Abstract
Objective
The left atrium (LA) is exposed to left ventricular pressure during diastole. Applying the 2016 American Society of Echocardiography left ventricular diastolic function (LVDF) guidelines, this study aims to investigate whether left atrial ejection fraction (LAEF) and left atrial active emptying fraction (LAAEF) are markers of diastolic dysfunction (LVDD).
Methods
Retrospective cohort of consecutive patients (n = 124) who underwent transthoracic echocardiography were studied. Doppler peak velocities of passive (MV E) and active filling (MV A) were measured and ratio E/A calculated. Tissue Doppler imaging parameters of peak early (e′) of the septal and lateral mitral annulus were measured, and average E/e′ ratio (E/e′) was calculated. Tricuspid regurgitation velocity, left atrial maximum volume, left atrial minimum volume and LA volume pre-contraction were measured, allowing calculation of LAEF and LAAEF. Subjects were assigned LVDF categories.
Results
Binomial logistic regression model (X2(2) = 48.924, P < 0.01) determined that LAEF and LAAEF predicted diastolic dysfunction with sensitivity 85.5% and specificity 78%. ROC curves determined good diagnostic accuracy for LAEF and LAAEF to predict LVDD, AUC 0.826 and 0.861 respectively. Logistic regression model (X2(2) = 39.525, P < 0.01) predicted those patients with E/e′ ≥14 using LAEF and LAAEF with sensitivity 51.6% and specificity 92.4%. Moderate correlations were found between E/e′ and log derivatives of LAEF and LAAEF.
Conclusions
A decline in LAAEF and LAEF is associated with worsening LVDD.
Keywords: echocardiography, left ventricular diastolic dysfunction, left atrial function
Introduction
The cardiac cycle is a complex process of tri-phasic relaxation/filling and bi-phasic ejection. The left ventricular (LV) diastolic process consists of four phases: isovolumic relaxation, passive early rapid ventricular filling and active atrial contraction. The primary role of the left atrium (LA) is to assist LV filling in three phases. A reservoir for pulmonary venous return (PVr) during ventricular systole; a conduit to the LV for PVr during early ventricular diastole and a pump to augment LV filling in late diastole (1, 2). The LA is therefore exposed to LV diastolic pressures via the open mitral valve (MV) throughout most of diastole (3, 4) and is susceptible to remodelling through increasing pressure and volume. LA size and volume (indexed to body surface area) is therefore considered a marker for left ventricular diastolic function (LVDF) and is included in the LVDF echocardiographic protocol set out by the American Society of Echocardiography (ASE) (5).
LA function (LAf) using two-dimensional (2D) transthoracic echocardiography (TTE) has been assessed previously using a variety of measures, including: the Manning method of LA systolic force (6), measurement of LA kinetic energy (7), LA ejection fraction (LAEF) (8) and left atrial expansion index (8).
It has long been known that as vascular resistance increases through reduced arterial compliance, ventricular output falls (9). By the same principle, if LA afterload increases through reduced LV compliance, LA output and consequently left atrial active emptying fraction (LAAEF) and LAEF may also fall. Although LAEF has been previously researched as a measure of LAf in a small number of studies (1, 10, 11, 12, 13, 14), Hsaio et al. are the only group to have correlated LAAEF (volume at atrial systole (preAv) minus LA minimum volume (LAmin) divided by preAv), with LVDF (8). Using the new ASE LVDF guidelines (5), this study aims to determine if LAAEF or LAEF correlate with or predict the presence of LVDF and therefore provide additional indicators of LVDF severity.
