Abstract
Mouse models have been invaluable for delineating the contributions of specific genes and signaling pathways to the pathogenesis of cardiac arrhythmias. Considering that there are important differences between mice and humans, here we discuss the strengths and limitations of mouse models of cardiac arrhythmias.
Keywords: Atrial fibrillation, Arrhythmias, Gene targeting, Inherited arrhythmia disorders, Mouse models
Subject codes: Arrhythmias, electrophysiology, animal models of human disease, gene therapy
Cardiac arrhythmias are a major cause of morbidity and sudden cardiac death, yet often difficult to treat due to an incomplete understanding of the underlying mechanisms. Various genetic and acquired factors can contribute to classic arrhythmia mechanisms such as abnormal impulse formation caused by triggered activity or enhanced automaticity, or reentry caused by altered conduction or enhanced heterogeneity of conduction and excitability.1 The presence of one or more of such factors can lead to transient (paroxysmal) or persistent forms of arrhythmias.
A major advance in our understanding of arrhythmia mechanisms resulted from pioneering human genetic studies that identified primary defects in genes encoding ion channel subunits.2 In the mid to late nineties, experiments to establish causality between ion channel mutations and inherited arrhythmia syndromes often involved expressing recombinant proteins in cell lines and Xenopus (X. leavis) oocytes.3 During the ensuing years, numerous studies revealed that cultures cells often lack the correct ion channel subunit stoichiometry and subcellular organization found in native cardiac myocytes. Moreover, with the additional discovery of mutations in adaptor proteins, signaling molecules, and even transcription factors as genetic causes of arrhythmias, it became clear that whole animal models offer unique benefits over cell culture studies (e.g., HL-1 cells). Although induced pluripotent stem cells (iPSC) are evolving as a powerful tool for preclinical investigations relevant to the study of arrhythmia mechanisms and therapeutics, the electrophysiological and Ca2+-handling properties of iPSC-derived cardiomyocytes do not fully recapitulate those of the adult cardiomyocytes.
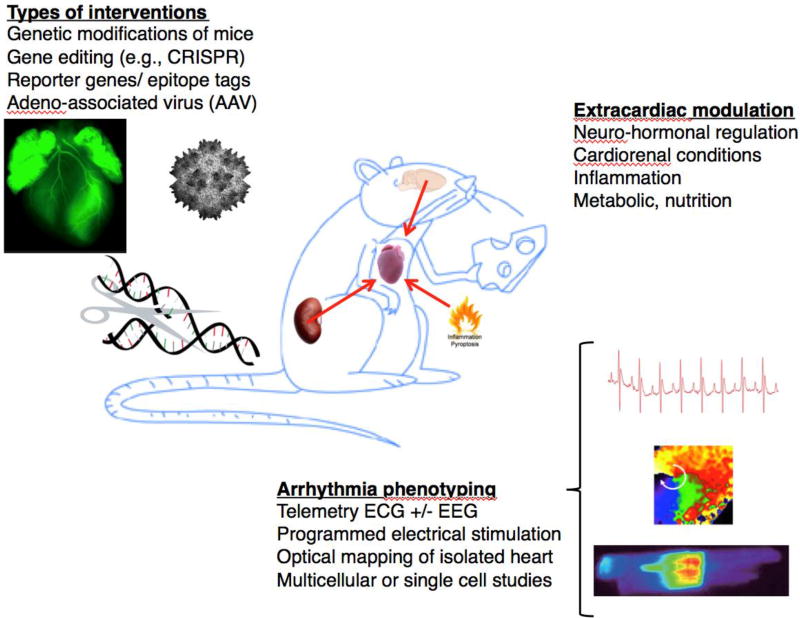
Since many genes have a high homology with the corresponding human genes, mice can be used to validate the genetic basis of human arrhythmia syndromes.4 While initially it was very time consuming to generate transgenic or knockout mice, targeted genetic modifications are now much easier to generate at relatively low costs. Recent technical innovations such as CRISPR/Cas9 genome editing offer the opportunity to create floxed or knockin mouse alleles within weeks. Moreover, cardiotropic adeno-associated viruses (AAV) have emerged as a great alternative to transgenic and tissue-specific knockout mice. Combining AAV and CRISPR/Cas9 technologies has even created the opportunity to perform therapeutic genome editing for cardiac arrhythmia disorders or other cardiovascular diseases (Figure 1).5
Figure 1.
