Abstract
To understand how the brain regulates behavior, many variables must be taken into account, with sex as a prominent variable. In this review, we will discuss recent human and rodent studies showing the sex-specific involvement of the neuropeptides vasopressin and oxytocin in social and anxiety-related behaviors. We discuss that sex differences can be evident at pre-pubertal ages as seen in the sex-specific regulation of social recognition, social play, and anxiety by the vasopressin system in juvenile rats. We further discuss that the oxytocin system in humans and rodents alters brain activation, anxiety, and sociosexual motivation in sex-specific ways. Finally, we propose that knowledge of vasopressin and oxytocin mediated sex-specific brain mechanisms can provide essential insights into how these neuropeptide systems contribute to sex-specific vulnerability as well as resilience to perturbations, with subsequent relevance to social and emotional disorders.
1. Studying both sexes provides a more complete understanding of how the brain modulates behavior
The 2014 National Institutes of Health policy of implementing sex as biological variable has stimulated a lot of discussion, with pros and cons of the policy voiced by a wide variety of scientists [1–5]. There is a strong tendency of simplifying and standardizing experimental designs and methods, including using a limited number of model organisms, contexts, and behavioral tests, and limiting studies to one sex [6–9]. This approach has been essential to gain a basic understanding of how the brain modulates behavior. Yet, we have obtained a very narrow and incomplete view of brain function [6]. In a first step to gain a more complete and meaningful understanding of how the brain mediates behavior, both sexes must be studied. Although males and females may be similar at the behavioral level, they often use different mechanisms to respond to social and emotional challenges and opportunities [10, 11]. To illustrate the importance of studying both sexes, this mini-review will highlight a few recent studies that have provided insights into the behavioral roles of the neuropeptides vasopressin (AVP) and oxytocin (OXT) in males and females, often with intriguing sex-specific outcomes.
2. Involvement of the AVP system in the sex-specific regulation of social and anxiety-related behaviors (Fig. 1)
Figure 1. Sex differences in the vasopressin (AVP) system in the rat brain and sex-specific regulation of behavior by the lateral septum (LS) AVP system in rats.
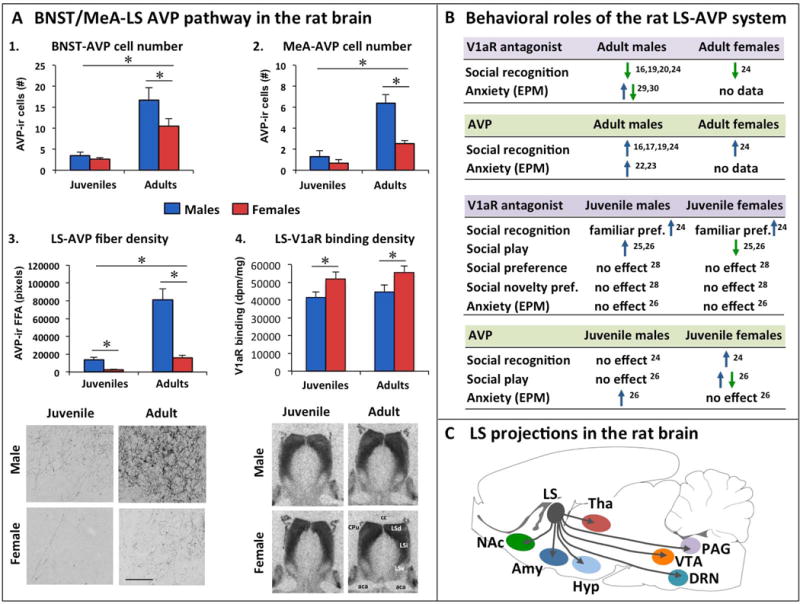
(A) Sex differences are found in the AVP pathway from bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA) to the lateral septum (LS): 1. Adult male rats have more AVP-immunoreactive (AVP-ir) cells in the posterior BNST than adult female rats, while there are fewer cells and no sex difference in juvenile rats [adapted from 16]. 2. Adult male rats have more AVP-immunoreactive (AVP-ir) cells in the posterodorsal MeA than adult female rats, while there are fewer cells and no sex difference in juvenile rats [adapted from 16]. 3. In the ventral caudal part of the LS, adult and juvenile male rats show denser AVP-ir fibers than females, and adults show denser AVP-ir fibers than juveniles [adapted from 16]; Photomicrographs (scale bar = 100 μm) depict AVP-ir fibers in the ventral caudal part of the LS of a juvenile and adult male and female rat [adapted from 16]. 4. In the dorsolateral LS, juvenile female rats have denser AVP V1a receptor (V1aR) binding than juvenile male rats, and adults show denser V1aR binding than juveniles [adapted from 17]; Autoradiographs show dense V1aR binding in the dorsolateral LS, which includes the dorsal part of the LS (LSd) and the lateral portion of the intermediate part of the LS (LSi) [adapted from 17]. (B) Pharmacological studies in adult rats demonstrate that, despite sex differences in the LS-AVP system, V1aR antagonist impairs and exogenous AVP improves social recognition in both male [17, 19, 20, 22, 23] and female [17] rats. The role of the LS-AVP system is more complicated in juvenile rats: the LS-AVP system regulates social play (V1aR antagonist, exogenous AVP) [27, 28] and social recognition (exogenous AVP) [17] in sex-specific ways. It should be noted that the same dose of the V1aR antagonist d(CH2)5[Tyr(Me)2]AVP (10 ng/0.5 μl) and the same dose of AVP (200 pg/0.5μl) were used in [27, 28], suggesting age differences in the role of the LS-AVP system regulating social recognition. Social recognition is reflected by the ability to discriminate between a novel and a familiar same-sex 3-week-old stimulus rat; Social preference is reflected by the preference to investigate a novel conspecific over a novel object; Social novelty preference is reflected by the preference to investigate a novel conspecific over a cage mate. The role of the LS-AVP system in anxiety-related behavior as determined on the elevated plus-maze (EPM) reveals for the most part anxiogenic effects of LS-AVP in adult and juvenile