Abstract
Essentials.
The standard of care for patients with TTP remains daily plasma exchange in addition to immune suppressive therapy.
Despite the improved treatment options for TTP, the acute mortality of TTP remains between 15‐20%.
Caplacizumab reduces the time to platelet recovery and the exacerbation rate in acute TTP.
A better understanding of the cause and treatments of long‐term complications of TTP are needed.
Thrombotic thrombocytopenic purpura (TTP) is characterized by microangiopathic hemolytic anemia and a consumptive thrombocytopenia, as a result of severe deficiency of ADAMTS13. The standard of care of the acute episode is treatment with plasma exchange and immunosuppression. After the acute episode is resolved, patients face a significant risk of relapse and long‐term complications associated with significant morbidity and even mortality. Novel treatments have been under development and will be discussed in this review. Caplacizumab, a nanobody that blocks the interaction between VWF and platelets, has shown promising results in decreasing the time to recover from the acute events that will hopefully translate into long‐term clinical benefit for patients. In addition, identifying biomarkers to allow us to better predict the risk for relapse and the development of these long‐term complications in patients with TTP are a few of the challenges that require our attention moving forward.
Keywords: ADAMTS13, caplacizumab, long‐term complications, splenectomy, thrombocytopenic purpura
1. OVERVIEW
Thrombotic thrombocytopenic purpura (TTP) is a rare and potentially fatal hematologic disease. It is defined as microangiopathic hemolytic anemia and consumptive thrombocytopenia without an alternative explanation, confirmed by severely reduced ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13) activity to less than 10%.1 Under normal circumstances, ADAMTS13 is responsible for cleaving ultra large VWF multimers (ULVWF). In TTP, ULVWF will not be cleaved due to a congenital deficiency of the protease (congenital TTP, cTT) or autoantibodies against it (immune mediated TTP, iTTP). The interaction between ultra large VWF (ULVWF) multimers, platelets and red blood cells, will lead to widespread microvascular thrombosis and hemolysis.
The current standard of treatment for the acute episode of TTP involves plasma exchange (PEX) and steroids.2, 3 Patients that are refractory to this treatment are offered rituximab most commonly,4, 5 although other therapies including cyclosporine and cyclophosphamide have been used successfully in the past. For cTTP (confirmed by negative antibody testing and the identification of biallelic mutations of ADAMTS13), the treatment consists of regular plasma infusions to prevent future relapses.6 cTTP is even less common than iTTP and usually presents at birth or early childhood. However, physicians must have a high index of suspicion in patients that experience their first episode of TTP during pregnancy. This was evidenced by a study from the French Reference Center for Thrombotic Microangiopathies, in which 10 of their 42 (24%) patients diagnosed with a first TTP episode during pregnancy were found to have cTTP.7
In patients diagnosed with iTTP, close monitoring after the acute episode is required, since one‐third of patients may suffer an early recurrence or exacerbation in the first 30 days after stopping PEX. In addition, at least one‐half of patients if not more will be at risk for future relapse of the disease.8, 9, 10, 11 Patients that are diagnosed with TTP, even if only one acute episode in the distant past are prone to long‐term complications that include hypertension, depression, headaches, and neurocognitive impairment.12, 13, 14, 15 These complications can lead to significant morbidity and are associated with a decreased life expectancy.
We have come a long way since the first case report of the disease in 1924,16 particularly in our understanding of the pathophysiology of the disease (Figure 1). The introduction of PEX for the treatment of iTTP decreased the mortality from an acute TTP episode from nearly 100% to 15‐20%.17, 18 Even though steroids have been routinely used in the treatment of iTTP for a long time, their efficacy in helping to reduce the ADAMTS13 autoantibodies and improving the ADAMTS13 activity was documented only recently.19 In this review, we will discuss novel therapies directed at improving the treatment of the acute episodes, but also hopefully decreasing the relapse rate and long‐term complications associated with TTP (Table 1).
Figure 1.
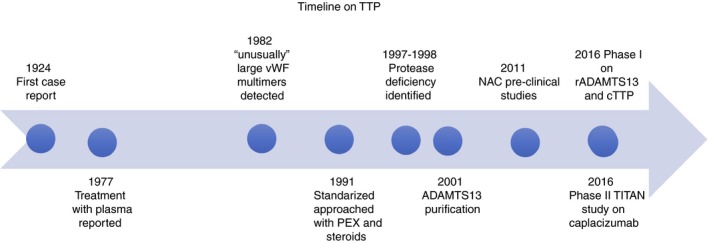
Thrombotic thrombocytopenic purpura timeline showing the different discoveries in pathophysiology and treatment since the initial case report almost 100 years ago. cTTP, congenital TTP; iTTP, immune TTP; NAC, N acetlylcysteine; PEX, plasma exchange therapy; rADAMTS13, recombinant ADAMTS13
Table 1.
