Abstract
Essentials.
Little evidence exists to guide procedural interruption of direct oral anticoagulants (DOACs).
Conducted a meta‐analysis of the interruption of DOACs in patients with atrial fibrillation (AF).
The 30‐day risk for thromboembolic and major bleeding events were 0.4% and 1.8%, respectively.
Perioperative interruption of DOACs in patients with AF appears to be safe and effective.
Background
Patients with atrial fibrillation (AF) frequently undergo invasive procedures that require temporary interruption of anticoagulation. There is little evidence to guide the perioperative interruption of direct oral anticoagulants (DOACs).
Methods
A systematic literature search including studies that evaluated the perioperative interruption of DOACs for non‐emergent invasive procedures in patients with AF was performed. The primary outcomes of interest were the 30‐day risk of thromboembolic events and major bleeding. Secondary outcomes of interest included the 30‐day risk of minor bleeding and overall mortality. The systematic review protocol and search strategy were registered online (PROSPERO January 27th 2017:CRD42017056124).
Results
A total of 8 publications encompassing 14 446 patients and 17 107 periprocedural interruptions were included in our study. Our analysis revealed a pooled postoperative 30‐day thromboembolic complication risk of 0.41% (95% CI 0.29‐ 0.54), and a pooled 30‐day postoperative major bleeding risk of 1.81% (95% CI 0.84‐3.13). Pooled 30‐day postoperative risks of minor bleeding and overall mortality were 3.08% (95% CI 1.02‐6.20) and 0.67% (95% CI 0.29‐1.23), respectively. Meta‐analysis of the included comparative studies did not reveal any significant differences in these postoperative outcomes following the perioperative interruption of DOACs or vitamin K antagonists.
Conclusions
The perioperative interruption of DOACs in patients with AF was associated with 0.4% thromboembolic and 1.8% major bleeding events at 30 days post surgery. These findings seem reassuring, but require validation in large prospective management studies where pre‐operative DOAC levels are measured and compared with clinical outcomes in this patient population.
Keywords: anticoagulants, atrial fibrillation, hemorrhage, meta‐analysis, review
1. INTRODUCTION
Atrial fibrillation (AF) is increasingly common as our population ages, with estimates indicating that the number of affected patients will reach 5.6 million by the year 2050.1 Atrial fibrillation has become a cardiovascular epidemic, and is associated with significant morbidity and mortality, with considerable implications for population disease burden and medical costs.2, 3, 4 Oral anticoagulants, which include vitamin K antagonists (VKA) and direct oral anticoagulants (DOACs), are used to prevent thromboembolism including stroke and systemic embolism in this patient population.5, 6, 7, 8, 9, 10, 11, 12 Patients on oral anticoagulants frequently undergo invasive procedures that require temporary interruption of anticoagulation. The increasing prevalence of AF, as well as the need to interrupt anticoagulation for invasive procedures poses a growing problem for a wide variety of clinicians.13 Previous expert narrative reviews have provided guidance to clinicians on the management of DOACs in the perioperative period.14, 15 In order to update these expert reviews with clinical data, we conducted a systematic review and meta‐analysis of the literature on the perioperative management of DOACs in patients with AF. Our aim was to assess risk of perioperative thromboembolism and bleeding following the perioperative interruption of DOACs.
2. METHODS
2.1. Search strategy
We conducted a systematic literature search using EMBASE, MEDLINE, and the Cochrane Central Register of Controlled Trials. The PICO question was: In patient with AF on DOACs for stroke prevention requiring perioperative temporary interruption of their anticoagulation regimen for a procedure, what is the 30‐day risk for thromboembolic and major bleeding events? The full search strategy is available in the supporting information. References of included studies, narrative reviews and recent conference proceedings of major international conferences were reviewed for additional studies. There were no restrictions with respect to date of publication or language. The systematic review protocol and search strategy were registered online (PROSPERO January 27, 2017: CRD42017056124).
2.2. Study selection
Two authors (JS and JW) independently identified studies eligible for inclusion based on an initial screen of reference titles and abstracts. Articles were included for further review if they evaluated the perioperative interruption of a DOAC (dabigatran, rivaroxaban, apixaban, or edoxaban) in patients with AF, and reported both postoperative thromboembolic and bleeding outcomes. Randomized controlled trials, prospective and retrospective studies were included. Studies that exclusively evaluated the perioperative interruption of DOACs for cardiac ablation were excluded, as well as those exclusively evaluating urgent/emergent procedures. Article records were independently reviewed for inclusion in duplicate, and discrepancies were resolved by consensus.