male rats. It should be noted that an increase in anxiety was seen after chronic V1aR antagonist application in the LS [32], while a decrease in anxiety was seen after a single V1aR antagonist application in the LS [31]. (C) The LS has many projections to telencephalon, diencephalon and mesencephalon, with the most notable output to the ventral tegmental area (VTA), nucleus accumbens (NAc), periaqueductal grey (PAG), dorsal raphe nucleus (DRN), hypothalamus (HYP), amygdala (AMY), and thalamus (THA) [12, 35]. It is possible that sex differences in the behavioral effects of LS-AVP manipulations can be, in part, attributed to sex differences in the recruitment of specific LS outputs to mediate behavior. These can include LS-induced changes in mesolimbic reward systems (through e.g., VTA and Nac), neuroendocrine and autonomic projections (through e.g., HYP, PAG, and indirectly via AMY), alterations in monoamine functioning (serotonin through DRN connection and norepinephrine through LC connection, not shown). aca, anterior part of the anterior commissure; cc, corpus collosum; Cpu, caudate putamen; EPM, elevated plus-maze; F, female; FFA, fiber fractional area; LSd, dorsal part of the lateral septum; LSi, intermediate part of the lateral septum; LSv, ventral part of the lateral septum; M, male; *p < 0.05.
AVP is synthesized in several hypothalamic and extrahypothalamic regions and can modulate the activation of numerous brain regions through the AVP V1a receptor (V1aR). In this section, we will discuss recent studies that focused on the behavioral roles of the AVP system in the lateral septum (LS), a key brain region involved in the regulation of emotion, reward, and social behavior [12]. The LS receives vasopressinergic innervations from the bed nucleus of the stria terminalis (BNST) and medial amygdala (MeA) [13, 14]. The LS-AVP system in the rat shows complex sex differences: compared to females, adult males have denser AVP axonal fibers, but less V1aR binding [15–18] (Fig 1A). Many studies have shown an important role of the LS-AVP system in the regulation of various social behaviors in adult male rodents [19–26]. Recent comparative studies have demonstrated the involvement of the LS-AVP system in social behavior regulation in females as well. In detail, application of a V1aR antagonist into the LS impaired social recognition in both adult male and female rats [17] (Fig 1D). Likewise, administration of AVP into the LS prolonged social recognition in both adult male and female rats [17] (Fig. 1D). Together, these findings indicate that, despite sex differences in AVP fiber and V1aR densities, the LS-AVP system in adult rats seems to play a similar role in the regulation of social recognition in males and females.
Interestingly, a similar analysis of juvenile (5-week-old) rats revealed sex differences in the function of the LS-AVP system. LS-AVP fiber density is significantly lower in juveniles of both sexes compared with adults; yet, juvenile males have denser LS-AVP fibers than females [16] (Fig 1B, C). In contrast, LS-V1aR binding is very dense at both ages, but here, juvenile males show lower V1aR binding density than females (Fig 1B, C) [17]. With regards to the functional implications, pharmacological blockade of V1aR in the LS did not impair social recognition in juvenile males and females, but instead, induced a preference to investigate the previously encountered stimulus rat over the novel stimulus rat [17]. Moreover, administration of AVP into the LS improved social recognition in female, but not male, juveniles [17]. These results demonstrate that the regulation of social recognition by the LS-AVP system and sex differences in this regulation are age-dependent.
Analysis of other behaviors in juvenile rats revealed that the sex-specific role of the LS-AVP system is highly behavior specific. Here, pharmacological blockade of V1aR in the LS increased social play behavior in juvenile males, but decreased social play behavior in females [27, 28]. Social play is a highly rewarding behavior predominantly displayed by juveniles and social play interactions contribute to the development of social skills [29]. LS-V1aR blockade did not alter social preference (i.e., preference to investigate a novel conspecific over a novel object) [30], social novelty preference (i.e., preference to investigate a novel conspecific over a cage mate) [30], or anxiety-related behavior [28] in either sex. Together, these findings demonstrate that the LS-V1aR in juvenile male and female rats is important for the regulation of specific types of behavior, i.e., social recognition and social play behavior. Intriguingly, LS-V1aR blockade alters social recognition in a similar direction in males compared to females, but alters social play in an opposite direction in males compared to females. This implies that the LS-V1aR-activated pathways mediating social recognition are likely similar between the sexes, while the LS-V1aR-activated pathways mediating social play are likely distinct between the sexes.
