Abstract
Background
Major depressive disorder (MDD) is a chronic, life-threatening, highly disabling disease. Standardized treatment with fewer adverse effects, quick onset, and long-term maintenance of the effects of brief treatment for MDD is always being pursued. Long non-coding RNAs (lncRNAs) are highly expressed in the central nervous system and are involved in the occurrence and development of neurodegenerative and psychiatric diseases. This study aimed to investigate whether the overexpression and interference of 3 differentially down-regulated lncRNAs (NONHSAT142707, NONHSAG045500, and ENST00000517573) in MDD can affect the expression of central neurotransmitter serotonin (5-hydroxytryptamine) transporter (SERT) in vitro.
Material/Methods
First, we synthesized and validated the effect of 3 lncRNA plasmids and small interfering RNAs (siRNAs); next, we transfected the plasmids and siRNAs that caused significant overexpression or interference in SK-N-SH cells, and tested the expression of SERT by qRT-PCR.
Results
The results showed that 3 lncRNA plasmids and siRNAs2 caused overexpression and interference, respectively. Only the overexpression of NONHSAG045500 could significantly inhibit the expression of SERT; interference with NONHSAG045500 could significantly strengthen the expression of SERT.
Conclusions
This study indicated that the expression of SERT could be regulated by up-regulating or down-regulating NONHSAG045500 expression and suggested that NONHSAG045500 could potentially be established as a new therapeutic target of MDD. Future work may be needed to definitively determine the correlation between NONHSAG045500 and SERT in vivo.
MeSH Keywords: Depressive Disorder, Major; RNA, Long Noncoding; Serotonin Plasma Membrane Transport Proteins
Background
Major depressive disorder (MDD) is a severe, chronic, and life-threatening disease with a high incidence. MDD is now the leading cause of disability and lost productivity globally [1], and is predicted to be the second-leading cause of disease burden worldwide just after coronary heart disease by the year of 2030 [2]. Because antidepressant drug therapy is more easily available and lower in price than psychotherapy, antidepressant drug therapy is the most commonly used treatment in China, especially selective serotonin reuptake inhibitors (SSRIs). SSRIs act on the midbrain serotonin (5-hydroxytryptamine (5-HT)) system, which plays an important role in many brain functions, including mood control. 5-HT reuptake is mediated by 5-HT transporter (5-hydroxytryptamine transporter; 5-HTT; SERT). Chronic, but not acute, SSRI treatment down-regulates SERT, increasing forebrain serotonergic neurotransmission and neuronal plasticity in the hippocampus [3–5]. Thus, these drugs need to be administered for a long time before clinical improvement emerges, and they cause full remission of depressive symptoms in only one-third of patients and leave a large proportion of people with partial or incomplete clinical responses [6,7]. Therefore, the identification of new drug types to induce SERT down-regulation may be a new target for the development of fast-acting antidepressants.
The modern accepted pathogenesis of depression is that psychological and social factors as well as biological and genetic factors can cause dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis, inflammatory pathways [8], the extracellular signal-regulated kinase/CREB/BDNF pathway [9], autophagy [10], and apoptosis [11]. This evidence supports the hypothesis that risk factors, which disrupt neuronal function and morphology resulting in dysfunction of the neural circuitry underlying mood regulation and cognitive function through converging molecular and cellular mechanisms, are potential targets of antidepressants [12]. Recent studies have established that environmental events and behavioral experience induce epigenetic changes at specific gene loci, and these changes help shape neuronal plasticity, function, and behavior, hence contributing to the pathogenesis of depression [13]. Long non-coding RNAs (lncRNAs), one of the major types of epigenetic regulation that utilizes functionally untranslated RNA species, are non-coding RNAs with a length greater than 200 nucleotides. lncRNAs play versatile roles in many aspects of gene regulation, including mRNA splicing, transcription, epigenetic silencing, translation, X-chromosome inactivation, and the processing of small ncRNAs [14,15]. lncRNAs associated with cognitive disorders and synaptic plasticity may also contribute to the pathophysiology of major depression. For example, lncRNA non-protein-coding RNA repressor of NFAT (NRON), which interacts with multiple proteins including members of the importin-beta superfamily, could suppress nuclear factor of activated T cells (NFAT) signaling via regulating NFAT nuclear-cytoplasmic trafficking [16]; COX-2-lncRNA (PACER) was found to activate COX-2 expression through occluding repressive NF-κB complexes, and had a significant correlation with cognitive function in recurrent depressive disorder [17]; and BDNF-AS discovered in mice was shown to prevent BDNF transcription by recruiting EZH2, a key component of the epigenetic silencing complex, PRC2. Therefore, lncRNA regulation is considered to have therapeutic potential for use in MDD [18].