2.3. Data extraction and quality assessment
Two authors (JS and JW) independently extracted data using a standardized form. Primary outcomes of interest included 30‐day risk of perioperative thromboembolic and major bleeding. Secondary outcomes of interest included 30‐day risk of perioperative minor bleeding and all‐cause mortality. Outcomes were defined according to what was used in the included studies. Thromboembolic events were defined as stroke or systemic embolism. Major bleeding was defined according to the criteria of the International Society on Thrombosis and Haemostasis.16, 17, 18 Minor bleeding was defined as bleeding events not meeting the major bleeding criteria. The quality of randomized controlled trials was assessed using the Cochrane Collaboration's tool for assessing risk of bias. The quality of cohort/case‐control studies was assessed using the Newcastle‐Ottawa Scale.
2.4. Statistical analysis
Outcomes were reported per perioperative interruption. Pooled percentages and 95% confidence intervals of primary and secondary outcomes were generated using Stat Direct 3.1.11 software (Cheshire, UK). Forest plots of randomized controlled data (DOACs vs. VKAs) were also generated using Revman 5.3 software (London, UK). Analyses were conducted using a random effects model (DerSimonian‐Laird analysis). Subgroup analysis of studies assessing interruption of dabigatran was conducted. The I 2 statistic was used to estimate total variation among the pooled estimates across studies. An I 2 of <25% was considered as low‐level heterogeneity, 25% to 50% was moderate level and higher than 50% was considered as high level.19
3. RESULTS
3.1. Study characteristics
Our literature search identified an initial 917 records, of which 46 studies met our preliminary inclusion criteria based on title and abstract screening. A total of 8 publications including 14 446 patients and 17 107 periprocedural interruptions met full eligibility criteria (Table 1, Figure 1).20, 21, 22, 23, 24, 25, 26, 27 Four studies consisted of post‐hoc retrospective analyses of prospectively collected randomized controlled trial data and the remaining four studies were prospective or retrospective cohort studies. Five studies evaluated the perioperative interruption of dabigatran, whereas there was one study for each of rivaroxaban, apixaban and edoxaban.
Table 1.
Study characteristics
| References | Year | Author | Design | DOAC/VKA | CHADS2 (mean ± SD) (median (IQR)) | No. of Patients | No. of Interruptions |
|---|---|---|---|---|---|---|---|
| 20 | 2012 | Healey | RCT |
Dabigatran 150 Dabigatran 110 Warfarin |
2.1 ± 1.1 2.1 ± 1.1 2.1 ± 1.1 |
4591 | 4591 |
| 21 | 2014 | Sherwood | RCT |
Rivaroxaban Warfarin |
3.40 ± 0.95 3.42 ± 0.96 |
2130 | 2980 |
| 22 | 2014 | Garcia | RCT |
Apixaban Warfarin |
2.1 ± 1.1 2.1 ± 1.1 |
3930 | 5741 |
| 23 | 2015 | Douketis | RCT |
Edoxaban 60 Edoxaban 30 Warfarin |
2.8 ± 0.9 2.9 ± 0.9 2.8 ± 1.0 |
3116 | 3116 |
| 24 | 2015 | Schulman | Prospective Cohort | Dabigatran/‐ | – | 531 | 531 |
| 25 | 2014 | Kosiuk | Prospective Cohort | Dabigatran/‐ | 4 (3‐5) | 85 | 85 |
| 26 | 2014 | Terekhov | Prospective Cohort | Dabigatran/‐ | – | 16 | 16 |
| 27 | 2016 | Madan | Retrospective Cohort | Dabigatran/‐ | – | 47 | 47 |
DOAC, Direct Oral Anticoagulant; RCT, Randomized Controlled Trial; VKA, Vitamin K Antagonist.
Figure 1.
