Abstract
In patients with thrombocytopenia, it can be difficult to predict a patient’s bleeding risk based on platelet count alone. Platelet reactivity may provide additional information; however, current clinical assays cannot reliably assess platelet function in the setting of thrombocytopenia. New methods to study platelet reactivity in thrombocytopenic samples are needed. In this study, we sought to develop a laboratory model of thrombocytopenia using blood from healthy subjects that preserves the whole blood environment and reproducibly produces samples with a specific platelet count and hematocrit. We compared the activation state of unstimulated and agonist-stimulated platelets in thrombocytopenic samples derived from this method with normocytic controls. Whole blood was diluted with autologous red blood cell concentrate and platelet-poor plasma, which were obtained via centrifugation, in specific ratios to attain a final sample with a predetermined platelet count and hematocrit. P-selectin exposure and GPIIbIIIa activation in unstimulated platelets and platelets stimulated with collagen-related peptide (CRP) or adenosine diphosphate (ADP) in thrombocytopenic samples and the normocytic control from which they were derived were quantified by flow cytometry. Our methodology reliably produced thrombocytopenic samples with a platelet count ≤50,000/µL and an accurately and precisely controlled hematocrit. P-selectin exposure and GPIIbIIIa activation on unstimulated platelets or on ADP- or CRP-stimulated platelets did not differ in thrombocytopenic samples compared to normocytic controls. We describe a new method for creating thrombocytopenic blood that can be used to better understand the contributions of platelet number and function to hemostasis.
Keywords: Blood platelets, Flow cytometry, Platelet activation, Platelet aggregation, Thrombocytopenia
Introduction
Hypoproliferative thrombocytopenia is common in patients receiving chemotherapy or it can be caused by diseases such as aplastic anemia or myelodysplasia. While a low platelet count increases bleeding risk, not all patients with severe thrombocytopenia experience bleeding [1, 2]. It can be difficult to predict a patient’s bleeding risk based on platelet count alone [3, 4]; therefore, clinicians often administer prophylactic platelet transfusions or other hemostatic therapies to all patients with severe thrombocytopenia [5, 6].
Platelet dysfunction likely contributes to bleeding in thrombocytopenic patients [7, 8]. Assessment of platelet function in patients with thrombocytopenia may help to identify patients who are at risk for bleeding and therefore requires a prophylactic platelet transfusion. Clinical tests that are commonly used to diagnose platelet dysfunction include the Platelet Function Analyzer-100 (PFA-100), whole blood and light transmittance aggregometry, and viscoelastic assays [9–11]. All of these tests require a normal platelet count; therefore, in the setting of thrombocytopenia, they cannot distinguish qualitative platelet function defects from quantitative ones [9, 12]. New platelet function tests are needed to measure platelet function in the setting of thrombocytopenia.
A source of thrombocytopenic blood is required for the validation of platelet function tests that could diagnose dysfunction in the setting of thrombocytopenia. Blood from patients with thrombocytopenia may not be useful for this purpose because platelet function may not be normal. The ability to create thrombocytopenic whole blood from healthy volunteers is therefore essential to the development and validation of these new assays. However, currently published methods of creating thrombocytopenic blood take a long time, lead to platelet activation, replace plasma with buffer, and do not enable precise control over the platelet count and hematocrit in the final sample [13, 14]. A new method for making thrombocytopenic blood is needed.
Flow cytometry offers a method to test platelet function and is considered relatively insensitive to thrombocytopenia [12, 15]. It can be used to evaluate resting and agonist-stimulated platelets by measuring physical characteristics such as size and granularity and surface expression of integrins, granule contents, and bound ligands [15, 16]. Flow cytometry has been used to investigate platelet activation in patients with idiopathic thrombocytopenia purpura (ITP) and chemotherapy-induced thrombocytopenia [8, 17]. However, the ability of flow cytometry to measure platelet function irrespective of platelet count has not been thoroughly tested.
In this study, we describe the development of a laboratory model of thrombocytopenia using blood from healthy subjects that preserves the whole blood environment and reproducibly produces samples with a specific platelet count and hematocrit. We used the model to characterize resting and agonist-stimulated platelets by flow cytometry. We found that thrombocytopenia did not affect the extent of platelet activation as measured by flow cytometry in response to adenosine diphosphate (ADP) or collagen-related peptide (CRP).
Thrombocytopenic whole blood samples created from normal subjects could be useful in many applications, including development and validation of novel platelet function tests that are insensitive to platelet count, as an experimental model for testing the efficacy of interventions used for treatment of thrombocytopenia, or as a background for testing the function of small numbers of platelets (e.g., those produced from hematopoietic stem cells in vitro) in a whole blood environment.
Methods
Preparation of thrombocytopenic whole blood samples
Using a protocol approved by the local institutional review board, whole blood was collected from healthy adult volunteers (n = 18) into 3.2 % sodium citrate. For evaluation of the model in a different anticoagulant, samples were collected into hirudin (final concentration >15 µg/mL, Verum Diagnostica, Munich, Germany).