Finally, we determined the effects of AVP administered into the LS on social recognition, social play, and anxiety-related behavior in juvenile rats. Application of AVP into the LS improved social recognition in female, but not male, juveniles [17]. Moreover, LS-AVP administration increased social play in female, but not male, juveniles when tested in the home cage [27, 28]. Along with the LS-V1aR blockade-induced decrease in social play behavior, this suggests that social play in a familiar setting is facilitated by the LS-AVP system. In contrast, LS-AVP administration decreased social play behavior in female, but not male, juveniles when tested in a novel cage [28]. This indicates that the effects of LS-AVP in female juveniles are strongly dependent on the familiarity of the environment. Interestingly, AVP increased anxiety-related behavior on the elevated plus-maze in male, but not female, juveniles [28]. This anxiolytic effect of LS-AVP in male juvenile rats is in line with studies in adult male rodents (adult virgin female rodents have not been tested) [22, 25, 31, 32]. Overall, these comparative studies in juvenile rats suggest that AVP applied to the LS changes primarily social behaviors in juvenile females, while altering primarily anxiety-related behavior in juvenile males.
To summarize, the LS-AVP system in rats is involved in the sex-specific regulation of social recognition, social play, and anxiety-related behavior in juveniles as well as in the age-specific regulation of social recognition (Fig 1B). Although the V1aR antagonist and AVP may have some affinity for the OXT receptor (OTR) [33], it seems unlikely that this contributed to the observed sex-specific effects, because parallel studies administering OTR antagonist or OXT into the LS yielded different behavioral effects than those mediated by V1aR antagonist or AVP, respectively [27, 28]. Two main questions that need to be addressed are how this sex-specific regulation (1) corresponds with sex differences in LS-AVP fibers and LS-V1aR binding and (2) affects downstream pathways. It is likely that LS-V1aR activation modulates the activation of other neurotransmitter systems in the LS. The LS receives input from major neurotransmitter cell groups, including glutamatergic, GABAergic, serotoninergic, dopaminergic, and norepinephrinergic input [12]. We recently showed that blockade of ionotropic glutamate receptors in the LS decreased social play behavior in juvenile female, but not male, rats [34]. It would be interesting to determine whether LS-V1aR activation modulates LS-glutamate activity to regulate social play in sex-specific ways. Finally, LS-V1aR activation likely induces sex-specific changes in the activation of downstream pathways to modulate social recognition, social play, and anxiety in sex-specific ways. These could include intracellular pathways as well as LS projection regions important for learning and memory (such as hippocampus, indirect projection via hypothalamus [35]) to mediate social recognition, regions important for motivation and reward (such as the ventral tegmental area and nucleus accumbens [12]) to mediate social play behavior, and regions important for emotion (such as the amygdala and hypothalamus [12, 35] to mediate anxiety-related behavior (see Fig. 1C).
3. Involvement of the OXT system in the sex-specific regulation of social and anxiety-related behaviors (Fig. 2)
Figure 2. Sex differences in the oxytocin (OXT) system in the rat brain and sex-specific effects on brain activation and behavior by the OXT system.
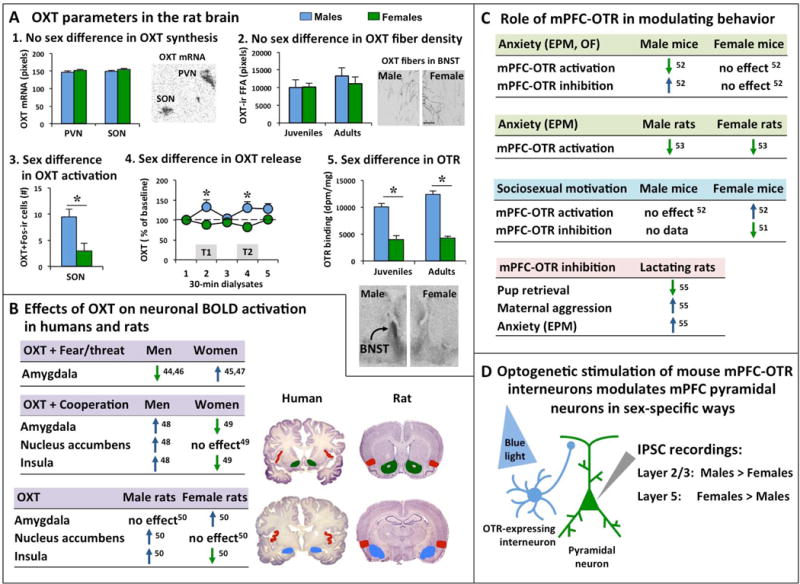
(A) Analysis of the rat OXT system reveals sex differences in some, but not all OXT parameters: 1. No sex differences are found in OXT mRNA expression in the paraventricular nucleus (PVN) and supraoptic nucleus (SON) of the hypothalamus in adult rats [adapted from 41]. The autoradiogram depicts OXT mRNA expression (black) in the left PVN and left SON in a 16-μm coronal brain section of an adult male rat [adapted from 41]. 2. No sex differences are found in OXT-immunoreactive (OXT-ir) fiber density (expressed as fiber fractional area or FFA) in the bed nucleus of the stria terminalis (BNST) of juvenile and adult rats [adapted from 16]. Photomicrographs depict OXT-ir fibers in the BNST of a male and female adult rat [adapted from 16]; Scale bar indicates 100 μm. 3. Juvenile male rats have more Fos-positive OXT-ir neurons in the SON than females [38]; Fos is an immediate early gene used as marker for neuronal activation. 4. Adult male rats show higher extracellular OXT release (calculated as percentage of baseline OXT release) in the posterior BNST compared to females during both trials of the social discrimination tests in which the rats were exposed to an unfamiliar sex-matched juvenile rat during trial 1 (T1) and the same previously encountered unfamiliar juvenile rat (now familiar) along with a second unfamiliar sex-matched juvenile rat during trial 2 (T2) [adapted from 39]. 