Novel therapies in TTP
| Novel therapies for TTP | Mechanism | Evidence |
|---|---|---|
| Caplacizumab | VWF A1 antagonist ‐ blocks VWF and platelet interaction | Phase II showed decrease time to recover from acute episodes and lower exacerbation rate, Phase III completed |
| Anfibatide | Platelet GpIb antagonist ‐ block VWF and platelet interaction | Inhibits adhesion and thrombus formation in murine models |
| rADAMTS13 | Restoration of ADAMTS13 function | Phase I in cTTP have shown good safety and tolerability |
| N‐acetylcysteine | Reduce the ULVWF | Pre‐clinical studies showing mechanism. Case reports with mixed results. |
| Bortezumib | Eliminates plasma cells, thereby decreases the production of autoantibodies against ADAMTS13 | Case reports and case series showing clinical improvement for refractory TTP |
| Splenectomy | Reduce the autoimmune response/Eliminate B‐cell reservoir | Case reports and Case series for its use in prevention of relapses |
| Rituximab | Aim at decreasing the production of anti‐ADAMTS13 autoantibodies by B‐cell depletion | Cohort and Case control studies for its use as first line therapy (with fewer and later relapses than PEX and steroids alone) and also for prevention of relapses. |
2. CAPLACIZUMAB
Caplacizumab is a bivalent nanobody that inhibits UL‐VWF mediated platelet aggregation by blocking the interaction between the A1 domain of VWF and the platelet receptor GPIb‐IX‐V. Nanobodies are proteins derived from the variable domains of heavy‐chain‐only immunoglobulin from animals of the Camelidae family. Preclinical studies have shown that the bivalency of caplacizumab allows a tight interaction with VWF, as well as inhibition of platelet adhesion at high‐shear rates, but not at low‐shear rates. This is expected, as the A1 domain of VWF is exposed only under high‐shear conditions seen in small vessels, suggesting that there may be lower bleeding risk when compared to other antithrombotic agents such as aspirin, clopidogrel and abciximab.20, 21
A randomized, multicenter, phase II trial (TITAN study), included 75 patients with a clinical diagnosis of iTTP (36 randomized to receive 10 mg of subcutaneous caplacizumab daily during plasma exchange and 30 days additional days, and 39 to placebo). The time to normalization of platelet count in the caplacizumab group was significantly decreased compared to patients receiving placebo (39% reduction in median time, P = .005). In addition, only three patients on the caplacizumab arm versus 11 on the placebo arm suffered an early recurrence or exacerbation of TTP that was a direct result of the continued caplacizumab treatment in the first 30 days after stopping PEX. After stopping the caplacizumab however, eight patients in the study drug group had a relapse, compared to none in the placebo group. Seven of the eight patients that relapsed had a severely deficient ADAMTS13 activity to less than 10%, suggesting a persistent autoimmune activity. These important data demonstrate both the protective effect of caplacizumab, but also emphasize that the drug does not alter the underlying disease process driven by the persistent ADAMTS13 deficiency. Addressing the immune‐mediated ADAMTS13 deficiency with immune suppressive therapy to restore the ADAMTS13 protease function may be equally important as the initial therapy with PEX and caplacizumab in patients with immune‐mediated TTP.
When compared to placebo, caplacizumab‐treated patients had more bleeding‐related adverse events (38% and 54%, respectively). The majority (83%) of bleeding events were mild and did not require the administration of the antidote (plasma‐derived VWF/factor VIII concentrates).22
A post hoc analysis of the phase II trial found a significantly reduced risk of major thromboembolic events, exacerbation and TTP related mortality with caplacizumab compared to placebo.23 The HERCULES study, a randomized, multicenter, phase III trial has already completed recruitment with results that should be reported soon (NCT0255331).
3. RECOMBINANT ADAMTS13 (RADAMTS13, BAX930)
Since the identification of ADAMTS13 protease,24 increased attention was directed to the cloning and expression of the protease to better evaluate its function and therapeutic opportunities. Antoine et al., showed the normalization of the ADAMTS13 activity in two brothers with congenital TTP when their plasma was mixed with rADAMTS13 obtained from transfected HEK293 cells.25 A preclinical model using ADAMTS13 knockout mice infused with high dose of recombinant human VWF to trigger the disease showed that prophylactic and therapeutic use of rADAMTS13 improved the hematological and pathologic parameters of TTP.26 A phase 1, multicenter, open label, dose‐escalation study involving 15 patients with cTTP between the ages of 12 to 65, with ADAMTS13 activity <6% showed that ADAMTS13 was well tolerated at the three doses tested (5, 20, or 40 U/kg). No anti‐ADAMTS13 antibodies were detected with the treatment although the follow‐up was relatively short (30 days). There was, however, a decrease in the ULVWF after the drug was administered that was accompanied by an increase in the fraction of intermediate size VWF multimers, an increase in the platelet count, and a decrease in the LDH, demonstrating the functional effects of the rADAMTS13.27
Even though the more obvious application would be cTTP, there may be the potential for the use of rADAMTS13 for the treatment iTTP. Plaimauer et al. showed that rADAMTS13 can neutralize autoantibodies and reconstitute the ADAMTS13 activity in vitro.28 Moreover, pre‐clinical studies using Sprague‐Dawley rats, showed that rADAMTS13 can restore the ADAMTS13 activity by forming ADAMTS‐13 specific immune complexes.29, 30 These data collectively suggest a role for rADAMTS13 in iTTP and may be the rationale for future studies.