Dilution method
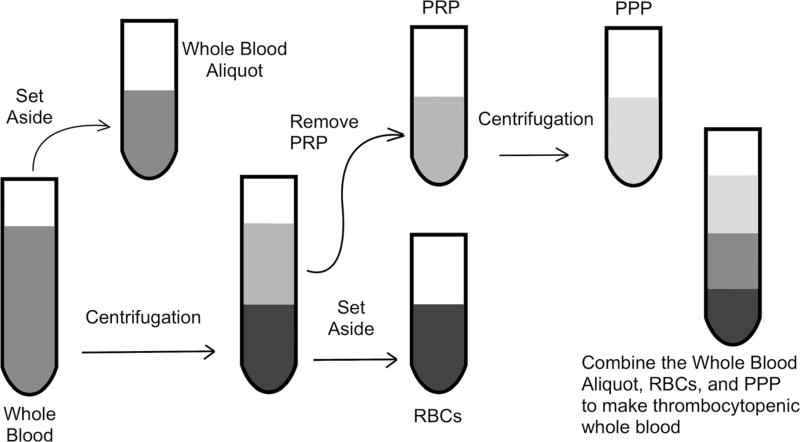
An aliquot of the starting whole blood was set aside (Fig. 1). The remainder was centrifuged for 8 min at 300g. The fraction containing the platelet-rich plasma (PRP) and buffy coat was removed from the red blood cell (RBC) concentrate and centrifuged for 10 min at 3800g to prepare platelet-poor plasma (PPP). Platelet counts (C) and hematocrits (H) of the starting unmanipulated whole blood (W) and RBCs (R, diluted 1:1 with normal saline) were measured using the Scil Vet ABC Hematology Analyzer (Scil Animal Care Company, Gurnee, IL).
Fig. 1.
Dilution method of creating thrombocytopenic whole blood. From the starting whole blood, a small aliquot is set aside. The remainder of the whole blood is centrifuged at 300g for 8 min to separate the platelet-rich plasma (PRP) from the red blood cells (RBCs). The PRP is subsequently centrifuged at 3800g for 10 min, and the platelet-poor plasma (PPP) is removed from the platelet pellet. The PPP, RBCs, and previously set aside whole blood aliquot are mixed together in a ratio based on goal platelet count and hematocrit
The amounts of whole blood (VW), RBCs (VR, undiluted), and PPP (VP) that were added to make the final thrombocytopenic sample were determined using the following equations:
where V = volume, C = platelet count, H = hematocrit, and W = whole blood, R = RBCs, P = PPP, and G = goal. A spreadsheet into which these equations are incorporated is provided in the Supplemental Information as is the derivation of these equations.
Centrifugation method
A centrifugation method for preparing thrombocytopenic blood has been previously described by Larsen et al. [13]. Briefly, whole blood was centrifuged for 15 min at 100g to separate RBCs from PRP, and PRP was subsequently centrifuged for 25 min at 3300g to produce PPP that was then mixed with RBCs to produce thrombocytopenic whole blood. After each cycle, platelet count and hematocrit were measured using the Scil Hematology Analyzer. This process was repeated up to five times to achieve reconstituted whole blood with a final platelet count of approximately 20,000 or 50,000 platelets/µL.
Whole blood aggregometry
Whole blood aggregometry was used to determine whether restoration of a normal platelet count to thrombocytopenic whole blood restored full platelet function. To this end, thrombocytopenic samples with platelet counts of 20,000 and 100,000/µL and a hematocrit of 30–33 % were created from a given healthy subject. Platelet concentrate was made from the same subject [18]. Briefly, whole blood was centrifuged at room temperature at 200g for 10 min, and the resultant PRP was centrifuged at 400g for 10min, after which approximately three quarters of the plasma was removed and the platelet pellet was resuspended in the remaining plasma for a goal platelet count between 1,200,000 and 1,700,000/µL. Platelet concentrate was added to the thrombocytopenic samples to restore the platelet count to that of the original unmanipulated whole blood (~220,000/µL).
Aggregation in response to ADP 10 µM (DiaPharma, West Chester, OH) in the unmanipulated whole blood (normocytic control), thrombocytopenic samples, and thrombocytopenic samples in which platelet counts were restored to that of the normocytic control were analyzed on the multiplate aggregometer (DiaPharma).
Assessment of surface expression of P-selectin and GPIIbIIIa activation on resting and agonist-stimulated platelets
The mAb used to label GPIIbIIIa for platelet identification was 312.2 (Hybridoma Core, BloodCenter of Wisconsin [BCW], Milwaukee, WI) [19] conjugated to AF660 (Invitrogen) per manufacturer’s instructions. The mAb used to label surface P-selectin was anti-CD62P-PE (BioLegend, San Diego, CA), and the mAb used to label activated GPIIbIIIa was PAC-1-FITC (BD Biosciences, San Jose, CA).