Methods
Study population
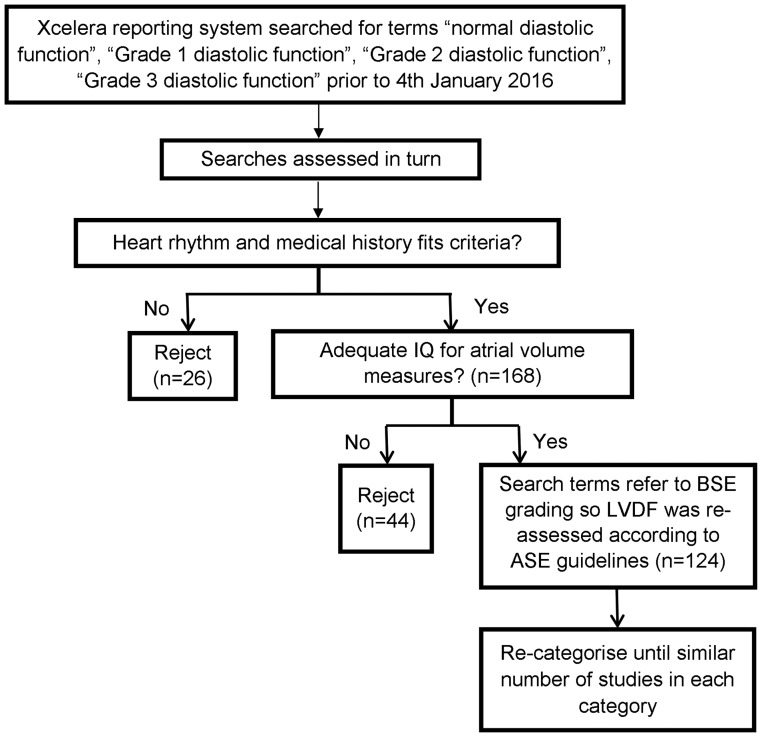
This was a retrospective cohort study of patients referred for TTE at Royal Papworth Hospital NHS Foundation Trust prior to January 2016. Patients were undergoing TTE for either: open access referral made by their General Practitioner (GPOA) due to breathlessness or by a cardiothoracic surgeon prior to coronary artery bypass graft (CABG) surgery. Data were stored on the Philips Xcelera system (version 3.2.1.712, Koninklijke Philips Electronic N.V2011). The study aimed to collect the data of 100 subjects spread throughout the LVDF categories, indeterminate cases were excluded from statistical analysis. TTE studies were assessed using a purposive sampling method of consecutive studies analysed in reverse chronological order until each ASE-defined diastolic category had a comparable number of studies (Fig. 1). Grade III diastolic dysfunction (restrictive filling) category was underpopulated due to lack of patients who fulfilled the inclusion criteria.
Figure 1.
Study population selection methods.
The study was approved by Royal Papworth Hospital NHS Foundation Trust Research and Development Department.
Inclusion/exclusion criteria
All subjects referred via GPOA or pre-op assessments routes and who underwent a full TTE at Royal Papworth Hospital NHS Foundation Trust prior to January 2016 were considered for this study. Additionally, inpatients requiring full TTE prior to CABG were also considered. The minimum age was 16 years; there was no maximum age cut-off, as although age impacts LV relaxation, it does not affect interpretation of LVDF.
Subjects were excluded from the study, if at the time of the TTE they were in atrial fibrillation, atrial flutter or sinus tachycardia; had evidence of multiple ectopic beats on saved loops or if image quality was inadequate for accurate measurement. The following exclusion criteria were applied: 1) ≥moderate left-sided valvular heart disease, 2) previous MV surgery, prosthetic valves or annular rings, 3) atrial septal closure device, 4) pericardial effusion, 5) primary pulmonary hypertension (eccentricity index >1), 6) LV outflow tract obstruction (either at rest or with provocation), 7) patients receiving dialysis, haemofiltration or inotrope infusions, 8) prior heart transplantation and 9) history of AF ablation.
Echocardiographic assessment
LAEF measurements
TTEs performed on the Philips IE33 or CX50 were retrieved from the Philips Xcelera system. All measures of LA volume were performed by British Society of Echocardiography accredited sonographers (15).
Left atrial volume (LAV) was measured at three points in the cardiac cycle, in both apical four chamber (A4C) and apical two chamber views: left atrial maximum volume (LAmax) at end systole, LAmin at end-diastole, frame before MV closure and preAv, frame before MV re-opens for atrial kick.
The parameters used in analyses were:
LAAEF: (preAv − LAmin)/preAv
LAEF: (LAmax − LAmin)/LAmax
LVDF measurements
The ASE protocol for measurement of LVDF parameters was applied (5) to archived A4C images of trans-mitral inflow and mitral annular velocities from pulsed-tissue Doppler imaging (TDI). Peak velocities of the early (MV E) and late (MV A) phases of the mitral inflow from the Doppler recordings were measured and their ratio (E/A) calculated. Peak early diastolic velocity (e′) of the septal and lateral mitral annulus was measured. The E/e′ was calculated in each case. Additionally, tricuspid regurgitation velocity and LAmax indexed by body surface area were measured. LVDF category was then determined according to the algorithms within the ASE protocol.