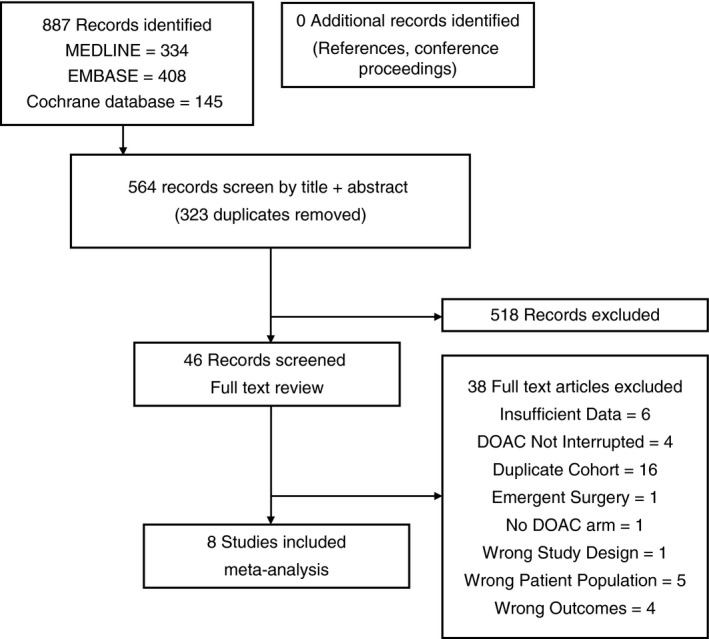
Study flow diagram
3.2. Procedural characteristics
Three cohort studies exclusively included patients undergoing permanent pacemaker insertion or implantable cardioverter defibrillator procedures. Five studies included patients undergoing a variety of procedures (Table 2 and Table 3). Of the eight included studies, five had defined perioperative anticoagulation protocols. Timing of anticoagulation discontinuation/resumption and the use of bridging anticoagulation were variable across studies (Table 2).
Table 2.
Procedural characteristics
| References | Author | Defined Perioperative Anticoagulation Protocol | DOAC/VKA | Emergent %/(n) | Bridging %/(n) | Procedural Type (%) |
|---|---|---|---|---|---|---|
| 20 | Healey | Yes |
Dabigatran 150 Dabigatran 110 Warfarin |
9.1 (141) 4.2 (63) 7.1 (111) |
17.0 (263) 15.3 (228) 28.5 (444) |
PPM/ICD (10.3) Dental (10.0) Diagnostic (10.0) Cataract Removal (9.3) Colonoscopy (8.6) Arthroplasty (6.2) Other (45.6) |
| 21 | Sherwood | Yes |
Rivaroxaban Warfarin |
Unknown | Unknown |
Colonoscopy (17.0) Dental (17.0) Abdominal/Thoracic/ Orthopedic (13.0) Dermatological (11.0) Unknown (11.0) Electrophysiology (9.0) Ocular (8.0) Other (8.0) Angiography/PCI (6.0) Urologic (4.0) CABG (1.0) |
| 22 | Garcia | Yes |
Apixaban Warfarin |
2.9% (266) |
11.7 (548) 11.7 (548) |
Dental (14.6) Colonoscopy (9.9) Ocular (8.0) EGD (7.6) PPM (3.5) Cystoscopy (3.2) PCI (2.8) AV Node Ablation (1.0) Pulmonary Vein Isolation (0.8) ICD (0.8) |
| 23 | Douketis | No |
Edoxaban 60 Edoxaban 30 Warfarin |
0% (0) |
4.5 (47) 4.7 (48) 5.2 (55) |
Angiography/PCI (9.6) GI Endoscopy (12.0) Dental (13.6) PPM/ICD (12.7) Ocular (10.8) Orthopedic Surgery (8.0) Genitourinary Surgery (3.9) General Surgery (5.6) Superficial Procedure (7.5) Invasive Diagnostic (11.4) Other (4.9) |
AV, Atrioventricular; CABG, Coronary Artery Bypass Grafting; DOAC, Direct Oral Anticoagulant; EGD, Esophagogastroduodenoscopy; PCI, Percutaneous Coronary Intervention; TURP, Transurethral Resection of the Prostate; TURBT, Transurethral Resection of Bladder Tumor; VKA, Vitamin K Antagonist.
Table 3.