Whole blood samples were diluted tenfold with HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)-buffered Tyrode’s solution containing 0.001 % bovine serum albumin (BSA) (HEPES-Tyrode’s-BSA) [20]. The diluted whole blood was incubated in the dark at room temperature for 20 min with 312.2-AF660, anti-CD62P-PE, and PAC-1-FITC or their respective isotype controls. Agonist—either ADP at 1.25 or 5 µM (Chrono-log Corp., Havertown, PA)—or CRP (2.5 µg/mL, Protein Core, BCW) was added 10 min into the 20-min incubation period. To mimic platelets unable to respond to an agonist, some samples were pre-treated with apyrase, which inactivates ATP and ADP by converting them to AMP. For these samples, apyrase was added at the same time as the antibodies and then ADP was added after 10 min. Following the incubation period, samples were diluted tenfold with the HEPES-Tyrode’s-BSA buffer to stop the platelet activation (final dilution of 1:100).
Statistical analyses
Platelet count accuracy
Platelet count and hematocrit are expressed as mean ± standard deviation (SD). To compare whether platelet count goal (20,000, 50,000, or 100,000/µL) had an impact on hematocrit accuracy, ANOVA was performed for samples with a goal hematocrit of 25 and 30 % and a t test was used to compared the samples with a goal of 35 %.
Comparison of two methods to make thrombocytopenic blood
A t test was used to compare the amount of time needed to make the thrombocytopenic samples using either the dilution method or the centrifugation method. Repeated measures ANOVA with Bonferroni correction for multiple comparisons was used to compare P-selectin exposure on resting platelets in normocytic controls versus thrombocytopenic samples made using the dilution versus centrifugation method.
Characterization of platelets in activated thrombocytopenic samples
A power analysis determined that 14 pairs of thrombocytopenic samples and normocytic controls, which were the whole blood samples from which the thrombocytopenic samples were made, would give 81 % power to detect a 10 % difference between the two samples at a significance level of 0.05. Sample size calculation was performed using SAS Studio 3.4. All other statistical analyses were performed using Prism 6.0g for Mac (GraphPad Software). All tests were performed with a two-sided alpha of 0.05 or a family alpha of 0.05 in the case of multiple comparison analyses.
For whole blood aggregometry assays, data were analyzed using repeated measures ANOVA with Bonferroni correction for multiple comparisons with thrombocytopenic and transfused samples compared to the normocytic control. A paired t test was performed to compare thrombocytopenic and normocytic samples with respect to P-selectin exposure and activated GPIIbIIIa expression as well as forward and side scatter (FSC and SSC).
Results
Preparation of thrombocytopenic samples
The healthy adult volunteers had normal hematocrits (39.2 ± 3.3 %, mean ± SD) and platelet counts (242,000 ± 60,000/µL). Efficient separation of the RBCs from the PRP was essential to minimize the number of centrifuged platelets in the final thrombocytopenic sample. Methods to separate PRP from RBCs are well described in the literature [13, 21]; however, at these lower centrifugal forces, there were a significant number of platelets in the remaining RBC concentrate (data not shown). Centrifugation at 300g for 8 min produced concentrated RBCs (hematocrit 86.9 ± 8.7 %) with minimal platelets (21,000 ± 7000/µL). Platelets from the RBCs comprised of 5–18 % of the total platelets in the final thrombocytopenic sample; final percent was dependent on the platelet counts and hematocrits of the whole blood, the concentrated RBCs, and the final goal of the thrombocytopenic sample. There were no platelets in the platelet-poor plasma. When comparing normocytic with thrombocytopenic samples, there was no difference in the FSC (104,614 ± 13,717 versus 102,905 ± 13,673, respectively, P = 0.1523) or SSC (7620 ± 947 versus 7962 ± 887, respectively, P = 0.0754), indicating similar size and granularity of the platelets in both samples.