Mouse models provide excellent opportunities to gain new insights into cardiac arrhythmias. Various types of modifications and interventions are easily employed in mice. Gain- and loss-of-function modifications and gene editing using for example the CRISPR/Cas9 system can be used. Reporter genes and epitope tags can be inserted in the mouse genome for advanced phenotyping. Gene therapy studies such as those with adeno-associated virus (AAV) are very feasible in mice. Studies in mice offer the opportunity to study how other organ systems and conditions influence cardiac function. Arrhythmias can be studies at various levels using mice and tissues obtained from mice such as perfused hearts, multicellular or single cell preparations. These studies can provide invaluable insights into arrhythmia mechanisms.
Knockin mice containing a single amino acid point mutation cannot only be used as a model of inherited disease-causing mutations, but also to model epigenetic or posttranslational modifications associated with acquired arrhythmia conditions, respectively.6 Such mouse models are well suited to reveal the causal involvement of specific genes or protein residues in arrhythmia formation. In addition to providing basic mechanistic insights, mouse models can also be helpful for drug development efforts and offer the opportunity to perform affordable preclinical evaluation in vivo and ex vivo in isolated hearts or cardiac myocytes isolated from mutant mice.7 Mouse models are superior to cell culture models since intact mouse hearts contain all relevant types of specialized cells including nodal and conduction system cells, endothelial cells and fibroblasts. Mice also offer human-like cardiac anatomy not seen in simpler animal models such as zebrafish (Danio rerio) that lack the right atrium and ventricle.
In addition, the mouse heart is fully innervated which allows for the evaluation of extra-cardiac influences on cardiac arrhythmias (Figure 1). Nervous system abnormalities have been shown to cause cardiac remodeling associated with arrhythmias. For example, we studied cardiac electrophysiology in mice carrying a mutation in methyl-CpG-binding protein 2 (MECP2), the gene that is defective in Rett syndrome, a neurodevelopmental disorder leading to sudden death in about 26% of patients.8 These mice exhibited prolonged QT-intervals and developed ventricular tachycardia (VT), leading to sudden death. Interestingly, however, removal of the MECP2 gene in the nervous system alone also caused abnormal QT-intervals and VT, suggesting that arrhythmias are secondary to nervous system deficits.8 Thus, mouse models offer the opportunity to test and validate disease-causing mechanism originating outside the cardiovascular system. These concepts may become very critical for the translation of experimental results to the clinical setting.
It is becoming increasingly clear that arrhythmias are also a major contributor to ‘sudden unexpected death in epilepsy’ (SUDEP). Many variants identified in SUDEP victims affect ion channel subunits expressed both in the brain and heart. We studied cortical excitability in a mouse model of CPVT caused by ryanodine receptor type-2 (RyR2) mutation R176Q. Simultaneous in vivo EEG and ECG monitoring revealed spontaneous bilateral cortical epileptiform spike discharges in R176Q mice, as well as episodes of bradycardia and ventricular fibrillation.9 R176Q mice were found to be more sensitive to seizure-induced death as a result of spreading depolarization in the brainstem, resulting in hypoxia resulting from peri-ictal cardiorespiratory instability. Clearly, this novel arrhythmia mechanism involving impaired brainstem autonomic regulation could have only been discovered in an animal model carrying a disease-causing human disease mutation. Complex studies as the ones described above are now possible in small rodents due to the availability of small (1 F) catheters for programmed electrical stimulation (PES), dual EEG/ECG monitors, and miniature cardiac pacemakers.10
Compared to mouse models, large animal models have heart rates, action potential shapes and durations, ion-channel profiles, and intracellular Ca2+-handling systems with dynamics that are more similar to those seen in humans.11 Therefore, large-animal models play a key role in preclinical studies, although it remains very difficult and expensive to perform gene-targeting in such models, even using AAV for example. Transgenic-rabbit models of long-QT syndrome and a knockin pig model of Brugada syndrome have been described, but their use has been very limited for financial and technical reasons.12 Non-genetic large-animal models are used more commonly, particularly for the study of acquired arrhythmias such as atrial fibrillation (AF) and ventricular fibrillation following myocardial infarction. Although these models allow control over co-morbidities and experimental conditions, the time-course of substrate development is rather short and usually monofactorial, with only a few studies combining two risk factors in the same animal model.
