Abstract
Essentials.
Overview of the three commercial thrombin generation methods.
Description of the sample preparation, data management, and analysis.
Description of the similarities and differences in regards to substrates results.
Discussion of advantages and disadvantages of the three approaches.
Currently there are three commercially available thrombin generation methods. These methods help detect the levels of thrombin generated in patient samples by the use of chromogenic or fluorogenic substrates in plasma or whole blood. Determining the rate of thrombin generation can help indicate if patients are at risk of clotting or bleeding. This review discusses two fluorogenic and one chromogenic method and focuses on similarities and differences of these three methods. The review specifically focuses on the accuracy of commercial substrates used in thrombin generation, and interference that can occur by various plasma proteins, as well as on evaluating the advantages and disadvantages of each method. The commercial chromogenic assay and both fluorogenic assays are able to monitor the rate of thrombin generation and can give indications towards potential coagulation abnormalities. Overall, the main differences between the thrombin generation methods are based on the type of substrate used, sample preparation, and data processing. Despite advancement in this field there are still technical challenges that preclude the widespread use of thrombin generation in clinical applications.
Keywords: substrate, thrombin, thrombin generation, thrombogram
1. METHODS
This review was conducted using Pubmed, Medline, SciFinder, and Google Scholar databases (January 1, 1956 to March 22, 2017). The initial searches focused on a general overview of the thrombogram methods and then focused on specifics of the kinetics and substrates using the following search terms: (commercial thrombography), (technothrombin AND/OR thrombinoscope), (thrombin generation assay calibration) AND (chromogenic OR fluorogenic), (thrombin substrate) AND (selectivity AND/OR kinetics OR evaluation), (Calibrated Automated thrombography), (selectivity of) AND (commercially available thrombin substrates OR commercial thrombin substrates), (thrombin generation) AND (assay review), (Z‐Gly‐Gly‐Arg‐AMC), (ZGGR‐AMC), (H‐β‐Ala‐Gly‐Arg‐pNA).
Literature from commercial manufacturers for equipment, software packages and chemical producers were also reviewed.
2. THROMBIN GENERATION METHODS
Continuous monitoring of thrombin was first introduced by Hemker et al.1 in 1993, which laid the groundwork for what is now known as calibrated automated thrombography (CAT) in 2003.2 Previously to this, thrombin generation assay (TGA) was a laborious, time‐consuming task that required individual time point sampling from the original sample, also known as subsampling, in order to generate a snapshot picture of thrombin formation. With a limited view by subsampling due to a small number of data points, the overall picture of thrombin generation was incomplete.3 The introduction of the CAT protocol allowed for rapid, continuous measurement of multiple samples, greatly improving the efficiency and accuracy of the thrombin generation assay.
The advancement of the TGA led to the production of the commercially available fluorogenic thrombin assays. Since then, there has been a bifurcation in the commercial TGA into using either chromogenic or fluorogenic assays to determine the levels of thrombin in a sample, whether it be a platelet poor (PPP) or platelet rich (PRP) plasma. PPP is used frequently since it has defined concentrations of tissue factor and phospholipids in each plasma sample, allowing for easy standardization.4 This standardization of thrombograms has resulted in three commercial automated thrombin assays and corresponding software packages: The Behring Coagulation System (BCS), which utilizes chromogenic substrate, and the Technothrombin TGA by Technoclone and Thrombinoscope originally by Thrombinoscope BV and now owned by Diagnostica Stago, Inc. (Asnieres sur Seine, France), which utilize fluorogenic substrates. The main characteristics of each of these methods are summarized in Table 1.
Table 1.