4. N‐ACETYLCYSTEINE
N‐acetylcysteine (NAC) is a compound use for the treatment of acetaminophen toxicity and as a mucolytic agent in patients with lung diseases such as chronic bronchitis and cystic fibrosis. It decreases mucin size and viscosity, helping with airway congestion and ventilation. Chen et al. noted that the structure of VWF is very similar to mucin, and they postulated that NAC could, in the same way as mucin, reduce the size of ULVWF. In vitro, NAC was able to reduce VWF multimers under static conditions, specifically disrupting disulfide bonds in the VWF A1 domain.31 A recent study using a baboon model also showed reduction in size and activity of VWF in plasma. However, NAC as a single therapy, was unable to reverse TTP signs nor dissolve preexisting VWF‐rich thrombi.32 Case reports have shown successful use of NAC in patients with refractory TTP as evidenced by normalization of platelet function and ADAMTS13 activity in four patients33, 34 but was unsuccessful in three others.35, 36, 37 A pilot study to evaluate the use of NAC in suspected TTP is underway (NCT01808521).
5. ANFIBATIDE
Anfibatide is a snake‐venom derived protein in the pre‐clinical phase of development. It inhibits platelet aggregation by binding to GpIb and inhibiting its interaction with VWF. In VWF (−/−) deficient mice, it inhibited ristocetin‐induced aggregation of washed murine platelets and recombinant murine VWF. In an ex‐vivo perfusion chamber model at high and low shear rates, pretreatment with anfibatide inhibited platelet adhesion, aggregation, and thrombus formation at both high and low shear rates, but the inhibition was stronger as the shear rates increased. Moreover, anfibatide dissolved pre‐formed thrombi in the same model. In an in‐vivo mouse model using Ferric Chloride (FeCl3−) induced thrombosis in a mesenteric arteriole, anfibatide inhibited platelets interaction and adhesion in the vascular wall, requiring a longer time for thrombus formation and delaying vessel occlusion.38 These studies were followed by a murine model study, using Adamts13 ‐/‐ mice treated with an intravenous injection of Stx‐2. Mice pretreated with anfibatide prior to the Stx‐2 injection showed decreased rates of thrombocytopenia, compared to the ones not treated with anfibatide, as well as fewer microvascular thrombosis on histological analysis.39
6. BORTEZOMIB
Bortezomib is a proteasome inhibitor used extensively in multiple myeloma. Its mechanism of action in TTP is thought to be due to the elimination of plasma cells that produce the autoantibodies against ADAMTS13. The evidence for the use of this drug is still limited to case reports35, 40, 41, 42, 43, 44, 45 and a case series37 that reported 14 cases of TTP that were refractory to PEX, steroids, and rituximab. All but one patient recovered from their acute episode of TTP. The optimal dose and schedule of administration however is still unclear (varied between 4 and 13 doses), as well as the time required to improve the ADAMTS13 activity after the initiation of therapy.
7. NEW DEVELOPMENTS ON OLDER THERAPIES
7.1. Splenectomy
The use of splenectomy for treatment of TTP is certainly not a recent one. Before the use of PEX, splenectomy and steroids were the treatment of choice for acute TTP.46, 47 Splenectomy has been shown to be effective in relapsing iTTP and, although more controversial, also in refractory iTTP.48, 49 A retrospective study of 24 patients with relapsing TTP showed a decrease in the relapse rate from 0.74 relapses/patient‐year to 0.1 relapses/patient‐year after splenectomy after a median follow up of 115 months.50 It could also be argued that the long‐term remission rates that have been reported after splenectomy are comparable to those seen with rituximab.51, 52 The complications related to splenectomy are minimal, but are increased when splenectomy is used for refractory TTP. Although difficult to accomplish, a prospective trial comparing rituximab and splenectomy to prevent relapses would be important to directly compare the long‐term relapse rates with these two approaches to secondary prevention of iTTP.
The mechanism of action of splenectomy to prevent relapses of TTP however is not known. Does a splenectomy remove a large burden of antibody producing cells, or rather are memory B‐cells that may be responsible for the chronic relapsing phenotype of iTTP being removed? Can splenectomy alone lead to the recovery of previously severely deficient ADAMTS13 activity? In one case studied carefully at our institution, we followed the ADAMTS13 activity pre and post‐splenectomy of a 25‐year‐old woman diagnosed with iTTP at the age of 20 that suffered an episode of iTTP at least once a year since her initial diagnosis. She was intolerant of cyclosporine and rituximab when they were used prophylactically. She was noted to have persistent, severely deficient ADAMTS13 activity during outpatient follow‐up and underwent a prophylactic, laparoscopic splenectomy. At the time of the surgery, she was asymptomatic and both platelets are LDH were within normal limits. The surgery went well without complications and her ADAMTS13 activity increased to 67.5% at her first follow‐up eight measurement weeks after surgery (Figure 2). At 14 months of follow‐up, she has not had any recurrences of TTP and has continued to demonstrate normal ADAMTS13 activity.