Statistical analysis
Data are presented as mean ± standard deviation for continuous variables. Initially, ordinal logistic regression analysis was run to predict LVDF category according to LAAEF and LAEF. However, assumptions necessary for the test were violated, this test was therefore invalidated. Alternatively, binomial logistic regression analyses were run to determine the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of predicting LVDF using LAAEF and LAEF. ROC curves were used to determine the strength of the predictive tests; accuracy of parameters for predicting LVDF and to define measurement values producing the highest sensitivity and specificity to predict left ventricular diastolic dysfunction (LVDD). Univariate correlations between log LA variables and E/e′ were assessed using Pearson’s correlation co-efficient (r) for normally distributed data. Reproducibility of the measurements was assessed by inter-class correlation coefficients (ICCs). For all statistical analyses, a two-tailed P < 0.01 was considered significant. Statistical analyses were performed using SPSS software, V.21.0 (SPSS Inc.).
Reproducibility of LA volumes
Reproducibility of LA volumes was assessed in ten randomly selected subjects. One accredited supervisor re-measured the atrial volumes of the subjects, blinded to the original measurements, ICC = 0.959, P < 0.01. LA volumes for ten additional randomly selected subjects were re-measured by the primary operator, blinded to the initial results, ICC = 0.898.
Results
Echo characteristics of the sample
Impaired LVDF was present in 69 subjects (55.6%). LVDF was indeterminate in 14 subjects (11.3%). Of those with LVDF, 28 subjects had grade I LVDF (22.6%), 25 subjects had grade II LVDF (20.2%) and 16 subjects had grade III LVDF (12.9%). The main LVDF and LA parameters by ASE guidelines are displayed in Table 1.
Table 1.
Mean values for the LVDF and LA parameters by LVDF category.
| Normal (n = 41) | Grade I (n = 28) | Grade II (n = 25) | Grade III (n = 16) | Indeterminate (n = 14) | |
|---|---|---|---|---|---|
| Age | 46.3 ± 18.2 | 51.3 ± 16.1 | 65.3 ± 9.1 | 59.3 ± 15.7 | 61.1 ± 12.8 |
| Gender % male | 31.7% | 82.1% | 64.0% | 81.2% | 85.7% |
| BSA (m2) | 1.8 ± 0.3 | 2.0 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.2 |
| HR (bpm) | 70.9 ± 11.7 | 62.4 ± 10.0 | 61.7 ± 11.0 | 72.4 ± 10.2 | 61.7 ± 11.7 |
| sBP (mmHg) | 132.4 ± 20.5 | 136.3 ± 29.1 | 133.1 ± 26.2 | 134.7 ± 19.7 | 126.2 ± 23.4 |
| dBP (mmHg) | 74.7 ± 11.4 | 78.7 ± 14.7 | 69.6 ± 12.2 | 79.1 ± 10.0 | 71.6 ± 14.9 |
| LVEF (%) | 61.4 ± 4.0 | 47.7 ± 11.1 | 53.1 ± 14.2 | 34.6 ± 12.5 | 56.4 ± 10.7 |
| LV mass indexed (g/m2) | 73.3 ± 18.4 | 99.8 ± 34.1 | 105.6 ± 39.6 | 120.7 ± 35.7 | 85.8 ± 29.7 |
| LA mini (mL/m2) | 11.0 ± 3.0 | 14.5 ± 5.9 | 23.2 ± 7.1 | 37.1 ± 12.1 | 17.8 ± 6.2 |
| LApreAvi (mL/m2) | 19.3 ± 4.6 | 22.8 ± 7.9 | 33.3 ± 7.8 | 44.1 ± 12.3 | 26.6 ± 7.2 |
| LAmaxi (mL/m2) | 26.7 ± 4.4 | 29.5 ± 8.5 | 42.9 ± 9.3 | 48.3 ± 11.9 | 35.1 ± 10.3 |
| MV E velocity (cm/s) | 74.5 ± 15.1 | 59.5 ± 16.2 | 82.1 ± 23.3 | 98.5 ± 17.0 | 74.2 ± 11.0 |
| E/A ratio | 1.3 ± 0.5 | 1.0 ± 0.3 | 1.2 ± 0.4 | 3.0 ± 1.1 | 1.3 ± 0.4 |
| Septal e′ (cm/s) | 8.8 ± 2.9 | 6.1 ± 2.1 | 5.2 ± 1.4 | 4.6 ± 1.7 | 5.7 ± 1.1 |
| Lateral e′ (cm/s) | 12.8 ± 4.2 | 8.1 ± 2.9 | 6.4 ± 2.2 | 5.7 ± 2.6 | 7.9 ± 2.0 |
| E/e′ average | 7.6 ± 2.0 | 9.1 ± 2.3 | 14.9 ± 3.4 | 21.9 ± 6.5 | 11.6 ± 2.4 |
| LAAEF (%) | 44 ± 6.0 | 37 ± 8.0 | 30.0 ± 9.0 | 17 ± 6.0 | 34.0 ± 10.0 |
| LAEF (%) | 59 ± 8.0 | 52 ± 9.0 | 46.0 ± 10.0 | 24 ± 8.0 | 49.0 ± 7.0 |
BSA, body surface area; dBP, diastolic blood pressure; HR, heart rate; LAAEF, left atrial active emptying fraction; LAEF, left atrial ejection fraction; LAmaxi, maximum left atrial volume indexed; LAmini, minimum left atrial volume indexed; LApreAvi, left atrial volume pre-contraction indexed; LVEF, left ventricular ejection fraction; MV E, mitral valve E wave; sBP, systolic blood pressure.