5. Juvenile and adult male rats show denser OXT receptor binding in the posterior BNST compared to females [adapted from 18]. Autoradiograms show representative OXT receptor binding in the posterior BNST of an adult male and adult female rat [adapted from 18]. (B) Intranasal application of OXT in humans and intracerebroventricular administration of OXT in rats induce sex-specific blood-oxygen-level dependent (BOLD) activation of the amygdala, nucleus accumbens, and insula [44–50]. In the human studies, men and women were exposed to fearful and/or threatening images or scenes (Fear/threat) or were exposed to an interactive social game (the Prisoner’s Dilemma Game) to examine cooperative interactions (Cooperation). Images depict coronal sections of the human brain (source: https://msu.edu/user/brains/brains/human/) and rat brain [source: 64]. Colors in the coronal brain section depict the amygdala (blue), nucleus accumbens (green), and insula (red). (C) Studies in mice show that the OXT receptor (OTR) in the medial prefrontal cortex (mPFC) regulates anxiety-related behavior and sociosexual motivation in sex-specific ways [51, 52]. In contrast, the mPFC-OTR in rats regulates anxiety-related behavior similiary in males and females [53]. Moreover, the mPFC-OTR is involved in maternal care (pup retrieval), maternal aggression, and anxiety in lactating rats [55]. (D) Studies in mice have shown that in vitro optogenetic stimulation of OTR-expressing interneurons induces a stronger inhibitory postsynaptic current (IPSC) in layer 2/3 pyramidal neurons (important for intra-mPFC connectivity) in males compared to females and a stronger IPSC in layer 5 pyramidal neurons (important for output to subcortical regions) in females compared to males [52]. * p<0.05 versus females.
OXT modulates social and anxiety-related behaviors in males and females of various species including humans, rats, and mice, and often does so in sex-specific ways (for comprehensive reviews see [36, 37]. Yet, in most rodent and primate species (including humans) examined, there are no sex differences in OXT synthesis or number of OXT neurons in the brain [reviewed in 36, 37] (Fig 2A). Furthermore, in juvenile and adult rats, there are no sex differences in OXT fiber density in forebrain regions encompassing the social behavior neural network, including the LS, BNST, MeA, medial preoptic area, anterior hypothalamus, and ventromedial hypothalamus [16] (Fig. 2A). However, a sex difference was found in Fos-activated OXT neurons in the supraoptic nucleus of the hypothalamus (SON) in juvenile rats. Here, juvenile male rats showed higher Fos expression in SON-OXT neurons than juvenile female rats, a sex difference that was independent on whether the juveniles were exposed to a sex- and age-matched play mate for 10 min or nothing 90 min before perfusion [38]. These results suggest the potential for higher OXT release in male compared to female juveniles. Interestingly, a sex difference was found in the extracellular release of OXT in response to social stimuli in adult rats. In detail, extracellular OXT release (expressed as percentage from baseline OXT release) in the posterior BNST was higher in adult male rats compared to females during exposure to a social recognition test [39] (Fig 2A). Finally, sex differences have been found in OTR expression in the rodent brain. For example, adult male rats showed higher OTR mRNA expression [40] and higher OTR binding density [18, 41] than females in the ventromedial hypothalamus. Furthermore, juvenile and adult male rats showed higher OTR binding density than females in the posterior BNST [18, 41] (Fig 2A) and medial amygdala [18, 41]. In contrast, lower OTR binding density was found in adult male prairie and montane voles compared to females in the medial prefrontal cortex (mPFC) [42]. These examples illustrate the complex pattern of sex differences in the OXT system, which are highly species-, and brain region-specific [reviewed in 26, 37]. Further research is required to unravel how sex differences in the OXT system are involved in enabling males and females to display species-appropriate behaviors in similar or sex-specific ways.
Recent studies in humans and rats suggest that the sex-specific behavioral regulation by the OXT system may involve activation of sex-specific neural circuitries (Fig. 2B). For example, young adult women respond to intranasal OXT by a strengthening of resting-state amygdala-mPFC functional connectivity, an effect that was not seen in young adult men or older adult women [43]. It should be noted, however, that young adult men had greater amygdala–mPFC connectivity strength than women under placebo, which could have favored an effect of OXT in women only. Yet, these findings indicate that the OXT system plays a role in modulating connectivity strength between subcortical and cortical regions that may have implications for sex-specific behaviors. Furthermore, intranasal OXT decreased fear and threat-induced amygdala blood oxygen level-dependent (BOLD) activation in men, but increased it in women [44–47] (Fig. 2B). Interestingly, the opposite effect was found during human cooperation. Here, intranasal OXT administration increased amygdala BOLD activation in men, but decreased it in women [48, 49] (Fig 2B). Intranasal OXT further induced sex-specific BOLD activation in the nucleus accumbens (increased activation in men, no change in women), and insular cortex (increased activation in men, decreased activation in women [48, 49]) (Fig. 2B). Strikingly, intracerebroventricular application of OXT in awake adult rats induced sex differences in BOLD activation in the same brain regions as in humans, i.e., more OXT-induced activation in the amygdala of females, less OXT-induced activation in the insular cortex in females, and more OXT-induced activation in the nucleus accumbens and insular cortex of males [50] (Fig. 2B). This implies that there are some similarities between humans and rats in the sex-specific effects of OXT on brain activation and may provide an opportunity to use rats as model organism to investigate the underlying mechanisms.