Our previous studies showed that 6 lncRNAs (TCONS_00019174, ENST00000566208, NONHSAG045500, ENST00000517573, NONHSAT034045, and NONHSAT142707) had diagnostic value, therapeutic evaluation, differential diagnosis, and participated in many functions in the central nervous system [19]. The expression of 6 lncRNAs was significantly correlated with suicide risk [20]. This study aimed to investigate whether 3 candidate differentially expressed lncRNAs (NONHSAT142707, NONHSAG045500, ENST00000517573) in MDD, which have much higher expression in the brain, can internalize SERT and reduce SERT-binding sites through mediating SERT mRNA levels in SK-N-SH cells. In addition, we aimed to determine the mechanism, which may lead to novel therapeutics.
Material and Methods
Cell culture and transfection
Human neuroblastoma cell line SK-N-SH cells were purchased from the Chinese Academy of Sciences (Beijing, China). SK-N-SH cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA) containing 1% penicillin (100 U/mL) and 10% fetal bovine serum (FBS, Gibco, USA) at 37°C in a 5% CO2 humidified atmosphere. When the SK-N-SH cells were 70–80% confluent, the cell culture medium was replaced with OptiMEM 2 h prior to transfection.
The 3 lncRNAs plasmid or siRNAs were transfected into SK-N-SH cells to up-regulate or down-regulate corresponding lncRNAs expression. lncRNAs plasmid, lncRNA siRNAs, and their negative control lncRNAs were produced by Genscript Co., Ltd. (Nanjing, China). We then seeded cells in 6-well plates overnight before transfection. lncRNAs plasmid (or negative control, pre-NC) and lncRNAs siRNAs (or negative control, NC) were transfected into cells using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, California, USA) according to the manufacturer’s instructions.
qRT-PCR test for lncRNAs and SERT
Quantitative RT-PCR was used to detect lncRNAs and SERT in SK-N-SH cells. Total RNA was extracted from SK-N-SH cells using TRIzol reagent (Invitrogen, Carlsbad, California, USA) following the manufacturer’s protocols. Reverse transcription of lncRNAs and SERT was performed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative PCR was performed using SYBR® Green MasterMix in an ABI PRISM® 7500 Sequence Detection System (Applied Biosystems Inc., Foster City, CA). All the primers specific for 3 lncRNAs, SERT, U6, and b-Actin were synthesized by Genscript Co., Ltd. (Nanjing, China). Samples were amplified for an initial denaturation at 95°C for 10 min, followed by 40 cycles each consisting of denaturation at 95°C for 15 s, annealing at 60°C for 1 min, and extension at 82°C for 30 s. Each sample was assayed in triplicate. The quantification of gene expression was performed using the ΔΔCT calculation with CT as the threshold cycle. The relative levels of target genes, normalized to the sample with the lowest CT, are given as 2−ΔΔCT.
Each lncRNA was designed with 2 siRNAs, and we validated the one with a significant interfering effect. The sequence for NONHSAG045500 siRNA1 was UGUAGUUCAGCCUCCAUGGUU, and siRNA2 was AAAUCAUUUAAAAGAUGGCCU; the sequence for NONHSAT142707 siRNA1 was UUUUUAAGGC AAUUUGAGGUC, and siRNA2 was AUUUUUAAGGCAAUU UGAGGU; ENST00000517573 siRNA1 sequence was UAAAUGAAUUAAUGAAUAGAU, and siRNA2 was ACAAAUAAAUG AAUUAAUGAA. The overexpression plasmids vector for the 3 lncRNAs were all pcDNA3.1 (+) vector, and the vector map and promoter element are included in the Supplementary Figure 1.