Cohort study procedural characteristics
| References | Author | Defined Perioperative Anticoagulation Protocol | DOAC | Emergent (%) | Bridging (%) | Procedural Type (%) |
|---|---|---|---|---|---|---|
| 24 | Schulman | Yes | Dabigatran | 0 | 1.7 |
Endoscopy/Bronchoscopy (21.8) EPS/Ablation (14.6) Cardiac Catheterization (12.4) ICD/PPM (9.6) Joint Surgery (5.4) Abdominal Surgery (5.2) Biopsy (4.4) Ocular (3.9) Vascular (3.5) TURP/TURBT (3.1) Other (16.1) |
| 25 | Kosiuk | Yes | Dabigatran | 0 | 0 |
PPM (65.0) ICD (35.0) |
| 26 | Terekhov | No | Dabigatran | 0 | 0 | PPM (100.0) |
| 27 | Madan | No | Dabigatran | 0 | 0 |
PPM (55.3) ICD (44.7) |
DOAC, Direct Oral Anticoagulant; EPS, Electrophysiology Procedure; ICD, Implantable Cardioverter Defibrillator; PPM, Permanent Pacemaker; TURP, Transurethral Resection of the Prostate; TURBT, Transurethral Resection of Bladder Tumor.
3.3. Postoperative thromboembolic events
Among the eight included studies, there were 38 postoperative thromboembolic events during 9939 DOAC interruptions, yielding a pooled 30‐day postoperative thromboembolic risk of 0.41% (95% CI 0.29‐0.54, I 2 = 0%) (Tables 4 and 5). The pooled risk of postoperative thromboembolic events among studies evaluating dabigatran was similar at 0.44% (95% CI 0.26‐ 0.68, I 2 = 0%) (Table 5). The meta‐analysis including the 4 randomized controlled trials is reported in Figure 2A.20, 21, 22, 23 There were no significant differences between postoperative thromboembolic risk following procedural interruption of DOACs as compared to VKA.
Table 4.
Postoperative outcomes
| References | Author | DOAC/Warfarin | 30‐Day Thromboembolic (events/Interruptions, %) | 30‐Day major bleeding (Events/Interruptions, %) | 30‐day Minor Bleeding (events/Interruptions, %) | 30‐Day Overall Mortality (events/Interruptions, %) |
|---|---|---|---|---|---|---|
| 20 | Healey |
Dabigatran Warfarin |
14/3033 (0.46) 7/1558 (0.45) |
135/3033 (4.45) 72/1558 (4.62) |
259/3033 (8.54) 122/1558 (7.83) |
– |
| 21 | Sherwood |
Rivaroxaban Warfarin |
4/1297 (0.31) 8/1683 (0.48) |
14/1297 (1.08) 18/1683 (1.07) |
20/1297 (1.54) 24/1683 (1.43) |
1/1297 (0.08) 3/1683 (0.18) |
| 22 | Garcia |
Apixaban Warfarin |
9/2877 (0.31) 10/2864 (0.35) |
46/2792 (1.65) 35/2774 (1.26) |
26/2792 (0.93) 26/2774 (0.94) |
30/2877 (1.04) 15/2864 (0.52) |
| 23 | Douketis |
Edoxaban Warfarin |
10/2053 (0.49) 6/1063 (0.56) |
23/2053 (1.12) 11/1063 (1.03) |
56/2053 (2.73) 30/1063 (2.82) |
22/2053 (1.07) 13/1063 (1.22) |
| 24 | Schulman | Dabigatran | 1/531 (0.19) | 10/531 (1.88) | 24/531 (4.52) | 4/531 (0.75) |
| 25 | Kosiuk | Dabigatran | 0/85 (0) | – | – | 0/85 (0) |
| 26 | Terekhov | Dabigatran | 0/16 (0) | 0/16 (0) | 1/16 (6.25) | 0/16 (0) |
| 27 | Madan | Dabigatran | 0/47 (0) | 0/47 (0) | 0/47 (0) | 0/47 (0) |
| Total | – |
DOAC Warfarin |
38/9939 (0.38) 31/7168 (0.43) |
228/9769 (2.33) 136/7078 (1.92) |
386/9769 (3.95) 202/7078 (2.85) |
57/6906 (0.83) 31/5610 (0.55) |
DOAC, Direct Oral Anticoagulant.
Table 5.
All DOACs and dabigatran postoperative outcomes pooled incidence analysis
| 30‐day thromboembolic % [95% CI; I 2] | 30‐day major bleeding % [95% CI; I 2] | 30‐day minor bleeding % [95% CI; I 2] | 30‐day overall mortality % [95% CI] | |
|---|---|---|---|---|
| Overall | 0.41% [0.29‐0.54; 0%] | 1.81% [0.84‐3.1; 92.4%] | 3.08% [1.02‐6.20; 97.7%] | 0.67% [0.29‐1.23] |
| Dabigatran | 0.44% [0.26‐0.68; 0%] | 2.56% [0.92‐4.99; 76.9%] | 5.15% [2.21‐9.25; 85.2%] | 0.74% [0.24‐1.53] |
DOAC, Direct Oral Anticoagulant.
