Summary
Leishmania are kinetoplastid parasites that cause the sandfly-transmitted disease leishmaniasis. To maintain fitness throughout their infectious life cycle, Leishmania must undergo rapid metabolic adaptations to the dramatically distinct environments encountered during transition between sandfly and vertebrate hosts. We performed proteomic and immunoblot analyses of attenuated L. major strains deficient for LACK, the Leishmania ortholog of the mammalian receptor for activated c kinase (RACK1), that is important for parasite thermotolerance and virulence. This approach identified cytochrome c oxidase (LmCOX) subunit IV as a LACK-dependent fitness protein. Consistent with decreased levels of LmCOX subunit IV at mammalian temperature, and in amastigotes, LmCOX activity and mitochondrial function were also impaired in LACK-deficient L. major under these conditions. Importantly, overexpression of LmCOX subunit IV in LACK-deficient L. major restored thermotolerance and macrophage infectivity. Interestingly, overexpression of LmCOX subunit IV enhanced LmCOX subunit VI expression at mammalian temperature. Collectively, our data suggest LACK promotes Leishmania adaptation to the mammalian host environment by sustaining LmCOX subunit IV expression and hence energy metabolism in response to stress stimuli such as heat. These findings extend the repertoire of RACK1 protein utility to include a role in mitochondrial function.
Introduction
Leishmania are trypanosomatid protozoa transmitted via the bite of phlebotomine sandflies into vertebrate hosts, establishing chronic infection as they replicate in the phagolysosomal compartment of macrophages, their primary host cells. Alternating between the dramatically distinct environments of insect and mammalian host, Leishmania undergo striking morphological changes that accompany significant alterations in gene expression between the insect (promastigote) and mammalian (amastigote) stages (Thiel and Bruchhaus, 2001; McNicoll et al., 2006; Cohen-Freue et al., 2007; Tsigankov et al., 2012). Intriguingly, Leishmania genes lack conventional RNA polymerase II promoters (Campbell et al., 2003; Clayton and Shapira, 2007), and stage-specific changes in gene expression do not rely heavily upon transcriptional regulation and transcript abundance (Cohen-Freue et al., 2007). Rather, stage-specific changes in Leishmania gene expression are controlled largely by post transcriptional mechanisms (Clayton and Shapira, 2007; Lahav et al., 2011).
Among the critical changes that occur during promastigote to amastigote differentiation are metabolic adaptations to their distinct niches. These include a shift from glycolysis to β-oxidation of fatty acids, amino acid utilization and mitochondrial electron transport (Opperdoes and Coombs, 2007; Depledge et al., 2009; McConville and Naderer, 2011; Saunders et al., 2014). Such changes are considered to reflect transition from the sugar-rich environment of the sandfly midgut to the sugar-deficient, amino acid and lipid-rich environment of the macrophage phagolysosome (Burchmore and Barrett, 2001; McConville et al., 2007; Naderer and McConville, 2008; Melo and Dvorak, 2012).
Despite these insights, molecular mechanisms driving cellular and molecular changes required for adaptation of Leishmania promastigotes to residence in the mammalian host remain poorly characterized. During our investigations to understand these mechanisms, we previously generated gene-targeted hypomorphic strains of L. major deficient for LACK, the Leishmania ortholog of the receptor for activated c kinase (RACK1) (Kelly et al., 2003). The genome of wildtype (WT) L. major encodes four copies of the LACK gene, organized as two tandemly repeated gene copies on each allele within the L. major diploid genome. One two-copy allele can be deleted without impacting parasite viability or pathogenicity (Kelly et al., 2003). These parasites, previously termed lack++/−− (Kelly et al., 2003), have one endogenous two-copy allele remaining and are designated LACK/LACK in this report. Deletion of a third copy of LACK results in parasites with a single haploid copy of the LACK gene. These single haploid LACK parasites, previously termed lack+−/−− (Kelly et al., 2003), and denoted as LACK/− in this report, show decreased levels of LACK, reduced viability at mammalian temperatures and severely attenuated virulence (Kelly et al., 2003). Replacement of the third copy of LACK with an XhoI restriction site-tagged LACK gene results in fully viable, virulent parasites (Kelly et al., 2003). This latter strain, maintaining LACK expression from two gene copies, contains one native LACK gene copy and one targeted LACK copy [previously designated lack++Δ/— (Kelly et al., 2003)]; this targeted gene copy encodes the wildtype LACK coding sequence cloned immediately 3’ of the puromycin drug-selection cassette (Kelly et al., 2003) and is termed LACK/LACK-Addbackin this study. Thus, threshold levels of LACK, expressed from a minimum of two gene copies, are critical for parasite fitness in the mammalian host. Molecular mechanisms that underlie LACK’s virulence-associated function, however, are poorly understood.
In other eukaryotes, including the trypanosomatid pathogen Trypanosoma brucei, RACK1 proteins have critical functions in a variety of biological processes. These include cellular signaling, environmental responses and translation (McCahill et al., 2002; Rothberg et al., 2006; Regmi et al., 2008; Adams et al., 2011). Indeed, a number of studies suggest a major function of RACK1 proteins is to coordinate cellular signaling pathways with translational control (Nilsson et al., 2004; Coyle et al., 2009). Previously, we also demonstrated that although LACK is functionally distinguishable from other RACK1 orthologs, it too has translational functions (Choudhury et al., 2011).
During infection, Leishmania promastigotes mount a heat stress response to elevated temperatures encountered following inoculation from the sandfly into the mammalian host. Such stress responses typically result in a generalized decrease in protein synthesis (Hunter et al., 1984; Shapira et al., 1988; Zilberstein and Shapira, 1994), albeit with an induction of specific proteins, such as heat shock proteins (Shapira et al., 2001). Significantly, this heat stress alone represents the primary physiologic signal for amastigote development (Zilberstein and Shapira, 1994; Alcolea et al., 2010), suggesting a link between heat stress and translational control in amastigote differentiation. Given the evidence supporting LACK’s functions in temperature tolerance, translation and virulence (Kelly et al., 2003; Choudhury et al., 2011), LACK may play a role coordinating environmental cues with amastigote development and/or replication. Based on these collective findings, we speculated that threshold levels of LACK promote the expression of one or more virulence-associated proteins in the mammalian host. Expression of such proteins may enhance parasite fitness during Leishmania entry, differentiation and replication in the hostile mammalian environment.
To identify putative LACK-dependent fitness proteins, we used two-dimensional difference gel electrophoresis (2D-DIGE) proteomics and mass spectrometry to compare the proteomes of virulent LACK/LACK with attenuated LACK/− parasites that were exposed to mammalian temperature. This approach identified cytochrome c oxidase subunit IV (termed LmCOX4) as the major resolvable species that was down-modulated under these conditions in LACK/− L. major. LmCOX4 is a component of cytochrome c oxidase, an enzyme complex located in the inner mitochondrial membrane of eukaryotic cells. This complex, made up of three mitochondrial-encoded and at least 11 nuclear-encoded subunits, is a key terminal constituent of eukaryote electron transport chains required to generate a mitochondrial membrane potential for adenosine triphosphate (ATP) synthesis (Horvath et al., 2005; Dey et al., 2010).
Immunoblotting was used to confirm that LmCOX4 levels are significantly decreased in LACK/−promastigotes exposed to mammalian temperature and, importantly, in LACK/− lesion-derived amastigotes obtained after high-dose infection of BALB/c mice. Consistent with these observations, mitochondrial functions, including cytochrome c oxidase activity and ATP levels, are also decreased in LACK/− parasites incubated at mammalian temperature. Significantly, exogenous expression of LmCOX4 in the LACK/− line restored the ability to withstand mammalian temperature and parasitize macrophages. Interestingly, exogenous expression of LmCOX4 also enhanced LmCOX6 levels under heat stress. These data suggest that upon Leishmania entry into the mammalian host, threshold levels of LACK, expressed from two LACK gene copies, are required to sustain LmCOX4 expression for efficient mitochondrial function and virulence.
Results
Identification of proteins down-modulated in LACK-deficient L. major under mammalian conditions
In previous studies we determined that LACK associates with actively translating ribosomes and, compared with the virulent control, the attenuated LACK/− line was highly sensitive to the translation initiation inhibitor hippuristanol (Choudhury et al., 2011). These findings indicate a function for LACK in Leishmania protein synthesis.
The expression of many cellular proteins can also be inhibited via activation of stress response pathways by stimuli including elevated temperature (Wek and Anthony, 2006; Wek et al., 2006; Wek and Cavener, 2007; Xu et al., 2011). Such pathways are present in all known eukaryotes including Leishmania (Gosline et al., 2011). Intriguingly, LACK/− L. major also show elevated sensitivity to mammalian temperatures (Kelly et al., 2003; Choudhury et al., 2011), thus providing a potential link between LACK’s protein synthesis functions and parasite viability under heat stress conditions. Based on these collective findings, we speculated that at least two LACK copies are required for the expression of key fitness proteins under the translationally restrictive temperature conditions imposed upon Leishmania by its mammalian host.