| Methods | Technothrombin | Thrombinoscope | Innovance ETP (BCS) |
|---|---|---|---|
| Company | Technoclone | Stago | Siemens healthcare |
| Analysis method | Fluorogenic | Fluorogenic | Chromogenic |
| Wavelength (nm) | 390 (excitation), 460 (emission) | 390 (excitation), 460 (emission) | 405 (absorption) |
| Recommended Spectrometera | Ceveron Alpha TGA | Fluoroskan Ascent | BCS XP System |
| Substrate | Z‐Gly‐Gly‐Arg‐AMC8 | Z‐Gly‐Gly‐Arg‐AMC8 | H‐β‐Ala‐Gly‐Arg‐pNA9 |
| Calibration | Human thrombin (1 μmol L−1) in buffer with BSA | Human α2M‐thrombin calibrator (0.5‐1.0 μmol L−1) | INNOVANCE ETP Standard (proprietary) |
| Coagulation activator |
15 mmol L−1 CaCl2
10‐50 pmol L−1 TF |
0.1 mol L−1 CaCl2
6‐30 pmol L−1 TF |
0.25 mol L−1 CaCl2
5.208‐7.3610 nmol L−1 TF |
| Scan time | 50‐120 minutes | 50‐120 minute | 20 minute |
| Fibrin inhibitor | No | No | Yes |
| Inner filter correction | No | Yes | N/A |
| α2M correction | No | Yes | Yes |
| Continuous measurements | Yes | Yes | Yes |
| Total sample volume | 100 μL | 120 μL | 260 μL |
| Plasma volume | 40 μL | 80 μL | 135 μL |
| Substrate volume/Conc | 50 μL (0.5 mmol L−1) | 20 μL (0.42 mmol L−1) | 40 μL (1 mmol L−1) |
| CaCl2 volume/Conc | −(1.5 mmol L−1)b | −(16.7 mmol L−1)c | 15 μL (14.4 mmol L−1) |
| TF volume/Conc | 10 μL (1‐5 pmol L−1) | 20 μL (1‐5 pmol L−1) | 30 μL (600‐850 pmol L−1) |
| Buffer volume | – | – | 40 μL |
| %v/v Plasma | 40% | 67% | 52% |
| Technical repeats | 3 | 3 | 3 |
α2M, alpha‐2‐macroglobulin; AMC, 7‐amino‐4‐methylcoumarin; BSA, bovine serum albumin; Conc, concentration; ETP, endogenous thrombin potential; pNA, para‐nitroaniline; TF, Tissue Factor; TGA, Thrombin generation assay; v, volume.
Based on manufacturer's recommendation.
Volume included in TF.
Volume included in substrate.
At the most basic level, the three systems are similar in that they continuously measure the generation of thrombin via production of an indicator through the cleavage of a substrate. This reaction specifically occurs through the active site between the 60‐loop and the γ‐loop of thrombin.5, 6
The preparation of the plasma samples for the three systems is similar in that plasma is obtained from 9:1 blood to sodium citrate (3.2 or 3.8%) tubes, which are then centrifuged at 1500 g to 2500 g for 10 to 15 minutes to generate PPP. The three systems use different amounts of PPP in the assay, ranging from 40 μL to 135 μL (40–67% total volume), with individual samples assayed in triplicate. To initiate coagulation, 10–20 μL of recombinant tissue factor (TF) is added in the concentration final range from 1 pmol L−1 to 5 pmol L−1, which contains both a low or high concentration of phospholipids in the fluorogenic assay; and 30 μL of 5.20–7.36 nmol L−1 TF in the chromogenic assay.8, 9, 10 To this plasma:TF mixture a substrate is added along with calcium chloride (CaCl2). In the BCS‐XP system the substrate H‐β‐Ala‐Gly‐Arg‐pNA is used in 1 mmol L−1 concentration along with a fibrin inhibitor and various undisclosed salts for stability, in conjunction with 14.4 mmol L−1 CaCl2.11 For the Thrombinoscope, the Z‐Gly‐Gly‐Arg‐AMC substrate is used in 0.42 mmol L−1 concentration along with 16.7 mmol L−1 CaCl2.2 In the Technothrombin, the substrate 0.5 mmol L−1 Z‐Gly‐Gly‐Arg‐AMC is in solution with 1.5 mmol L−1 CaCl2.12 The calibration for the Technothrombin is performed using 40 μL of 1 μmol L−1 human thrombin of various dilutions in 50 μL of the substrate. The Thrombinoscope uses 20 μL of between 0.5 and 1.0 μmol L−1 of an alpha‐2‐macroglobulin (α2M) thrombin complex in 80 μL plasma, depending on batch to batch variation of the thrombin calibrator. The advantage of using α2M‐thombin complex as a calibrator that it is not influenced by factors in the plasma that affect thrombin. The BCS system uses a proprietary standard calibrator in accordance with the sample scheme.