Accuracy and precision of the dilution method for preparation of thrombocytopenic whole blood samples
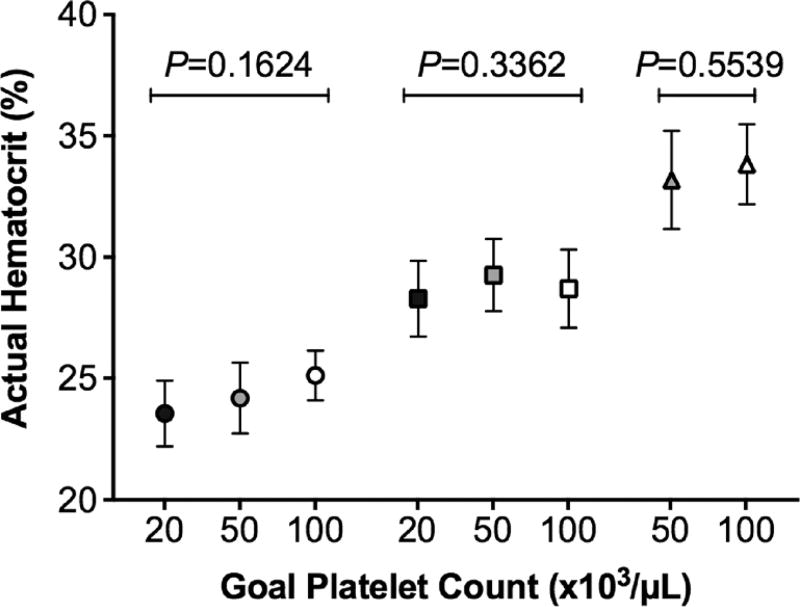
Our goal was to develop a model that would reliably produce thrombocytopenic whole blood samples with discrete platelet counts and hematocrits. We first evaluated our ability to hit a series of platelet count targets in whole blood using the dilution method. For samples prepared with a target platelet count of 20,000/µL, the actual mean platelet count of prepared samples was 24,000 ± 4000/µL. When the target platelet count was 30,000/µL, the actual mean platelet count of the final sample was 33,000 ± 5000/µL. In samples prepared with a target platelet count of 50,000/µL, the mean platelet count was 51,000 ± 5000/µL (with hirudin as the anticoagulant, this value was 48,000 ± 5000/µL). The mean platelet count of samples prepared with a target platelet count of 100,000/µL was 98,000 ± 9000/µL. We next evaluated our ability to hit a series of hematocrit targets in whole blood using the dilution method. Samples prepared with a target hematocrit of 25 % had a final mean hematocrit of 24.2 ± 1.4 %. In samples where the target was 30 %, the actual mean hematocrit was 28.7 ± 1.6 % (with hirudin, this value was 29.8 ± 1.5 %). When the goal hematocrit was 35 %, the mean hematocrit was 33.5 ± 1.8 %. Importantly, hematocrits did not differ significantly among samples with different goal platelet counts (Fig. 2). We conclude that the dilution method enables preparation of whole blood samples with discrete platelet counts and hematocrits with reasonable accuracy irrespective of anticoagulant used.
Fig. 2.
Hematocrit accuracy based on goal platelet count. Hematocrit is not affected by platelet count in the thrombocytopenic samples with platelet counts of 20,000/µL (black symbols), 50,000/µL (gray), and 100,000/µL (white). Data are expressed as mean ± standard deviation
Comparison of dilution and centrifugation methods for preparation of thrombocytopenic whole blood samples
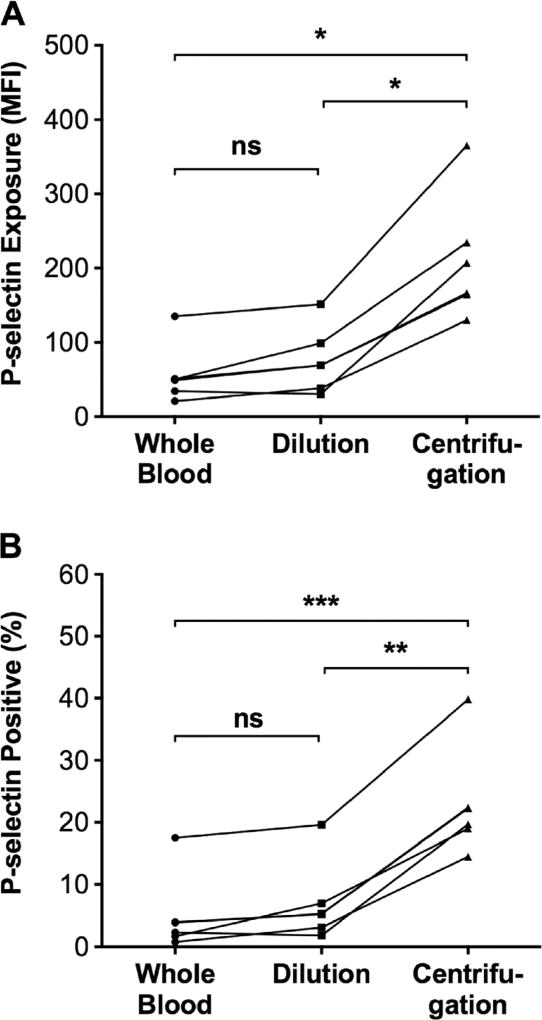
The dilution method was compared with the previously published centrifugation method [13] on the basis of several factors, including precision in achieving the targeted platelet count, rapidity, and ability to preserve platelets in an inactive state. The dilution method was more precise than the centrifugation method in that it exhibited less variability in achieving platelet counts of 20,000 and 50,000/µL. Specifically, the dilution method produced thrombocytopenic samples with platelet counts of 22,000 ± 2000 and 51,000 ± 3000/µL, whereas the centrifugation method produced samples with platelet counts of 17,000 ± 4000 and 54,000 ± 14,000/µL. The increased variability in platelet count in samples prepared using the centrifugation method was likely due to variability in the number of cycles required to achieve the goal platelet count. Thus, it took 1–2 cycles of centrifugation to obtain a platelet count of ~50,000/µL and 2–4 cycles to obtain a platelet count of ~20,000/µL. The dilution method was also quicker than the centrifugation method. It took significantly less time (P < 0.0001) to prepare a sample with a platelet count of 20,000/µL using the dilution method (51.3 ± 13.0 min) than the centrifugation method (139.8 ± 39.8 min). Finally, the dilution method maintained platelets in an inactive state better than did the centrifugation method. Thus, both the level of P-selectin exposure (211 ± 84 median fluorescence intesity [MFI]) and the percent of platelets that were P-selectin positive (23.0 ± 8.7 %) were significantly higher in thrombocytopenic samples prepared by the centrifugation method than in either thrombocytopenic samples prepared with the dilution method (P-selectin MFI = 57 ± 40; P-selectin positive = 5.1 ± 6.2 %) or unmanipulated normocytic starting whole blood samples (P-selectin MFI = 76 ± 40; P-selectin positive 6.9 ± 6.4%), the latter two of which samples did not differ significantly from each other (Fig. 3). On the basis of these findings, we conclude that the dilution method is superior to the previously described centrifugation method [13] because it is faster, less variable, and preserves platelets in an inactive state better during preparation of the thrombocytopenic sample.