To identify proteins whose levels under heat stress depend upon at least two LACK copies, we used 2D-DIGE to compare proteomes of LACK/LACK and LACK/− L. major incubated at 35°C. Unexpectedly, despite their contrasting virulence phenotypes, few major differences in protein profiles between LACK/−and LACK/LACKL. major were repeatedly detected under these conditions. Indeed, in four separate 35°C proteomics replicates, while over 2000 protein spots were detected (Table S1), we consistently identified only one resolvable protein, LmCOX4 (see Table 1 and Fig. S2 for LmCOX4 peptides identified by mass spectrometry), as being down-modulated 1.42-fold (P< 0.001) in LACK/− L. major compared with LACK/LACKL. major (Fig. 1, Table 1). For all experiments, parasites were enumerated using a hemocytometer and, where appropriate, protein concentration was determined by bicinchoninic acid (BCA) assay. At 35°C, the percentage of viable cells within each culture, as evaluated by fluorescein diacetate (FDA) staining, was comparable (Fig. S1). For all experiments, statistical significance was determined by the two-tailed Student’s t-test.
Table 1.
The DeCyder BVA threshold ratio and t-test P-values for LmCOX4 (see also Fig. 1E).
| Accession number | Protein name | LACK/LACK: LACK/−ratio | P-value* | Unique peptide matches |
|---|---|---|---|---|
| LmjF12.0670 | Cytochrome c oxidase subunit IV (LmCOX4) | 1.42 | 0.00036 | 6 |
The number of unique peptides from mass spectrometric interrogation are listed.
P < 0.05
Fig. 1.
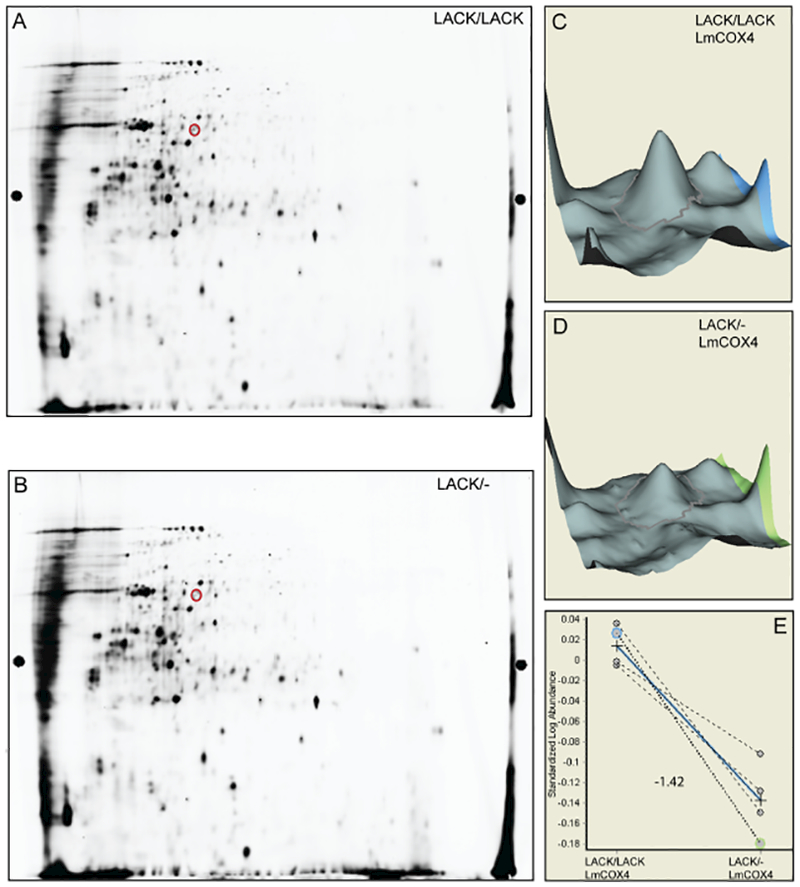
Cytochrome c oxidase subunit LmCOX4 is decreased in LACK/− L. major at 35°C.
A and B. 2D-DIGE gels of LACK/LACK and LACK/− L. major lysates, respectively, obtained from parasites cultured at 35°C for 4 days (representative of four separate replicates). Red circles denote LmCOX4 as determined by mass spectrometry.
C and D. 3-dimensional representation of the volume of the LmCOX4 spots from parts A and B, respectively, using the DeCyder Biological Variation Analysis (BVA) module. </p/>E. Differences in the LmCOX4 spot abundance, across four independent experiments, between LACK/LACK and LACK/− parasites. The blue line indicates the average difference in abundance, and the number indicates the fold change in abundance of LACK/− to LACK/LACK.
LmCOX4 is down-modulated in LACK/− L. major incubated at mammalian temperature and in amastigotes
To confirm our proteomic findings, we performed immunoblot analyses of the LACK/LACK, LACK/− and LACK/LACK-Addback lines using antisera against T. brucei cytochrome c oxidase subunit IV (COIV). We also used antisera against T brucei cytochrome c oxidase subunit VI (COVI) to assess whether or not any effects of LACK deficiency were specific to LmCOX4, or whether it also impacted other COX subunits. Fig. 2A shows a representative immunoblot. To determine relative expression of these proteins normalized to α-tubulin, between three and four immunoblots were quantified using ImageJ (Abramoff et al., 2004), as indicated in Fig. 2B and C. LACK/− L. major displayed moderately decreased LmCOX4 levels at27°C relative to LACK/LACK L. major (Fig. 2A and B). At 35°C, there was a pronounced decrease in LmCOX4 levels in LACK/− versus LACK/LACK parasites (Fig. 2A and B). Interestingly, the COVI antisera detected levels of LmCOX6 that were comparable with LmCOX4 in LACK/− L. major and LACK/LACK L. major at 27°C and 35°C (Fig. 2A and C). The LACK/LACK-Addback line in these experiments expressed LmCOX4 and LmCOX6 at levels comparable with the LACK/LACK line. Importantly, in lesion amastigotes, LmCOX4 was also dramatically decreased in LACK/−compared with LACK/LACKL. major (Fig. 2A and B). Unexpectedly, unlike LmCOX4, the abundance of LmCOX6 in lesion amastigotes was not decreased in LACK/−compared with LACK/LACKL. major (Fig. 2A and C).
Fig. 2.
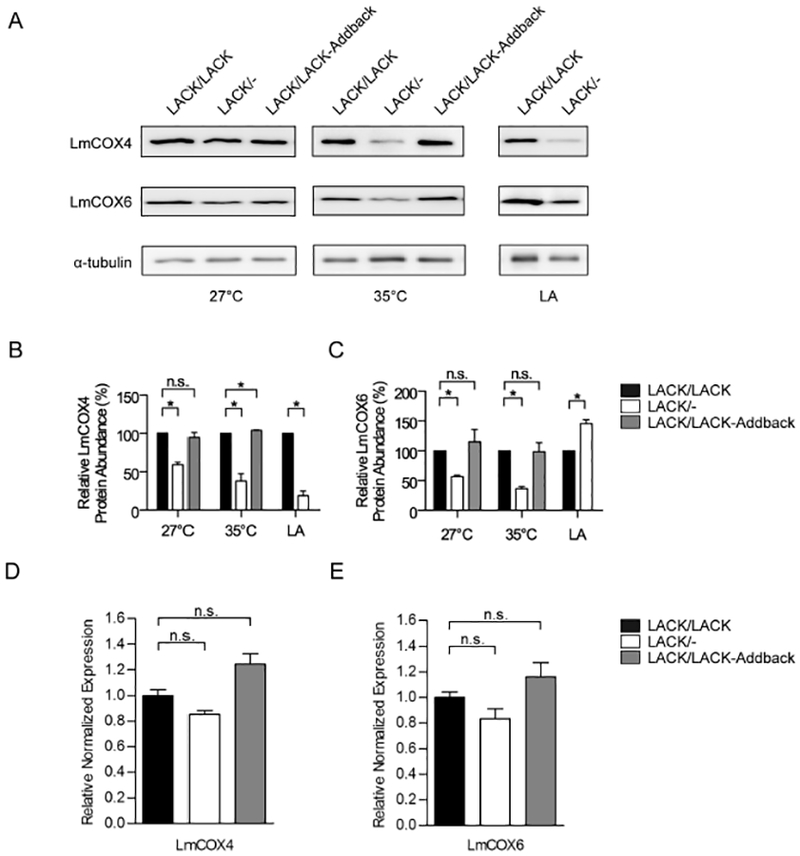
LmCOX4 is decreased in LACK/− L. major incubated at mammalian temperature and in amastigotes.
A. Immunoblot analysis of lysates from L. major lines, as indicated. Left and middle panels: lysates obtained from parasites incubated for 4 days at 27°C or 35°C as previously described (Choudhury et al., 2011). Right panel: L. major lesion-derived amastigotes (LA), as indicated, were purified from the footpads of infected BALB/c mice. The immunoblots were probed with antisera raised against trypanosomatid COIV (LmCOX4), trypanosomatid COVI (LmCOX6) and α-tubulin as denoted.