Recent studies in mice provide evidence for the involvement of OTR in the mPFC in mediating sex differences in anxiety-related behavior (Fig. 2C, D). OTRs are expressed by a small population of interneurons in the mouse mPFC, with equal expression in males and females [51]. However, optogenetic stimulation of these OTR-expressing interneurons decreased anxiety-related behavior in males, while it did not alter anxiety-related behavior in females [52] (Fig 2C). Likewise, viral vector-mediated knockdown of the OTR in the mPFC increased anxiety-related behavior in males, but not in females [52]. Although electrophysiological recordings of OTR-expressing interneurons in the mPFC did not show differences between males and females, mPFC pyramidal neurons (that likely receive input from OTR-expressing interneurons) responded in a sex-specific way. Here, optogenetic stimulation of OTR-expressing interneurons induced a stronger inhibitory effect in layer 2/3 pyramidal neurons in males and a stronger inhibitory effect in layer 5 pyramidal neurons in females [52] (Fig. 2D). Layer 2/3 pyramidal neurons are important for intraregional connectivity while layer 5 pyramidal neurons have axons projecting to regions outside the cortex. Moreover, OTR-expressing interneurons show a remarkably different gene expression profile in male versus female mice (shown in Table S2 in [52]). Together, this demonstrates the potential of OTR-expressing interneurons in the mPFC to be part of a distinct neural network in male versus female mice that, in turn, may underlie the observed sex differences in anxiety-related behavior mediated by activation or inhibition of mPFC OTR-expressing interneurons.
Interestingly, the mPFC-OTR in female mice plays a role in sociosexual motivation [51]. In detail, impairing OTR function in the mPFC (either by chronic silencing of OTR-expressing interneurons, viral vector-mediated knockdown of the OTR gene in OTR-expressing interneurons, or administration of an OTR antagonist into the mPFC) reduced the preference of estrus female mice to investigate a novel adult male mouse over a novel object [51] (Fig 2C). In contrast, the preference to investigate a novel female over a novel object was not altered upon silencing mPFC-OTR in estrus female mice [51]. This indicates that activation of mPFC-OTR mediates sociosexual motivation rather than general social motivation in female mice. Furthermore, optogenetic stimulation of OTR-expressing interneurons in the mPFC increased the preference of female mice to investigate a male mouse over a novel object, while it didn’t alter the preference of male mice to investigate a female mouse over a novel object [52] (Fig 2C). This suggests a sex-specific regulation of sociosexual motivation by mPFC-OTR. However, social investigation was higher in male than female mice [52]. Thus, a ceiling effect may have prevented optogenetic mPFC-OTR activation to further increase social interest in male mice. An essential experiment would be to determine whether sociosexual motivation could be decreased in male mice by blocking mPFC-OTR function. Unfortunately, this experiment has yet to be performed. If further research were to find that impairing mPFC-OTR function in males does not alter sociosexual motivation, then it would be highly interesting to investigate the sex-specific mechanisms underlying mPFC-OTR-mediated sociosexual motivation.
In contrast to mice, OXT application to the mPFC of rats reduced anxiety-related behavior (as measured on the elevated plus-maze) in both sexes [53], indicating a species-specific role of mPFC-OTR in modulating anxiety (Fig. 2C). The OXT effects on anxiety in rats were restricted to the prelimbic mPFC, required OTR (not V1aR) activation, and were likely mediated via activation of GABAergic interneurons [54]. Furthermore, mPFC-OTR blockade impaired pup retrieval behavior, increased maternal aggression, and increased anxiety in lactating rats [55] (Fig. 2C), suggesting an extended role for mPFC-OTR in not only sociosexual motivation, but more broadly in reproductive behaviors (mating and maternal care) in female rodents. Given the possible sex differences in sociosexual motivation mediated by mPFC-OTR in mice [52], it would be interesting to determine whether the mPFC-OTR is involved in sex-specific reproductive behaviors in rats.
To summarize, we discussed that OXT application induced sex differences in the activation of similar brain regions in humans and rats [44–50]. Furthermore, the mPFC-OTR is involved in the sex-specific regulation of anxiety-related behavior in mice but not in rats [52–55]. Finally, the mPFC-OTR was shown to regulate sociosexual motivation in female mice [51]. It is yet unclear to what extent these sex-specific effects mediated by the OXT system occurred due to sex differences in OXT neurotransmission and/or sex differences in downstream targets. Studies in mice have started to shed light on such potential mechanisms by showing sex differences in gene expression of mPFC-OTR interneurons and sex differences in the strength of inhibition by mPFC-OTR interneurons on specific subsets of pyramidal mPFC neurons [52], which both may enable the sex-specific regulation of anxiety by mPFC-OTR.
4. Sex differences in behavior mediated by AVP and OXT: Basis for sex differences in social and emotional disorders? (Fig. 3)
Figure 3. Unraveling the neuronal mechanisms that makes one sex vulnerable and the other sex resilient may help to improve treatment options for social and emotional disorders.
