombophilia, inflammation, or active cancer. High risk thrombophilia were defined as protein S or C deficiency, homozygous factor V‐Leiden mutation, antithrombin deficiency, or antiphospholipid antibody syndrome.21 Antithrombin deficiency was defined as functional antithrombin <70%.22 Protein S deficiency was defined as free protein S below reference range (<2.5th percentile), and protein C deficiency was defined as protein C below reference range (< 2.5th percentile)—both in the absence of vitamin K‐deficiency.23 Inflammation was defined as the presence of an systemic inflammatory disease such as Crohn's disease, ulcerative colitis, or connective tissue disease. The presence of elevated D‐dimer, high factor VIII, and persistent elevated CRP was considered for risk assessment 1 month after stop anticoagulation only.
Figure 1.
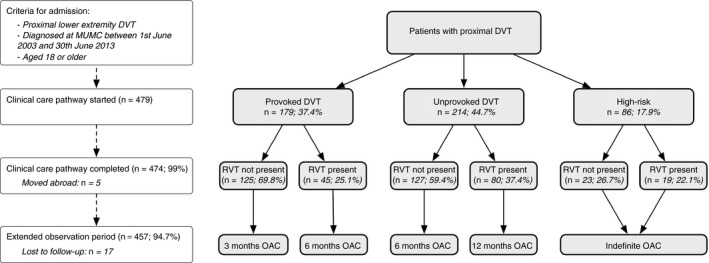
Flow of patients within the clinical care pathway and study cohort
Presence of RVO at the time of planned treatment discontinuation is the primary risk factor upon which treatment duration is further tailored. If no RVO is present at this time point, anticoagulation treatment will be stopped. In the case of detected RVO, anticoagulation will be prolonged for another 3 months (provoked DVT), or 6 months (unprovoked), respectively. Treatment will only be prolonged once. Deviations from the protocol could be made at the discretion of the treating physician to address patients preferences.
2.4. Assessment of RVO
RVO was assessed using compression ultrasound (CU) as previously described24 and a protocol has been implemented for conducting a series of standardized ultrasound measurements as follows. Measurements were taken at: (i) the common femoral vein, just below the inguinal ligament, and (ii) at the popliteal vein. No iliacal or calf veins were assessed. B‐mode images were taken in a transverse plane. RVO was defined according to the definition of Prandoni as residual vein diameter during compression of more than 2 mm.24 Several studies confirmed an acceptable accuracy and inter‐observer reproducibility of this method24, 25, 26, 27 and agreement between observers was achieved by close teamwork among team members. A formal assessment of the inter‐observer agreement was not conducted.
2.5. Collection of data
All data were prospectively recorded as part of routine clinical practice. A structured database was implemented. Outcome data were also documented as part of clinical routine. After completion of 2 years of follow‐up in the course of CCP, outcome data were additionally collected over the course of further outpatient visits and accessing MUMC and general practitioner records.
2.6. Determination of laboratory tests
Laboratory data were determined in a certified MUMC+ laboratory using established methods as previously described.28 Venous blood samples were collected in commercially available tubes with and without citrate 0.106 mol/l as appropriate following an established protocol to ensure adequate preanalytic conditions. Samples were centrifuged according to recent guidelines (10 minutes at 1500 g or 5 minutes at 2500 g, respectively). D‐dimer and CRP was measured after centrifugation. Plasma for factor VIII measurements were snap‐frozen at −80°C. D‐dimer were determined using the Vidas assay until May 2008 (bioMérieux Clinical Diagnostics, Marcy‐l'Etoile, France) and the Innovance assay from June 2008 (Siemens Healthcare, Marburg, Germany). Factor VIII was determined using a one‐stage coagulometric assay (Actin FS; Siemens Healthcare, Marburg, Germany). Both assays were run on a Sysmex CA7000 (Siemens, Marburg, Germany). CRP was determined with a turbidimetric test (Synchron LX Systems, Beckman Coulter Inc., Fullerton, CA, USA).
2.7. Outcomes, predictor variables, and co‐variables
We defined the time to recurrent VTE as the primary outcome. Recurrent VTE was defined as objectively confirmed proximal or distal DVT, PE, or other venous thrombosis as determined by CU, spiral computed tomography, or ventilation‐perfusion lung scanning. Recurrent DVT was defined as: (i) a new non‐compressible vein in the contralateral leg, (ii) a new non‐compressible vein of the same leg as the first event (previously unaffected), (iii) a clear proximal extension of the known thrombus, or (iv) a new non‐compressible site of a vein that was effected but previously re‐canalized.11, 26, 29
Secondary outcome was time to death from any cause. A number of variables previously identified or suspected as predictors for recurrent VTE were recorded as a potential predictor or a co‐variable, respectively (Table 1).4, 5, 30, 31, 32 Periods of oral anticoagulation administration were recorded and represented a time‐varying co‐variate. D‐dimer, CRP, and factor VIII were measured one month after cessation of anticoagulation therapy. All other variables were considered at the time of diagnosis. D‐dimer was considered positive if ≥ 500 ng/mL, CRP if ≥ 5.0 mg/L (according to recent recommendations33) and factor VIII if ≥ 213% (corresponding to the 80th percentile of the study population). Due to organizational reasons, bleeding events were not recorded.
Table 1.