B and C. Quantification of LmCOX4 and LmCOX6 band intensities, respectively, from LACK/LACK, LACK/− and LACK/LACK-Addback lines at different conditions, as denoted in the figure. Averaged band intensities from three to four immunoblots were analyzed using ImageJ, normalized to α-tubulin and displayed as a percentage of the intensity of its corresponding band in LACK/LACK L. major. Data are displayed as a mean; error bars represent standard error of the mean (SEM) [n = 3 for LmCOX4 (Fig. 2B) and n = 4 for LmCOX6 (Fig. 2C)].
D and E. Reverse transcriptase quantitative PCR analysis of LmCOX4 and LmCOX6 expression at 35°C respectively. Relative expression was normalized to α-tubulin. Data are displayed as a mean; error bars represent SEM (n = 3). *P< 0.05. Two independently derived clones of each parasite line were used in these experiments.
To assess whether these changes in protein abundance correspond to alterations in mRNA levels, we performed reverse transcriptase quantitative PCR analyses of total RNA isolated from LACK/LACK, LACK/− or LACK/LACK- Addback lines incubated at 35°C. As shown in Fig. 2D and E, both LmCOX4 and LmCOX6 mRNAs were detected at comparable levels among all three parasite lines. These data indicate that the differential abundance of LmCOX4 and LmCOX6 observed at 35°C in LACK/− versus control lines occurs via a posttranscriptional mechanism.
Cytochrome c oxidase activity and oxygen consumption are decreased in LACK/− L. major at mammalian temperature
COX4 and COX6 are subunits of the cytochrome c oxidase enzyme complex (COX). This enzyme constitutes complex IV of the electron transport chain located in the inner mitochondrial membrane of eukaryote mitochondria. As the terminal complex in the electron transport chain, COX is critical for mitochondrial energy generation.
Having established that LmCOX4 protein levels are decreased in LACK/− L. major under mammalian conditions (Fig. 2), we then sought to determine the biochemical consequences of this depletion by comparing LmCOX activity in mitochondrial extracts of LACK/− with control strains that had been incubated at 27°C or 35°C. Isolation of mitochondria and LmCOX activity assays were performed as previously described (Horvath et al., 2005). As indicated in Fig. 3A, mitochondrial extracts isolated from 27°C cultures showed no significant LmCOX activity differences among all three lines, with activities of approximately 5 mU mg−1 of protein. In contrast, when cultured at 35°C, LACK/− L. major showed a threefold decrease in cytochrome c oxidase activity compared with LACK/LACK and LACK/LACK-Addback lines, with activities of 1.2, 0.4 and 1.2mUmg−1 of mitochondrial protein for the LACK/LACK, LACK/− and LACK/LACK- Addback lines respectively.
Fig. 3.
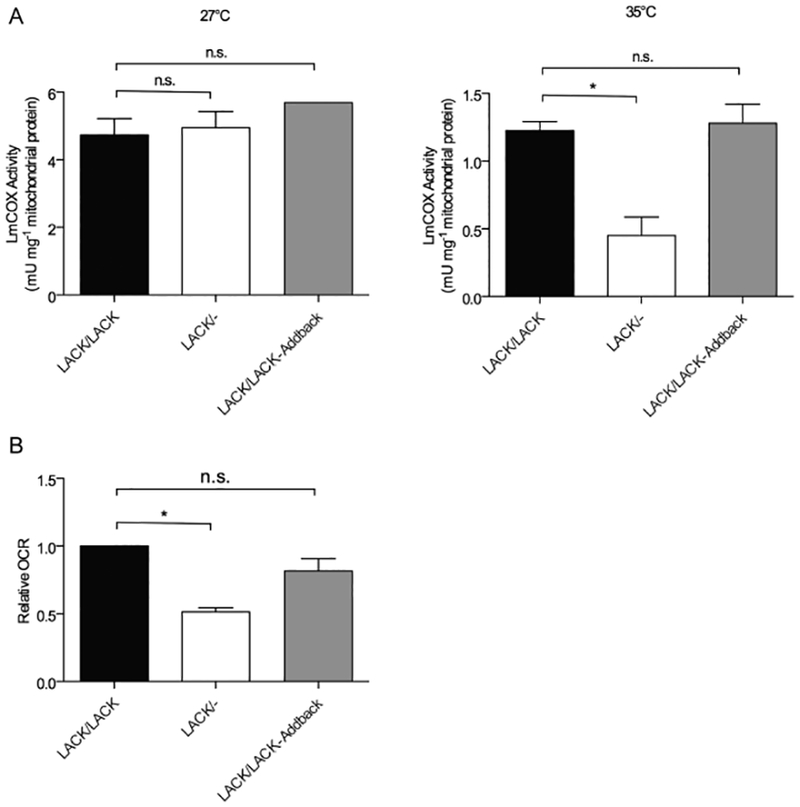
Cytochrome c oxidase activity and oxygen consumption are decreased in LACK/− L. major at mammalian temperature.
A. Mitochondrial preparations from the indicated parasite lines cultured for 4 days at either 27°C (left panel) or 35°C (right panel) and were assayed for cytochrome c oxidase activity as described in Experimental procedures.
B. The indicated lines of L. major were incubated at 35°C for 4 days and analyzed for oxygen consumption rate relative to LACK/LACK L. major as detailed in Experimental procedures. Data are displayed as a mean; error bars represent SEM (n = 3). *P < 0.05. Two independently derived clones of each parasite line were used in these experiments.
As cytochrome c oxidase activity is a major consumer of oxygen, we further confirmed our findings in Fig. 3A by monitoring oxygen consumption at 35°C using an oxygen-quenched fluorescent reagent assay. Briefly, whole cells were incubated with MitoXpress probe (Luxcel Biosciences, Cork, Ireland) in a microtiter plate and fluorescence was monitored. As the cells consume oxygen, MitoXpress becomes unquenched and the fluorescence intensity increases. As indicated in Fig. 3B, oxygen consumption at 35°C was significantly decreased in the LACK/− line, which showed approximately 50% of the oxygen consumption of the control LACK/LACK line. Conversely, the LACK/LACK-Addback line showed approximately 80% of the control.
Mitochondrial membrane potential and ATP production are impaired in LACK/− L. major at mammalian temperature
As a critical component of the electron transport chain, cytochrome c oxidase activity drives extrusion of H+ ions across this enzyme complex located in the inner mitochondrial membrane, thus establishing a mitochondrial membrane potential (ΔΨm) for the generation of ATP. Based on our findings in Fig. 3A, we predicted that the impaired cytochrome c oxidase activity observed in LACK/− L. major would result in a decreased ΔΨm.
To measure mitochondrial membrane potential, we used an assay based on the fluorescent dye JC-1. The JC-1 dye is driven into the mitochondria in a membrane potential-dependent manner. A high ΔΨm causes the dye to aggregate in the mitochondria where it fluoresces red, whereas mitochondrial depolarization causes JC-1 to remain outside and fluoresce green. We found that, compared with LACK/LACK and LACK/LACK-Addback, the ΔΨm of LACK/− L. major was approximately 50% decreased at 35°C, with no differences observed at 27°C (Fig. 4A).
Fig. 4.
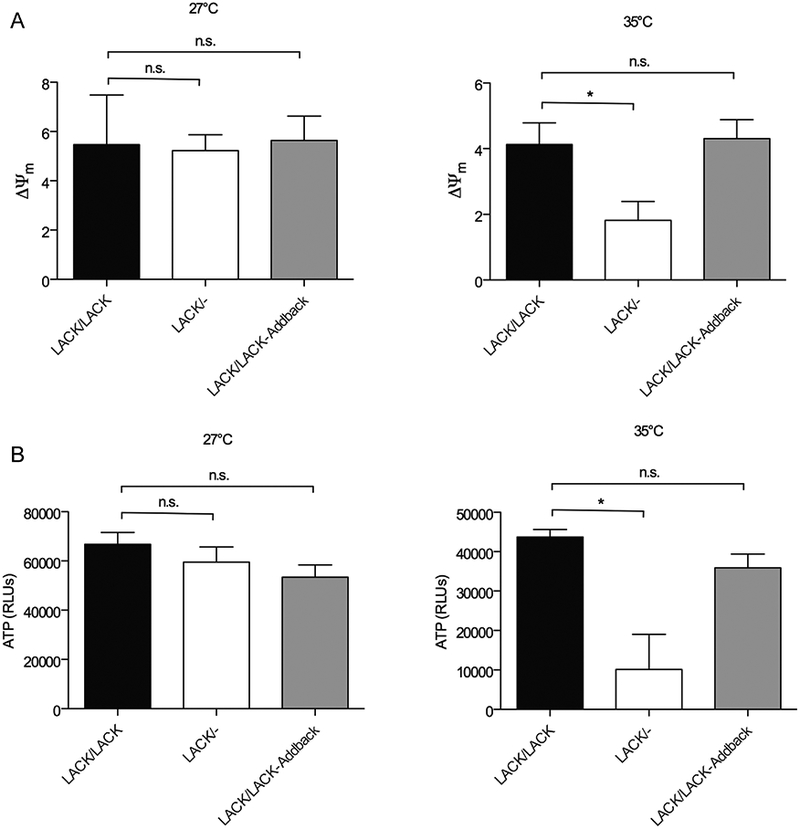
Mitochondrial membrane potential and ATP production are impaired in LACK/− L. major at mammalian temperature.