Figure 2.
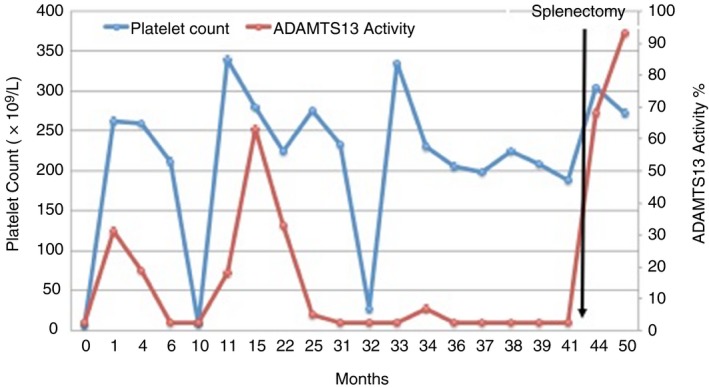
Serial measurement of the ADAMTS13 activity, platelet count and lactate dehydrogenase (LDH)pre‐ and post‐splenectomy in a patient with recurrent TTP with relapses of TTP demonstrated by the acute drops in the platelet count
Modern technology has allowed us to be able to better characterize the splenic anti‐ADAMTS13 antibody response in patients with TTP. Researchers from Bern, Switzerland, have published their findings on spleens from two patients with recurrent iTTP. They found that splenic B cells can produce anti‐ADAMTS13 autoantibodies that can inhibit the protease in the same way as those present in plasma. Interestingly, these two unrelated patients shared four heavy‐chain complementarity determining region 3 motifs.53 At the ISTH Congress in 2017, the same group reported additional findings from two patients that had a splenectomy for iTTP. They found both inhibitory and non‐inhibitory antibodies in the spleens from iTTP patients that mirrored the antibodies found in the plasma. The predicted epitope of the non‐inhibitory antibodies involved the heavy chain‐derived residues with stronger prediction scores, whereas the predicted epitope of the inhibitory antibodies involved heavy and light chains‐derived residues.54
7.2. Rituximab
The first case reports describing the use of rituximab in TTP date back to 15 years ago.55, 56 Since then, different groups have reported their experience with the use of rituximab for refractory, first line, and prevention of relapses in TTP. To date, rituximab is recommended for refractory TTP, based on the results of multiple case reports and case series. Scully et al. reported their experience treating 25 patients with refractory TTP with rituximab, all of which recovered completely after a median of 11 days after starting rituximab.57
The use of rituximab as part of the upfront therapy for the treatment of TTP is still debated. Three different groups have evaluated its use as addition to PEX and steroids in the acute episode of TTP.5, 8, 52 All three of them reported fewer and later relapses in the rituximab treated patients. Despite these results, its use its still controversial because it is estimated that 50% of patients will have a relapse after their initial episode and we may be over treating a significant group of patients with this approach. Moreover, an ISTH abstract regarding a retrospective review of 88 patients with a median follow up of 8.1 years, showed no difference in relapse rate in patients that received rituximab during an acute episode compared to those that did not.58
The rationale behind the use of rituximab for prevention of relapses is that rituximab will deplete B cells, thereby decreasing the production of anti‐ADAMTS13 antibodies and increasing the ADAMTS13 activity. The French Thrombotic Microangiopathies Reference Centre published an observational study comparing 30 patients treated with preemptive rituximab. The relapse rate decreased from 0.57 episodes per year to 0 episodes per year with a median follow up of 36 months after the first rituximab infusions.51 With these results, our approach has been to consider the use of rituximab for patients with a history of one or more relapses and at least two consecutive severely deficient ADAMTS13 activity measurements.
7.3. Long‐term complications in TTP
Despite the presumptions in the past that a patient with a prior history of iTTP was ‘normal’ if the platelet count had recovered, patients with a prior history of iTTP are more prone to develop hypertension and depression, and have a shorter life‐expectancy independent of future relapses of iTTP.15 The quality of life for recovered iTTP patients is also significantly impacted by the development of headaches12 and neurocognitive impairment.13, 14 There are now increasing numbers of TTP survivors that may be at risk for these complications. Similar findings have also been reported in congenital TTP, including those on regular plasma infusions.59 For these reasons, TTP must now be considered a chronic disorder, with regular follow up required both to judge the risk of relapse, but more importantly to monitor for the development of long‐term complications. The etiology of these long‐term complications is not completely understood, but they do not appear to be related to the number of prior TTP episodes nor with the severity of the episodes. Prospective studies to better characterize these long‐term complications and their etiology, and to determine who is at greatest risk of developing them are needed.