Patient characteristics
| Characteristics | Clinical care pathway (n = 479) | Extended observation period (n = 474) | ||
|---|---|---|---|---|
| Frequency (%) | Missing values | Frequency (%) | Missing values | |
| Observation period | 876 patient‐years (median 2) | 5a | 2231 patient‐years (median 5) | 17b |
| Age (median, IQR) | 58.0 (46.1, 71.1) | 0 | 58.0 (46.1, 71.0) | 0 |
| Females | 242 (50.5) | 0 | 239 (50.4) | 0 |
| Duration of anticoagulation (mutually exclusive groups) | ||||
| 3 months | 75 (15.7) | 0 | 75 (15.8) | 0 |
| 6 months | 230 (48.0) | 0 | 228 (48.10) | 0 |
| 12 months | 95 (19.8) | 0 | 95 (20.1) | 0 |
| Indefinite | 79 (16.5) | 0 | 76 (16.0) | 0 |
| Provoking risk factors (mutually exclusive groups) | ||||
| Provoked by surgeryc | 95 (19.9) | 0 | 95 (20.0) | 0 |
| Non‐surgical transient risk factorc | 107 (22.3) | 0 | 106 (22.4) | 0 |
| Unprovoked VTEc | 265 (55.3) | 0 | 261 (55.1) | 0 |
| Active cancerc | 12 (2.5) | 0 | 12 (2.5) | 0 |
| Other risk factors | ||||
| Pregnancyd | 6 (1.3) | 0 | 6 (1.3) | 0 |
| Contraceptive use at the time of thrombosisd | 50 (10.4) | 6 | 50 (10.6) | 6 |
| Traveld | 34 (7.1) | 0 | 34 (7.2) | 0 |
| Immobilizationd | 40 (8.4) | 6 | 39 (8.2) | 6 |
| Inflammationd | 64 (13.4) | 7 | 63 (13.3) | 7 |
| Previous VTEd | 91 (19.0) | 0 | 90 (19.0) | 0 |
| Cardiovascular diseased | 115 (24.0) | 5 | 115 (24.3) | 5 |
| Heart failured | 6 (1.3) | 6 | 6 (1.3) | 6 |
| Known thrombophiliad | 19 (4.0) | 7 | 18 (3.8) | 7 |
| Venous insufficiencyd | 31 (6.5) | 8 | 31 (6.5) | 8 |
| Varicosisd | 22 (4.6) | 177 | 22 (4.6) | 173 |
| Residual thrombosisd , e | 144 (30.1) | 60 | 141 (29.8) | 58 |
| Smokingd | 107 (22.3) | 29 | 106 (22.4) | 26 |
| Family historyd | 140 (29.2) | 14 | 138 (29.1) | 14 |
| Risk factors not assessed at baseline | ||||
| Elevated D‐dimerd , f | 112 (23.4) | 122 | 112 (23.6) | 119 |
| Elevated CRPd , f | 109 (22.8) | 174 | 109 (23.0) | 171 |
| Elevated FVIIId& | 65 (13.6) | 157 | 65 (13.7) | 153 |
| Elevated Villalta scored , g , h | 78 (16.3) | 96 | 78 (16.5) | 94 |
CRP, C‐reactive protein; IQR, inter‐quartile range; VTE, venous thromboembolism.
Moved abroad.
Lost to follow‐up.
Mutually exclusive groups.
Patients can be subject to more than one risk factor.
One week before intended stop of OAC.
One month after stopping OAC.
At six months.
≥5 points & ≥213%; corresponding to the 80th percentile of the study population.
2.8. Statistical analysis
Incidence rates of recurrent VTE per 100 patient‐years and mortality rates were reported for all subgroups. Cumulative incidences of recurrent VTE in patients with and without risk factors were compared using Kaplan–Meier curves and the log‐rank test. A Cox proportional hazards model was used to calculate associations between predictor variables and recurrent VTE. Estimates were adjusted for factors that were previously identified as relevant predictors4, 5, 30, 31, 32: age, sex, surgery, pregnancy, contraceptive use at the time of thrombosis, traveling, inflammation, previous VTE, D‐dimer, and periods of anticoagulation as time‐varying covariate. Observations with missing values were excluded from analysis; a sensitivity analysis was conducted after multiple imputation. All analyses were conducted using Stata statistical software (StataCorp, College Station, TX, USA).
3. RESULTS
3.1. Patient characteristics
Four‐hundred and seventy‐nine patients diagnosed with proximal lower extremity DVT were treated within the CCP; the flow of the patients is shown in Figure 1, detailed patient characteristics are reported in Table 1. During the two‐year CCP following diagnosis, five patients moved abroad (1.0%). After finishing CCP, 17 patients were lost to extended follow‐up (3.6%). Finally, 457 patients were followed for a median of 4.6 years equivalent to 2231.2 patient‐years (Table 1). The median age was 58.0 years (inter‐quartile range [IQR] 46.1, 71.1; 50.5% of the patients were female (n = 242). DVT was unprovoked in 55.3% of the cases (n = 265). RVO was observed in 30.1% of the patients (n = 144). Duration of anticoagulation therapy was three months in 15.7% of the patients (n = 75), six months in 48.0% (n = 230), 12 months in 19.8% (n = 95) and indefinite in 16.5% (n = 79). All patients received vitamin K antagonist therapy (predominantly acenocoumarol). RVO was present in 45 patients with provoked DVT (25.1%), in 80 patients with unprovoked DVT (37.4%), and in 19 patients with high risk DVT (22.1%; see Figure 1).