Figure 2.
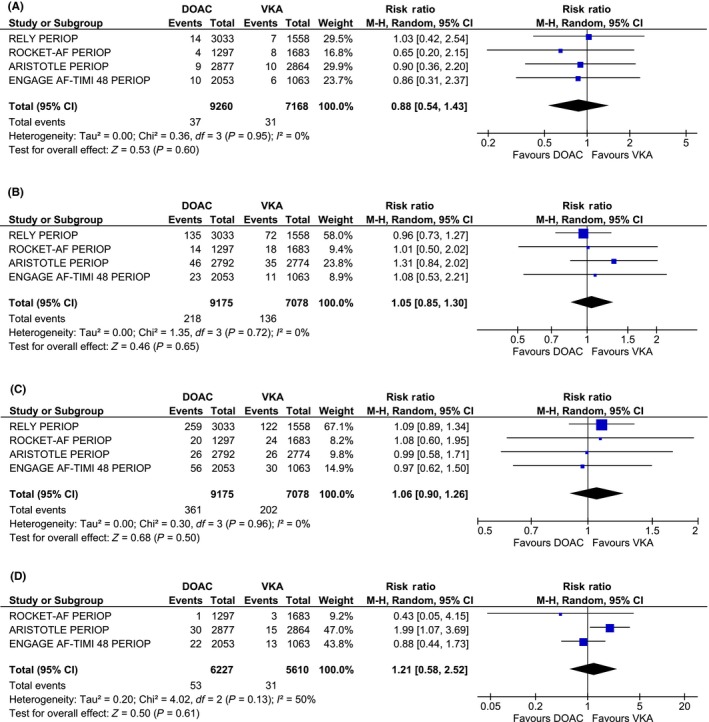
Randomized controlled trial meta‐analyses . Meta‐analyses of 30‐day event rates (A) thromboembolic events (B) major bleeding (C) minor bleeding (D) overall mortality. DOAC, direct oral anticoagulant; VKA, vitamin K antagonist
3.4. Postoperative major bleeding
Seven studies were included in the pooled analysis of major bleeding events. There were a total of 228 major bleeding events during 9769 DOAC interruptions, yielding a pooled 30‐day postoperative major bleeding risk of 1.81% (95% CI 0.8‐3.13, I 2 = 92.4%) (Table 4 and 5). The pooled percentage of postoperative major bleeding risk among patients using dabigatran was similar at 2.56% (95% CI 0.92‐4.99, I 2 = 76.9%) (Table 5). The majority of major bleeding events occurred in the perioperative analysis of one randomized trial.20 The meta‐analysis including the four randomized controlled trials is reported in Figure 2B.20, 21, 22, 23 There were no significant differences between postoperative major bleeding episodes following procedural interruption of DOACs as compared to VKA.
3.5. Postoperative minor bleeding
Seven of eight studies reporting minor bleeding events were included in the pooled analyses. There were a total of 386 minor bleeding events occurring during 9769 DOAC interruptions, yielding a pooled 30‐day postoperative minor bleeding risk of 3.08% (95% CI 1.02‐6.20, I 2 = 97.7%) (Tables 4 and 5). Similarly, there were no significant differences between postoperative minor bleeding episodes following procedural interruption of DOACs as compared to VKA (Figure 2C).
3.6. Postoperative all‐cause mortality
Seven out of the eight studies were included in the pooled analysis of 30‐day all‐cause mortality. There were a total of 57 postoperative deaths over 6906 DOAC interruptions, yielding a pooled 30‐day postoperative overall mortality risk of 0.67% (95% CI 0.29‐1.23, I 2 = 71.3%) (Tables 4 and Table 5). These results were similar to the pooled all‐cause mortality for dabigatran studies, with a 30‐day overall mortality of 0.74% (95% CI 0.24‐1.53, I 2 = 0%). There were no significant differences between postoperative overall mortality following procedural interruption of DOACs as compared to VKA (Figure 2D).