A. L. major lines, as indicated, were incubated at either 27°C (left panel) or 35°C (right panel) for 4 days, then analyzed for mitochondrial membrane potential (ΔΨm) as described in Experimental procedures. Data are displayed as a mean; error bars represent SEM (n = 2) for the left panel and (n = 5) for the right panel.
B. ATP content was determined as described in Experimental procedures for L. major lines, as denoted in the figure, at either 27°C (left panel) or 35°C (right panel). Data are displayed as a mean; error bars represent SEM (n = 4) for the left panel and (n = 3) for the right panel. *P < 0.05. Two independently derived clones of each parasite line were used in these experiments.
Next, we sought to determine how these phenotypes impact overall parasite energy production. We therefore compared ATP content, using a luciferase-based luminescent assay, in these lines at 27°C and 35°C. As shown in Fig. 4B, although comparable levels ofATP were observed among all three lines at 27°C, at 35°C they were decreased in LACK/− L. major to approximately 25% of LACK/LACK and LACK/LACK-Addback lines. These results indicate a significant loss of energy production under these conditions, consistent with the decreased LmCOX activity phenotype we found associated with the depletion of LmCOX4 observed in LACK/− L. major.
Exogenous expression of LmCOX4 in LACK/− L. major promotes thermotolerance and intracellular parasitization
The LACK/− phenotypes we have identified could be a direct consequence of the LmCOX4 depletion we observed in this line, or alternatively, the perturbation of other LACK-dependent proteins that we could not unambiguously detect by proteomics. To discriminate between these two possibilities, we therefore tested whether exogenous expression of LmCOX4 in LACK-deficient parasites restored any aspects of the virulent LACK/LACK phenotype.
To generate LACK/− parasites expressing exogenous LmCOX4, we transfected LACK/− L. major using the expression plasmid pXG-LmCOX4. As shown in Fig. 5A, we demonstrated that, compared with LACK/LACK and LACK/− L. major transfected with pXG-GFP, as a control, the LACK/− +pXG-LmCOX4 line showed elevated levels of LmCOX4 at 35°C. Using ImageJ software (Abramoff et al., 2004), band intensities were quantified, normalized to α-tubulin and averaged from four immunoblots (Fig. 5B). These data show that LACK/− +pXG-LmCOX4 transfectants express elevated levels of LmCOX4 compared with the LACK/LACK +pXG-GFP and LACK/− +pXG-GFP lines (Fig. 5B). Furthermore, compared with the LACK/− +pXG-GFP control, LACK/− +pXG-LmCOX4 showed a moderately higher cell density at 35°C, as indicated by FDA fluorescence (Fig. 5C). These findings indicate overexpression of LmCOX4 partially compensates for the thermotolerance defect of the LACK/− line.
Fig. 5.
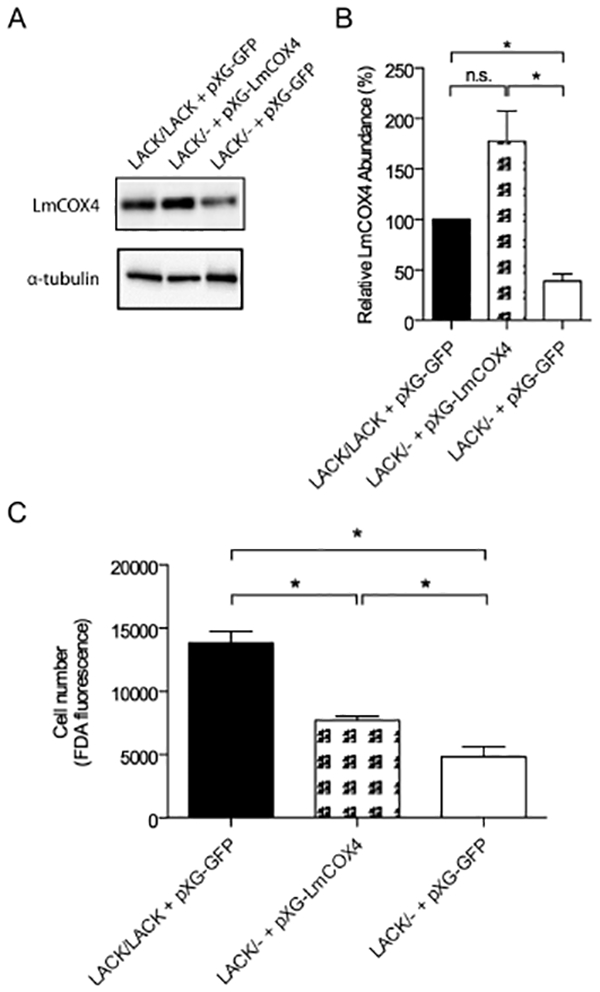
Exogenous expression of LmCOX4 enhances thermotolerance in LACK/− L. major.
A. Immunoblot analysis of LACK/− L. major transfected with expression plasmid pXG-LmCOX4 or LACK/LACK and LACK/controls transfected with pXG expressing green fluorescent protein (pXG-GFP), as indicated. Parasites were cultured at 35°C.
B. Quantification of LmCOX4 abundance at 35°C, in the parasite lines indicated. Band intensities were normalized to α-tubulin and displayed relative to the levels observed in LACK/LACK L. major. Data are displayed as a mean; error bars represent SEM (n = 4).
C. Cell density of L. major transfectants shown in Fig. 5A incubated at 35°C for 4 days, determined by fluorescein diacetate (FDA) esterase assay, as described in Experimental procedures. Data are displayed as a mean; error bars represent SEM (n = 2). *P < 0.05. Parasite lines used for these experiments were derived from a pool of uncloned transfectants.
Previously, we demonstrated that the attenuated LACK/− L. major line showed decreased parasite burden in macrophages (Kelly et al., 2003). We therefore determined whether overexpression of LmCOX4 would overcome the intracellular parasitization defect of this parasite line. We therefore infected macrophages by co-incubating them with parasites at a parasite to macrophage ratio of 10:1 for 4 h. After washing, the infections were allowed to proceed for 16 or 96 h. The macrophages were stained with Hoechst 33342 (Life Technologies, Carlsbad, CA, USA), and intracellular parasite burden was determined by immunofluorescence microscopy. As shown in Fig. 6A and B, after 16 h of infection, parasite burdens were comparable among all parasite lines. In contrast, after 96 h of infection, the LACK/− +pXG-GFP infection was significantly diminished, with a parasite burden of approximately 20% of the LACK/LACK +pXG-GFPcontrol (Fig. 6A and B, right panels). These findings are consistent with the intracellular infectivity defects observed previously in this LACK-deficient line (Kelly et al., 2003). Conversely, infection with the LACK/− +pXG-LmCOX4 line showed an elevated parasite burden at 96 h similar to that of LACK/LACK +pXG-GFP(Fig. 6A and B). These data indicate that overexpression of LmCOX4 overcomes the intracellular virulence defect of LACK/− L. major.
Fig. 6.
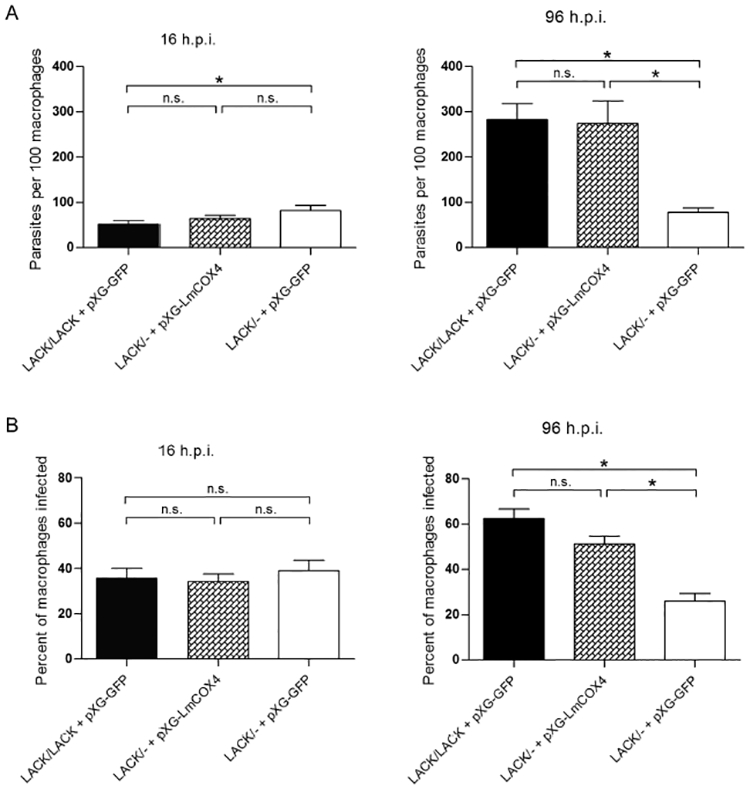
Exogenous expression of LmCOX4 in LACK/− L. major promotes virulence in macrophages. The L. major lines described in Fig. 5 were used in macrophage infection assays of parasite intracellular burden as indicated and detailed in Experimental procedures.