3.2. VTE recurrence
Thirty seven recurrent VTE were observed within the first two years of CCP follow‐up and 64 VTE during the extended follow‐up. Type of event was DVT in 39 patients (60.9%), PE in 20 patients (31.3%), and other venous thromboembolism in five patients (7.8%; cerebral venous sinus thrombosis [n = 1; 1.6%] and upper extremity DVT [n = 4; 6.3%]). Ipsilateral DVT was observed in 29 patients (45.3%).
The overall incidence rate was 4.2 per 100 patient‐years for the first two years and 2.9 for the extended observation period (Table 2). The Kaplan‐Meier estimate of overall recurrence is shown in Figure 1. In patients with DVT provoked by surgery, incidence rate was 1.1 per 100 patient‐years (CCP) or 1.3, respectively (extended observation period). For patients with non‐surgical transient risk factors, the incidence rate was 3.1 or 2.1, respectively. In contrast, the incidence rate was 6.1 or 4.0, respectively, in patients with unprovoked VTE. The cumulative incidence according to these groups is illustrated in Figure 3. Subgroup analyses revealed higher incidence rates for the following risk factors: male sex, traveling history, inflammatory disease, elevated D‐dimer, high factor VIII and elevated CRP (Table 2). Kaplan–Meier estimates are shown in Figure 3. No recurrent VTE events occurred during anticoagulation treatment.
Table 2.
Incidence rate of recurrent venous thromboembolic events by risk factors
| Risk factors | Clinical care pathway | Extended observation period | ||||
|---|---|---|---|---|---|---|
| Events frequency | Observation time patient‐years | Incidence rate (95% CI) per 100 patient‐years | Events frequency | Observation time patient‐years | Incidence rate (95% CI) per 100 patient‐years | |
| Total | 37 | 876 | 4.2 (3.1, 5.8) | 64 | 2231 | 2.9 (2.2, 3.7) |
| Provoking risk factors (mutually exclusive groups) | ||||||
| Provoked by surgery | 2 | 186 | 1.1 (0.3, 4.3) | 6 | 475 | 1.3 (0.6, 2.8) |
| Non‐surgical transient risk factor | 6 | 192 | 3.1 (1.4, 7.0) | 11 | 519 | 2.1 (1.2, 3.8) |
| Unprovoked VTE | 29 | 478 | 6.1 (4.2, 8.7) | 47 | 1186 | 4.0 (3.0, 5.3) |
| Active cancer | 0 | 21 | N/A | 0 | 52 | N/A |
| Other risk factors | ||||||
| Male sex | 26 | 429 | 6.1 (4.1, 8.9) | 41 | 1042 | 3.9 (2.9, 5.3) |
| Female sex | 11 | 447 | 2.5 (1.4, 4.4) | 23 | 1190 | 1.9 (1.3, 2.9) |
| Pregnancy | 0 | 11 | N/A | 1 | 30 | 3.3 (0.5, 23.7) |
| Contraceptive use at the time of thrombosis | 0 | 94 | N/A | 1 | 270 | 0.4 (0.0, 2.6) |
| Travel | 4 | 60 | 6.6 (2.5, 17.7) | 8 | 175 | 4.6 (2.2, 3.7) |
| Immobilization | 2 | 69 | 2.9 (0.7, 11.5) | 2 | 168 | 1.2 (0.3, 4.8) |
| Inflammation | 8 | 117 | 6.8 (0.3, 13.7) | 15 | 317 | 4.7 (2.9, 7.9) |
| Previous VTE | 2 | 175 | 1.1 (0.3, 4.6) | 10 | 492 | 2.0 (1.1, 3.8) |
| Cardiovascular disease | 10 | 209 | 4.8 (2.6, 8.9) | 17 | 467 | 3.6 (2.3, 5.9) |
| Heart failure | 0 | 12 | N/A | 1 | 33 | 3.0 (0.4, 21.6) |
| Known thrombophilia | 0 | 36 | N/A | 1 | 91 | 1.1 (0.2, 7.8) |
| Venous insufficiency | 0 | 59 | N/A | 0 | 106 | N/A |
| Varicose veins | 1 | 43 | 2.3 (0.3, 16.4) | 3 | 139 | 2.2 (0.7, 6.7) |
| Smoking | 7 | 200 | 3.5 (1.7, 7.4) | 13 | 524 | 2.5 (1.4, 4.3) |
| Family history | 10 | 260 | 3.9 (2.1, 7.2) | 17 | 659 | 2.6 (1.6, 4.1) |
| Risk factors not assessed at baseline | ||||||
| Residual vein obstructiona | 12 | 270 | 4.4 (2.5, 7.8) | 22 | 689 | 3.2 (2.1, 4.8) |
| Elevated Villalta scoreb ,d | 6 | 148 | 4.0 (1.8, 9.0) | 14 | 362 | 3.9 (2.3, 6.5) |
| Elevated D‐dimerc | 17 | 197 | 8.6 (5.4, 13.9) | 26 | 514 | 5.1 (3.4, 7.4) |
| High factor VIIIc | 9 | 120 | 7.5 (3.9, 14.5) | 17 | 329 | 5.2 (3.2, 8.3) |
| Elevated CRPc | 11 | 201 | 5.5 (3.0, 9.9) | 22 | 537 | 4.1 (2.7, 6.2) |
Assessed one week before intended cessation of anticoagulation treatment.