8. FUTURE DIRECTIONS
It is an exciting time in the field of TTP. A better understanding of the pathophysiology of the disease has been accompanied by newer therapies. At the ISTH 2017 Congress, there were some interesting abstracts presented describing basic science advances in TTP, such as the different conformation of ADAMTS13 in patients with iTTP compared to patients with sepsis and hemodialysis,60 which can affect the autoantibody binding and subsequent development of disease,61 or the contribution of peptidyl arginine deiminase type IV (PAD4) to inactivating ADAMTS13 in vitro.62 These important discoveries are some of the many contributions that will help in the development of therapeutic interventions for the disease.
Immune‐mediated TTP still carries a 10‐20% mortality risk from each acute episode, but the therapies discussed potentially will alter this persistent mortality rate. Of the novel therapies described, caplacizumab is furthest along in development. Caplacizumab, however, does not affect the autoimmune pathobiology of the disease, therefore, its use in combination with steroids or other types of immunosuppression will be required. Studies to optimize the role and utility of this novel therapy will be required.
Recombinant ADAMTS13 has shown promising results in cTTP, but there is also the potential for its use in iTTP by its ability to neutralize the ADAMTS13 inhibitor in vitro. These findings are important and may lead to future studies combining rADAMTS13 with immune‐suppressive therapy, or caplacizumab to decrease or eliminate the need for PEX, decreasing the potential risks of line insertion, infection, as well as complications of the infused plasma.
Even though ADAMTS13 is uniformly severely deficient during an acute episode, there is significant variability of this biomarker during remission, with deficient ADAMTS13 during remission not uniformly being accompanied by relapse.63, 64, 65 Being able to determine those at greatest risk of relapse, and other potential risk factors in addition to the ADAMTS13 activity will be key. Different enzymes (including thrombin, plasmin, cathepsin G, neutrophil elastase, proteinase 3, matrix metalloproteinase 9, and granzyme M and B) have been associated with the regulation of VWF and platelet interaction.44 Recent studies have also shown that plasmin can degrade VWF in microthrombi and break platelet‐VWF complexes in vitro,66, 67 as well as stimulate ADAMTS13 activity in plasma.68 In addition, recent work has demonstrated the interaction ULVWF multimers and the activation of the alternative pathway of complement, as well as the interactions of the complement system and the cleavage of ULVWF multimers. At the ISTH 2017, an in vitro study aiming to determine the anti‐spacer immunoprofiles in plasma from patients with iTTP suggests that this tool can be used to identify patients with a higher risk for relapse.60 Collectively these recently described findings require further study as potential predictors for relapse in patients already at risk due to severely deficient ADAMTS13 activity.
AUTHOR CONTRIBUTION
CM and SRC jointly wrote and edited the manuscript.
RELATIONSHIP DISCLOSURE
Dr. Cataland reports personal fees from Ablynx and personal fees from Shire Pharmaceuticals. Dr. Masias has nothing to disclose.
Masias C, Cataland SR. Novel therapies in thrombotic thrombocytopenic purpura. Res Pract Thromb Haemost. 2018;2:19–26. 10.1002/rth2.12066
REFERENCES
- 1. Scully M, Cataland S, Coppo P, et al. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312–22. [DOI] [PubMed] [Google Scholar]
- 2. Kremer Hovinga JA, Coppo P, Lammle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3:17020. [DOI] [PubMed] [Google Scholar]
- 3. Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129:2836–46. [DOI] [PubMed] [Google Scholar]
- 4. Coppo P, Froissart A. Treatment of thrombotic thrombocytopenic purpura beyond therapeutic plasma exchange. Hematology / the Education Program of the American Society of Hematology American Society of Hematology Education Program. 2015;2015:637–43. [DOI] [PubMed] [Google Scholar]
- 5. Froissart A, Buffet M, Veyradier A, et al. Efficacy and safety of first‐line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit Care Med. 2012;40:104–11. [DOI] [PubMed] [Google Scholar]
- 6. von Krogh AS, Quist‐Paulsen P, Waage A, et al. High prevalence of hereditary thrombotic thrombocytopenic purpura in central Norway: from clinical observation to evidence. J Thromb Haemost. 2016;14:73–82. [DOI] [PubMed] [Google Scholar]