A. Intracellular parasites per 100 macrophages were enumerated at 16 (left panel) and 96 (right panel) hours postinfection.
B. The percent of infected macrophages was measured at 16 (left panel) and 96 (right panel) hours postinfection. For each experiment, at least 300 macrophages in > 20 fields were analyzed. Data are displayed as a mean; error bars represent SEM (n = 2). *P< 0.05. Parasite lines used for these experiments were derived from a pool of uncloned transfectants.
Exogenous expression of LmCOX4 promotes expression of LmCOX6 in LACK/− L. major at mammalian temperature
As we demonstrated in Fig. 2A, although both LmCOX4 and LmCOX6 are decreased in LACK/− L. major promastigotes grown at 35°C, only LmCOX4 is decreased in lesion amastigotes. Furthermore, exogenous expression of LmCOX4 alone is sufficient to substantially rescue the virulence defects of LACK/− L. major. Although these findings indicate the importance of LmCOX4 in virulence, they do not exclude an important function for LmCOX6, especially because these proteins are both subunits of the same functional complex. Indeed, the abundance of one subunit may impact the other under certain conditions. To test this possibility, we determined whether levels of LmCOX6 were enhanced by exogenous expression of LmCOX4 in LACK/− L. major under mammalian conditions. As shown in Fig. 7, compared with LACK/− +pXG-GFP and also LACK/LACK +pXG- GFP L. major, LmCOX6 levels were enhanced in the LACK/− +pXG-LmCOX4 line at 35°C. These findings indicate that, under such conditions, LmCOX6 levels are influenced by LmCOX4.
Fig. 7.
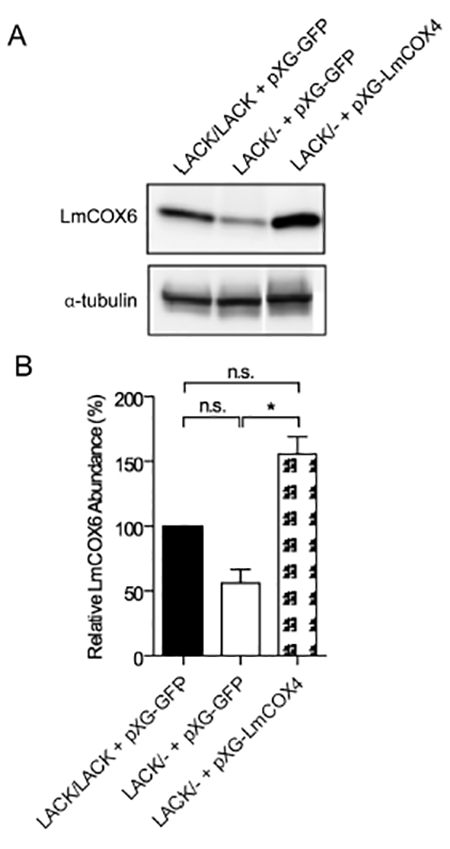
Exogenous expression of LmCOX4 enhances LmCOX6 expression in LACK/− L. major at mammalian temperature.
A. Immunoblot analysis of lysates from L. major lines, as indicated. Lysates were obtained from parasites incubated for 4 days at 35°C. The blot was probed with antisera raised against T. brucei COVI (LmCOX6) and α-tubulin as denoted.
B. Quantification of LmCOX6 abundance at 35°C in the lines indicated; normalized to α-tubulin and relative to the levels observed in LACK/LACK L. major. Data are displayed as a mean; error bars represent SEM (n = 3). *P < 0.05. Parasite lines used for these experiments were derived from a pool of uncloned transfectants.
Discussion
We previously determined that L. major requires a minimum of two LACK copies for robust virulence in vivo and thermotolerance in vitro (Kelly et al., 2003; Choudhury et al., 2011). To investigate molecular mechanisms underlying this threshold requirement for LACK in these pathogenic processes, we sought to identify proteins whose abundance under mammalian temperature conditions depends upon two LACK copies. Such proteins may represent parasite virulence or fitness factors critical for parasitism of the mammalian host. We therefore compared the proteomes of virulent LACK/LACK and attenuated LACK/− L. major, incubated at 35°C. This temperature represents a typical mammalian skin temperature and has previously been used for mammalian heat stress and differentiation studies in axenically cultured Leishmania and in macrophage infections (Zilberstein and Shapira, 1994; Kelly et al., 2003; Naderer et al., 2011).
LmCOX4 and LmCOX6 are nuclear-encoded subunits of the Leishmania mitochondrial cytochrome c oxidase complex. The activity of this enzyme is critical for efficient electron transport in the mitochondrion to generate a membrane potential for ATP synthesis. Significantly, although these COX subunits are conserved among trypanosomatids (Fig. S3), they are extremely divergent compared with their mammalian counterparts (Maslov et al., 2002; Horvath et al., 2005; Zikova et al., 2008; Dey et al., 2010); this makes them potentially attractive as candidates for therapeutic targeting.
In agreement with our results, depletion of COX subunits has previously been shown to significantly impair COX activity and mitochondrial function in trypanosomatids. Indeed, RNAi knockdown of COVI in T brucei substantially abrogated parasite oxygen consumption rate and replication (Horvath et al., 2005). Similarly in L. donovani, deletion of the novel COX subunit, p27, attenuated COX activity, ATP production and virulence (Dey et al., 2010).
Consistent with our finding that exogenous expression of LmCOX4 also rescued LmCOX6 abundance in LACK/− L. major at 35°C, studies of mammalian COX indicate that targeted loss of one COX subunit leads to depletion of other COX subunits (Galati et al., 2009). In T brucei, substantial depletion of the COX-associated mitochondrial protein X (MIX) impairs COX activity and moderately abrogates COIV and COVI levels (Zikova et al., 2008). Conversely, depletion of COVI itself does not appear to impact COIV levels in T. brucei (Horvath et al., 2005). Furthermore, in L. donovani, deletion of the p27 COX subunit had little effect upon COX4 or COX6 levels (Dey et al., 2010). Collectively, these data indicate substantial variability of subunit co-dependence for COX and its associated proteins, both within and between different eukaryote species. These conclusions are further corroborated by our findings that, in LACK-deficient L. major promastigotes, LmCOX4 and LmCOX6 show similar expression patterns at 35°C, yet are distinct in lesion amastigotes.
Although our observations suggest that, under mammalian conditions, LACK supports Leishmania virulence by sustaining LmCOX4 levels, it is also possible that other LmCOX subunits are dependent upon LACK. Interestingly, our data suggest LmCOX6 expression may be influenced more by LmCOX4 levels rather than directly by LACK. However, it remains unknown whether LACK modulates LmCOX4 expression via a direct mechanism.
Regardless of specific relationships that may exist between LACK, LmCOX4 and LmCOX6, our findings indicate that a critical pathogenic function of LACK stems from its ability to maintain LmCOX4 expression following entry into the mammalian host. By obtaining data from both promastigote and amastigote forms of LACK-deficient L. major, we propose that mammalian temperature is the principal stimulus that raises the threshold requirement for LACK in LmCOX4 expression. Thus, dependence of LmCOX4 expression upon two LACK copies likely begins following promastigote entry into the mammalian host, then persists after amastigote differentiation is completed. Intriguingly, LmCOX6 expression appears to be linked to LmCOX4 expression in LACK/− L. major at 35°C, yet unexpectedly, not in lesion amastigotes. This may reflect a transient requirement for LmCOX4 in LmCOX6 expression during, but not after, amastigote differentiation. Heat stress encountered by promastigotes is especially relevant to the Leishmania infectious life cycle because this parameter is considered to be the initiating signal for amastigote differentiation (Zilberstein and Shapira, 1994; Naderer et al., 2011). Importantly, associations between disrupted COX subunits and temperature sensitivity have also been described in other eukaryotes (Eccleshall et al., 1978; Nargang and Bertrand, 1978; Lightowlers et al., 1991; Liu et al., 2007; McConville and Naderer, 2011). Unexpectedly, although overexpression of LmCOX4 overcomes the virulence defect of LACK/− L. major in infected macrophages, it only partially overcomes their thermosensitivity. This may be because the elevated expression levels of LmCOX4 from pXG are suboptimal for thermotolerance, or that additional LmCOX4-independent, LACK-dependent pathways may contribute to this trait.