β-Actin and U6 small nuclear RNA served as internal normalized references of lncRNAs and SERT, respectively.
qRT-PCR for SERT was performed with the following primers:
SERT-FWD CAATTACTTCTCCGAGGACAAC
SERT-REV GTGGCGCGTGTAAAATTCTTC
SERT-SEQ TCACCTGGACCCTCCATTCCACG
Statistical analysis
SDS 2.3 software (Applied Biosystems, Inc.) and DataAssist v3.0 software were used to collect data. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). After normalization of β-Actin, the expression levels of lncRNAs were calculated using the 2−ΔΔCt method. The paired t test was used to compare the difference between the interfering effects of 2 siRNAs on 3 lncRNA (NONHSAT142707, NONHSAG045500, ENST00000517573) groups and the corresponding control group, as well as the difference between plasmid overexpression effects on the 3 lncRNA (NONHSAT142707, NONHSAG045500, ENST00000517573) groups and the corresponding control group. The Wilcoxon signed-rank test was used to compare the difference between the effects of siRNAs2 on SERT in the 3 lncRNA groups and the corresponding control group. A p-value of <0.05 (two-tailed) was considered statistically significant.
Results
Validating the overexpression effect of 3 lncRNA plasmids
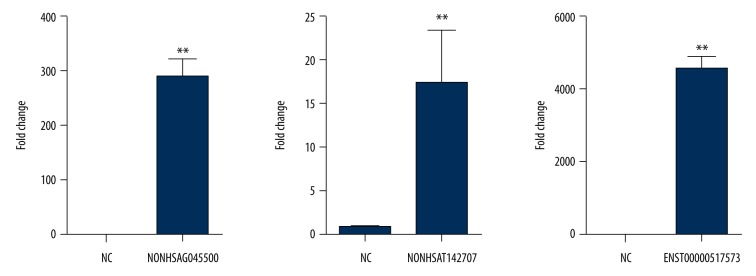
The expression of 3 lncRNAs (NONHSAT142707, NONHSAG045500, ENST00000517573) in the SK-N-SH cells after transfection with plasmid were studied by real-time PCR. SK-N-SH cells were seeded in 6-well plates, with 2×105 cells for each well. After approximately 24 h of cell adherence, 1 μg overexpression plasmid for each lncRNA was transfected. Lipo 2000 null vector transfection was used as the control. According to the Wilcoxon signed-rank test, the results from these 3 experiments showed that compared to the control group, the expression of NONHSAG045500 was up-regulated by 2898 times (P=0.0039), the expression of NONHSAT142707 was up-regulated by 17 times (P=0.0039), and the ENST00000517573 was up-regulated by 3600 times (p=0.0039). The results indicated that 3 lncRNAs were all significantly up-regulated, which indicated a significant difference between the overexpression effect of 3 lncRNA plasmids and the control (Figure 1).
Figure 1.
The effect of 3 lncRNA overexpression plasmids. Three lncRNAs were all overexpressed after plasmid transfection. Each column represents the mean ±S.E.M. Data were analyzed using the Mann-Whitney test. ** P<0.01 compared with the control group.
Overexpression effect of 3 lncRNAs on SERT expression
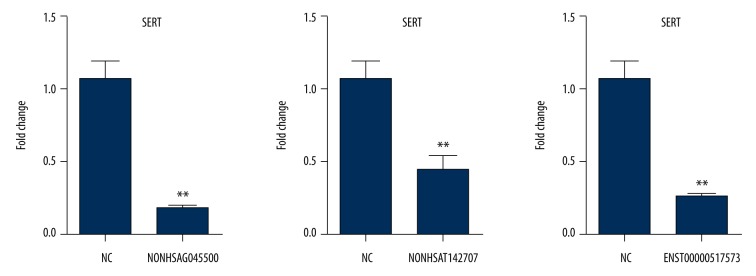
The expression of SERT in the SK-N-SH cells was analyzed by real-time PCR. SK-N-SH cells were seeded in 6-well plates, 2×105 cells for each well. After 24 h of cell adherence, 1 μg for each lncRNA overexpression plasmid was transfected. The control group was the lipo 2000 empty transfected group. According to the Wilcoxon signed-rank test, the results indicated that the expression of SERT was significantly down-regulated in the NONHSAT142707 (P=0.0043), NONHSAG045500 (P=0.0039), and ENST00000517573 (P=0.0039) groups compared with that in the control group, which demonstrated that the overexpression of 3 lncRNAs could inhibit the expression of SERT (Figure 2).