- 7. Moatti‐Cohen M, Garrec C, Wolf M, et al. Unexpected frequency of Upshaw‐Schulman syndrome in pregnancy‐onset thrombotic thrombocytopenic purpura. Blood. 2012;119:5888–97. [DOI] [PubMed] [Google Scholar]
- 8. Scully M, McDonald V, Cavenagh J, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118:1746–53. [DOI] [PubMed] [Google Scholar]
- 9. Willis MS, Bandarenko N. Relapse of thrombotic thrombocytopenic purpura: is it a continuum of disease? Semin Thromb Hemost. 2005;31:700–8. [DOI] [PubMed] [Google Scholar]
- 10. Bandarenko N, Brecher ME. United States Thrombotic Thrombocytopenic Purpura Apheresis Study Group (US TTP ASG): multicenter survey and retrospective analysis of current efficacy of therapeutic plasma exchange. J Clin Apher. 1998;13:133–41. [DOI] [PubMed] [Google Scholar]
- 11. Shumak KH, Rock GA, Nair RC. Late relapses in patients successfully treated for thrombotic thrombocytopenic purpura. Canadian Apheresis Group. Ann Intern Med. 1995;122:569–72. [DOI] [PubMed] [Google Scholar]
- 12. Saultz JN, Wu HM, Cataland S. Headache prevalence following recovery from TTP and aHUS. Ann Hematol. 2015;94:1473–6. [DOI] [PubMed] [Google Scholar]
- 13. Kennedy AS, Lewis QF, Scott JG, et al. Cognitive deficits after recovery from thrombotic thrombocytopenic purpura. Transfusion. 2009;49:1092–101. [DOI] [PubMed] [Google Scholar]
- 14. Cataland SR, Scully MA, Paskavitz J, et al. Evidence of persistent neurologic injury following thrombotic thrombocytopenic purpura. Am J Hematol. 2011;86:87–9. [DOI] [PubMed] [Google Scholar]
- 15. Deford CC, Reese JA, Schwartz LH, et al. Multiple major morbidities and increased mortality during long‐term follow‐up after recovery from thrombotic thrombocytopenic purpura. Blood. 2013;122:2023–9; quiz 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moschcowitz E. Hyaline thrombosis of the terminal arteriorles and capillaries: a hitherto undescribed disease. Proc NY Pathol Soc. 1924;24:21–4. [Google Scholar]
- 17. Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura‐hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325:398–403. [DOI] [PubMed] [Google Scholar]
- 18. Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–7. [DOI] [PubMed] [Google Scholar]
- 19. Cataland S, Yang S, Masias C, et al., eds. A Prospective, Randomized Study of Cyclosporine or Corticosteroids As an Adjunct to Plasma Exchange for the Treatment of Thrombotic Thrombocytopenic Purpura. American Society of Hematology 58th Annual Meeting and Exposition; 2016 Dec 3‐6 2016, San Diego, CA.
- 20. Ulrichts H, Silence K, Schoolmeester A, et al. Antithrombotic drug candidate ALX‐0081 shows superior preclinical efficacy and safety compared with currently marketed antiplatelet drugs. Blood. 2011;118:757–65. [DOI] [PubMed] [Google Scholar]
- 21. Callewaert F, Roodt J, Ulrichts H, et al. Evaluation of efficacy and safety of the anti‐VWF Nanobody ALX‐0681 in a preclinical baboon model of acquired thrombotic thrombocytopenic purpura. Blood. 2012;120:3603–10. [DOI] [PubMed] [Google Scholar]
- 22. Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374:511–22. [DOI] [PubMed] [Google Scholar]
- 23. Peyvandi F, Scully M, Kremer Hovinga JA, et al. Caplacizumab reduces the frequency of major thromboembolic events, exacerbations and death in patients with acquired thrombotic thrombocytopenic purpura. J Thromb Haemost. 2017;15:1448–52. [DOI] [PubMed] [Google Scholar]
- 24. Plaimauer B, Zimmermann K, Volkel D, et al. Cloning, expression, and functional characterization of the von Willebrand factor‐cleaving protease (ADAMTS13). Blood. 2002;100:3626–32. [DOI] [PubMed] [Google Scholar]
- 25. Antoine G, Zimmermann K, Plaimauer B, et al. ADAMTS13 gene defects in two brothers with constitutional thrombotic thrombocytopenic purpura and normalization of von Willebrand factor‐cleaving protease activity by recombinant human ADAMTS13. Br J Haematol. 2003;120:821–4. [DOI] [PubMed] [Google Scholar]
- 26. Schiviz A, Wuersch K, Piskernik C, et al. A new mouse model mimicking thrombotic thrombocytopenic purpura: correction of symptoms by recombinant human ADAMTS13. Blood. 2012;119:6128–35. [DOI] [PubMed] [Google Scholar]
- 27. Scully M, Knobl P, Kentouche K, et al. A recombinant human ADAMTS‐13: first‐in‐human study evaluating pharmacokinetics, safety and tolerability in cTTP patients. Blood. 2017;130:2055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Plaimauer B, Kremer Hovinga JA, et al. Recombinant ADAMTS13 normalizes von Willebrand factor‐cleaving activity in plasma of acquired TTP patients by overriding inhibitory antibodies. J Thromb Haemost. 2011;9:936–44. [DOI] [PubMed] [Google Scholar]