Energy metabolism in Leishmania plays a central role in pathogenesis. A dramatic metabolic shift accompanies differentiation of the insect procyclic promastigotes to the vertebrate intracellular amastigote forms (McConville and Naderer, 2011). Rapidly growing promastigotes are heavily dependent on glycolysis for energy generation. In contrast, slower-growing intracellular amastigotes rely more on the tricarboxylic acid cycle and oxidative phosphorylation to generate energy. Our studies indicate that LACK/− parasites do not display a substantial difference compared with virulent controls during promastigote growth at 27°C. However, they are hampered in their growth and survival under heat stress and within macrophages. Empirically, our observations indicate that the functions of LACK that permit this crucial shift in energy metabolism represent a novel therapeutic target. Our conclusion that RACK1 proteins have important functions in energy metabolism coincides with a recent proteomic survey in Saccharomyces cerevisiae predicting that RACK1 facilitates expression of proteins involved in both aerobic respiration and fermentation (Rachfall et al., 2013). Our results demonstrate for the first time in any eukaryote system that a RACK1 ortholog can promote mitochondrial function by sustaining cytochrome c oxidase activity.
Compared with other studies of RACK1 biology, our findings are unique in two respects. First, we show that expression of COX subunits, COX enzymatic function and ATP synthesis can be dependent upon threshold levels of a RACK1 protein. Indeed, as far as we are aware, our data represent the most definitive evidence to date suggesting that RACK1 proteins can impact the energy balance in a eukaryote cell. Second, this relationship is only manifest under a specific set of environmental conditions and cellular differentiation state.
Maintenance of LmCOX4 levels by LACK in the mammalian host may reflect adaptations of a digenetic protozoan pathogen to the peculiar stresses imposed by its parasitic lifestyle, transitioning between sandfly and mammalian hosts. Importantly, these adaptations may not be shared with mammals, where a constant body temperature around 33–37°C maintains an environment to support mammalian cell physiology. Such contextual differences between mammalian and trypanosomatid cells, and the distinct environmental demands imposed upon their molecular machinery, have important implications for the development of anti-parasitic therapies and hence warrant further investigation.
Experimental procedures
Ethics statement
All animal infections were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The infection protocol was approved by the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee (IACUC), IACUC protocol number 2584. All infections were performed under isoflurane anesthesia, and efforts were made to mitigate discomfort. Macrophages were isolated from previously euthanized mice kindly provided by Dr. Matthew Whim [Department of Cell Biology and Anatomy, Louisiana State University Health Sciences Center (LSUHSC)] according to a protocol approved by the LSUHSC IACUC, IACUC protocol number 3134.
Parasites
All L. major lines used were derived from WT L. major strain WHOM/IR/-/173 and cultured at 27°C or 35°C in medium 199 (M199), 10% heat-inactivated fetal bovine serum (FBS) as previously described (Kelly et al., 2003). Where appropriate, parasites were evaluated for viability by staining with FDA (Sigma-Aldrich Co., St. Louis, MO, USA) as previously described (Sacks and Melby, 2001) or by enumeration using a hemocytometer with a Nikon Eclipse Ti fluorescence microscope (Nikon Instruments Inc, Melville, NY, USA) equipped with an EXFO X-Cite UV light source (EXFO Photonics Solutions Inc., Ontario, Canada). To obtain LACK/LACK lesion amastigotes, female BALB/c mice (The Jackson Laboratory, Bar Harbor, ME, USA) were inoculated subcutaneously in the left hind footpad with 4 × 106 stationary phase LACK/LACK promastigotes, prepared as previously described (Kelly et al., 2003). To isolate sufficient numbers of LACK/− lesion amastigotes, female BALB/c mice were inoculated with 8 × 107 stationary phase promastigotes. Amastigotes were harvested from infected mouse footpads as follows: footpad cell suspensions were prepared in Dulbecco’s Modified Eagle Medium (DMEM) with 10% FBS (eight footpads per 25 ml medium). The suspensions were centrifuged at 100 × g for 5 min. The supernatant was retained, and saponin (final concentration: 0.05%) was added. After a 5 min incubation at room temperature, the sample was centrifuged at 2000 × g for 10 min. The resulting pellet was washed twice with fresh medium, lysed with 2× sample buffer then submitted for immunoblot analysis.
2D gel electrophoresis
2D-DIGE analysis was performed by the LSUHSC Proteomics Core facility. Briefly, virulent LACK/LACK and attenuated LACK/− L. major cultures were seeded into 50 ml volumes of M199 at a density of 2.5 × 106 parasites ml−1 and cultured for 4 days at 35°C. After harvesting, the cells were washed twice with PBS, lysed in IEF sample buffer (7.0 M urea, 2.0 M thiourea, 4.0% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 20% glycerol and 20 mM Tris, pH 8.5) and normalized for total protein using the Bradford Assay (BioRad, Hercules, CA, USA). Proteins were then labeled for quantitative 2D-DIGE analysis as previously described (Alban et al., 2003). Gels were scanned using a Typhoon 9400 (GE Healthcare: Life Sciences, Piscataway, NJ, USA). Spot detection and quantification were performed using DeCyder Differential In-gel Analysis software (GE Healthcare: Life Sciences), and individual gels were imported into DeCyder Biological Variation Analysis for t-test analysis. Spots chosen for mass spectrometric protein identification were processed using an Ettan Spot Handling Workstation (GE Healthcare: Life Sciences) and subjected to tryptic digestion. Peptide mass was determined using a LTQ-XL linear ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) coupled to a nanoLC (Eksigent, Redwood City, CA, USA). Peptide samples were loaded onto an Acclaim PepMap100 C18 trap column with dimensions of 300 μm (inside diameter) × 5 mm (Dionex, Sunnyvale, CA, USA) and were separated by a reversed-phase C18 PicoFrit emitter with dimensions of 75 μm (inside diameter) × 10 cm (bed length) with 15 μm tip size (part number PF7515–100-H002) (New Objective, Woburn, MA, USA). Peptides were loaded at 500 nl min−1 using a mobile phase of 2% acetonitrile and 0.1% formic acid and then eluted using a gradient of 5–40% acetonitrile and 0.1% formic acid over 35 min, with a ramp to 60% acetonitrile and 0.1% formic acid for 10 min, and finally a ramp to 95% acetonitrile and 0.1% formic acid for 10 min. A top-five data-dependent scan strategy was utilized. The MS1 scan range is between m/z 300 and 2000. The top five most abundant peptides in this MS1 scan were chosen for MS/MS. The MS/MS parameters were the defaults; in brief, the isolation window is set to 2 Da, 35% relative collision energy [collision-induced dissociation (CID)], dynamic exclusion enabled with repeat count set to 1, repeat duration of 30 s and an exclusion size of 100 with an exclusion duration of 75 s. Raw data were then analyzed by Proteome Discoverer 1.4 (Thermo, Waltham, MA, USA) using SEQUEST search against the Leishmania major (Friedlin strain) gene database (www.genedb.org/Homepage/Lmajor). Database search was performed for b and y ion series and allowed for up to one missed trypsin cleavages. Precursor Mass Tolerance was set to 1.2 Daltons and Fragment Mass Tolerance set to 0.8 Daltons. Possible modifications of a methionine oxidation and cysteine carbamidomethyl were considered. Xcorr values were used to assert high confidence versus modest confidence peptides. As Xcorr values are search dependent, high confidence peptides had an Xcorr value of greater than 1.71 for singly charged, greater than 2.44 for doubly charged, and greater than 2.6 for triply charged peptides.
Immunoblot analysis
Lysates from 2 × 107 parasites per lane were prepared using 2× Laemmli buffer and run on 12% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) gels and blotted onto PVDF membranes (BioRad) in accordance with manufacturer’s instructions. Following overnight blocking (4°C) in 5% milk powder in PBS with 0.05% Tween 20 (PBS-T), blots were incubated with 1:1000 rabbit anti-COIV (COX4), 1:1000 rabbit anti-COVI (COX6) or 1:2000 mouse anti-α-tubulin for 2 h at room temperature. After washing in PBS-T, blots were incubated with 1:3000 goat anti-rabbit Ig or 1:6000 goat anti-mouse Ig, conjugated with horseradish peroxidase for 1 h at room temperature. The blots were washed and developed using ECL chemiluminescence reagents (GE Healthcare: Life Sciences) according to the manufacturer’s instructions. Quantification of bands was performed using ImageJ software (Abramoff et al., 2004).