Fig. 3.
Effect of dilution versus centrifugation method for preparation of thrombocytopenic whole blood on the activation state of unstimulated platelets. P-selectin exposure on resting platelets was quantified in normocytic control samples (black circle) and thrombocytopenic samples in samples prepared using the dilution method (black square) or the centrifugation method (black triangle) using flow cytometry. Thrombocytopenic samples prepared using the centrifugation method had a higher levels of P-selectin exposure on platelets as indicated by median fluorescence intensity (MFI) and b a higher percent of platelets that were positive for P-selectin compared to both the normocytic control and platelets in thrombocytopenic samples prepared using the dilution method. The platelet activation state did not differ significantly in normocytic control samples and thrombocytopenic samples prepared using the dilution method. *P ≤ 0.01, **P ≤ 0.001, and ***P < 0.0001
Validation of the dilution method as a model of thrombocytopenia
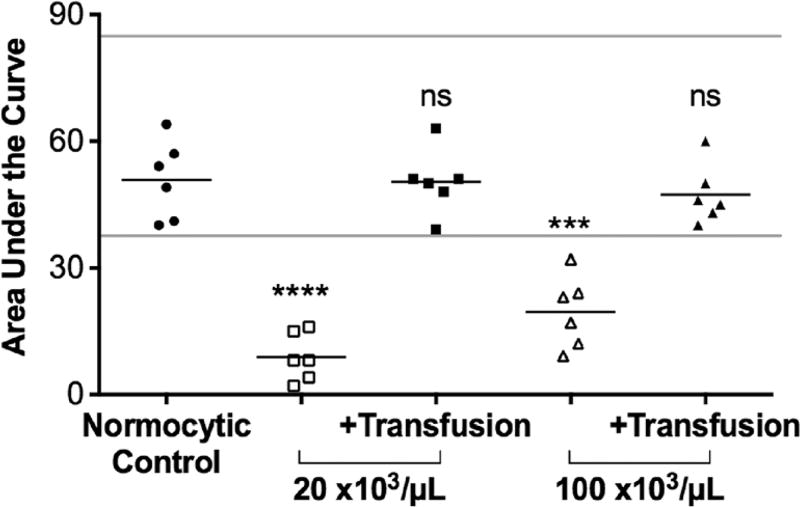
One setting in which a whole blood model of thrombocytopenia would be useful is to enable ex vivo testing of the efficacy of hemostatic interventions such as platelet transfusions. To determine the utility of thrombocytopenic whole blood samples prepared using the dilution method for this purpose, we used whole blood aggregometry to measure platelet aggregation in thrombocytopenic samples before and after restoration of a normal platelet count (~220,000/µL) by ex vivo transfusion. The area under the curve (AUC) in response to ADP (Fig. 4) was significantly decreased in both thrombocytopenic samples compared to the normocytic controls (for 20,000/µL, P < 0.0001, and for 100,000/µL, P = 0.0003). Note that P values were adjusted for multiple comparisons between normocytic samples and thrombocytopenic samples pre- and post-transfusion. Restoration of the platelet count in thrombocytopenic samples to a normal level restored agonist-induced platelet aggregation to levels that did not differ significantly from those observed in unmanipulated normocytic controls. We conclude from these findings that thrombocytopenic whole blood samples prepared using the dilution method can be useful for testing the potential efficacy of platelet transfusions.
Fig. 4.