Assessed at six months.
Assessed one month after stopping OAC.
≥5 points.
Figure 3.
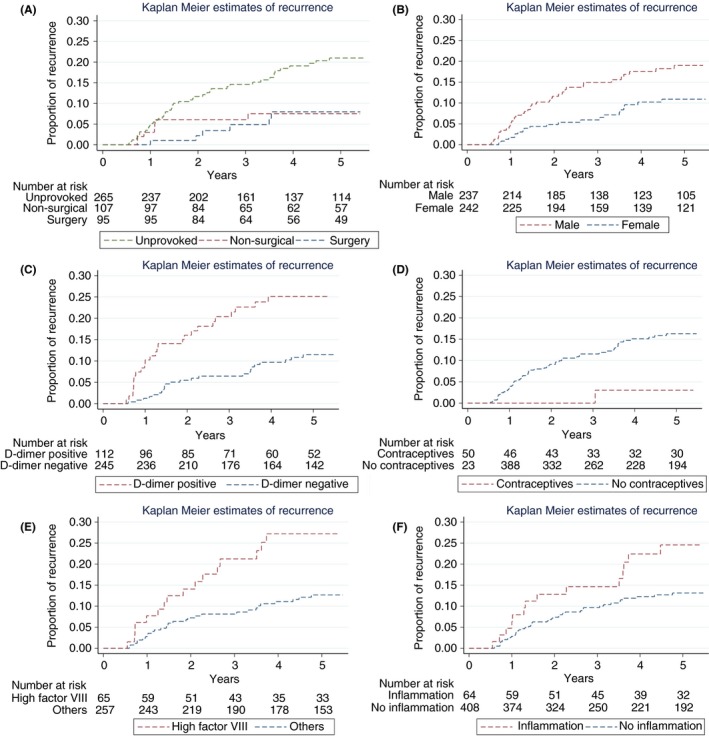
Cumulative incidence of VTE recurrence according to the presence of risk factors. (A) Unprovoked VTE, non‐surgical transient risk factor, provoked by surgery (mutually exclusive groups); Incidence rates were 1.3 per 100 patient‐years if provoked by surgery, 2.1 if a non‐surgical transient risk factor was present and 4.0 if unprovoked; (B) Sex; (C) Elevated D‐dimer one month after cessation of anticoagulant treatment; (D) Use of contraceptives at the time of thrombosis; (E) High factor VIII; and (F) Presence of inflammation
Figure 2.
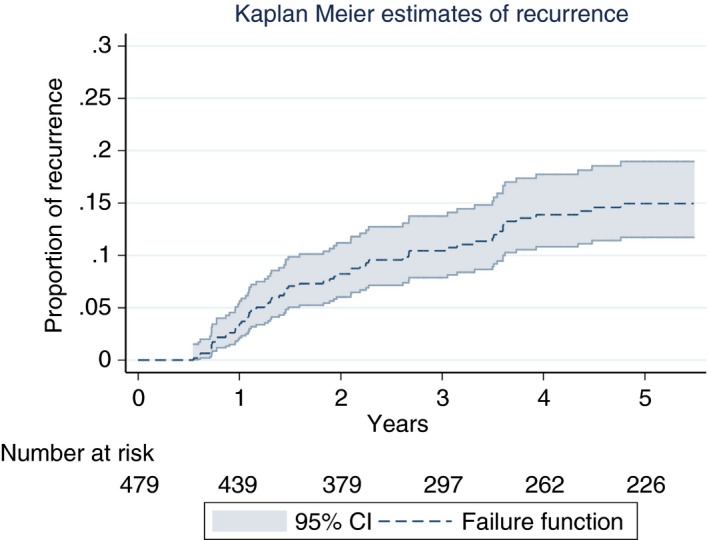
Cumulative incidence of VTE recurrence in patients managed in a clinical care pathway incorporating the presence of residual vein obstruction. The overall incidence rate was 2.9 per 100 patient‐years
3.3. Mortality
Twenty‐one patients died during the observation period resulting in a mortality rate of 2.4 per 100 patient‐years (CCP), or 1.5 (extended follow‐up), respectively. The highest mortality rates were observed among patients with active cancer (14.6 or 17.4 per 100 patient‐years, respectively). In contrast, low mortality rates (below 2.0 and 1.5 per 100 patient‐years respectively) were recorded in patients with non‐surgical transient risk factors, namely, previous VTE, venous insufficiency, a family history of VTE, residual thrombosis or high factor VIII. Details for all subgroups are presented in Table S1 (Supplemental Material).
3.4. Risk factors for recurrent VTE
Unadjusted and adjusted hazard ratios (HR) are reported in Table 3. Compared to patients with VTE provoked by surgery, the adjusted HR was 1.6 for non‐surgical transient risk factors (95% CI: 0.5, 5.5), and 4.6 for unprovoked VTE (95% CI: 1.7, 11.9). The association between active cancer and recurrent VTE could not be determined due to a very low number of patients in this subgroup. A statistically significant association with recurrence of VTE was observed for a number of additional predictors (Table 3): male sex (HR 2.8; 95%CI: 1.5, 5.1), use of contraceptives at the time of thrombosis (HR 0.1; 95%CI 0.0, 0.9), elevated D‐dimer (HR 3.3; 95% CI 1.8, 6.1); and high factor VIII (HR 2.2; 95%CI: 1.2, 4.0).