3. SUBSTRATES AND DATA PROCESSING
The main differences between the three systems are the use of either UV‐Vis spectroscopy or fluorimetry to detect the generation of thrombin. The BCS system utilises a chromogenic substrate H‐β‐Ala‐Gly‐Arg‐pNA (Pefachrome TG or Innovance ETP). The molecule pNA or para‐nitroaniline is a chromophore with a strong absorbance at 405 nm, which is generated when the thrombin cleaves the substrate. The fluorogenic assays use the Z‐Gly‐Gly‐Arg‐AMC (or ZGGR‐AMC) substrate. The molecule AMC or 7‐Amino‐4‐methylcoumarin is a fluorophore that generates a signal by being excited at 390 nm light producing measured fluorescence at approximately 460 nm. By taking the integral (dx/dt) of the relative change in absorption or fluorescence it is possible to determine the Endogenous Thrombin Potential or ETP, which corresponds to the area under the curve (AUC). From the resulting curve, it is possible to determine: lag time, the time it takes for thrombin generation to first occur; time to peak, the amount of time it takes from the start of the assay to reach the maximal rate of thrombin generation; the velocity index, the rate of thrombin generation, and the maximal amount of thrombin generated (Figure 1). Since the onset of thrombin generation is significantly faster in the chromogenic assay, the complete assay run is on average 20 minutes, whereas the fluorogenic assay requires between 50 to 120 minutes to complete.
Figure 1.
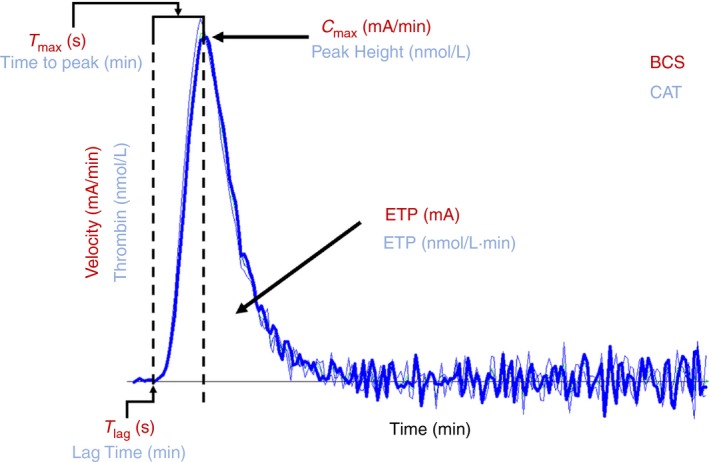
BCS and CAT thrombogram outlining major components of the reaction curve
The fluorogenic and chromogenic systems perform similar mathematical calculations in order to obtain the ETP, by integrating the AUC. The BCS and Thrombinoscope systems account for the presence of the α2M‐thrombin complex, whereas the Technothrombin system does not perform this mathematical calculation. By factoring in a standard amount of α2M‐thrombin complex there is a smaller AUC for both the BCS and Thrombinoscope in comparison with the Technothrombin, since α2M‐thombin complex adds approximately 20% to the total AUC.13 However, in the case of fluorogenic assays there is a significant difference in the mathematical processing of the inner filter effect, also known as fluorescence quenching. Fluorescence quenching occurs when the fluorophore absorbs light generated from fluorescence. As the concentration of fluorophore increases, there is a non‐linear increase in the quenching. This is tied with a continuous decrease in the substrate concentration as it is being consumed by the thrombin generated in the PPP. These two factors combine to cause a rapid decrease in the rate of change (dF/dt) in the overall fluorescence after the initial “burst” of fluorescence. Using a mathematical calculation known as an H‐transform, it is possible to take the acquired data and to generate a diagnostic plot that accounts for the inner filter effect and the substrate consumption.14 However, even with this correction there can also be quenching from various compounds in the plasma that can change from sample to sample and can in turn affect the results of the plot.15 For the Technothrombin system there is no correction for this inner filter effect, resulting in a lower overall ETP and shorter peak height versus that of the Thombinoscope which does factor the inner filter effect into the calculations.16 In the case of the BCS‐XP there is no need for this correction as the inner filter effect only applies to fluorescence.