RNA isolation and reverse transcriptase quantitative PCR (RT-qPCR)
Total RNA was extracted from LACK/LACK, LACK/− and LACK/LACK-Addback lines cultured at 35°C for 4 days using a Direct-zol RNA isolation kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s directions. RNA samples were treated with TURBO DNase (Life Technologies, Carlsbad, CA, USA) following the manufacturer’s protocols. One microgram of each RNA sample was subsequently used to template cDNAsynthesis using TaqMan Reverse Transcription Reagents (Life Technologies) according to the manufacturer’s instructions. RT-qPCR was performed for each cDNA sample in triplicate using the iTaq Universal SYBR Green Supermix (BioRad) and primer sets for LmCOX4 (forward primer AACCTTAACACGGAGCTACAC and reverse primer GGAAGTCGTCGAAGTACACAAT), LmCOX6 (forward primer AACGGGATTCTGGCCTACTA and reverse primer CTCGACATACCAACGCATCTT) and α-tubulin (forward primer TTCAGTTCGTGGACTGGTG and reverse primer GAGTTGGCAATCATGCACAC). Each reaction mixture contained 10 μl of 2 × supermix, 2 μl of each 300 nM primer, 100 ng of template cDNA and water to a final volume of 20 μl. For each primer set, a reaction without template was run as a control. RT-qPCR was performed on a C1000 Touch Thermal Cycler with CFX96 Real-Time System (BioRad) with the following cycling conditions: 95°C for 5 min, followed by 40 cycles of two steps consisting of 95°C for 10 s and 57°C for 30 s. Melting curve analysis was performed to confirm the specificity of the amplicons. Amplification efficiency was determined for each primer set. Relative quantification of LmCOX4 and LmCOX6, normalized to α-tubulin, was attained using CFX Manager Software (BioRad).
Isolation of mitochondria and cytochrome c oxidase activity assay
Mitochondrial vesicles were isolated by hypotonic lysis as described previously (Horvath et al., 2005). Protein concentration was determined by BCA Protein assay (Pierce, Rockford, IL, USA). Cytochrome c oxidase activity was measured by adding 100 μg of mitochondrial protein to 1 ml of COX buffer (40 mM sodium phosphate buffer, pH 7.4; 0.5 mM ethylenediaminetetraacetic acid (EDTA); 20 μM horse heart cytochrome c; 30 μM sodium ascorbate; 0.005% w/v dodecyl maltoside) containing antimycin A at a final concentration of 300 ng ml−1 to inhibit cytochrome c reductase activity in a 1 ml cuvette. The change in absorbance at 550 nm was measured every 20 s for 10 min and used to determine LmCOX activity. A unit of LmCOX activity was defined as the amount of enzyme that catalyzes the oxidation of 1 μmol of ferrocytochrome c to ferricytochrome c per minute, assuming an extinction coefficient of 21.1 mM−1 cm−1 for LmCOX (Horvath et al., 2005).
Measurement of oxygen consumption
Relative oxygen consumption was measured using MitoXpress oxygen probe (Luxcel Biosciences, Cork, Ireland) according to manufacturer’s instructions. Briefly, 10 μl of 1 μM MitoXpress was added to 150 μl of parasites in a black 96-well microtiter plate. A layer (100 μl) of mineral oil was added over the cells to prevent diffusion of atmospheric oxygen. MitoXpress signal was measured at 90 s intervals for 120–200 min using excitation/emission wavelengths of 380 nm/650 nm. The rate of signal increase was used to determine relative oxygen consumption rates.
Measurement of mitochondrial membrane potential
Mitochondrial membrane potentials were determined by incubating live parasites at 27°C or 35°C, using the fluorescent probe JC-1 (Cayman Chemical, Ann Arbor, MI, USA), according to manufacturer’s instructions.
Analysis of ATP levels
Parasite ATP levels were determined by luminometer using the Cell Titer-Glo (Promega, Madison, WI, USA) in accordance with manufacturer’s instructions.
Exogenous expression of LmCOX4
The LmCOX4 open reading frame was PCR-amplified from WT L. major genomic DNA using forward primer 5’-CTCCCC GGGATGCTTACGCGTCGTGCCG-3’ [tagged with a SmaI restriction enzyme site (underlined)] and reverse primer 5’-CTCGGATCCTTACAATTTGCTCTCGTTCTTGG-3’ [tagged with a BamHI restriction enzyme site (underlined)] and cloned into pGEM-T Easy (Promega, Madison, WI, USA). The insert was then excised from pGEM-T Easy with SmaI and BamHI and cloned into pXG (Ha et al., 1996) cut with SmaI and BamHI. As previously described, 4 × 107 parasites were transfected with pXG-LmCOX4 by electroporation (Kelly et al., 2003). Transfectants were transferred to 10 ml M199 and incubated at 27°C for 1 day before being selected in liquid media with 20–200 μg ml−1 G418.
Macrophage infections
Peritoneal macrophages, isolated by peritoneal lavage from C57BL/6 mice, were incubated in RPMI 1640 with 5% FBS, 100 units ml−1 penicillin, 100 μg ml−1 streptomycin, 2 mM L-glutamine and 2 mM nonessential amino acids using four-chambered chamber slides [(Nalge Nunc International) Thermo Fisher Scientific, Waltham, MA, USA] at 2–3 × 105 cells per chamber for 24 h at 35°C in 5% CO2. After nonadherent cells were removed by washing with PBS, 2–3 × 106 stationary phase promastigotes were added to the macrophages followed by a 4 h incubation at 35°C in 5% CO2. The cells were washed three times with warm (37°C) PBS to remove free parasites, then 0.8 ml fresh media was added to each chamber and the infection allowed to proceed for 16 or 96 h. The slides were then washed three times in warm PBS and sequentially incubated in 4% paraformaldehyde for 20 min, 0.1% Triton X-100 for 4 min and 1:3000 diluted Hoechst 33342 in PBS for 2 min (slides were washed twice with PBS between each step). The slides were mounted using Fluoromount-G mounting media (Southern Biotech, Birmingham, AL, USA). Macrophages and parasites were detected using an Axio Observer.Z1 fluorescent microscope (Carl Zeiss AG, Oberkochen, Germany) with a 63× objective. The infection status of the macrophages was measured by enumerating intracellular parasites per at least 300 macrophages from over 20 random fields.
Statistical analysis
For all experiments, P values were determined by the twotailed Student’s f-test, using Prism software (GraphPad, La Jolla, CA, USA). A result of P< 0.05 was considered significant and denoted with an asterisk.
Supplementary Material
Acknowledgements
We thank Drs. Joy Sturtevant, Glen Palmer and Andy Catling (LSUHSC, New Orleans) for helpful suggestions throughout this work. We thank Drs. Joy Sturtevant, Lyndsey Buckner Baiamonte and Alison Quayle for help with microscopy. We also thank Dr. Matthew Whim for help with macrophage isolation. We thank Dr. Steve Beverley (Washington University, Saint Louis) for kindly providing the pXG plasmid vector. We thank The Louisiana State University Health Sciences Center Proteomics Core (supported in part by grants from the National Center for Research Resources (5 P20 RR018766-09) and the National Institute of General Medical Sciences (8 P20 GM103514–10) from the National Institutes of Health) for technical expertise with 2D-DIGE and mass spectrometry. We also thank Dr. Julius Lukes (University of South Bohemia, Czech Republic) for providing antisera against T. brucei cytochrome c oxidase subunits COIV and COVI, and Drs. Anton Horváth and Zdenek Verner (Comenius University, Bratislava, Slovakia) for helpful technical suggestions with the cytochrome c oxidase activity assay. This work was supported in part by the National Institutes of Health (NIH) (Grant AI055172 awarded to B.L.K. and A.A.) and the Louisiana Vaccine Center funded by the Louisiana Board of Regents.
Footnotes
The authors have no conflict of interest to declare.