Figure 2.
The effect of overexpression of 3 lncRNAs on SERT expression. The expression of SERT was inhibited by the overexpression of 3 lncRNAs. Data are reported as the mean ±S.E.M. and were analyzed using the Mann-Whitney test. **P<0.01 compared with the control group.
Validating the interfering effect of 3 lncRNAs siRNAs
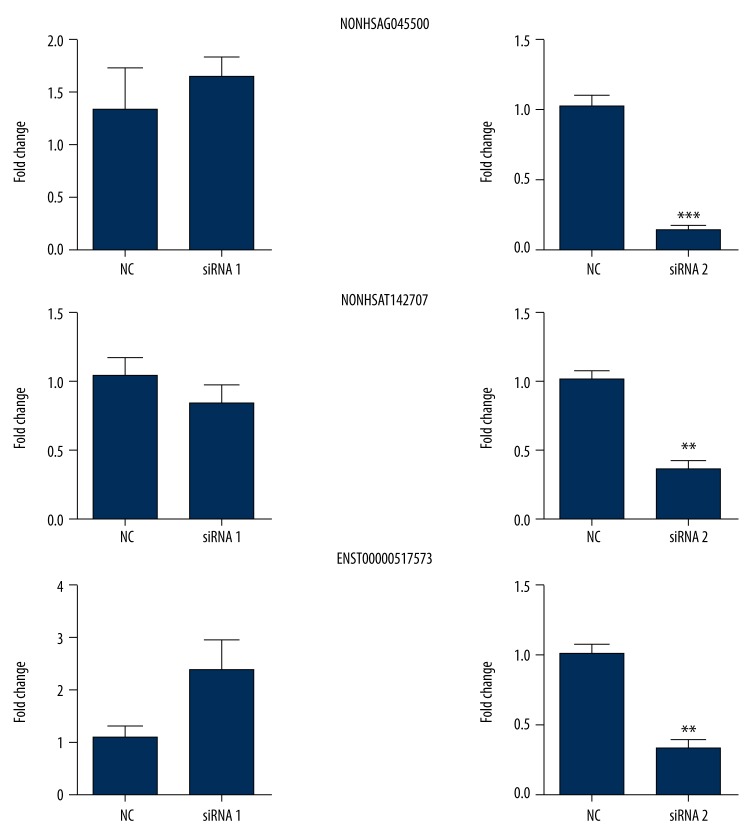
Since every lncRNA had been designed with 2 siRNAs, we attempted to determine which one had a significant and better interfering effect. SK-N-SH cells were seeded in 6-well plates, 1.5×105 cells for each well. After 24 h of cell adherence, 50 nM siRNA for each lncRNA was transfected into the cells. The control group was the lipo 2000 empty transfected group. As illustrated in Figure 3, according to the paired t test, the expression of 3 lncRNAs were significantly down-regulated after transfection with siRNA2 for each lncRNA compared with those after transfection with control; moreover, the expression of NONHSAG045500 was reduced by 86% (t=11.84, P<0.0001), the expression of NONHSAT142707 was reduced by 63% (t=6.010, P=0.0018), and the expression of ENST0000051757 was reduced by 68% (t=6.339, P=0.0014). However, there was no significant difference in siRNA1 for each lncRNA (P>0.05), which indicated that compared with the control, NONHSAG045500 siRNA2, NONHSAT142707 siRNA2, and ENST00000517573 siRNA2 had a remarkable interfering effect, but siRNA1 for 3 lncRNAs had no such effect.
Figure 3.
The interfering effect of 3 lncRNA siRNAs. Every lncRNA had 2 siRNAs. Only siRNA2 for every lncRNA had significant interfering effect. Each column represents the mean ±S.E.M. Data were analyzed using the Mann-Whitney test. ** P<0.01, *** P<0.001 compared with the control group.