After the raw data are collected, the mathematical calculations convert the results into the thrombogram that is used to evaluate the thrombin potential of a sample (Figure 1). In the case of the BCS method the results are provided as milliabsorption units (mA), based on the absorbance of the chromophore. The same parameters are determined by the two fluorogenic assays, except the results are expressed in nanomolar thrombin, since the use of an internal standard allows for conversion of fluorescence to the corresponding concentration of thrombin.15 Both the CAT and BCS methods measure the thrombogram over the course of minutes though the BCS often reports its data in seconds rather than minutes. Therefore, the Y‐axis in the BCS the measurement is in milliabsorbance per minute (mA/min) and is described as the velocity while the CAT uses nanomolar (nmol L−1) concentration of thrombin. The onset time of thrombin generation is measured as T lag in seconds in the BCS and Lag time measured in minutes for the CAT. The maximal height of the thrombogram is measured as C max in the BCS and measured in mA/min and Peak Height in the CAT measured in nmol L−1 of thrombin. The time to reach the maximal height is T max for the BCS measured in seconds and Time to Peak measured in minutes for the CAT. Both the BCS and CAT used the term Endogenous Thrombin Potential or ETP to describe the AUC for thrombin in milliabsorbance units and nanomolar concentration times minute (nmol L−1 min−1) respectively.
When focusing on the effectiveness of a substrate, the two constants that are most often referred to are the K m and k cat. The K m is the Michaelis constant, which indicates how much affinity the enzyme has for the substrate, thus the lower the number, the higher the affinity. Whereas the k cat is the “turn over” rate at which the enzyme consumes the substrate, thus the large the number the more substrate is consumed per second. The kcat/Km describes the enzymatic efficiency the enzyme has for the particular substrate and can denote specificity when compared to other enzymes, with the higher the number the higher the specificity. One minor factor that could potentially cause a small, but not insignificant difference in lag time and time to peak between the chromogenic and fluorogenic methods is the difference in the substrates themselves (Figure 2). The substrates are similar in that they are both tri‐peptides with a Glycine‐Arginine‐Reporter group motif, but beyond that they have some noticeable differences. The most obvious of these differences is that of the reporting group on the substrate. The chromogenic substrate utilises the para‐nitroaniline (4‐nitroaniline, or pNA) chromophore, whereas the fluorogenic assays use the 7‐Amino‐4‐methylcoumarin (AMC) fluorophore. These two different substrates are cleaved from the peptide at different rates and as such have different limits of detection. This would partly account for the difference in detection of thrombin generation, though the level of tissue factor is the main driver for the rate of thrombin generation in regards to the two commercial substrates.
Figure 2.
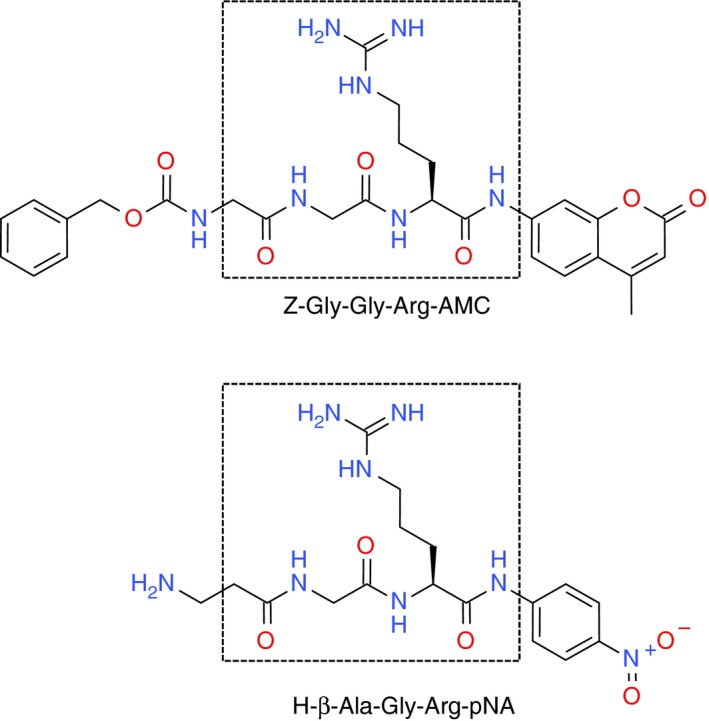
Comparison of fluorogenic (top) and chromogenic (bottom) substrates, boxes represent area of similar structure