Whole blood aggregometry in thrombocytopenic samples before and after ex vivo transfusion. Area under the curve (AUC) in response to stimulation with ADP was significantly decreased in samples with a platelet count of 20,000 or 100,000/µL compared to normocytic controls. There was no significant difference in the AUC following ADP stimulation between thrombocytopenic samples to which platelets were added back to achieve a platelet count similar to the normocytic control and the unmanipulated normocytic control. Lines (gray) indicated normal range as supplied by the multiplate manufacturer. ***P ≤ 0.001, ****P < 0.0001, and ns not significant when compared to control
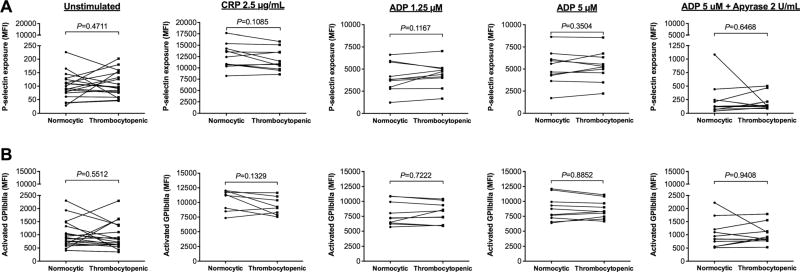
Thrombocytopenic whole blood samples prepared from healthy subjects would also be useful as controls for studies of the platelet activation state in blood samples obtained from patients with thrombocytopenia. Assessment of P-selectin exposure and GPIIbIIIa activation by flow cytometry is an accepted approach for quantifying platelet activation, and this approach has been used to characterize platelet activation in patients with thrombocytopenia [8, 17]. Interpretation of the results of such studies is, however, hampered by failure to include controls with similarly low numbers of normally functioning platelets. To determine the utility of thrombocytopenic whole blood samples prepared using the dilution method for assessment of the platelet activation state by flow cytometry, we compared levels of P-selectin exposure and GPIIbIIIa activation on the surfaces of platelets in thrombocytopenic samples versus unmanipulated normocytic controls both before and after agonist stimulation. Platelets in thrombocytopenic samples that were left unstimulated exhibited low levels of P-selectin exposure (Fig. 5a) and GPIIbIIIa activation (Fig. 5b) that did not differ significantly from those observed in normocytic control samples.
Fig. 5.
P-selectin exposure and GPIIbIIIa activation in normocytic controls (black circle) and thrombocytopenic (black square) samples prepared using the dilution method There was no significant difference in a P-selectin exposure or b GPIIbIIIa activation in (from left to right) unstimulated platelets nor in platelets stimulated with CRP, low-(1.25 µM) and high-dose (5 µM) ADP, or samples pre-treated with apyrase prior to addition of high-dose ADP
Similarly, platelets in thrombocytopenic samples that were stimulated with low- or high-dose ADP or CRP exhibited high levels of P-selectin exposure (Fig. 5a) and GPIIbIIIa activation (Fig. 5b) that did not differ from levels observed in normocytic samples. Both normocytic and thrombocytopenic samples that were pre-treated with apyrase and then activated by ADP showed minimal P-selectin exposure (Fig. 5a) and GPIIbIIIa activation (Fig. 5b) and were not significantly different from each other. Additionally, when thrombocytopenic samples were made using blood anticoagulated with hirudin, there was no difference in P-selectin exposure or activated GPIIbIIIa on the surfaces of unstimulated platelets compared to the normal control (mean difference in MFI = 48 ± 40 [P = 0.1771] and 18 ± 93 [P = 0.7702], respectively). When stimulated with an agonist (either ADP or CRP), platelets in thrombocytopenic samples anticoagulated with hirudin exhibited levels of P-selectin exposure and activated GPIIbIIIa expression that were not significantly different from levels observed in normocytic controls (mean difference in MFI = −637 ± 1341 [P = 0.3477] and −1148 ± 940 [P = 0.0524], respectively). We conclude from these findings that thrombocytopenic whole blood samples prepared from healthy subjects can be useful as controls for studies of platelet activation as measured by flow cytometry.
Discussion
In this study, we describe a method for making thrombocytopenic whole blood samples from healthy subjects using dilution of whole blood with autologous platelet-reduced RBCs and platelet-poor plasma. We demonstrate the utility of this method for assessment of platelet activation and aggregation in the setting of thrombocytopenia. Our dilution method is superior to previously published methods [13, 14, 22] because it quickly, accurately, and reproducibly produces whole blood samples with discrete platelet counts and hematocrits while keeping the platelets quiescent and enabling them to be fully responsive to agonist stimulation. We directly compared our dilution method with a previously published method that relied on serial centrifugations to replace platelet-rich plasma with platelet-poor plasma [13] and found that our method took less than half the time (an average of 51 versus 139 min) to obtain a sample with a platelet count of approximately 20,000/µL and resulted in significantly less platelet activation as indicated by P-selectin exposure. We also compared our dilution method with another previously published method for making whole blood thrombocytopenic that relied on recombining platelet-poor plasma, RBCs, and buffy coat [22]. The recombination model had been used to demonstrate a change in platelet aggregation following in vitro addition of platelets; however, samples in which a normal platelet count had been restored exhibited a greater than 50 % reduction in aggregation compared to the unmanipulated control [22]. In contrast, using our dilution method, samples with platelet counts of 20,000 and 100,000/µL that were subsequently restored to a platelet count of approximately 200,000/µL exhibited no difference in aggregation compared to the unmanipulated control sample with a similar platelet count. On the basis of these comparisons, we conclude that the novel dilution method described herein represents a significant improvement over other previously methods for preparing thrombocytopenic whole blood samples.