LA function and LVDF
The relationship between LAAEF and LAEF with LVDF was assessed using the most recent ASE guidelines. Subjects with indeterminate LVDF described by ASE guidelines were removed from the following statistical tests. A binomial logistic regression was performed to ascertain the effects of LAAEF and LAEF on the likelihood that patients have LVDD. The logistic regression model was statistically significant X 2(2) = 48.924, P < 0.01, of the two predictor variables, LAAEF was statistically significant (P < 0.01). Sensitivity, specificity, PPV and NPV are shown in Table 2. This indicates that 86.8% of patients who have LVDD were also predicted to have LVDD by this model (true positives). Additionally, 76.2% of patients who have normal LVDF were predicted to have normal LVDF by this model (true negatives).
Table 2.
Results of binominal logistic regression model ascertaining effects of LAAEF and LAEF on the likelihood that patients have LVDD.
| LV diastolic dysfunction (%) | |
|---|---|
| Sensitivity | 85.5 |
| Specificity | 78 |
| PPV | 86.8 |
| NPV | 76.2 |
NPV, negative predictive value; PPV, positive predictive value.
ROC curves were performed to determine the diagnostic accuracy of using LAAEF and LAEF to predict LVDD (Fig. 2). AUC 0.861 and 0.826 suggest that LAAEF and LAEF respectively are strong indicators of the presence of LVDD. When considering an optimal balance between maximum sensitivity and specificity, LAAEF ≤38.5% and LAEF ≤52.5% predicts diastolic dysfunction with sensitivity 79.7% and 72.5%, and specificity 82.9% and 73.2% respectively (Table 3).
Figure 2.
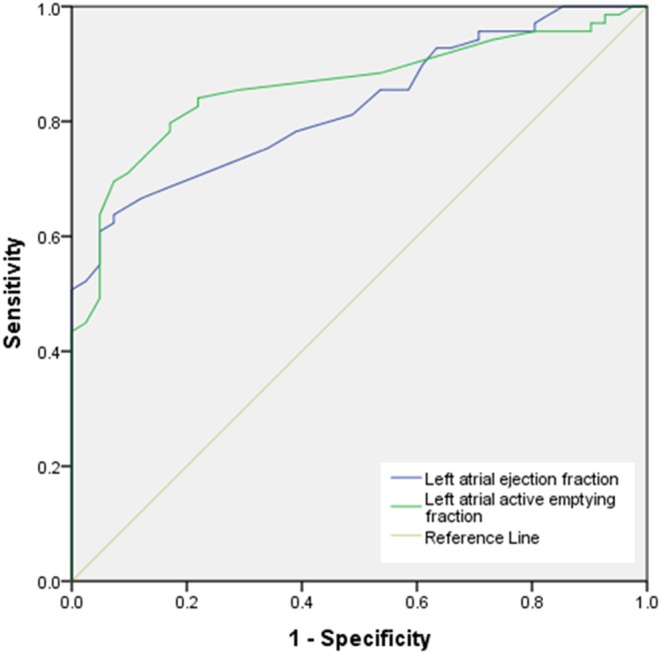
ROC curve displaying diagnostic accuracy of using LAAEF and LAEF to predict LVDD.
Table 3.
ROC curve co-ordinate results to determine the diagnostic accuracy of using LAAEF and LAEF to predict LVDD.
| Predictor | AUC | P-Value | Sensitivity (%) | Specificity (%) | Predictor value for given sensitivity and specificity (%) |
|---|---|---|---|---|---|
| LAAEF | 0.861 | <0.01 | 79.7 | 82.9 | ≤38.5 |
| LAEF | 0.826 | <0.01 | 72.5 | 73.2 | ≤52.5 |
AUC, area under curve; LAAEF, left atrial active emptying fraction; LAEF, left atrial ejection fraction.