Mouse models have also been very helpful in uncovering new mechanistic insights into AF, the most common cardiac arrhythmia. Some experts argued that mice would not be suitable to study AF pathophysiology in the belief that their small atria could not accommodate reentrant circuits. However, this theoretical prediction has been proven false in various mouse models of AF, in which reentrant arrhythmias have indeed been demonstrated.13 The majority of currently available mouse models require arrhythmia induction using PES to uncover an increased susceptibility to AF.11 Such models are suitable to study the contribution of specific gene defects or signaling pathways to the development of a proarrhythmia substrate that enables AF maintenance once induced by PES. The duration of AF episodes in those mice is typically rather short (seconds to a few minutes), resembling the clinical presentation of some patients with paroxysmal AF.
There are fewer examples of mouse models of spontaneous AF. For example, transgenic mice with cardiomyocyte-restricted overexpression of cAMP-response element (CREM) develop spontaneous atrial ectopy, followed by progressively longer episodes of spontaneous AF at an older age.13 Like other mouse models of spontaneous AF, the CREM-transgenic mice have a predisposition to focal ectopic firing, exhibit electrophysiological abnormalities that promote reentry due to abnormal atrial repolarization, and display atrial conduction abnormalities that produce a reentrant substrate. Such mouse models mimic the progressive nature of AF observed in many patients, who progress from paroxysmal AF to persistent AF forms over time. These models are well suited to test the effectiveness of genetic perturbations or therapeutic approaches at various stages of the disease progression. It is interesting to note that most of these mouse models exhibit features of atrial cardiomyopathy and structural remodeling similar to those we have seen in atrial tissue from AF patients.
Mouse models of AF have uncovered mechanistic insights that were previously not identified in human or large animal studies. For example, the CREM transgenic mice revealed that abnormal RyR2-mediated Ca2+ handling is directly responsible for the progression of paroxysmal AF to more persistent forms.13 Evidence for the causal involvement of RyR2 in the atrial remodeling process was obtained by crossing CREM-transgenic mice with knockin mice carrying a single amino acid point mutation in RyR2 that protects against SR Ca2+ leak.13 Another example of a new mechanistic finding is our recent work demonstrating the causal association between enhanced activity of the NLRP3 inflammasome and the spontaneous development of AF. Knock-in mice with cardiomyocyte-restricted constitutively-enhanced activity of ‘NACHT, LRR and PYD domains-containing protein 3’ (NLRP3) developed AF. This is the first study to show that the NLRP3 inflammasome plays a role within adult cardiac myocytes, from both mice and humans, rather than in immune cells typically associated with this pathway.14 Atrial hypertrophy, abnormal diastolic Ca2+ leak, action potential shortening along with altered gene transcription were typical findings in mice with constitutive NLRP3 activation. These data position cardiomyocyte NLRP3-mediated inflammatory signaling as a previously unrecognized nodal point in the creation of the vulnerable substrate for AF development, promoting both electrical, Ca2+-handling and structural remodeling.
Finally, mouse models can be helpful in elucidating the mechanisms by which genetic variants uncovered by genome-wide association studies (GWAS) increased AF susceptibility. Recent GWAS have uncovered 111 loci and 165 candidate genes potentially associated with AF, which provides a major challenge for validation studies in model systems. Single-nucleotide polymorphisms (SNPs) on 4q25 near the PITX2 gene have shown the highest statistical association with AF risk in patients. Studies in mouse models have been instrumental in uncovering potential mechanisms by which alterations in PITX2 might promote AF. For example, PITX2 deficiency in mice – which mimics the situation in some AF patients – led to de-repression of sinoatrial node-specific genes in the left atrium and an enhanced AF susceptibility.15 However, SNPs identified in GWAS are typically found between protein-encoding genes and a variety of approaches including mouse models may be required to determine the functional impact of those SNPs on AF susceptibility.
In conclusion, we believe that mouse models provide unique opportunities to perform mechanistic and preclinical therapeutic studies related to cardiac arrhythmias. Recent advances in gene editing approaches have not only made gene targeting more affordable but also allow for chamber-specific studies or therapeutic gene-editing approaches. Mouse models also provide opportunities to determine the effects of other organ systems or environmental factors on cardiac arrhythmogenesis. Although human iPSC-derived cell lines and large-animal models can yield complementary insights, mice are expected to remain a preferred model system for arrhythmia studies in the foreseeable future.
Acknowledgments
SOURCES OF FUNDING
D.D. is supported by the German Research Foundation DFG (Do 769/4-1) and NIH grants R01-HL131517 and R01-HL136389. X.H.T.W. is supported by NIH grants R01-HL089598, R01-HL091947, R01-HL117641.