Currently there are only a few reports focusing on the difference in selectivity, specificity or kinetics for identical peptide sequences that have different chromophores or fluorophores. Kanaoka, et al. observed that replacing AMC with a more hydrophilic 7‐amino‐4‐methylsulfonyl coumarin doubled the K m but decreased the k cat by a factor of 10 000 for thrombin, indicating that the fluorophore was interfering with hydrolysis of the substrate.16 Work by Butenas, et al. synthesized various substituted aminonaphthalenesulfonamides that were used as fluorescent labels on peptides and showed improved selectivity of the substrate to thrombin over Factor Xa by up to a factor of 30, when compared to the Chromozyme TH (Tos‐GPR‐pNA).17
Another difference between the two commercial thrombin substrates is that the fluorogenic substrate Z‐Gly‐Gly‐Arg‐AMC, uses a carboxybenzyl (‘Z’ or ‘Cbz’ in shorthand) protecting group on the N‐terminus of the peptide, while the H‐β‐Ala‐Gly‐Arg‐pNA (Figure 2) has a free amine. Work by Berkel, et al. showed that when the carboxylbenzyl group was removed from the Z‐Gly‐Gly‐Arg‐AMC and replaced with the free amine to generate H‐Gly‐Gly‐Arg‐AMC, both the K m and k cat increased by a factor of 6 and 3, respectively. This modification also resulted in a ninefold increase in selectivity of thrombin over Factor Xa.18 While the core of the Z‐Gly‐Gly‐Arg‐AMC and H‐β‐Ala‐Gly‐Arg‐pNA substrates are fairly similar, it is possible that the bulkier Cbz substituent on the fluorogenic substrate is a poor fit in the active site of thrombin, leading to a significantly slower rate of hydrolysis than that of the unprotected variant.
When comparing the chromogenic to fluorogenic substrate, the kinetics are fairly similar, with the k cat/K m, being about the same (Table 2). However, some of the original data specify that the k cat of the fluorogenic substrate is 310 s−1, which is unlikely, since it would rapidly use up all the substrate in an assay,18 while more recent analysis specifies a significantly lower kcat of 1.86 s−1.8 While the kinetics do play a role in the lag time and time to peak, the TF and presence of a fibrin inhibitor have a much greater effect on the rate thrombin generation. Adding the TF and fibrin inhibitor results in an assay taking approximately 20 minutes to perform on the BCS system compared to approximately 60 minutes for the fluorogenic methods.19 Furthermore, the higher concentrations of TF forces the thrombin generation to solely extrinsic (except Factor XI), where Factor V and Factor VII dominate. Since fibrin causes turbidity in the sample and interferes with absorption on a spectrometer, a fibrin inhibitor is needed in order to accurately measure thrombin potential. However, the use of fibrin inhibitors along with the higher tissue factor concentration causes a decrease in T lag and Tmax.20 In the future if substrates with greater catalytic efficiency (k cat/K m) are synthesized that also retain the selectivity and specificity it is possible that the speed of the fluorogenic assay could increase. Fibrin inhibition in conjunction with the very high TF concentration causes the chromogenic assay to be faster than the fluorogenic assays with shorter lag times and time to peak (Table 3).21
Table 2.
Comparison of substrate kinetics for the two main thrombin generation substrates
Table 3.
| BCS/CAT Terms | Chromogenic Assay (BCS) | Fluorogenic Assay (CAT) |
|---|---|---|
| Substrate | H‐β‐Ala‐Gly‐Arg‐pNA | Z‐Gly‐Gly‐Arg‐AMC |
| Scan time | 20 minute | 50‐120 minute |
| ETPa | 450‐550 mA | 1200‐2400 nmol L−1 min−1 |
| Cmax/Peak heighta | 135‐180 mA/min | 200‐450 nmol L−1 |
| T lag/Lag time | 15‐20 seconds | 2.5‐4.5 minute |
| T max/Time to peak | 35‐55 seconds | 5‐8 minute |
AMC‐ 7‐amino‐4‐methylcoumarin; ETP, endogenous thrombin potential; CAT, calibrated automated thrombogram; pNA, para‐nitroaniline.
Comparison of absolute numbers of different units.