Table 3.
Hazard ratios of recurrent VTE by risk factors
| Risk factor | Hazard ratioa | 95% CI | Hazard ratioa | 95% CI |
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Provoking risk factors (mutually exclusive groups) | ||||
| Provoked by surgeryc | 1.0 | 1.0 | ||
| Non‐surgical transient risk factorc | 1.8 | 0.7, 4.8 | 1.6 | 0.5, 5.5 |
| Unprovoked VTEc | 3.1 | 1.3, 7.4 | 4.6 | 1.7, 11.9 |
| Active cancerc | N/A | N/A | ||
| Other risk factors | ||||
| Male sex | 2.0 | 1.2, 3.3 | 2.8 | 1.5, 5.1 |
| Pregnancy | 1.1 | 0.2, 8.1 | 1.9 | 0.2, 15.1 |
| Contraceptive use at the time of thrombosis | 0.1 | 0.0, 0.9 | 0.1 | 0.0, 0.9 |
| Traveling | 1.8 | 0.9, 3.8 | 1.5 | 0.6, 3.8 |
| Immobilization | 0.4 | 0.1, 1.6 | 0.2 | 0.0, 1.5 |
| Inflammation | 1.9 | 1.1, 3.4 | 1.8 | 0.9, 3.4 |
| Previous VTE | 0.7 | 0.3, 1.3 | 0.8 | 0.3, 1.8 |
| Cardiovascular disease | 1.3 | 0.7, 2.2 | 1.1 | 0.6, 2.2 |
| Heart failure | 1.0 | 0.1, 7.4 | 2.0 | 0.3, 15.2 |
| Known thrombophilia | 0.4 | 0.1, 2.7 | 0.6 | 0.1, 4.8 |
| Varicose veins | 0.8 | 0.3, 2.6 | 1.0 | 0.3, 3.4 |
| Smoking | 0.8 | 0.4, 1.5 | 0.7 | 0.4, 1.5 |
| Family history | 0.8 | 0.5, 1.5 | 0.9 | 0.5, 1.7 |
| Risk factors not assessed at baseline | ||||
| Residual thrombosisd | 1.0 | 0.6, 1.7 | 1.1 | 0.6, 2.0 |
| Elevated Villalta scoree , f | 1.6 | 0.9, 3.0 | 1.6 | 0.8, 3.5 |
| Elevated D‐dimerb, g | 2.5 | 1.5, 4.3 | 3.3 | 1.8, 6.1 |
| High factor VIIIb, g | 2.3 | 1.3, 4.2 | 2.2 | 1.2, 4.0 |
| Elevated CRPb, g | 1.8 | 1.0, 3.2 | 1.5 | 0.8, 2.7 |
Cox proportional hazards model.
Adjusted by anticoagulation (time‐varying co‐variable), age, surgery, pregnancy, contraceptive use at the time of thrombosis, travel, inflammation, sex, previous VTE, D‐dimer, and Factor VIII levels.
Groups are mutually exclusive, reference is provoked by surgery.
Assessed one week before intended cessation of anticoagulation treatment.
Assessed at six months.
≥5 points.
Assessed one month after stop anticoagulation treatment.
4. DISCUSSION
In our study of a cohort of patients with proximal DVT who were managed with a CCP that incorporated the presence of RVO, we found that predictors of recurrent VTE were unprovoked VTE, elevated D‐dimer 1 month after anticoagulant treatment was stopped, male sex, and high factor VIII. We also documented that 99% of patients diagnosed with proximal DVT and treated within the CCP completed the 2 year treatment protocol, the overall rate of recurrence was relatively low, and recurrence rates were lowest in women with VTE provoked by oral contraceptives.
Minimal data is available on clinical outcomes of patients treated within a particular CCP or treatment program, and research to date has instead focused on the short‐term management. Tillman and colleagues evaluated an outpatient DVT treatment program in 391 patients19 resulting in a VTE incidence rate of 6.0 per 100 patient‐years. An elevated risk of mortality was stated in a different cohort of 131 patients, but no incidence rates were reported.34 Equivalent numbers of recurrent VTE compared to usual care were recorded in another CCP in the community setting, but only short‐term effects were observed.17 There have been several other investigations studying small cohorts, but few or no clinical outcomes were reported.17, 35, 36, 37, 38 In contrast, we studied the long‐term clinical outcomes in large cohort of patients treated within a defined CCP considering risk assessment and long‐term treatment strategies.32 In accordance with previous investigations, patients with unprovoked VTE were found to carry a high risk of recurrence.32 Both observational data39, 40, 41 and interventional studies have also confirmed the predictive value of elevated D‐dimer 1 month after cessation of treatment for VTE recurrence.42 Men had a higher risk of recurrence than women, in our setting as well as in others.43, 44 In contrast to earlier cohorts, risk of recurrence was very low in patients taking estrogen containing contraceptives at the time of thrombosis.45 This difference is most probably reflected by the increased awareness of the associated risk and the subsequent strict avoidance of estrogen‐containing drugs in patients that have suffered from VTE. In line with previous data, high factor VIII was associated with recurrent thromboembolism.46, 47 In addition, inflammatory conditions, as well as elevated CRP were associated with VTE recurrence, at least in the univariate analysis.48 RVO was not associated with recurrent VTE,11 perhaps because the duration of anticoagulation was tailored according to the presence of RVO in this CCP.