- 29. Tersteeg C, Schiviz A, De Meyer SF, et al. Potential for Recombinant ADAMTS13 as an effective therapy for acquired thrombotic thrombocytopenic purpura. Arterioscler Thromb Vasc Biol. 2015;35:2336–2242. [DOI] [PubMed] [Google Scholar]
- 30. Plaimauer B, Schiviz A, Kaufmann S, Hollriegl W, Rottensteiner H, Scheiflinger F. Neutralization of inhibitory antibodies and restoration of therapeutic ADAMTS‐13 activity levels in inhibitor‐treated rats by the use of defined doses of recombinant ADAMTS‐13. J Thromb Haemost. 2015;13:2053–62. [DOI] [PubMed] [Google Scholar]
- 31. Chen J, Reheman A, Gushiken FC, et al. N‐acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J Clin Invest. 2011;121:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tersteeg C, Roodt J, Van Rensburg WJ, et al. N‐acetylcysteine in preclinical mouse and baboon models of thrombotic thrombocytopenic purpura. Blood. 2017;129:1030–8. [DOI] [PubMed] [Google Scholar]
- 33. Li GW, Rambally S, Kamboj J, et al. Treatment of refractory thrombotic thrombocytopenic purpura with N‐acetylcysteine: a case report. Transfusion. 2014;54:1221–4. [DOI] [PubMed] [Google Scholar]
- 34. Rottenstreich A, Hochberg‐Klein S, Rund D, Kalish Y. The role of N‐acetylcysteine in the treatment of thrombotic thrombocytopenic purpura. J Thromb Thrombolysis. 2016;41:678–83. [DOI] [PubMed] [Google Scholar]
- 35. Acedillo RR, Govind M, Kashgary A, Clark WF. Treatment of severe, refractory and rapidly evolving thrombotic thrombocytopenic purpura. BMJ Case Rep. 2016. 10.1136/bcr-2016-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chapin J, Weksler B, Magro C, Laurence J. Eculizumab in the treatment of refractory idiopathic thrombotic thrombocytopenic purpura. Br J Haemat. 2012;157:772–724. [DOI] [PubMed] [Google Scholar]
- 37. Patriquin CJ, Thomas MR, Dutt T, et al. Bortezomib in the treatment of refractory thrombotic thrombocytopenic purpura. Br J Haematol. 2016;173:779–85. [DOI] [PubMed] [Google Scholar]
- 38. Lei X, Reheman A, Hou Y, et al. Anfibatide, a novel GPIb complex antagonist, inhibits platelet adhesion and thrombus formation in vitro and in vivo in murine models of thrombosis. Thromb Haemost. 2014;111:279–89. [DOI] [PubMed] [Google Scholar]
- 39. Zheng L, Mao Y, Abdelgawwad MS, et al. Therapeutic efficacy of the platelet glycoprotein Ib antagonist anfibatide in murine models of thrombotic thrombocytopenic purpura. Blood Adv. 2016;1:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shortt J, Oh DH, Opat SS. ADAMTS13 antibody depletion by bortezomib in thrombotic thrombocytopenic purpura. N Engl J Med. 2013;368:90–2. [DOI] [PubMed] [Google Scholar]
- 41. Yates S, Matevosyan K, Rutherford C, Shen YM, Sarode R. Bortezomib for chronic relapsing thrombotic thrombocytopenic purpura: a case report. Transfusion. 2014;54:2064–7. [DOI] [PubMed] [Google Scholar]
- 42. Mazepa MA, Raval JS, Moll S, Ma A, Park YA. Bortezomib induces clinical remission and reduction of ADAMTS13 inhibitory antibodies in relapsed refractory idiopathic thrombotic thrombocytopenic purpura. Br J Haematol. 2014;164:900–2. [DOI] [PubMed] [Google Scholar]
- 43. Van Balen T, Schreuder MF, de Jong H, van de Kar NC. Refractory thrombotic thrombocytopenic purpura in a 16‐year‐old girl: successful treatment with bortezomib. Eur J Haematol. 2014;92:80–2. [DOI] [PubMed] [Google Scholar]
- 44. Patel PP, Becker J, Freyer C, Griffiths E, Thompson JE, Wang ES. Rituximab‐refractory thrombotic thrombocytopenic purpura responsive to intravenous but not subcutaneous bortezomib. Transfusion. 2016;56:970–4. [DOI] [PubMed] [Google Scholar]
- 45. Pandey MR, Vachhani P, Ontiveros EP. Remission of severe, relapsed, and refractory TTP after multiple cycles of bortezomib. Case Rep Hematol. 2017;2017:9681832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shapiro HD, Doktor D, Churg J. Thrombotic thrombocytopenic purpura (Moschcowitz's disease): report of a case with remission after splenectomy and steroid therapy. Ann Intern Med. 1957;47:582–5. [DOI] [PubMed] [Google Scholar]
- 47. Meacham GC, Orbison JL, Heinle RW, Steele HJ, Schaefer JA. Thrombotic thrombocytopenic purpura, a disseminated disease of arterioles. Blood. 1951;6:706–19. [PubMed] [Google Scholar]
- 48. Veltman GA, Brand A, Leeksma OC, ten Bosch GJ, van Krieken JH, Briet E. The role of splenectomy in the treatment of relapsing thrombotic thrombocytopenic purpura. Ann Hematol. 1995;70:231–6. [PubMed] [Google Scholar]