Interfering effect of 3 lncRNAs siRNA2 on SERT expression
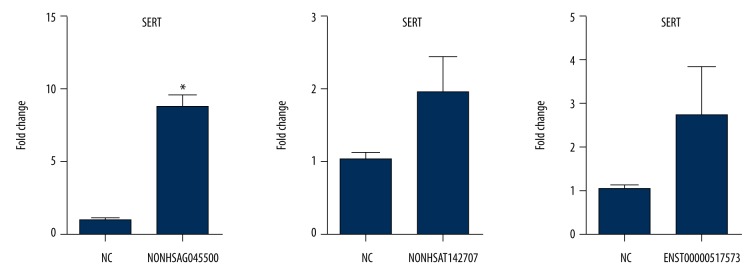
NONHSAG045500 siRNA2, NONHSAT142707 siRNA2, and ENST00000517573 siRNA2 were used to test the effect on SERT expression. SK-N-SH cells were seeded in 6-well plates, 1.5×105 cells for each well. After 24 h of cell adherence, 50 nM siRNA2 for the 3 lncRNAs were transfected into cells. Lipo 2000 null vector transfection was used as the control. According to the Wilcoxon signed-rank test, the results revealed that the expression of SERT was significantly higher in the NONHSAG045500 siRNA2 group (P=0.0313) than in the control group, but no significant difference was found between the control group and the NONHSAT142707 siRNA2 group (P=0.0625) and the ENST00000517573 siRNA2 groups (P=0.2188). This result suggested that the interference of NONHSAG045500 could enhance the expression of SERT (Figure 4).
Figure 4.
The effect of the interference of 3 lncRNA siRNA2 on SERT expression. The expression of SERT in NONHSAG045500 siRNA2 was higher than that in the control, and no significant difference was observed in the other 2 lncRNA groups. Interference with NONHSAG045500 expression could strength the expression of SERT. Data are reported as the mean ±S.E.M. and were analyzed using the Mann-Whitney test. * P<0.05 compared with the control group.
Discussion
The present study indicated that the expression of 5-HT transporter SERT could be enhanced by interfering lncRNA NONHSAG045500 and inhibited by NONHSAG045500 overexpression in SK-N-SH cells in vitro. Due to the high expression of NONHSAG045500 in the human brain, we could regulate the expression of NONHSAG045500 by intravenously injecting NONHSAG045500 plasmid or siRNA2 to regulate SERT expression, eventually changing the content of 5-HT in synaptic cleft and depressive symptoms.
The pathogenesis of MDD remains elusive, and the treatment involves 1 major neurotransmitter, 5-HT. Generally, antidepressants elicit their therapeutic effects only after long-term treatment and are not effective in all patients [21]. Because epigenetic regulation, such as DNA methylation and histone modification, plays an important role in the development of the embryo and nervous system, it has increasingly become the new direction of disease treatment [22]. Existing pharmaceutical agents to treat diseases through epigenetic mechanisms are non-specific, and a multitude of challenges and opportunities exist for the future pharmacological manipulation of lncRNAs. RNA therapeutics can capitalize on various lncRNA cellular functions and target those pathways through structure disruption mechanisms and gene silencing. Extensive secondary structures and the long length of lncRNA may hinder the design of effective small-molecule inhibitors and small interfering RNAs (siRNAs) [23].