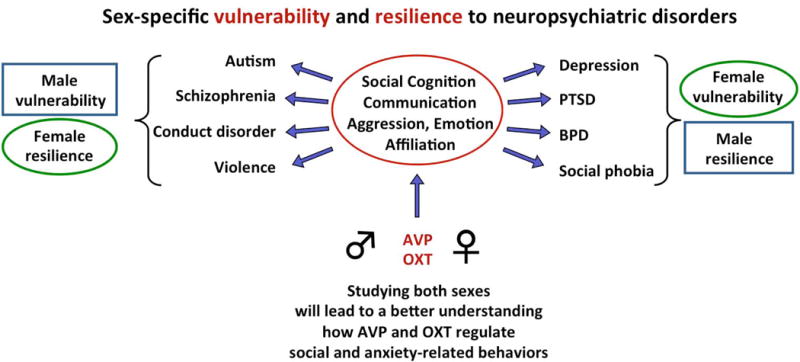
AVP and OXT regulate social behaviors in sex-specific ways, suggesting that perturbations of AVP/OXT systems have different behavioral consequences for males compared to females and could cause one sex to be more vulnerable and the other sex to be more resilient to the development of a specific social or emotional disorder. Studying the mechanisms underlying the sex-specific behavioral effects of AVP and OXT systems will not only provide a more complete understanding of the function of the brain for behavior, but also has the potential to provide insights into sex-specific vulnerability and resilience to these neuropsychiatric disorders. PTSD, posttraumatic disorder; BPD, borderline personality disorder.
The above-discussed studies in humans and rodents illustrate that the integration of sex as a variable provides unique and essential insights into the different ways AVP and OXT can regulate behavior, which, in turn, may have relevance for human health and disease. Specifically, the sex-specific regulation of behavior by AVP and OXT systems suggests that perturbations of these systems will have different consequences for males versus females. In support, several studies have shown sex-specific changes in AVP and OXT systems in response to various environmental challenges, especially when they occur in early life [56–59]. Moreover, AVP and OXT have been implicated in the pathophysiology and treatment of several social and emotional disorders [60, 61] and these disorders often show a strong sex bias in prevalence and treatment responses [62, 63]. Accordingly, if one sex is more vulnerable, then it follows that the other sex must be more resilient to the development of these disorders. Therefore, comparing the roles of AVP/OXT systems in males versus females may provide important insights into the mechanisms that mediate sex-specific vulnerability and resilience to social and emotional disorders in which AVP and/or OXT play a role (Fig. 3). Finally, the above-discussed studies have shown that the sex-specific regulation of social and anxiety-related behaviors by the AVP system is evident during early development [27, 28]. This may have relevance for understanding sex-biased social and emotional disorders that have an early onset, such as autism spectrum disorder. In closing, neuroscientists have only just begun to uncover the sex-specific involvement of the AVP and OXT systems in behavior and brain functions. These findings have reassured the importance of including both sexes in basic and preclinical research. Further research into the mechanisms and conditions by which the AVP and OXT systems regulate behavior differently in males compared to females is necessary in providing a more complete understanding of the various ways in which the brain regulates behavior which, in turn, will provide insights into the sex-biases observed in social and emotional disorders.
Highlights.
AVP regulates social recognition, social play, and anxiety in sex-specific ways in juvenile rats
OXT induces sex-specific activation of the amygdala, nucleus accumbens, and insular in humans and rats
OXT in the prefrontal cortex regulates anxiety in sex-specific ways in mice but not rats
OXT in the prefrontal cortex modulates sociosexual motivation in female mice
AVP/OXT may provide insights into sex-specific vulnerability and resilience to social/emotional disorders
Acknowledgments
We would like to thank the Veenema lab for critically reading the manuscript. This work was supported by NSF IOS 1253386, NIMH R15MH102807, and NIMH R01MH102456 to AHV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fields RD. NIH policy: mandate goes too far. Nature. 2014;510:340. doi: 10.1038/510340a. [DOI] [PubMed] [Google Scholar]
- 2.McCullough LD, McCarthy MM, de Vries GJ. NIH policy: status quo is also costly. Nature. 2014;510:340. doi: 10.1038/510340b. [DOI] [PubMed] [Google Scholar]
- 3.Clayton JA. Studying both sexes: a guiding principle for biomedicine. FASEB J. 2016 Feb;30(2):519–24. doi: 10.1096/fj.15-279554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eliot L, Richardson SS. Sex in Context: Limitations of Animal Studies for Addressing Human Sex Gender Neurobehavioral Health Disparities. J Neurosci. 2016 Nov 23;36(47):11823–11830. doi: 10.1523/JNEUROSCI.1391-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shansky RM, Woolley CS. Considering Sex as a Biological Variable Will Be Valuable for Neuroscience Research. J Neurosci. 2016 Nov 23;36(47):11817–11822. doi: 10.1523/JNEUROSCI.1390-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taborsky M, Hofmann HA, Beery AK, Blumstein DT, Hayes LD, Lacey EA, Martins EP, Phelps SM, Solomon NG, Rubenstein DR. Taxon matters: promoting integrative studies of social behavior: NESCent Working Group on Integrative Models of Vertebrate Sociality: Evolution, Mechanisms, and Emergent Properties. Trends Neurosci. 2015;38(4):189–91. doi: 10.1016/j.tins.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Donaldson ZR. We’re the Same… but Different: Addressing Academic Divides in the Study of Brain and Behavior. Front Behav Neurosci. 2010 Jul;21:4. doi: 10.3389/fnbeh.2010.00041. pii: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann HA, Renn SC, Rubenstein DR. Introduction to Symposium: New Frontiers in the Integrative Study of Animal Behavior: Nothing in Neuroscience Makes Sense Except in the Light of Behavior. Integr Comp Biol. 2016 Dec;56(6):1192–1196. doi: 10.1093/icb/icw127. [DOI] [PubMed] [Google Scholar]
- 9**.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011 Jan;35(3):565–72. doi: 10.1016/j.neubiorev.2010.07.002. The authors performed a large analysis of sex bias in biomedical research and found biases toward male animals in eight of the 10 fields with the highest male-to-female ratios in neuroscience (5.5:1), pharmacology (5:1), and physiology (3.7:1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bangasser DA, Wicks B. Sex-specific mechanisms for responding to stress. J Neurosci Res. 2017 Jan 2;95(1-2):75–82. doi: 10.1002/jnr.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Palanza P, Parmigiani S. How does sex matter? Behavior, stress and animal models of neurobehavioral disorders. Neurosci Biobehav Rev. 2017 May;76(Pt A):134–143. doi: 10.1016/j.neubiorev.2017.01.037. Excellent review emphasizing that males and females use different social strategies and that this is associated with marked differences in which males and females respond to social challenges. The authors argue that data obtained in males are often irrelevant for inferring psychopathology and efficacy of pharmacological treatments for females, and that female animal modeling should use an ethological approach and center around female reproduction given the higher parental investment and reproductive burden in females compared to males. [DOI] [PubMed] [Google Scholar]