References
- Abramoff MD, Magalhaes PJ, and Ram SJ (2004) Image processing with ImageJ. Biophot Int 11: 36–42. [Google Scholar]
- Adams DR, Ron D, and Kiely PA (2011) RACK1, a multifaceted scaffolding protein: structure and function. Cell Commun Signal 9: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alban A, David SO, Bjorkesten L, Andersson C, Sloge E, Lewis S, and Currie I (2003) A novel experimental design for comparative two-dimensional gel analysis: twodimensional gel electrophoresis incorporating a pooled internal standard. Proteomics 3: 36–44. [DOI] [PubMed] [Google Scholar]
- Alcolea PJ, Alonso A, Gomez MJ, Sanchez-Gorostiaga A, Moreno-Paz M, Gonzalez-Pastor E, et al. (2010) Temperature increase prevails over acidification in gene expression modulation of amastigote differentiation in Leishmania infantum. BMC Genomics 11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchmore RJ, and Barrett MP (2001) Life in vacuoles - nutrient acquisition by Leishmania amastigotes. Int J Parasitol 31: 1311–1320. [DOI] [PubMed] [Google Scholar]
- Campbell DA, Thomas S, and Sturm NR (2003) Transcription in kinetoplastid protozoa: why be normal? Microbes Infect 5: 1231–1240. [DOI] [PubMed] [Google Scholar]
- Choudhury K, Cardenas D, Pullikuth AK, Catling AD, Aiyar A, and Kelly BL (2011) Trypanosomatid RACK1 orthologs show functional differences associated with translation despite similar roles in Leishmania pathogenesis. PLoS ONE 6: e20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton C, and Shapira M (2007) Post-transcriptional regulation of gene expression in trypanosomes and leishmanias. Mol Biochem Parasitol 156: 93–101. [DOI] [PubMed] [Google Scholar]
- Cohen-Freue G, Holzer TR, Forney JD, and McMaster WR (2007) Global gene expression in Leishmania. Int J Parasitol 37: 1077–1086. [DOI] [PubMed] [Google Scholar]
- Coyle SM, Gilbert WV, and Doudna JA (2009) Direct link between RACK1 function and localization at the ribosome in vivo. Mol Cell Biol 29: 1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depledge DP, Evans KJ, Ivens AC, Aziz N, Maroof A, Kaye PM, and Smith DF (2009) Comparative expression profiling of Leishmania·. modulation in gene expression between species and in different host genetic backgrounds. PLoS Negl Trop Dis 3: e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey R, Meneses C, Salotra P, Kamhawi S, Nakhasi HL, and Duncan R (2010) Characterization of a Leishmania stage-specific mitochondrial membrane protein that enhances the activity of cytochrome c oxidase and its role in virulence. Mol Microbiol 77: 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleshall TR, Needleman RB, Storm EM, Buchferer B, and Marmur J (1978) A temperature-sensitive yeast mitochondrial mutant with altered cytochrome c oxidase subunit. Nature 273: 67–70. [DOI] [PubMed] [Google Scholar]
- Galati D, Srinivasan S, Raza H, Prabu SK, Hardy M, Chandran K, et al. (2009) Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex. implications in mitochondrial dysfunction and ROS production. Biochem J 420: 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosline SJ, Nascimento M, McCall LI, Zilberstein D, Thomas DY, Matlashewski G, and Hallett M (2011) Intracellular eukaryotic parasites have a distinct unfolded protein response. PLoS ONE 6: e19118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha DS, Schwarz JK, Turco SJ, and Beverley SM (1996) Use of the green fluorescent protein as a marker in transfected Leishmania. Mol Biochem Parasitol 77: 57–64. [DOI] [PubMed] [Google Scholar]
- Horvath A, Horakova E, Dunajcikova P, Verner Z, Pravdova E, Slapetova I, et al. (2005) Downregulation of the nuclear-encoded subunits of the complexes III and IV disrupts their respective complexes but not complex I in procyclic Trypanosoma brucei. Mol Microbiol 58: 116–130. [DOI] [PubMed] [Google Scholar]
- Hunter KW, Cook CL, and Hayunga EG (1984) Leishmanial differentiation in vitro. induction of heat shock proteins. Biochem Biophys Res Commun 125: 755–760. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Stetson DB, and Locksley RM (2003) Leishmania major LACK antigen is required for efficient vertebrate parasitization. J Exp Med 198: 1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahav T, Sivam D, Volpin H, Ronen M, Tsigankov P, Green A, et al. (2011) Multiple levels of gene regulation mediate differentiation of the intracellular pathogen Leishmania. FASEB J 25: 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightowlers R, Chrzanowska-Lightowlers Z, Marusich M, and Capaldi RA (1991) Subunit function in eukaryote cytochrome c oxidase. A mutation in the nuclear-coded subunit IV allows assembly but alters the function and stability of yeast cytochrome c oxidase. J Biol Chem 266: 7688–7693. [PubMed] [Google Scholar]
- Liu W, Gnanasambandam R, Benjamin J, Kaur G, Getman PB, Siegel AJ, et al. (2007) Mutations in cytochrome c oxidase subunit VIa cause neurodegeneration and motor dysfunction in Drosophila. Genetics 176: 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahill A, Warwicker J, Bolger GB, Houslay MD, and Yarwood SJ (2002) The RACK1 scaffold protein. a dynamic cog in cell response mechanisms. Mol Pharmacol 62: 1261–1273. [DOI] [PubMed] [Google Scholar]
- McConville MJ, and Naderer T (2011) Metabolic pathways required for the intracellular survival of Leishmania. Annu Rev Microbiol 65: 543–561. [DOI] [PubMed] [Google Scholar]
- McConville MJ, de Souza D, Saunders E, Likic VA, and Naderer T (2007) Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol 23: 368–375. [DOI] [PubMed] [Google Scholar]
- McNicoll F, Drummelsmith J, Muller M, Madore E,Boilard N, Ouellette M, and Papadopoulou B (2006) A combined proteomic and transcriptomic approach to the study of stage differentiation in Leishmania infantum. Proteomics 6: 3567–3581. [DOI] [PubMed] [Google Scholar]
- Maslov DA, Zikova A, Kyselova I, and Lukes J (2002) A putative novel nuclear-encoded subunit of the cytochrome c oxidase complex in trypanosomatids. Mol Biochem Parasitol 125: 113–125. [DOI] [PubMed] [Google Scholar]
- Melo RC, and Dvorak AM (2012) Lipid body-phagosome interaction in macrophages during infectious diseases: host defense or pathogen survival strategy? PLoS Pathog 8: e1002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naderer T, and McConville MJ (2008) The Leishmania- macrophage interaction: a metabolic perspective. Cell Microbiol 10: 301–308. [DOI] [PubMed] [Google Scholar]
- Naderer T, Dandash O, and McConville MJ (2011) Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Mol Microbiol 80: 471–480. [DOI] [PubMed] [Google Scholar]
- Nargang FE, and Bertrand H (1978) Nuclear mutants of Neurospora crassa temperature-sensitive for the synthesis of cytochrome aa3. I. Isolation and preliminary characterization. Mol Gen Genet 166: 15–23. [DOI] [PubMed] [Google Scholar]
- Nilsson J, Sengupta J, Frank J, and Nissen P (2004) Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep 5: 1137–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opperdoes FR, and Coombs GH (2007) Metabolism of Leishmania: proven and predicted. Trends Parasitol 23: 149–158. [DOI] [PubMed] [Google Scholar]
- Rachfall N, Schmitt K, Bandau S, Smolinski N, Ehrenreich A, Valerius O, and Braus GH (2013) RACK1/Asc1p, a ribosomal node in cellular signaling. Mol Cell Proteomics 12: 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi S, Rothberg KG, Hubbard JG, and Ruben L (2008) The RACK1 signal anchor protein from Trypanosoma brucei associates with eukaryotic elongation factor 1A: a role for translational control in cytokinesis. Mol Microbiol 70: 724–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Burdette DL, Pfannstiel J, Jetton N, Singh R, and Ruben L (2006) The RACK1 homologue from Trypanosoma brucei is required for the onset and progression of cytokinesis. J Biol Chem 281: 9781–9790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks DL, and Melby PC (2001) Animal Models for the Analysis of Immune Responses to Leishmaniasis. Current Protocols in Immunology. 28:19.2:19.2.1–19.2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EC, Ng WW, Kloehn J, Chambers JM, Ng M, and McConville MJ (2014) Induction of a stringent metabolic response in intracellular stages of Leishmania mexicana leads to increased dependence on mitochondrial metabolism. PLoS Pathog 10: e1003888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M, McEwen JG, and Jaffe CL (1988) Temperature effects on molecular processes which lead to stage differentiation in Leishmania. EMBO J 7: 2895–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M, Zilka A, Garlapati S, Dahan E, Dahan I, and Yavesky V (2001) Post transcriptional control of gene expression in Leishmania. Med Microbiol Immunol (Berl) 190: 23–26. [DOI] [PubMed] [Google Scholar]
- Thiel M, and Bruchhaus I (2001) Comparative proteome analysis of Leishmania donovani at different stages of transformation from promastigotes to amastigotes. Med Microbiol Immunol (Berl) 190: 33–36. [DOI] [PubMed] [Google Scholar]
- Tsigankov P, Gherardini PF, Helmer-Citterich M, and Zilberstein D (2012) What has proteomics taught us about Leishmania development? Parasitology 139: 1146–1157. [DOI] [PubMed] [Google Scholar]
- Wek RC, and Anthony TG (2006) EXtENDINg beta cell survival by UPRegulating ATF4 translation. Cell Metab 4: 333–334. [DOI] [PubMed] [Google Scholar]
- Wek RC, and Cavener DR (2007) Translational control and the unfolded protein response. Antioxid Redox Signal 9: 2357–2371. [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, and Anthony TG (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11. [DOI] [PubMed] [Google Scholar]
- Xu X, Gupta S, Hu W, McGrath BC, and Cavener DR (2011) Hyperthermia induces the ER stress pathway. PLoS ONE 6: e23740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikova A, Panigrahi AK, Uboldi AD, Dalley RA, Handman E, and Stuart K (2008) Structural and functional association of Trypanosoma brucei MIX protein with cytochrome c oxidase complex. Eukaryot Cell 7: 1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D, and Shapira M (1994) The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol 48: 449–470. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