4. THROMBIN GENERATION IN CLINCAL SETTTINGS
In regards to the analysis that the three systems perform, there is no one system that is superior to the other and each has its own advantages and disadvantages. Currently, in regards to the three systems there are no clinical studies that directly compare the rates of thrombin generation to patient outcomes. Though the recommended detectors are not required, they are designed with the specific assays in mind, as well as being able to perform similar assays (APTT, PT, etc.). The main advantage of the BCS system over the fluorogenic systems is the lower overall scan time, allowing for more samples per hour to be run. However, one disadvantage is that in combination with the lack of an internal standard, the concentration of thrombin cannot be directly reported for the BCS system.22 The main advantage of the fluorogenic systems is their ability to directly quantify the exact amount of thrombin generated in a patient's plasma. The disadvantage however, is the longer amount of time required for sample analysis and the interference that can be caused by the IFE (Table 4).
Table 4.
Advantages and disadvantages of different automated thrombin generation methods
| Method/Company | Technothrombin Technoclone | Thrombinoscope Stago | Innovance ETP Siemens Healthcare |
|---|---|---|---|
| Advantages | Small plasma sample | IFE correction Standardized α2M‐thrombin calibrator |
Faster assay No IFE |
| Disadvantages |
Slower assay No IFE correction No α2M‐thrombin calibrator |
Slower assay |
Higher TF concentration Large plasma sample Fibrin inhibitor required No α2M‐thrombin calibrator |
Overall the three methods are somewhat similar in sample preparation, use of substrates, and data analysis. The main issue for analysing thrombin generation is that all three methods still use very laborious preparations and a multiple to perform a test that takes many minutes to complete and cannot be done on a large, rapid scale. The lack of consistent results is a problem for the TGA, as well as the fact that results can vary from location to location,11 with day‐to‐day variation also present, despite the same individual being tested.8 In addition, the inability of the substrates to differentiate free thrombin and α2M‐thrombin complex in the automated assays is an issue in that the assay doesn't reflect the true levels of free thrombin, though corrections do get it closer to the actual value. This particular problem can only be solved with the design of new substrates that are selective for free thrombin.
While two of the three commercially available TGA systems attempt to correct for the presence of the α2M‐thrombin complex, the Thombinoscope through a calibrator and the BCS system via mathematical calculations, there is a significant issue with this both approaches. The rate of α2M calculated for every system is constant regardless of the source. It has been shown that the levels of α2M can vary greatly by age,23, 24 and by clinical conditions such as hepatitis C,25 pancreatitis,26 or acute ischemic heart disease.27 However, with such a heterogeneity of α2M concentrations it would be nearly impossible to predict an accurate amount of α2M in each individual sample without exhaustive analysis. An ideal solution to this situation would be to design a substrate that could specifically detect thrombin without detecting α2M‐thrombin complexes. This goal is particularly challenging since α2M‐thrombin complex contains the same active site as the free thrombin that hydrolyses the available substrates. To design a substrate that could discriminate between thrombin and α2M‐thrombin complex will require a significant amount of resources. This could lead to more consistent and physiologically relevant results by eliminating a major variable that is a part of all currently available commercial thrombin generation methods.
While the total volume of plasma is minimal and could easily be run multiple times on standard 3 mL sample of whole blood, the sample volume can be an issue when dealing with neonates or pre‐term neonates. With the BCS requiring 130 μL of plasma, a typical neonate whole blood only consists of 32–45% plasma28 it would require approximately 300–400 μL of neonate whole blood per test, while the Thrombinoscope would require approximately 175–250 μL of neonate whole blood and approximately 90–125 μL neonate whole blood per test for the Technothrombin system. With a 1 kg neonate, the maximum daily limit of blood withdrawal is between 1 and 4 mL depending on guidelines,29 which greatly restricts the number of test that can be performed, especially when a typical thrombogram is run in triplicate.