Because lncRNAs have species diversities and differences, a lncRNA cannot always be expressed in both human and animals. Therefore, in this study, we did not explore lncRNAs from lncRNA databases but from previous human studies. Liu et al. [24] used microarray-based genome-wide analysis to determine the association of lncRNAs and depression, and found 2007 lncRNAs differentially expressed in MDD patients compared with those in controls. However, they did not explore whether lncRNA expression can vary following antidepressant treatment or if it can be used to distinguish MDD from other mental diseases. In our previous studies, we first screened 8 candidate differentially expressed lncRNAs in MMD patients and compared the expression of the 8 lncRNAs before and after standardized antidepressant treatment. We found 6 lncRNAs (TCONS_00019174, ENST00000566208, NONHSAG045500, ENST00000517573, NONHSAT034045, and NONHSAT142707) with significant differences in expression levels that returned to normal when depressive symptoms went into remission [20]. Finally, we cross-validated the 6 MDD lncRNAs with 3 differentially expressed schizophrenia lncRNAs and 3 differentially expressed generalized anxiety disorder lncRNAs. This result indicated that these 6 lncRNAs had specificity for MDD. Thus, we concluded that these 6 lncRNAs have value for diagnosis, therapy evaluation, and differential diagnosis, and play a critical role in the central nervous system, as determined using Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway analysis [25]. On this basis, we analyzed the effect of the interference and overexpression of 3 lncRNAs (NONHSAT142707, NONHSAG045500, and ENST00000517573), which are much more highly expressed in the human brain than the other 3 lncRNAs, on SERT expression, which is the key target of antidepressant therapy. First, we validated the overexpression effect of 3 lncRNAs plasmid, and found that the 3 lncRNAs were all significantly overexpressed after transfecting plasmid (Figure 1). Then, the 3 lncRNAs plasmid were administered to SK-N-SH cells and indicated that the overexpression of NONHSAT142707, NONHSAG045500, and ENST00000517573 could inhibit the expression of SERT (Figure 2). Second, we validated the effect of the 3 lncRNAs siRNAs. Every lncRNA had 2 siRNAs, and results showed that only siRNA2 for each lncRNA had a remarkable interference effect compared with that of the control (Figure 3). Then, we transfected SK-N-SH cells with the 3 lncRNA siRNA2, and the results demonstrated that only the expression of SERT in NONHSAG045500 siRNA2 was much higher than that in the control, indicating that interfering with NONHSAG045500 expression could strengthen the expression of SERT. In conclusion, NONHSAG045500 has therapeutic antidepressant potential through regulating serotonin transporter SERT in MDD patients.
There is already evidence proving that lncRNAs can regulate the expression of another major central neurotransmitter dopamine (DA) in psychiatric disorders. BC1, one of the first annotated lncRNAs in the central nervous system, displayed significant upregulation of D2R protein in the striatum and potentiated responses to D2R agonists in knockout mice [26]. In Gomafu (a schizophrenia-associated lncRNA) knockout mice, a significant increase in dopamine levels was detected in the brain after exposure to the psychostimulant methamphetamine (MAP), and the increased dopamine was correlated with hyperactivity of knockout mice [27]. AS-Uchl1, a lncRNA antisense to the Uchl1 gene, is repressed in dopaminergic neurons of Parkinson’s disease models and regulated by Nurr1, a core transcription factor involved in the maturation and viability of dopamine neurons [28]. In addition, microRNA (miRNA), another kind of non-coding RNA, can also mediate the expression of dopamine and serotonin. miR-132, miR135a2, and miR-218 are known to direct the fate and survival of dopaminoceptive neurons (DN) by binding to key neuronal transcription factors, including Nurr1 [29–31]. Luciferase reporter assay confirmed that miR-24 inhibitor acted as a promoter to up-regulate the mRNA and protein expression of SERT in human intestinal epithelial cells [32]. An animal study involving anti-miR-16 intracerebroventricular injection was performed to confirm the role of CSF miR-16 in MDD, and found that anti-miR-16-treated rats had extremely lower CSF miR-16, significantly higher CSF serotonin, and obviously higher raphe SERT protein than control rats [33]. Furthermore, a study involving siRNA treatment of disease in live rats has been conducted. After treatment with NONRATT021972 siRNA by sublingual injection, the serum norepinephrine and epinephrine concentrations, up-regulated P2X7 mRNA (involved in myocardial ischemic injury), and proteins were decreased in myocardial ischemic rats. In addition, their increased systolic blood pressure, diastolic blood pressure, low-frequency power, heart rate, and LF/HF ratio were reduced to normal levels, suggesting that NONRATT021972 siRNA could decrease the upregulation of the P2X7 receptor and improve the abnormal changes in cardiac function after myocardial ischemia [34]. In the future, we plan to administer NONHSAG045500 siRNA2 in MDD model rats and observe the alteration of SERT expression and depressive symptoms.