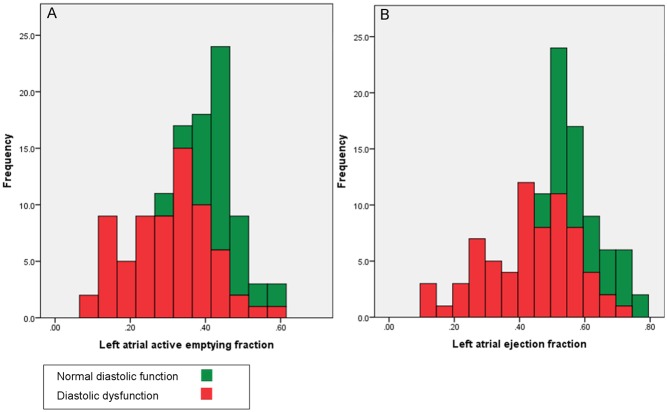
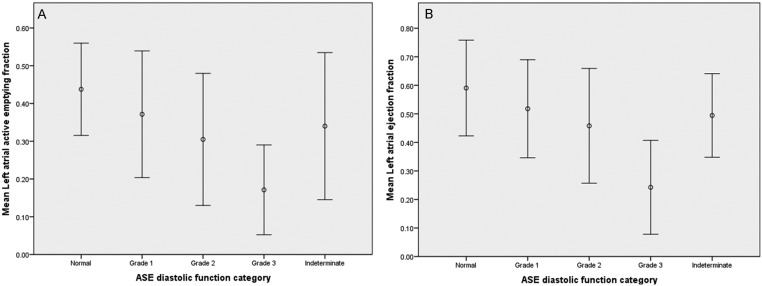
Bar graphs and box plots display the relationship between LAAEF and LAEF, with LVDF. These demonstrate that as LVDF worsens, LAAEF and LAEF decrease (Figs 3 and 4).
Figure 3.
Bar graphs demonstrating the relationship between the predictors and LVDF. (A) Relationship between LAAEF and LVDF. (B) Relationship between LAEF and LVDF.
Figure 4.
Box plots displaying the mean ± 2 s.d. LAAEF and LAEF for each LVDF category. (A) Mean LAAEF ± 2 s.d. for each LVDF category. (B) Mean LAEF ± 2 s.d. for each LVDF category
Relationship between average E/e′ and LA function
A binomial logistic regression was performed to ascertain the effects of LAAEF and LAEF on the likelihood that patients have E/e′ ≥14. The logistic regression model was statistically significant X 2(2) = 39.525, P < 0.01; results are shown in Table 4. Of the two predictor variables none were statistically significant.
Table 4.
Binomial logistic regression results ascertaining effects of LAAEF and LAEF on the likelihood that patients have E/e′ >14.
| Average E/e′ (%) | |
|---|---|
| Sensitivity | 51.6 |
| Specificity | 92.4 |
| PPV | 72.7 |
| NPV | 83.0 |
NPV, negative predictive value; PPV, positive predictive value.
Additionally, ROC curves were performed to determine the diagnostic accuracy of LAAEF and LAEF to predict average E/e′ (Fig. 5). AUC 0.828 and 0.846 respectively identify these measures as good indicators of LVDD. Co-ordinates are summarised in Table 5.
Figure 5.
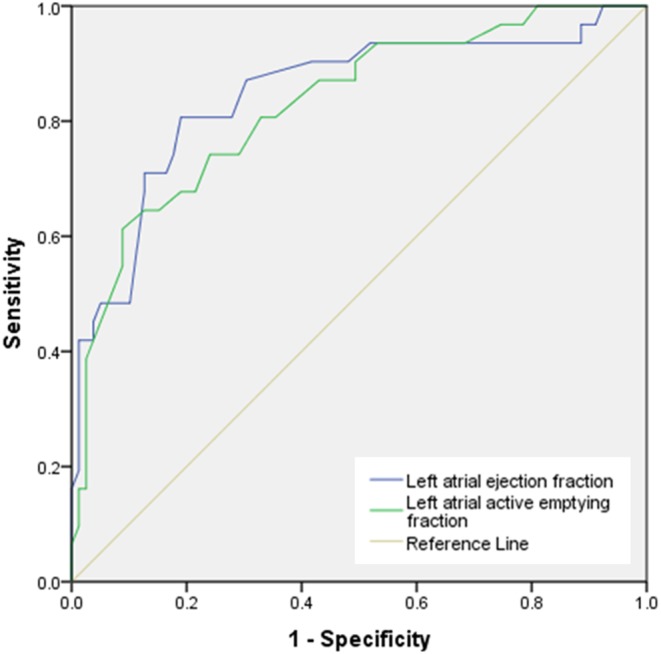
ROC curve displaying diagnostic accuracy of using LAAEF and LAEF to predict average E/e′.