The role of thrombin generation assays in the clinical settings is uncertain. Ten Huge and van Veen et al. have studied thrombin generation and found that while useful for many disorders such as venous thrombosis, arterial disease, and various bleeding disorders, there is a significant issue with standardization between centers.15, 30 Tripodi points out that while automated thrombography has helped in elucidating the clotting mechanism to many diseases that were previously unknown, there have been insufficient studies of the clinical use of these assays.31 Campo et al. explains that the difference in substrates, non‐standardized calibrators and equipment between laboratories further complicates the issue.21 To mitigate some of these issues, steps are underway to standardize thrombin generation among multiple medical centres. Dargaud et al. has shown that by standardizing protocols for the Thrombinoscope, through training along with use of standard materials, could help transition thrombin generation from the laboratory to general clinical practice.7 Further research shows that using normalized, standard reference plasma samples between multiple centers along with strict adherence to protocols is critical to obtain clinically valid results.32, 33
5. CONCLUDING REMARKS
The three commercially available thrombin generation methods can detect the continuous generation of thrombin in a patient sample. The major differences between the methods originate from the substrates themselves, the sample preparation required for the different substrates, and the mathematical formulations needed to process the data. None of the three systems are superior to each other, with all three methods having their own advantages and disadvantages. The decision of which system to use comes down to personal preference, laboratory requirements and/or the compatibility with already established spectrometers or fluorimeters. The current and future clinical applicability of the TGA is dependent on controlling/standardising the variables associated with the assays themselves; as well as the correlation of thrombin generation results with clinical outcomes and understanding the underlying mechanisms of disease progression in relation to thrombin. Once these questions are answered, the TGA will have significant clinical relevance.
AUTHOR CONTRIBUTIONS
J. Kintigh drafted and reviewed literature for this manuscript. P. Monagle reviewed and edited the manuscript. V. Ignjatovic reviewed and edited this manuscript.
ACKNOWLEDGMENTS
No funding was received for the production of this manuscript.
RELATIONSHIP DISCLOSURE
None of the authors have any disclosures relevant to this paper.
Kintigh J, Monagle P, Ignjatovic V. A review of commercially available thrombin generation assays. Res Pract Thromb Haemost. 2018;2:42–48. 10.1002/rth2.12048
REFERENCES
- 1. Hemker HC, Wielders S, Kessels H, Béguin S. Continuous registration of thrombin generation in plasma, it's use for the determination of the thrombin potential. Thromb Haemost. 1993;70:617–24. [PubMed] [Google Scholar]
- 2. Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurements in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. [DOI] [PubMed] [Google Scholar]
- 3. Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. 1953;6:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Loeffen R, Kleinegris MC, Loubele ST, et al. Preanalytic variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. 2012;10:2544–54. [DOI] [PubMed] [Google Scholar]
- 5. Parry MA, Stone SR, Hofsteenge J, Jackman MP. Evidence for common structural changes in thrombin induced by active‐site or exosite binding. Biochem J. 1993;290:665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huntington JA. Molecular recognition mechanisms of thrombin. J Thromb Haemost. 2005;3:1861–72. [DOI] [PubMed] [Google Scholar]
- 7. Dargaud Y, Luddington R, Gray E, et al. Standardisation of thrombin generation test–which reference plasam for TGT? An international multicentre study Thromb Res. 2010;125:353–6. [DOI] [PubMed] [Google Scholar]
- 8. Devreese K, Wijns W, Combes I, Van kerckhoven S, Hoylaerts MF. Thrombin generation in plasma of healthy adults and children: chromogenic versus fluorogenic thrombin analysis. Thromb Haemost 2007;98:600–13. [PubMed] [Google Scholar]
- 9. Lawrie AS, Gray E, Leeming D, et al. A multicentre assessment of the endogenous thrombin potential using a continuous monitoring amidolytic technique. Br J Haematol. 2003;123:335–41. [DOI] [PubMed] [Google Scholar]