3.7. Quality assessment
Quality assessment of the randomized trials were carried out using the Cochrane Risk of Bias tool (Supporting information). None of the randomized trials had pre‐defined planned perioperative analyses with defined postoperative outcome definitions (selective reporting). In addition, none of the trials explicitly reported the percentage of patients completing 30‐day following each procedure (incomplete outcome data), although the percentage of patients lost to follow up in the trials was low. The Newcastle‐Ottawa Scale was used to assess the quality of cohort studies (Supporting information). Three out of the 4 included cohort studies did not have adequacy of follow up. In addition, most studies lacked details surrounding derivation of the cohort (eg, sequential recruitment). Three out of four of these studies did not incorporate blinded assessments of the outcomes of interest.
4. DISCUSSION
The principal finding from our meta‐analysis, which involved over 14,000 patients with AF who had periprocedural VKA or DOAC interruption, is that postoperative adverse outcomes are uncommon following DOAC interruption, with 30‐day postoperative risks of thromboembolism and major bleeding of 0.4% and 1.8%, respectively.
Our pooled results are similar to other large studies evaluating the perioperative interruption of VKA in patients with AF. In a recent randomized controlled trial,28 the 30‐day postoperative thrombotic risks were 0.4% and 0.3% in the no‐bridging and bridging groups, respectively, which are similar to our pooled DOAC risk of 0.41%. Similarly, the risk of postoperative thromboembolism was 0.4% overall in a recently published prospective registry evaluating the perioperative interruption of VKA.29 Our pooled major bleeding risk of 1.8% is also comparable to the results of a previously published trial on VKAs, which demonstrated a major bleeding risk of 1.3% and 3.2% in the non‐bridging and bridging groups, respectively. Finally, our reported risk of bleeding events also align with the major bleeding results observed in the ORBIT‐AF registry.29 Therefore, the results from our meta‐analyses seem to be generalizable to current clinical practices.
The postoperative major and minor bleeding risk seems to be higher in the studies assessing dabigatran for patients with AF. This discrepancy might be accounted for by the higher rates of bridging anticoagulation compared to the other included studies (Table 2). In addition, the perioperative dabigatran protocol was modified during the conduct of the trials to account for newly available pharmacokinetic data on dabigatran. Initially, dabigatran was held for 24 hours prior to all procedures. The protocol was then amended to hold dabigatran for 24 hours prior low risk surgical procedures and between two to five days prior high risk surgical procedures depending on renal function.20 This standardized interruption protocol has been shown to yield approximately 85% of patients with no residual anticoagulant effect at the time of procedure and low risk of major bleeding.30 Therefore, it is possible that dabigatran interruptions that occurred early after initiation of the randomized trials may have contributed to a higher bleeding risk. Furthermore, additional studies on DOAC interruption for elective procedure are needed to confirm these findings.
Our study has potential limitations. First, although several randomized controlled trials are included in our meta‐analysis, it is important to consider that the included data are from post‐hoc analyses of the original clinical trials. None of the randomized trials’ protocols described pre‐defined perioperative analyses or postoperative outcome definitions prior to conducting these perioperative analyses.31, 32, 33, 34 Therefore, the reported pooled estimates might be subject to bias and may underestimate the true 30‐day complications associated with interruption of DOACs. However, these post‐hoc analyses all incorporate widely accepted postoperative outcome definitions based on standard 30‐day complication rates, which would serve to reduce bias. Second, there was significant variability among perioperative anticoagulation practices between the different studies. In particular, only five out of the eight included studies had defined perioperative anticoagulation protocols that clearly instructed physicians on when to stop and re‐start anticoagulation in the event of an invasive procedure (Tables 2 and 3). Rates of bridging anticoagulation with alternative parenteral anticoagulation also varied significantly between studies. Overall, the rates of bridging were low but varied between 4.5% to 17.0% for DOAC‐treated patients. The timing and dosing of bridging anticoagulation (ie, pre‐operative, peri‐operative or postoperative only) are also unknown.35 Third, we excluded studies that exclusively evaluated cardiac ablation, as this topic has been extensively studied36, 37 This may have biased our results towards lower bleeding rates, as cardiac ablation procedures possibly carry a lower postoperative bleeding risk as compared to major procedures. We also excluded studies that focussed exclusively on emergent procedures. Therefore, our results may not be generalizable to these populations, as emergent procedures likely carry a high bleeding risk. Fourth, we did not have access to patient level data. Therefore, it is possible that we included patients that had more than one DOAC interruption. Finally, we were unable to report results according to procedure types. It is likely that the risk of these clinical outcomes varies depending on the procedure, as there is considerable cross‐procedure variability in risks of bleeding and thromboembolism.38
5. CONCLUSIONS
Interruption of DOACs for invasive procedures is associated with a relatively low risk of postoperative thrombotic and bleeding complications in patients with AF. There is also no significant difference in these risks when compared to the interruption of VKAs. The perioperative interruption of DOACs in patients with AF appears to be safe and effective. These findings are re‐assuring, but require validation in prospective management studies in this patient population.