Table 5.
ROC curve co-ordinate results to determine the diagnostic accuracy of using LAAEF and LAEF to predict an E/e′ >14.
| Predictor | AUC | P-Value | Sensitivity (%) | Specificity (%) | Predictor value for given sensitivity and specificity (%) |
|---|---|---|---|---|---|
| LAAEF | 0.828 | <0.01 | 74.2 | 75.9 | ≤33.5 |
| LAEF | 0.846 | <0.01 | 80.6 | 81.0 | ≤46.5 |
AUC, area under curve; LAAEF, left atrial active emptying fraction; LAEF, left atrial ejection fraction.
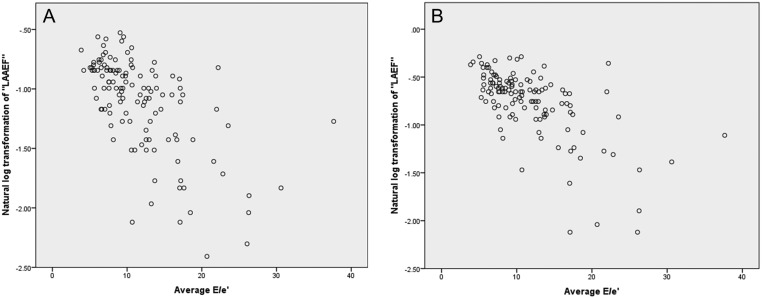
Pearson’s product–moment correlation was performed to assess the relationship between E/e′ and the natural log of LAAEF and LAEF. Statistically significant moderate correlations were found (Table 6) and are illustrated in scatterplots (Fig. 6).
Table 6.
Pearson’s product–moment correlation between E/e′ and the natural log of LAAEF and LAEF.
| R | P | |
|---|---|---|
| Ln LAAEF | −0.622 | <0.01 |
| Ln LAEF | −0.638 | <0.01 |
LAEF, natural log of left atrial ejection fraction; Ln LAEF, natural log of left atrial active emptying fraction.
Figure 6.
Scatterplots illustrating correlations between average E/e′; ln(LAAEF) and ln(LAEF). (A) Scatterplot demonstrating correlation between average E/e′ and ln(LAAEF). (B) Scatterplot demonstrating correlation between average E/e′ and ln(LAEF)
Discussion
Relationship between LVDF and LA function
When LA pre-load is stable, indexed LAVs are a robust marker for LV filling pressures (E/e′) (4). In this study, we assessed the relationship between LAAEF and LAEF with grades of LVDF as defined by recent ASE guidelines (5). Our findings demonstrate that both predictor variables are capable of predicting LVDD with high sensitivity and specificity. LAAEF ≤38.5% (sensitivity 79.4% and specificity 82.9%) was found to be more sensitive and specific than LAEF.
Filling of the LA during ventricular systole (atrial reservoir phase) is contributed through contraction of longitudinal fibres within the LV. Shortening of the LV towards the apex and mechanical traction of the mitral annulus stretches the LA, in turn decreasing mean LA pressure and augmenting flow from the pulmonary veins; LAmax is therefore achieved at the end of ventricular systole (16). However, despite being considered a key indicator of LVDF in current guidelines, not only is LAmax measured during ventricular systole, it is also influenced by both the degree of LV longitudinal contraction and the compliance of the LA itself. It is therefore clear that additional measures are required to improve the accuracy of TTE for the diagnosis LVDF. Only one other study was found in the literature that compared Simpsons method of discs derived LAAEF with LVDF (8). Hsiao et al. concluded that LA parameters offer a better estimation of LVDF than TDI and pulsed-wave Doppler measurements (8).
Similar findings to this study were also found by both Russo et al. and Scherr et al. (4, 17). Both studies assessed the relationship between LAAEF and LAEF and LVDF by real-time 3D echocardiography (4). Scherr et al. found a stronger relationship between LAAEF and LVDF than the one between LAmin and LVDF.
Teo et al. studied the impact of LVDF on LAf and volume and found that LA active contraction is augmented to compensate for changes in LV diastolic properties (18). However, they described this compensatory mechanism failing as the LA becomes dysfunctional and the severity of LVDD increases, causing a lower total emptying volume (18). This describes how the LA behaves similarly to the LV in regards to the Frank-Starling law. Output will increase with an increase in LA size, which aids maintenance of normal stroke volume. Eventually, the output plateaus with continued increase in LAV, until finally moving onto the descending limb of the Frank-Starling curve when contractile function may decrease in the presence of severe LA dilation (19).