- 10. Duckers C, Simioni P, Spiezia L, et al. Residual platelet factor V ensures thrombin generation in patients with severe congenital factor V deficiency and mind bleeding symptoms. Blood. 2010;115:879–86. [DOI] [PubMed] [Google Scholar]
- 11. Innovin ETP Datasheet Insert. Marburg, Germany: Siemens; 2010. [Google Scholar]
- 12. Technothrombin TGA Datasheet Insert. Vienna, Austria: Technoclone GmbH; 2007. [Google Scholar]
- 13. Chandler WL, Roshal M. Optimization of plasma fluorogenic thrombin‐generation assay. Am J Cin Pathol. 2009;123:169–79. [DOI] [PubMed] [Google Scholar]
- 14. Hemker HC, Kreemers R. Data management in thrombin generation. Thromb Res. 2013;131:3–11. [DOI] [PubMed] [Google Scholar]
- 15. van Veen JJ, Gatt A, Makris M. Thrombin generation testing in routine clinical practice: are we there yet? Br J Haematol. 2008;142:889–903. [DOI] [PubMed] [Google Scholar]
- 16. De Smedt E, Al Dieri R, Spronk HM, Hamulyak K, ten Cate H, Hemker HC. The technique of measuring thrombin generation with fluorogenic substrates: 1. Necessity of adequate calibration. Thomb Hamost. 2008;100:343–9. [PubMed] [Google Scholar]
- 17. Butenas S, Orfeo T, Lawson JH, Mann KG. Aminonaphthalenesulfonamides, a new class of modifiable fluorescent detecting groups and their use in substrates for serine protease enzymes. Biochem. 1992;31:5399–411. [DOI] [PubMed] [Google Scholar]
- 18. Sato E, Matsuhisa A, Sakashita M, Kanaoka Y. New water‐soluble fluorogenic amine.: 7‐Aminocoumarin‐4‐methanesulfonic acid (ACMS) and related substrates for proteinases. Chem Pharm Bull. 1988;36:3496–502. [DOI] [PubMed] [Google Scholar]
- 19. Dinkelaar J, Molenaar PJ, Ninivaggi M, de Laat B, Brinkman HJ, Leyte A. In vitro assessment, using thrombin generation, of the applicability of prothrombin complex concentrate as an antidote for Rivaroxaban. J Thromb Haemost. 2013;11:1111–8. [DOI] [PubMed] [Google Scholar]
- 20. Duchemin J, Pan‐Petesch B, Arnaud B, Blouch MT, Abgrall JF. Influence of coagulation factors and tissue factor concentration on the thrombin generation test in plasma. J Thromb Haemost. 2008;99:767–73. [DOI] [PubMed] [Google Scholar]
- 21. Baglin T. The measurement and application of thrombin generation. Br J Haematol. 2005;130:653–61. [DOI] [PubMed] [Google Scholar]
- 22. Campo G, Pavasini R, Pollina A, et al. Thrombin generation assay: a new tool to predict and optimize clinical outcome in cardiovascular patients? Blood Coagul Fibrinolysis. 2012;23:680–7. [DOI] [PubMed] [Google Scholar]
- 23. Tunstall AM, Merriman JM, Milne I, James K. Normal and pathological serum levels of α‐2‐macroglobulins in men and mice. J Clin Pathol. 1975;28:133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ignjatovic V, Greenway A, Summerhayes R, Monagle P. Thrombin generation: the functional role of alpha‐2‐macroglobulin and influence of developmental haemostasis. Br J Haematol. 2007;138:366–8. [DOI] [PubMed] [Google Scholar]
- 25. Ho AH, Cheng CC, Lee SC, et al. Novel biomarkers predict liver fibrosis in hepatitis C patients: alpha‐2‐macroglobulin, vitamin D binding protein and apolipoprotein AI. J Biomed Sci. 2010;17:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Banks RE, Evans SW, Alexander D, Van Leuven F, Whicher JT, McMahon MJ. Alpha‐2‐macroglobulin state in acute pancreatitis. Raised values of alpha‐2‐macroglobulin‐protease complexes in severe and mild attacks. Gut 1991;32:430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Losito R, Gattiker H, Bilodeau G, Verville N, Longpré B. Levels of antithrombin III, alpha 2‐macroglobulin, and alpha 1‐antitrypsin in acute ischemic heart disease. J Lab Clin Med. 1981;97:241–50. [PubMed] [Google Scholar]
- 28. Fauci AS, Braunwald E, Kasper DL, et al. Harrisons's Principles of Internal Medicine. 17th ed New York: McGraw‐Hill; 2008. [Google Scholar]
- 29. Howie SR. Blood sample volumes in child health research: review of safe limits. Bull World Health Organ. 2011;89:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ten Cate H. Thrombin generation in clinical conditions. Thromb Res. 2012;129:367–70. [DOI] [PubMed] [Google Scholar]
- 31. Tripodi A. Thrombin generation assay and its application in the clinical laboratory. Clin Chem. 2016;62:699–707. [DOI] [PubMed] [Google Scholar]
- 32. Perrin J, Depasse F, Lecompte T. Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. 2015;136:125–30. [DOI] [PubMed] [Google Scholar]
- 33. Bagot CE, Leishman E. Establishing a reference range for thrombin generation using a standard plasma significantly improves assay precision. Thromb Res. 2015;136:139–43. [DOI] [PubMed] [Google Scholar]