AUTHOR CONTRIBUTIONS
J. Shaw developed the study concept and design, performed the systematic review, data extraction, data analysis, and authored the manuscript. J. Woodfine performed the systematic review and data analysis, and provided critical revisions to the manuscript. J. Douketis resolved conflicts with regards to study selection, provided unpublished data from one of the included studies, and provided critical revisions to the manuscript. S. Schulman provided unpublished data from one of the included studies, and provided critical revisions to the manuscript. M. Carrier provided critical advice surrounding study design and data extraction, performed data analysis, and provided critical revisions to the manuscript.
RELATIONSHIP DISCLOSURES
J. Shaw and J. Woodfine reported no conflicts of interests. M. Carrier received research funding from Bristol‐Myers Squibb and Leo Pharma. M. Carrier has also received honorarium from Pfizer, Bayer, Sanofi, Leo Pharma, and Daiichi Sankyo. J. Douketis reports receiving fees for serving on advisory boards from Biotie Therapies, Portola Pharmaceuticals, and The Medicines Company; honoraria from Bristol‐Myers Squibb, Pfizer, and Sanofi‐Aventis; consulting fees from Boehringer Ingelheim, Bayer, Janssen, Bristol‐Myers Squibb, Daiichi Sankyo, and Actelion Pharmaceuticals; and grant support from Boehringer Ingelheim. S. Schulman reports receiving honoraria from Boehringer Ingelheim, Bayer HealthCare, Daiichi Sankyo, and Sanofi, and research support from Boehringer Ingelheim, Baxter, and Octapharma.
Supporting information
Shaw JR, Woodfine JD, Douketis J, Schulman S, Carrier M. Perioperative interruption of direct oral anticoagulants in patients with atrial fibrillation: A systematic review and meta‐analysis. Res Pract Thromb Haemost. 2018;2:282–290. 10.1002/rth2.12076
[Article updated on March 03, 2018 after first online publication on February 16, 2018: A reference to NFAF in the abstract was corrected to AF.]
Contributor Information
Joseph R. Shaw, https://twitter.com/JRand083.
Marc Carrier, Email: mcarrier@toh.ca, https://twitter.com/MarcCarrier1.
REFERENCES
- 1. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 2. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–57. [PubMed] [Google Scholar]
- 3. Lip GY, Tean KN, Dunn FG. Treatment of atrial fibrillation in a district general hospital. Br Heart J. 1994;71:92–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. [DOI] [PubMed] [Google Scholar]
- 5. Patel MR, Mahaffey KW, Garg J, et al. ROCKET AF Investigators . Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–91. [DOI] [PubMed] [Google Scholar]
- 6. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. [DOI] [PubMed] [Google Scholar]
- 7. Schulman S, Kearon C, Kakkar AK, et al. RE‐COVER Study Group . Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. [DOI] [PubMed] [Google Scholar]
- 8. Agnelli G, Buller HR, Cohen A, et al. AMPLIFY Investigators . Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. [DOI] [PubMed] [Google Scholar]
- 9. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 10. Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. [DOI] [PubMed] [Google Scholar]
- 11. EINSTEIN Investigators , Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 12. EINSTEIN–PE Investigators , Buller HR, Prins MH, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97. [DOI] [PubMed] [Google Scholar]
- 13. Douketis JD. Pharmacologic properties of the new oral anticoagulants: a clinician‐oriented review with a focus on perioperative management. Curr Pharm Des. 2010;16:3436–41. [DOI] [PubMed] [Google Scholar]
- 14. Ferrandis R, Castillo J, de Andres J, et al. The perioperative management of new direct oral anticoagulants: a question without answers. Thromb Haemost. 2013;110:515–22. [DOI] [PubMed] [Google Scholar]
- 15. Spyropoulos AC, Douketis JD. How I treat anticoagulated patients undergoing an elective procedure or surgery. Blood. 2012;120:2954–62. [DOI] [PubMed] [Google Scholar]
- 16. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 17. Schulman S, Angeras U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. J Thromb Haemost. 2010;8:202–4. [DOI] [PubMed] [Google Scholar]