- 12*.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46(1):71–117. doi: 10.1016/j.brainresrev.2004.04.009. Comprehensive review on the lateral septum highlighting its central role in mood and motivation through extensive connections with the mesocorticolimbic dopamine system, hippocampus, and hypothalamus, among others. [DOI] [PubMed] [Google Scholar]
- 13.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 14.Caffé AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- 15*.De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain–presence of a sex difference in the lateral septum. Brain Res. 1981;218(1–2):67–78. doi: 10.1016/0006-8993(81)90989-6. This study in rats is the first to report the existence of a sex difference in vasopressin fiber density in the brain. The finding was somewhat serendipitous; rats were separated by sex when the authors found a “disturbingly large variability”. Since these first findings, homologous sex differences have been found in many different species, in mammals as well as other vertebrates. [DOI] [PubMed] [Google Scholar]
- 16.DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 2017;525(11):2549–2570. doi: 10.1002/cne.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm Behav. 2012 Jan;61(1):50–6. doi: 10.1016/j.yhbeh.2011.10.002. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CJW, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct Funct. 2017;222(2):981–1006. doi: 10.1007/s00429-016-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dantzer R, Koob GF, Bluthé RM, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988 Aug;457(2)(1):143–7. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 20.Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994 Jan;55(1):145–9. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Ferris CF, De Vries GJ. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster) Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):400–4. doi: 10.1073/pnas.91.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CJ, Holsboer F, Engelmann M. V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci. 1995;15:4250–4258. doi: 10.1523/JNEUROSCI.15-06-04250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003 Jul;18(2):403–11. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- 24.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005 Aug 18;47(4):503–13. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 25.Beiderbeck DI, Neumann ID, Veenema AH. Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. Eur J Neurosci. 2007 Dec;26(12):3597–605. doi: 10.1111/j.1460-9568.2007.05974.x. [DOI] [PubMed] [Google Scholar]
- 26.Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010 Jul;58(2):273–81. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 27*.Veenema AH, Bredewold R, De Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38(11):2554–61. doi: 10.1016/j.psyneuen.2013.06.002. Demonstration that vasopressin regulates social play in opposite ways in male and female juvenile rats as shown by V1aR antagonist application intracerebroventricularly and locally in the lateral septum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bredewold R, Smith CJ, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Front Behav Neurosci. 2014;8:216. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trezza V, Baarendse PJ, Vanderschuren LJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–9. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith CJ, Wilkins KB, Mogavero JN, Veenema AH. Social Novelty Investigation in the Juvenile Rat: Modulation by the μ-Opioid System. J Neuroendocrinol. 2015;27(10):752–64. doi: 10.1111/jne.12301. [DOI] [PubMed] [Google Scholar]
- 31.Liebsch G, Wotjak CT, Landgraf R, Engelmann M. Septal vasopressin modulates anxiety-related behaviour in rats. Neurosci Lett. 1996 Oct 18;217(2-3):101–4. [PubMed] [Google Scholar]
- 32.Everts HG, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res. 1999 Feb 15;99(1):7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- 33.Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog Brain Res. 2008;170:473–512. doi: 10.1016/S0079-6123(08)00437-8. [DOI] [PubMed] [Google Scholar]
- 34.Bredewold R, Schiavo JK, van der Hart M, Verreij M, Veenema AH. Dynamic changes in extracellular release of GABA and glutamate in the lateral septum during social play behavior in juvenile rats: Implications for sex-specific regulation of social play behavior. Neuroscience. 2015;307:117–27. doi: 10.1016/j.neuroscience.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risold PY, Swanson LW. Connections of the rat lateral septal complex. Brain Res Brain Res Rev. 1997;24(2–3):115–95. doi: 10.1016/s0165-0173(97)00009-x. [DOI] [PubMed] [Google Scholar]
- 36*.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrinol. 2016a;40:1–23. doi: 10.1016/j.yfrne.2015.04.003. First comprehensive review on sex differences in vasopressin and oxytocin systems and in their regulation of social behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dumais KM, Veenema AH. Presence and absence of sex differences in structure and function of the brain oxytocin system: Implications for understanding social behavior. In: Shansky R, Johnson J, editors. Sex Differences in the Central Nervous System. Academic Press; 2016b. pp. 247–295. [Google Scholar]