Conclusions
The current experiments are, to the best of our knowledge, the first profile of lncRNA dysregulation associated with 5-HT function. In the present study, we identified lncRNA NONHSAG045500 as a regulator of serotonin transmission in vitro. The NONHSAG045500-dependent modulation of SERT might be involved in the modifications of the efficacy of 5-HT transmission seen in physiological and pathological conditions, indicating that dysregulation of NONHSAG045500 expression could mediate pathological alterations of 5-HT transmission. Moreover, normalizing NONHSAG045500 could have therapeutic antidepressant potential and provide a new therapeutic strategy for MDD.
Limitation
In this study, we examined the relationship between the change in NONHSAG045500 expression and SERT levels in vitro; however, our results were insufficient to determine whether NONHSAG045500 siRNAs or plasmids can be used clinically. Thus, there is still a great need for animal and human research before it can be applied in clinical practice. In addition, there are numerous challenges that must be overcome for wider use of lncRNAs.
Supplementary Figure
Gene control elements for lncRNAs.
Acknowledgments
We sincerely thank all the medical staff, including Ming Zhang, Jiandong Gu, and Hongyi Jiang, involved in collecting and dealing with the specimens.
Footnotes
Conflicts of interest
None.
Source of support: This work was supported by Changzhou City Health and Family Planning Commission (grant number WZ201720) and the project of the Provincial Key Research and Development Program (Social Development), which was funded by the Science and Technology Department of Jiangsu Province (grant number BE2017650)
References
- 1.Oved K, Morag A, Pasmanik-Chor M, et al. Genome-wide miRNA expression profiling of human lymphoblastoid cell lines identifies tentative SSRI antidepressant response biomarkers. Pharmacogenomics. 2012;13:1129–39. doi: 10.2217/pgs.12.93. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmansour S, Owens WA, Cecchi M, et al. Serotonin clearance in vivo is altered to a greater extent by antidepressant-induced downregulation of the serotonin transporter than by acute blockade of this transporter. J Neurosci. 2002;22:6766–72. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.David DJ, Samuels BA, Rainer Q, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–9. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 6.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–52. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 8.Martin C, Tansey KE, Schalkwyk LC, Powell TR. The inflammatory cytokines: Molecular biomarkers for major depressive disorder? Biomark Med. 2015;9:169–80. doi: 10.2217/bmm.14.29. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Zhang Y, Qiao M, et al. Mechanisms of extracellular signal-regulated kinase/cAMP response element-binding protein/brain-derived neurotrophic factor signal transduction pathway in depressive disorder. Neural Regen Res. 2013;8:843–52. doi: 10.3969/j.issn.1673-5374.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia J, Le W. Molecular network of neuronal autophagy in the pathophysiology and treatment of depression. Neurosci Bull. 2015;31:427–34. doi: 10.1007/s12264-015-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miguel-Hidalgo JJ, Whittom A, Villarreal A, et al. Apoptosis-related proteins and proliferation markers in the orbitofrontal cortex in major depressive disorder. J Affect Disord. 2014;158:62–70. doi: 10.1016/j.jad.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Luo YL, Mao YS, Ji JL. The link between long noncoding RNAs and depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017;73:73–78. doi: 10.1016/j.pnpbp.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Vialou V, Feng J, Robison AJ, Nestler EJ. Epigenetic mechanisms of depression and antidepressant action. Ann Rev Pharmacol Toxicol. 2013;53:59–87. doi: 10.1146/annurev-pharmtox-010611-134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karapetyan AR, Buiting C, Kuiper RA, Coolen MW. Regulatory roles for long ncRNA and mRNA. Cancers. 2013;5:462–90. doi: 10.3390/cancers5020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–61. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Willingham AT, Orth AP, Batalov S, et al. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science (New York, NY) 2005;309:1570–73. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]