In conclusion, our study has demonstrated that increasing LA afterload, secondary to worsening LVDD, results in deteriorating LAAEF and LAEF. Furthermore, our data suggest that LAAEF and LAEF values of ≤38.5% and ≤52.5% respectively indicate the presence of LVDD.
Limitations
Due to the retrospective nature of data collection, suboptimal image quality had to be accepted in some cases. Due to the size of our study sample and underpopulation of patients with grade III LVDD, results would need to be validated over a greater population.
Doppler derived velocities and flow ratios are often influenced by the respiratory cycle. Because respiration alters diaphragmatic traction, and therefore, the position of intra-cardiac structures in relation to a fixed Doppler sample, the angle of insonation between the ultrasound beam and area of interest is variable. This consequently leads to variations in peak velocity measurements and potentially variable parameters of LVDF (20). The phases of respiration also affect TDI in the same manner (20).
There is currently no echocardiographic ‘gold standard’ for quantifying LV end-diastolic pressure, potentially increasing the overall error and accuracy of this study. However, the measures used are validated against the ASE LVDF algorithm. Nevertheless, a raised average E/e′ is rarely seen in normal individuals, but a normal E/e′ can be seen in those with grade 2 and 3 LVDD. Consequently, this study may have excluded some subjects with grade 2 and 3 LVDD on the basis of normal E/e′ and discordant values; however, subjects with raised E/e′ are very likely to have high LV-filling pressures.
Suggestions for future research
Although further validation is required before LAAEF and LAEF can be considered an additional measurement for the assessment of LVDF, we believe that these measures could be useful parameters in subjects where LVDD cannot be confirmed by current guideline algorithms. In addition, subjects with significant valvular disease and recipients of heart transplants were excluded from this cohort due to the difficulties in assessing LVDF in these groups. In such patients, where LVDD may co-exist or potentially indicate allograft rejection, research into LAAEF and LAEF, correlations with the standard LVDF measurements is recommended.
There is a growing body of evidence to support the estimation of LA strain for the assessment LVDF. However, considering that reference values have not yet been established and that significant expertise is necessary for accurate measurement, LA strain is not yet recommended for use within daily practice. In contrast, estimation of chamber volumes and ejection fraction are familiar concepts and routine practice to all sonographers, they are also supported by recognised reference ranges for LA size. In view of this, this study sought to identify additional parameters of LVDF that could be routinely performed on a daily basis, without the need for extensive further training and experience.
Conclusion
LVDD has not previously been compared with LAAEF and LAEF within the same 2D TTE study, or using the 2016 ASE diastolic function guidelines. The findings of this study clearly show that LAAEF and LAEF are strong predictors of LVDD with high sensitivity and specificity and could be considered valid additional markers to the standard dataset for assessing LVDF; as LVDD worsens, LAAEF and LAEF decrease.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Hoit BD. Left atrial size and function: role in prognosis. Journal of the American College of Cardiology 2014. 63 493–505. ( 10.1016/j.jacc.2013.10.055) [DOI] [PubMed] [Google Scholar]
- 2.Prioli A, Marino P, Lanzoni L, Zardini P. Increasing degrees of left ventricular filling impairment modulate left atrial function in humans. American Journal of Cardiology 1998. 82 756–761. ( 10.1016/S0002-9149(98)00452-4) [DOI] [PubMed] [Google Scholar]
- 3.Patel DA, Lavie CJ, Milani RV, Shah S, Gilliland Y. Clinical implications of left atrial enlargement: a review. Ochsner Journal 2009. 9 191–196. (available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3096293/) [PMC free article] [PubMed] [Google Scholar]
- 4.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Left atrial minimum volume and reservoir function as correlates of left ventricular diastolic function: impact of left ventricular systolic function. Heart 2012. 98 813–820. ( 10.1136/heartjnl-2011-301388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2016. 29 277–314. ( 10.1016/j.echo.2016.01.011) [DOI] [PubMed] [Google Scholar]