We are, however, faced with some limitations. The number of patients and recurrent events were limited, resulting in imprecise estimations for some of the more infrequent predictor variables or those with a smaller effect. This effect was intensified by a relevant number of missing values with regard to the variables not assessed at baseline. However, we did not find apparent discrepancies in sensitivity analyses (eg, after multiple imputation). Another limitation is that only very few patients with active cancer were included in our cohort. Thus, the results of our investigation cannot be extended to this specific population.49 Also concerning the efficacy of the management strategy some limitations have to be considered. We were not able to record bleeding events due to organizational reasons. Therefore, our conclusions are limited to the efficacy of the CCP. Moreover, we did not implement a formal assessment of the compliance with the CCP. The risk of relevant protocol‐deviations is however estimated to be low because the duration of anticoagulation fits well with the risk categories (85% of patients with provoked DVT were treated three to six months, 94% of patients with unprovoked DVT were treated six to 12 month, and 92% of high‐risk patients were treated indefinitely). In this study, as in many others,7, 8, 10, 50, 51, 52, 53, 54, 55 we did not formally asses the inter‐observer agreement with regard to RVO. We cannot fully exclude that this might have introduced a certain variability of results, obscuring a possible effect on clinical outcomes. However, this reflects clinical practice of a clinical care pathway. Another limitation is that we did not record if the recurrent event was provoked or unprovoked.
Our investigation has several strengths. Firstly, it is one of the first studies investigating clinical outcomes of DVT patients treated within a CCP and the first study investigating a management incorporating RVO in clinical practice. Secondly, we have conducted a long‐term follow up, facilitating long‐term predictions and counseling of patients.
CCPs aim to translate evidence‐based medicine into clinical practice, improve collaboration among multiple specialized caregivers, and standardize health‐care procedures. In addition the structured management and follow up of a CCP allows for prospective collection of data on clinical outcomes and identification of predictive factors for outcomes of interest. Our data support previous studies suggesting an implementation of a CCP for treatment of DVT in clinical practice and suggest that management incorporating the presence of RVO might be beneficial.
In conclusion, in this study we have demonstrated that long‐term VTE recurrence rates and mortality are low in DVT patients managed within a CCP incorporating the presence of RVO. Recurrence rates were lowest in women with VTE provoked by oral contraceptives. Major risk factors for VTE recurrence were unprovoked VTE, male sex, elevated D‐dimer, as well as factor VIII one month after cessation of treatment, and inflammation.
AUTHOR CONTRIBUTIONS
MN and ATC developed the study design, collected the data, and wrote the manuscript. MN developed the analysis plan and conducted the statistical analysis. ATC developed and implemented the CCP, co‐developed and reviewed the analysis plan, reviewed the analysis and acted as principal investigator. MP and HTC reviewed the study design and statistical analysis and wrote the manuscript.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper
Supporting information
Nagler M, ten Cate H, Prins MH, ten Cate‐Hoek AJ. Risk factors for recurrence in deep vein thrombosis patients following a tailored anticoagulant treatment incorporating residual vein obstruction. Res Pract Thromb Haemost. 2018;2:299–309. 10.1002/rth2.12079
REFERENCES
- 1. ISTH Steering Committee for World Thrombosis Day . Thrombosis: a major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–90. [DOI] [PubMed] [Google Scholar]
- 2. Cohen AT, Agnelli G, Anderson FA, et al. VTE Assessment Group in (VITAE) . Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98:756–64. [DOI] [PubMed] [Google Scholar]
- 3. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kyrle PA, Rosendaal FR, Eichinger S. Risk assessment for recurrent venous thrombosis. Lancet. 2010;376:2032–9. [DOI] [PubMed] [Google Scholar]
- 5. Lijfering WM, Rosendaal FR, Cannegieter SC. Risk factors for venous thrombosis ‐ current understanding from an epidemiological point of view. Br J Haematol. 2010;149:824–33. [DOI] [PubMed] [Google Scholar]
- 6. European Genetics Foundation; Cardiovascular Disease Educational and Research Trust; International Union of Angiology . Thrombophilia and venous thromboembolism. International consensus statement. Guidelines according to scientific evidence. Int Angiol. 2005;24:1–26. [PubMed] [Google Scholar]