- 18. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S. Subcommittee on Control of Anticoagulation . Definition of clinically relevant non‐major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non‐surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13:2119–26. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 20. Healey JS, Eikelboom J, Douketis J, et al.; RE‐LY Investigators . Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) randomized trial. Circulation. 2012;126:343–8. [DOI] [PubMed] [Google Scholar]
- 21. Sherwood MW, Douketis JD, Patel MR, et al. Outcomes of temporary interruption of rivaroxaban compared with warfarin in patients with nonvalvular atrial fibrillation: results from the rivaroxaban once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation. 2014;129:1850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Garcia D, Alexander JH, Wallentin L, et al. Management and clinical outcomes in patients treated with apixaban vs warfarin undergoing procedures. Blood. 2014;124:3692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Douketis JD, Weitz JI, Murphy S, Deenadayalu N, Crompton AE. Perioperative adverse outcomes in patients with atrial fibrillation taking edoxaban or warfarin: analysis of the ENGAGE AF‐TIMI 48 trial Abstract. J Am Coll Cardiol. 2015;65:A2092. [Google Scholar]
- 24. Schulman S, Carrier M, Lee AY, et al.; Periop Dabigatran Study Group . Perioperative management of dabigatran: a prospective cohort study. Circulation. 2015;132:167–73. [DOI] [PubMed] [Google Scholar]
- 25. Kosiuk J, Koutalas E, Doering M, et al. Comparison of dabigatran and uninterrupted warfarin in patients with atrial fibrillation undergoing cardiac rhythm device implantations. Case‐control study. Circ J. 2014;78:2402–7. [PubMed] [Google Scholar]
- 26. Terekov D, Agapov V, Kulikov K, Zadorozhnaya S, Samitin V. Permanent pacemaker implantation during anticoagulant treatment with dabigatran etexilate (single center experience). Eur Heart J. 2014;35:266.23739243 [Google Scholar]
- 27. Madan S, Muthusamy P, Mowers KL, et al. Safety of anticoagulation with uninterrupted warfarin vs. interrupted dabigatran in patients requiring an implantable cardiac device. Cardiovasc Diagn Ther 2016;6:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Douketis JD, Spyropoulos AC, Kaatz S, et al. BRIDGE Investigators . Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373:823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Steinberg BA, Peterson ED, Kim S, et al. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation Investigators and Patients . Use and outcomes associated with bridging during anticoagulation interruptions in patients with atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT‐AF). Circulation. 2015;131:488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Douketis JD, Wang G, Chan N, et al. Effect of standardized perioperative dabigatran interruption on the residual anticoagulation effect at the time of surgery or procedure. J Thromb Haemost. 2016;14:89–97. [DOI] [PubMed] [Google Scholar]
- 31. Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE‐LY: randomized evaluation of long‐term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J 2009;157:805–10, 810 e1‐2. [DOI] [PubMed] [Google Scholar]
- 32. Investigators RAS . Rivaroxaban‐once daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J 2010;159:340–7.e1. [DOI] [PubMed] [Google Scholar]
- 33. Lopes RD, Alexander JH, Al‐Khatib SM, et al. ARISTOTLE Investigators . Apixaban for reduction in stroke and other ThromboemboLic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331–9. [DOI] [PubMed] [Google Scholar]
- 34. Ruff CT, Giugliano RP, Antman EM, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation‐Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF‐TIMI 48). Am Heart J. 2010;160:635–41. [DOI] [PubMed] [Google Scholar]
- 35. Spyropoulos AC, Al‐Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14:875–85. [DOI] [PubMed] [Google Scholar]
- 36. Vallakati A, Sharma A, Madmani M, et al. Efficacy and safety of novel oral anticoagulants for atrial fibrillation ablation: an updated meta‐analysis. Cardiol Ther. 2016;5:85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nairooz R, Ayoub K, Sardar P, et al. Uninterrupted new oral anticoagulants compared with uninterrupted vitamin k antagonists in ablation of atrial fibrillation: a meta‐analysis. Can J Cardiol. 2016;32:814–23. [DOI] [PubMed] [Google Scholar]
- 38. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e326S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