Using our dilutional method to create thrombocytopenic whole blood samples with platelet counts ≤50,000/µL, we compared the agonist-induced response of platelets in thrombocytopenic samples with normocytic controls. Platelet activation as measured by increases in P-selectin exposure and GPIIbIIIa activation did not differ in thrombocytopenic samples versus normocytic controls in response to low- and high-dose ADP and CRP. These findings suggest that neither the autologous dilution process nor the platelet count has an impact on activation as measured by these flow cytometry parameters in response ADP and CRP. It is important to note that activation by endogenous ADP and collagen does not require subsequent propagation. It is possible, however, that activation in response to other agonists mediated by subsequent propagation may be affected by platelet count.
Our method for creating thrombocytopenic samples using autologous dilution to control final platelet count and hematocrit offers the opportunity to test separately the effects of platelet count and platelet function on hemostasis. Thrombocytopenic samples prepared from normal blood can be used to validate novel assays that test platelet function in a manner that is insensitive to platelet count. Additionally, thrombocytopenic whole blood can be used to test therapeutic interventions such as platelet transfusions in in vitro models. These samples can also provide a whole blood environment into which other platelets can be added to evaluate their function such as is the case with platelets generated from induced pluripotent stem cells.
In conclusion, this study presents a reliable and accurate method to produce thrombocytopenic whole blood samples with or without anemia from healthy volunteers in whom platelet function is normal. This method does not alter baseline levels of exposed P-selectin and activated GPIIbIIIa in unstimulated platelets nor does it impair the ability of platelets to be activated by ADP or CRP. This model of thrombocytopenia has multiple potential applications that can aid efforts to understand how platelet count and function independently contribute to bleeding risk in patients with thrombocytopenia and that can be used to test the efficacy of new therapeutics.
Supplementary Material
Acknowledgments
This project was supported by a grant from Advancing a Healthier Wisconsin and the Clinical and Translational Institute of Southeastern Wisconsin through the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001436. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Additional resources were provided by the BloodCenter Research Foundation and Medical Sciences Institute at BloodCenter of Wisconsin.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00277-016-2777-9) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, Bakrania L, Llewelyn C, Littlewood T, Soutar R, Norfolk D, Copplestone A, Smith N, Kerr P, Jones G, Raj K, Westerman DA, Szer J, Jackson N, Bardy PG, Plews D, Lyons S, Bielby L, Wood EM, Murphy MF Investigators T. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771–1780. doi: 10.1056/NEJMoa1212772. [DOI] [PubMed] [Google Scholar]
- 2.Wandt H, Schaefer-Eckart K, Wendelin K, Pilz B, Wilhelm M, Thalheimer M, Mahlknecht U, Ho A, Schaich M, Kramer M, Kaufmann M, Leimer L, Schwerdtfeger R, Conradi R, Dolken G, Klenner A, Hanel M, Herbst R, Junghanss C, Ehninger G, Study Alliance L. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet. 2012;380(9850):1309–1316. doi: 10.1016/S0140-6736(12)60689-8. [DOI] [PubMed] [Google Scholar]
- 3.Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, Gernsheimer TB, Ness PM, Brecher ME, Josephson CD, Konkle BA, Woodson RD, Ortel TL, Hillyer CD, Skerrett DL, McCrae KR, Sloan SR, Uhl L, George JN, Aquino VM, Manno CS, McFarland JG, Hess JR, Leissinger C, Granger S. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362(7):600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Lindern JS, van den Bruele T, Lopriore E, Walther FJ. Thrombocytopenia in neonates and the risk of intraventricular hemorrhage: a retrospective cohort study. BMC Pediatr. 2011;11:16. doi: 10.1186/1471-2431-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lieberman L, Bercovitz RS, Sholapur NS, Heddle NM, Stanworth SJ, Arnold DM. Platelet transfusions for critically ill patients with thrombocytopenia. Blood. 2014;123(8):1146–1151. doi: 10.1182/blood-2013-02-435693. quiz 1280. [DOI] [PubMed] [Google Scholar]