- 17.Gałecki P, Talarowska M, Bobińska K, Szemraj J. COX-2 gene expression is correlated with cognitive function in recurrent depressive disorder. Psychiatry Res. 2014;215:488–90. doi: 10.1016/j.psychres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Modarresi F, Faghihi MA, Lopeztoledano MA, et al. Inhibition of natural antisense transcripts in vivo results in gene-specific transcriptional upregulation. Nat Biotechnol. 2012;30:453–59. doi: 10.1038/nbt.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xuelian C, Xinyang S, Niu W, et al. Long non-coding RNA: Potential diagnostic and therapeutic biomarker for major depressive disorder. Med Sci Monit. 2016;22:5240–48. doi: 10.12659/MSM.899372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuelian C, Wei N, Lingming K, et al. Long non-coding RNA expression in peripheral blood mononuclear cells and suicide risk in Chinese patients with major depressive disorder. Brain Behav. 2017;7:e00711. doi: 10.1002/brb3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–49. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karsli-Ceppioglu S. Epigenetic mechanisms in psychiatric diseases and epigenetic therapy. Drug Dev Res. 2016;77:407–13. doi: 10.1002/ddr.21340. [DOI] [PubMed] [Google Scholar]
- 23.Prabhakar B, Zhong XB, Rasmussen TP. Exploiting long noncoding RNAs as pharmacological targets to modulate epigenetic diseases. Yale J Biol Med. 2017;90:73–86. [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Z, Li X, Sun N, et al. Microarray profiling and co-expression network analysis of circulating lncRNAs and mRNAs associated with major depressive disorder. PLoS One. 2014;9:e93388. doi: 10.1371/journal.pone.0093388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui X, Niu W, Kong L, et al. Can lncRNAs be indicators for the diagnosis of early onset or acute schizophrenia and distinguish major depressive disorder and generalized anxiety disorder? – A cross validation analysis. Am J Med Genet B Neuropsychiatr Genet. 2017;174:335–41. doi: 10.1002/ajmg.b.32521. [DOI] [PubMed] [Google Scholar]
- 26.Centonze D, Centonze D, Rossi S, et al. The brain cytoplasmic RNA BC1 regulates dopamine D2 receptor-mediated transmission in the striatum. J Neurosci. 2007;27:8885–92. doi: 10.1523/JNEUROSCI.0548-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ip JY, Sone M, Nashiki C, et al. Gomafu lncRNA knockout mice exhibit mild hyperactivity with enhanced responsiveness to the psychostimulant methamphetamine. Sci Rep. 2016;6:27204. doi: 10.1038/srep27204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carrieri C, Forrest A, Santoro C, et al. Expression analysis of the long non-coding RNA antisense to Uchl1 (AS Uchl1) during dopaminergic cells’ differentiation in vitro and in neurochemical models of Parkinson’s disease. Front Cell Neurosci. 2015;9:114. doi: 10.3389/fncel.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderegg A, Lin HP, Chen JA, et al. An Lmx1b-miR135a2 regulatory circuit modulates Wnt1/Wnt signaling and determines the size of the midbrain dopaminergic progenitor pool. PLoS Genet. 2013;9:e1003973. doi: 10.1371/journal.pgen.1003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baek S, Choi H, Kim J. Ebf3-miR218 regulation is involved in the development of dopaminergic neurons. Brain Res. 2014;1587:23–32. doi: 10.1016/j.brainres.2014.08.059. [DOI] [PubMed] [Google Scholar]
- 31.Yang D, Li T, Wang Y, et al. miR-132 regulates the differentiation of dopamine neurons by directly targeting Nurr1 expression. J Cell Sci. 2012;125:1673–82. doi: 10.1242/jcs.086421. [DOI] [PubMed] [Google Scholar]
- 32.Liao XJ, Mao WM, Wang Q, et al. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun. 2015;469:288–93. doi: 10.1016/j.bbrc.2015.11.102. [DOI] [PubMed] [Google Scholar]
- 33.Song MF, Dong JZ, Wang YW, et al. CSF miR-16 is decreased in major depression patients and its neutralization in rats induces depression-like behaviors via a serotonin transmitter system. J Affect Disord. 2015;178:25–31. doi: 10.1016/j.jad.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 34.Tu G, Zou L, Liu S, et al. Long noncoding NONRATT021972 siRNA normalized abnormal sympathetic activity mediated by the upregulation of P2X7 receptor in superior cervical ganglia after myocardial ischemia. Purinergic Signal. 2016;12(3):521–35. doi: 10.1007/s11302-016-9518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene control elements for lncRNAs.