Footnotes
DISCLOSURES
X.H.T.W. is a founding partner of Elex Biotech, a start-up company that developed drug molecules that target ryanodine receptors for the treatment of cardiac arrhythmia disorders. D.D. is member of the scientific advisory board of OMEICOS Therapeutics GmbH, a company developing small molecules mimicking the effects of omega-3 fatty acids, and of Acesion Pharma, a company developing selective blockers of small-conductance calcium-dependent potassium channels.
References
- 1.Nattel S, Dobrev D. Controversies about atrial fibrillation mechanisms: Aiming for order in chaos and whether it matters. Circ Res. 2017;120:1396–1398. doi: 10.1161/CIRCRESAHA.116.310489. [DOI] [PubMed] [Google Scholar]
- 2.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 3.Splawski I, Tristani-Firouzi M, Lehmann MH, Sanguinetti MC, Keating MT. Mutations in the hmink gene cause long qt syndrome and suppress iks function. Nat Genet. 1997;17:338–340. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 4.Wehrens XH, Kirchhoff S, Doevendans PA. Mouse electrocardiography: An interval of thirty years. Cardiovasc Res. 2000;45:231–237. doi: 10.1016/s0008-6363(99)00335-1. [DOI] [PubMed] [Google Scholar]
- 5.Xie C, Zhang YP, Song L, Luo J, Qi W, Hu J, Lu D, Yang Z, Zhang J, Xiao J, Zhou B, Du JL, Jing N, Liu Y, Wang Y, Li BL, Song BL, Yan Y. Genome editing with crispr/cas9 in postnatal mice corrects prkag2 cardiac syndrome. Cell Res. 2016;26:1099–1111. doi: 10.1038/cr.2016.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum ca2+ leak and increased na+-ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li N, Wang Q, Sibrian-Vazquez M, Klipp RC, Reynolds JO, Word TA, Scott L, Jr, Salama G, Strongin RM, Abramson JJ, Wehrens XHT. Treatment of catecholaminergic polymorphic ventricular tachycardia in mice using novel ryr2-modifying drugs. Int J Cardiol. 2017;227:668–673. doi: 10.1016/j.ijcard.2016.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCauley MD, Wang T, Mike E, Herrera J, Beavers DL, Huang TW, Ward CS, Skinner S, Percy AK, Glaze DG, Wehrens XH, Neul JL. Pathogenesis of lethal cardiac arrhythmias in mecp2 mutant mice: Implication for therapy in rett syndrome. Sci Transl Med. 2011;3:113ra125. doi: 10.1126/scitranslmed.3002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aiba I, Wehrens XH, Noebels JL. Leaky ryr2 channels unleash a brainstem spreading depolarization mechanism of sudden cardiac death. Proc Natl Acad Sci U S A. 2016;113:E4895–4903. doi: 10.1073/pnas.1605216113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laughner JI, Marrus SB, Zellmer ER, Weinheimer CJ, MacEwan MR, Cui SX, Nerbonne JM, Efimov IR. A fully implantable pacemaker for the mouse: From battery to wireless power. PLoS One. 2013;8:e76291. doi: 10.1371/journal.pone.0076291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heijman J, Algalarrondo V, Voigt N, Melka J, Wehrens XH, Dobrev D, Nattel S. The value of basic research insights into atrial fibrillation mechanisms as a guide to therapeutic innovation: A critical analysis. Cardiovasc Res. 2016;109:467–479. doi: 10.1093/cvr/cvv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park DS, Cerrone M, Morley G, Vasquez C, Fowler S, Liu N, Bernstein SA, Liu FY, Zhang J, Rogers CS, Priori SG, Chinitz LA, Fishman GI. Genetically engineered scn5a mutant pig hearts exhibit conduction defects and arrhythmias. J Clin Invest. 2015;125:403–412. doi: 10.1172/JCI76919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao C, Veleva T, Scott L, Jr, Cao S, Li L, Chen G, Jeyabal P, Pan X, Alsina KM, Abu-Taha I, Ghezelbash S, Reynolds CL, Shen YH, LeMaire SA, Schmitz W, Muller FU, El-Armouche A, Eissa NT, Beeton C, Nattel S, Wehrens XH, Dobrev D, Li N. Enhanced nlrp3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018 doi: 10.1161/CIRCULATIONAHA.118.035202. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Klysik E, Sood S, Johnson RL, Wehrens XH, Martin JF. Pitx2 prevents susceptibility to atrial arrhythmias by inhibiting left-sided pacemaker specification. Proc Natl Acad Sci U S A. 2010;107:9753–9758. doi: 10.1073/pnas.0912585107. [DOI] [PMC free article] [PubMed] [Google Scholar]