- 6.Estcourt L, Stanworth S, Doree C, Hopewell S, Murphy MF, Tinmouth A, Heddle N. Prophylactic platelet transfusion for prevention of bleeding in patients with haematological disorders after chemotherapy and stem cell transplantation. Cochrane Database Syst Rev. 2012;5:CD004269. doi: 10.1002/14651858.CD004269.pub3. [DOI] [PubMed] [Google Scholar]
- 7.De Cuyper IM, Meinders M, van de Vijver E, de Korte D, Porcelijn L, de Haas M, Eble JA, Seeger K, Rutella S, Pagliara D, Kuijpers TW, Verhoeven AJ, van den Berg TK, Gutierrez L. A novel flow cytometry-based platelet aggregation assay. Blood. 2013;121(10):e70–e80. doi: 10.1182/blood-2012-06-437723. [DOI] [PubMed] [Google Scholar]
- 8.Frelinger AL, 3rd, Grace RF, Gerrits AJ, Berny-Lang MA, Brown T, Carmichael SL, Neufeld EJ, Michelson AD. Platelet function tests, independent of platelet count, are associated with bleeding severity in ITP. Blood. 2015;126(7):873–879. doi: 10.1182/blood-2015-02-628461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Israels SJ. Laboratory testing for platelet function disorders. Int J Lab Hematol. 2015;37(Suppl 1):18–24. doi: 10.1111/ijlh.12346. [DOI] [PubMed] [Google Scholar]
- 10.McGlasson DL, Fritsma GA. Whole blood platelet aggregometry and platelet function testing. Semin Thromb Hemost. 2009;35(2):168–180. doi: 10.1055/s-0029-1220325. [DOI] [PubMed] [Google Scholar]
- 11.Carcao MD, Blanchette VS, Stephens D, He L, Wakefield CD, Butchart S, Christie DJ, Rand ML. Assessment of thrombocytopenic disorders using the platelet function analyzer (PFA-100) Br J Haematol. 2002;117(4):961–964. doi: 10.1046/j.1365-2141.2002.03511.x. [DOI] [PubMed] [Google Scholar]
- 12.Vinholt PJ, Hvas AM, Nybo M. An overview of platelet indices and methods for evaluating platelet function in thrombocytopenic patients. Eur J Haematol. 2014;92(5):367–376. doi: 10.1111/ejh.12262. [DOI] [PubMed] [Google Scholar]
- 13.Larsen OH, Ingerslev J, Sorensen B. Whole blood laboratory model of thrombocytopenia for use in evaluation of hemostatic interventions. Ann Hematol. 2007;86(3):217–221. doi: 10.1007/s00277-006-0223-0. [DOI] [PubMed] [Google Scholar]
- 14.Tiedemann SM, Rubak P, Halfdan Larsen O, Hvas AM. Thrombocytopenia model with minimal manipulation of blood cells allowing whole blood assessment of platelet function. Platelets. 2015;4:1–6. doi: 10.3109/09537104.2015.1095873. [DOI] [PubMed] [Google Scholar]
- 15.Michelson AD, Barnard MR, Krueger LA, Frelinger AL, 3rd, Furman MI. Evaluation of platelet function by flow cytometry. Methods. 2000;21(3):259–270. doi: 10.1006/meth.2000.1006. [DOI] [PubMed] [Google Scholar]
- 16.Linden MD. Platelet flow cytometry. Methods Mol Biol. 2013;992:241–262. doi: 10.1007/978-1-62703-339-8_18. [DOI] [PubMed] [Google Scholar]
- 17.Lim YA, Cho SR, Lee WG, Park JS, Kim SW. Change of platelet activation markers using flow cytometry in patients with hematology/oncology disorders after transfusion. Platelets. 2008;19(5):328–334. doi: 10.1080/09537100802129867. [DOI] [PubMed] [Google Scholar]
- 18.Perez AG, Lana JF, Rodrigues AA, Luzo AC, Belangero WD, Santana MH. Relevant aspects of centrifugation step in the preparation of platelet-rich plasma. ISRN Hematol. 2014;2014:176060. doi: 10.1155/2014/176060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson JA, Visentin GP, Newman PJ, Aster RH. A recombinant soluble form of the integrin aIIbb3 (GPIIb-IIIa) assumes an active, ligand-binding conformation and is recognized by GPIIb-IIIa-specific monoclonal, allo-, auto-, and drug-dependent platelet antibodies. Blood. 1998;92(6):2053–2063. [PubMed] [Google Scholar]
- 20.Hagberg IA, Lyberg T. Blood platelet activation evaluated by flow cytometry: optimised methods for clinical studies. Platelets. 2000;11(3):137–150. doi: 10.1080/095371000403071. [DOI] [PubMed] [Google Scholar]
- 21.Miyakawa Y, Oda A, Druker BJ, Kato T, Miyazaki H, Handa M, Ikeda Y. Recombinant thrombopoietin induces rapid protein tyrosine phosphorylation of Janus kinase 2 and Shc in human blood platelets. Blood. 1995;86(1):23–27. [PubMed] [Google Scholar]
- 22.Ferrer-Marin F, Chavda C, Lampa M, Michelson AD, Frelinger AL, 3rd, Sola-Visner M. Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost. 2011;9(5):1020–1028. doi: 10.1111/j.1538-7836.2011.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.