- 38.Reppucci CJ, Gergely CK, Veenema AH. Activation patterns of vasopressinergic and oxytocinergic brain regions following social play exposure in juvenile male and female rats. J Neuroendocrinology. 2018 doi: 10.1111/jne.12582. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39*.Dumais KM, Alonso AG, Immormino MA, Bredewold R, Veenema AH. Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition. Psychoneuroendocrinology. 2016;64:79–88. doi: 10.1016/j.psyneuen.2015.11.007. First study to compare extracellular OXT release in male and female rodents showing that baseline OXT release in the BNST is similar between the sexes, but that males show higher BNST-OXT release than females when exposed to social stimuli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bale TL, Dorsa DM. Sex differences in and effects of estrogen on oxytocin receptor messenger ribonucleic acid expression in the ventromedial hypothalamus. Endocrinology. 1995;136(1):27–32. doi: 10.1210/endo.136.1.7828541. [DOI] [PubMed] [Google Scholar]
- 41.Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex-specific ways. Horm Behav. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–151. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 43.Ebner NC, Chen H, Porges E, Lin T, Fischer H, Feifel D, Cohen RA. Oxytocin’s effect on resting-state functional connectivity varies by age and sex. Psychoneuroendocrinology. 2016;69:50–9. doi: 10.1016/j.psyneuen.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domes G, Heinrichs M, Glascher J, Muchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62(10):1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 45**.Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35(1):83–93. doi: 10.1016/j.psyneuen.2009.06.016. First study in women studying the effects of intranasal oxytocin on social emotional stimuli and demonstrating that intranasal OXT increased fear-induced amygdala BOLD activation in women, an effect opposite to men. [DOI] [PubMed] [Google Scholar]
- 46.Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lischke A, Gamer M, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC, Domes G. Oxytocin increases amygdala reactivity to threatening scenes in females. Psychoneuroendocrinology. 2012;37:1431–1438. doi: 10.1016/j.psyneuen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 48.Rilling JK, DeMarco AC, Hackett PD, Thompson R, Ditzen B, Patel R, Pagnoni G. Effects of intranasal oxytocin and vasopressin on cooperative behavior and associated brain activity in men. Psychoneuroendocrinology. 2012;37(4):447–461. doi: 10.1016/j.psyneuen.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49**.Rilling JK, DeMarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022. First study to investigate the effect of intranasal oxytocin on brain function in human males and females within the same paradigm and showing sex-specific activation patterns in the nucleus accumbens, central amygdala, and insular cortex during cooperation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Dumais KM, Kulkarni PP, Ferris CF, Veenema AH. Sex differences in neural activation following different routes of oxytocin administration in awake adult rats. Psychoneuroendocrinology. 2017;81:52–62. doi: 10.1016/j.psyneuen.2017.04.003. First study to investigate the effects of central and peripheral application of oxytocin on brain activation in male and female rats using fMRI showing overall activation in more brain regions in males than in females and sex differences in activation in the nucleus accumbens, central amygdala, and insular cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakajima M, Görlich A, Heintz N. Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell. 2014;159(2):295–305. doi: 10.1016/j.cell.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52**.Li K, Nakajima M, Ibañez-Tallon I, Heintz N. A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors. Cell. 2016;167(1):60–72. doi: 10.1016/j.cell.2016.08.067. This study in mice revealed a potential mechanism by which the OTR in the mPFC regulates anxiety-related behavior in sex-specific ways. Despite the absence of a sex difference in the expression level of mPFC-OTR or in the response of mPFC-OTR neurons, sex differences in gene expression of mPFC-OTR interneurons and sex differences in the strength of inhibition of layer 2/3 and layer 5 pyramidal mPFC neurons by mPFC-OTR interneurons were found. These sex differences may contribute to a distinct OTR-mediated neural network in males versus females that, in turn, may underlie the observed sex differences in anxiety-related behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabihi S, Durosko NE, Dong SM, Leuner B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 2014a;45:31–42. doi: 10.1016/j.psyneuen.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabihi S, Dong SM, Maurer SD, Post C, Leuner B. Oxytocin in the medial prefrontal cortex attenuates anxiety: Anatomical and receptor specificity and mechanism of action. Neuropharmacology. 2017;125:1–12. doi: 10.1016/j.neuropharm.2017.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sabihi S, Dong SM, Durosko NE, Leuner B. Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front Behav Neurosci. 2014b;8:258. doi: 10.3389/fnbeh.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baran NM, Tomaszycki ML, Adkins-Regan E. Early Life Manipulations of the Nonapeptide System Alter Pair Maintenance Behaviors and Neural Activity in Adult Male Zebra Finches. Front Behav Neurosci. 2016 Mar 29;10:58. doi: 10.3389/fnbeh.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldsby JA, Wolstenholme JT, Rissman EF. Multi- and Transgenerational Consequences of Bisphenol A on Sexually Dimorphic Cell Populations in Mouse Brain. Endocrinology. 2017 Jan 1;158(1):21–30. doi: 10.1210/en.2016-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 2012;3(1):15. doi: 10.1186/2042-6410-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32(5):437–50. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011 Aug 19;12(9):524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 61.Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012 Nov;35(11):649–59. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 62.Rutter M, Caspi A, Moffitt TE. Using sex differences in psychopathology to study causal mechanisms: unifying issues and research strategies. J Child Psychol Psychiatry. 2003;44:1092–1115. doi: 10.1111/1469-7610.00194. [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. Gender Disparities in Mental Health. 2016 [Google Scholar]
- 64.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2007. [Google Scholar]