- 49. Outschoorn UM, Ferber A. Outcomes in the treatment of thrombotic thrombocytopenic purpura with splenectomy: a retrospective cohort study. Am J Hematol. 2006;81:895–900. [DOI] [PubMed] [Google Scholar]
- 50. Kappers‐Klunne MC, Wijermans P, Fijnheer R, et al. Splenectomy for the treatment of thrombotic thrombocytopenic purpura. Br J Haematol. 2005;130:768–76. [DOI] [PubMed] [Google Scholar]
- 51. Hie M, Gay J, Galicier L, et al. Preemptive rituximab infusions after remission efficiently prevent relapses in acquired thrombotic thrombocytopenic purpura. Blood. 2014;124:204–10. [DOI] [PubMed] [Google Scholar]
- 52. Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Rituximab reduces risk for relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2016;127:3092–4. [DOI] [PubMed] [Google Scholar]
- 53. Schaller M, Vogel M, Kentouche K, Lammle B, Kremer Hovinga JA. The splenic autoimmune response to ADAMTS13 in thrombotic thrombocytopenic purpura contains recurrent antigen‐binding CDR3 motifs. Blood. 2014;124:3469–79. [DOI] [PubMed] [Google Scholar]
- 54. Skowronoska M, Kremer Hovinga J, Schaller M. Splenic anti‐ADAMTS13 response in relapsing thrombotic thrombocytopenic purpura (iTTP) patients mirrors the acute immune response in plasma. Res Pract Thromb Haemost. 2017;124(Supl S1):1295. [Google Scholar]
- 55. Chemnitz J, Draube A, Scheid C, et al. Successful treatment of severe thrombotic thrombocytopenic purpura with the monoclonal antibody rituximab. Am J Hematol. 2002;71:105–8. [DOI] [PubMed] [Google Scholar]
- 56. Gutterman LA, Kloster B, Tsai HM. Rituximab therapy for refractory thrombotic thrombocytopenic purpura. Blood Cells Mol Dis. 2002;28:385–91. [DOI] [PubMed] [Google Scholar]
- 57. Scully M, Cohen H, Cavenagh J, et al. Remission in acute refractory and relapsing thrombotic thrombocytopenic purpura following rituximab is associated with a reduction in IgG antibodies to ADAMTS‐13. Br J Haematol. 2007;136:451–61. [DOI] [PubMed] [Google Scholar]
- 58. Herold S, Falter T, Schmitt V, et al. Retrospective analysis of the relapse rate in patients surviving acute acquired thrombotic thrombocytopenic purpura (TTP) treated with or without rituximab. Res Pract Thromb Haemost. 2017;1(Suppl. S1):1297. [Google Scholar]
- 59. Mansouri M, Matsumoto M, Cermakova Z, Friedman KD, George JN, Hrachovinova I, et al., editors. Hereditary TTP – a young patient population with high prevalence of arterial thromboembolic events: first results from the hereditary TTP registry. XXV Congress of the International Society on Thrombosis and Haemostasis; 2015; Toronto.
- 60. Roose E, Schelpe AS, Joly BS, Vandenbulcke A, Caron J, Pareyn I, et al. Conformation of ADAMTS13 is Altered in Acquired TTP Patients. Res Pract Thromb Haemost. 2017;1(Suppl. 1):254. [Google Scholar]
- 61. Underwood MI, Thomas MR, Scully M, Crawley TB. Autoantibody binding to ‘Open’ and ‘Closed’ ADAMTS13 in patients with acquired immune thrombotic thrombocytopenic purpura. Res Pract Thromb Haemost. 2017;1(Suppl. S1):254. [Google Scholar]
- 62. Sorvillo N, Martinod K, Staudinger C, Wagner DD. PAD4 Citrullination of ADAMTS13: a new link between NETosis and thrombosis. Res Pract Thromb Haemost. 2017;1(Suppl. 1):254. [Google Scholar]
- 63. Page EE, Kremer Hovinga JA, Terrell DR, Vesely SK, George JN. Clinical importance of ADAMTS13 activity during remission in patients with acquired thrombotic thrombocytopenic purpura. Blood. 2016;128:2175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jin M, Casper TC, Cataland SR, et al. Relationship between ADAMTS13 activity in clinical remission and the risk of TTP relapse. Br J Haematol. 2008;141:651–8. [DOI] [PubMed] [Google Scholar]
- 65. Peyvandi F, Lavoretano S, Palla R, et al. ADAMTS13 and anti‐ADAMTS13 antibodies as markers for recurrence of acquired thrombotic thrombocytopenic purpura during remission. Haematologica. 2008;93:232–9. [DOI] [PubMed] [Google Scholar]
- 66. Tersteeg C, de Maat S, De Meyer SF, et al. Plasmin cleavage of von Willebrand factor as an emergency bypass for ADAMTS13 deficiency in thrombotic microangiopathy. Circulation. 2014;129:1320–31. [DOI] [PubMed] [Google Scholar]
- 67. Brophy TM, Ward SE, McGimsey TR, et al. Plasmin cleaves von Willebrand factor at K1491‐R1492 in the A1‐A2 linker region in a shear‐ and glycan‐dependent manner in vitro. Arterioscler Thromb Vasc Biol. 2017;37:845–55. [DOI] [PubMed] [Google Scholar]
- 68. Mebius MM, Clark CC, de Maat S, et al. Plasmin cleavage of ADAMTS‐13 enhances its Activity. Res Pract Thromb Haemost. 2017;1(Suppl. S1):1293. [Google Scholar]