- 7. Cosmi B, Legnani C, Cini M, Guazzaloca G, Palareti G. D‐dimer levels in combination with residual venous obstruction and the risk of recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Haemost. 2005;94:969–74. [DOI] [PubMed] [Google Scholar]
- 8. Piovella F, Crippa L, Barone M, et al. Normalization rates of compression ultrasonography in patients with a first episode of deep vein thrombosis of the lower limbs: association with recurrence and new thrombosis. Haematologica. 2002;87:515–22. [PubMed] [Google Scholar]
- 9. Tan M, Mos IC, Klok FA, Huisman MV. Residual venous thrombosis as predictive factor for recurrent venous thromboembolim in patients with proximal deep vein thrombosis: a sytematic review. Br J Haematol. 2011;153:168–78. [DOI] [PubMed] [Google Scholar]
- 10. Prandoni P, Lensing AW, Prins MH, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137:955–60. [DOI] [PubMed] [Google Scholar]
- 11. Carrier M, Rodger MA, Wells PS, Righini M, Le Gal G. Residual vein obstruction to predict the risk of recurrent venous thromboembolism in patients with deep vein thrombosis: a systematic review and meta‐analysis. J Thromb Haemost. 2011;9:1119–25. [DOI] [PubMed] [Google Scholar]
- 12. Campbell H, Hotchkiss R, Bradshaw N, Porteous M. Integrated care pathways. BMJ. 1998;316:133–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Bleser L, Depreitere R, De Waele K, Vanhaecht K, Vlayen J, Sermeus W. Defining pathways. J Nurs Manag. 2006;14:553–63. [DOI] [PubMed] [Google Scholar]
- 14. Kinsman L, Rotter T, James E, Snow P, Willis J. What is a clinical pathway? Development of a definition to inform the debate. BMC Med. 2010;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kidney R, Hosny G, Canning MB, Kong V, Barton D, Wakai A. Implementation of a clinical pathway for emergency department out‐patient management of deep vein thrombosis. Irish Med J. 2010;103:246–8. [PubMed] [Google Scholar]
- 16. Spyropoulos AC. Outpatient‐based treatment protocols in the management of venous thromboembolic disease. Am J Managed Care. 2000;6:S1034–44. [PubMed] [Google Scholar]
- 17. Vinson DR, Berman DA. Outpatient treatment of deep venous thrombosis: a clinical care pathway managed by the emergency department. Ann Emerg Med. 2001;37:251–8. [DOI] [PubMed] [Google Scholar]
- 18. Lee M, Pao D, Hsu T, Sonderskov A. Cost savings and effectiveness of outpatient treatment with low molecular weight heparin of deep vein thrombosis in a community hospital. Can J Clin Pharmacol. 2004;11:e17–27. [PubMed] [Google Scholar]
- 19. Tillman DJ, Charland SL, Witt DM. Effectiveness and economic impact associated with a program for outpatient management of acute deep vein thrombosis in a group model health maintenance organization. Arch Int Med. 2000;160:2926–32. [DOI] [PubMed] [Google Scholar]
- 20. Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C. Definition of post‐thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7:879–83. [DOI] [PubMed] [Google Scholar]
- 21. Angelillo‐Scherrer A, Nagler M. Thrombophilieabklärung. Ther Umsch. 2016;73:626–34. [DOI] [PubMed] [Google Scholar]
- 22. Di Minno MN, Dentali F, Lupoli R, Ageno W. Mild antithrombin deficiency and risk of recurrent venous thromboembolism: a prospective cohort study. Circulation. 2014;129:497–503. [DOI] [PubMed] [Google Scholar]
- 23. Kyrle PA, Eichinger S. Hereditäre thrombophile Risikofaktoren In: Pötzsch B, Madlener K, editors. Hämostaseologie, 2nd edn Berlin: Springer Verlag; 2010; p. 395–403. [Google Scholar]
- 24. Lensing AW, Prandoni P, Brandjes D, et al. Detection of deep‐vein thrombosis by real‐time B‐mode ultrasonography. N Engl J Med. 1989;320:342–5. [DOI] [PubMed] [Google Scholar]
- 25. Prandoni P, Cogo A, Bernardi E, et al. A simple ultrasound approach for detection of recurrent proximal‐vein thrombosis. Circulation. 1993;88:1730–5. [DOI] [PubMed] [Google Scholar]
- 26. Prandoni P, Lensing AW, Bernardi E, et al. The diagnostic value of compression ultrasonography in patients with suspected recurrent deep vein thrombosis. Thromb Haemost. 2002;88:402–6. [PubMed] [Google Scholar]
- 27. Linkins LA, Stretton R, Probyn L, Kearon C. Interobserver agreement on ultrasound measurements of residual vein diameter, thrombus echogenicity and Doppler venous flow in patients with previous venous thrombosis. Thromb Res. 2006;117:241–7. [DOI] [PubMed] [Google Scholar]
- 28. Bouman AC, Smits JJ, Ten Cate H, Ten Cate‐Hoek AJ. Markers of coagulation, fibrinolysis and inflammation in relation to post‐thrombotic syndrome. J Thromb Haemost. 2012;10:1532–8. [DOI] [PubMed] [Google Scholar]
- 29. Kyrle PA, Kammer M, Eischer L, et al. The long‐term recurrence risk of patients with unprovoked venous thromboembolism: an observational cohort study. J Thromb Haemost. 2016;14:2402–9. [DOI] [PubMed] [Google Scholar]
- 30. Cannegieter SC, van Hylckama Vlieg A. Venous thrombosis: understanding the paradoxes of recurrence. J Thromb Haemost. 2013;11(Suppl 1):161–9. [DOI] [PubMed] [Google Scholar]
- 31. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 32. Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170:1710–6. [DOI] [PubMed] [Google Scholar]