- 6.Manning WJ, Silverman DI, Katz SE, Douglas PS. Atrial ejection force: a noninvasive assessment of atrial systolic function. Journal of the American College of Cardiology 1993. 22 221–225. ( 10.1016/0735-1097(93)90838-R) [DOI] [PubMed] [Google Scholar]
- 7.Dardas PS, Pitsis AA, Mezilis NE, Tsikaderis DD, Ninios VN, Boudoulas H. Left atrial function and work after surgical ventricular restoration in postmyocardial infarction heart failure. Journal of the American Society of Echocardiography 2008. 21 841–847. ( 10.1016/j.echo.2007.12.005) [DOI] [PubMed] [Google Scholar]
- 8.Hsiao SH, Lin KL, Chiou KR. Comparison of left atrial volume parameters in detecting left ventricular diastolic dysfunction versus tissue Doppler recordings. American Journal of Cardiology 2012. 109 748–755. ( 10.1016/j.amjcard.2011.10.040) [DOI] [PubMed] [Google Scholar]
- 9.Vincent JL. Understanding cardiac output. Critical Care 2008. 12 174 ( 10.1186/cc6975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appleton CP, Galloway JM, Gonzalez MS, Gaballa M, Basnight MA. Estimation of left ventricular filling pressures using two-dimensional and Doppler echocardiography in adult patients with cardiac disease. Additional value of analyzing left atrial size, left atrial ejection fraction and the difference in duration of pulmonary venous and mitral flow velocity at atrial contraction. Journal of the American College of Cardiology 1993. 22 1972–1982. ( 10.1016/0735-1097(93)90787-2) [DOI] [PubMed] [Google Scholar]
- 11.Kataoka A, Funabashi N, Takahashi A, Yajima R, Takahashi M, Uehara M, Takaoka H, Saito M, Yamaguchi C, Lee K, et al Quantitative evaluation of left atrial volumes and ejection fraction by 320-slice computed-tomography in comparison with three- and two-dimensional echocardiography: a single-center retrospective-study in 22 subjects. International Journal of Cardiology 2011. 153 47–54. ( 10.1016/j.ijcard.2010.08.036) [DOI] [PubMed] [Google Scholar]
- 12.Yoon YE, Kim HJ, Kim SA, Kim SH, Park JH, Park KH, Choi S, Kim MK, Kim HS, Cho GY. Left atrial mechanical function and stiffness in patients with paroxysmal atrial fibrillation. Journal of Cardiovascular Ultrasound 2012. 20 140–145. ( 10.4250/jcu.2012.20.3.140) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anwar AM, Soliman OI, Nemes A, Geleijnse ML, ten Cate FJ. An integrated approach to determine left atrial volume, mass and function in hypertrophic cardiomyopathy by two-dimensional echocardiography. International Journal of Cardiovascular Imaging 2008. 24 45–52. ( 10.1007/s10554-007-9224-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Govindan M, Borgulya G, Kiotsekoglou A, Saha SK, Camm AJ. Prognostic value of left atrial expansion index and exercise-induced change in atrial natriuretic peptide as long-term predictors of atrial fibrillation recurrence. Europace 2012. 14 1302–1310. ( 10.1093/europace/eus088) [DOI] [PubMed] [Google Scholar]
- 15.Wharton G, Steeds R, Allen J, Phillips H, Jones R, Kanagala P, Lloyd G, Masani N, Mathew T, Oxborough D, et al A minimum dataset for a standard adult transthoracic echocardiogram: a guideline protocol from the British Society of Echocardiography. Echo Research and Practice 2015. 2 G9–G24. ( 10.1530/ERP-14-0079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castello R, Pearson AC, Lenzen P, Labovitz AJ. Evaluation of pulmonary venous flow by transesophageal echocardiography in subjects with a normal heart: comparison with transthoracic echocardiography. Journal of the American College of Cardiology 1991. 18 65–71. ( 10.1016/S0735-1097(10)80219-0) [DOI] [PubMed] [Google Scholar]
- 17.Scherr J, Jung P, Schuster T, Pollmer L, Eisele G, Goss F, Schneider J, Halle M. Left ventricular diastolic function is strongly correlated with active emptying of the left atrium: a novel analysis using three-dimensional echocardiography. Cardiovascular Ultrasound 2016. 14 43 ( 10.1186/s12947-016-0085-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teo SG, Yang H, Chai P, Yeo TC. Impact of left ventricular diastolic dysfunction on left atrial volume and function: a volumetric analysis. European Journal of Echocardiography 2010. 11 38–43. ( 10.1093/ejechocard/jep153) [DOI] [PubMed] [Google Scholar]
- 19.Rosca M, Lancellotti P, Popescu BA, Pierard LA. Left atrial function: pathophysiology, echocardiographic assessment, and clinical applications. Heart 2011. 97 1982–1989. ( 10.1136/heartjnl-2011-300069) [DOI] [PubMed] [Google Scholar]
- 20.Ginghina C, Beladan CC, Iancu M, Calin A, Popescu BA. Respiratory maneuvers in echocardiography: a review of clinical applications. Cardiovascular Ultrasound 2009. 7 42 ( 10.1186/1476-7120-7-42) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a