- 33. Chernecky CC, Berger BJ. Laboratory Tests and Diagnostic Procedures. St. Louis: Elsevier Saunders; 2013. [Google Scholar]
- 34. Ang DT, Simpson JM, Stewart IC, Murchison JT, Lockman KA. Excess long‐term mortality in outpatient deep venous thrombosis patients managed in an ambulatory care setting. QJMs. 2016;110:149–53. [DOI] [PubMed] [Google Scholar]
- 35. Dunn AS, Schechter C, Gotlin A, et al. Outpatient treatment of deep venous thrombosis in diverse inner‐city patients. Am J Med. 2001;110:458–62. [DOI] [PubMed] [Google Scholar]
- 36. Rymes NL, Lester W, Connor C, Chakrabarti S, Fegan CD. Outpatient management of DVT using low molecular weight heparin and a hospital outreach service. Clin Lab Haematol. 2002;24:165–70. [DOI] [PubMed] [Google Scholar]
- 37. Davis KA, Miyares MA, Price‐Goodnow VS. Optimizing transition of care through the facilitation of a pharmacist‐managed deep vein thrombosis treatment program. J Pharm Pract. 2013;26:438–41. [DOI] [PubMed] [Google Scholar]
- 38. Spyropoulos AC, Kardos J, Wigal P. Outcomes analysis of the outpatient treatment of venous thromboembolic disease using the low‐molecular‐weight heparin enoxaparin in a managed care organization. J Manag Care Pharm. 2000;6:298–304. [Google Scholar]
- 39. Cushman M, Folsom AR, Wang L, et al. Fibrin fragment D‐dimer and the risk of future venous thrombosis. Blood. 2003;101:1243–8. [DOI] [PubMed] [Google Scholar]
- 40. Andreescu AC, Cushman M, Rosendaal FR. D‐dimer as a risk factor for deep vein thrombosis: the Leiden Thrombophilia Study. Thromb Haemost. 2002;87:47–51. [PubMed] [Google Scholar]
- 41. Eichinger S, Minar E, Bialonczyk C, et al. D‐dimer levels and risk of recurrent venous thromboembolism. JAMA. 2003;290:1071–4. [DOI] [PubMed] [Google Scholar]
- 42. Palareti G, Cosmi B, Legnani C, et al. PROLONG Investigators . D‐dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. 2006;355:1780–9. [DOI] [PubMed] [Google Scholar]
- 43. Roach RE, Lijfering WM, Rosendaal FR, Cannegieter SC, le Cessie S. Sex difference in risk of second but not of first venous thrombosis: paradox explained. Circulation. 2014;129:51–6. [DOI] [PubMed] [Google Scholar]
- 44. Roach RE, Lijfering WM, Tait RC, et al. Sex difference in the risk of recurrent venous thrombosis: a detailed analysis in four European cohorts. J Thromb Haemost. 2015;13:1815–22. [DOI] [PubMed] [Google Scholar]
- 45. Christiansen SC, Cannegieter SC, Koster T, Vandenbroucke JP, Rosendaal FR. Thrombophilia, clinical factors, and recurrent venous thrombotic events. JAMA. 2005;293:2352–61. [DOI] [PubMed] [Google Scholar]
- 46. Cosmi B, Legnani C, Cini M, Favaretto E, Palareti G. D‐dimer and factor VIII are independent risk factors for recurrence after anticoagulation withdrawal for a first idiopathic deep vein thrombosis. Thromb Res. 2008;122:610–7. [DOI] [PubMed] [Google Scholar]
- 47. Eischer L, Gartner V, Schulman S, Kyrle PA, Eichinger S, AUREC‐FVIII investigators . 6 versus 30 months anticoagulation for recurrent venous thrombosis in patients with high factor VIII. Ann Hematol. 2009;88:485–90. [DOI] [PubMed] [Google Scholar]
- 48. Horvei LD, Grimnes G, Hindberg K, et al. C‐reactive protein, obesity, and the risk of arterial and venous thrombosis. J Thromb Haemost. 2016;14:1561–71. [DOI] [PubMed] [Google Scholar]
- 49. Kuderer NM, Culakova E, Lyman GH, Francis C, Falanga A, Khorana AA. A validated risk score for venous thromboembolism is predictive of cancer progression and mortality. Oncologist. 2016;21:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Prandoni P, Prins MH, Lensing AW, et al. AESOPUS Investigators . Residual thrombosis on ultrasonography to guide the duration of anticoagulation in patients with deep venous thrombosis: a randomized trial. Ann Intern Med. 2009;150:577–85. [DOI] [PubMed] [Google Scholar]
- 51. Cosmi B, Legnani C, Iorio A, et al. PROLONG Investigators . Residual venous obstruction, alone and in combination with D‐dimer, as a risk factor for recurrence after anticoagulation withdrawal following a first idiopathic deep vein thrombosis in the prolong study. Eur J Vasc Endovasc Surg. 2010;39:356–65. [DOI] [PubMed] [Google Scholar]
- 52. Young L, Ockelford P, Milne D, Rolfe‐Vyson V, McKelvie S, Harper P. Post‐treatment residual thrombus increases the risk of recurrent deep vein thrombosis and mortality. J Thromb Haemost. 2006;4:1919–24. [DOI] [PubMed] [Google Scholar]
- 53. Poli D, Antonucci E, Ciuti G, Abbate R, Prisco D. Combination of D‐dimer, F1+2 and residual vein obstruction as predictors of VTE recurrence in patients with first VTE episode after OAT withdrawal. J Thromb Haemost. 2008;6:708–10. [DOI] [PubMed] [Google Scholar]
- 54. Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim TM, Kim JS, Han SW, et al. Clinical predictors of recurrent venous thromboembolism: a single institute experience in Korea. Thrombs Res. 2009;123:436–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


