Summary
Tryptamine, a tryptophan-derived monoamine similar to 5-hydroxytryptamine (5-HT), is produced by gut bacteria and is abundant in human and rodent feces. However, the physiologic effect of tryptamine in the gastrointestinal (GI) tract remains unknown. Here, we show that the biological effects of tryptamine are mediated through 5-HT4 receptor (5-HT4R), a G-protein coupled receptor (GPCR) uniquely expressed in the colonic epithelium. Tryptamine increases ionic flux across the colonic epithelium and increases fluid secretion in colonoids from germ free (GF) and humanized (ex-GF colonized with human stool) mice consistent with increased intestinal secretion. The secretory effect of tryptamine is dependent on 5-HT4R activation and is blocked by 5-HT4R antagonist and absent in 5-HT4R−/− mice. GF mice colonized by Bacteroides thetaiotaomicron engineered to produce tryptamine exhibit accelerated gastrointestinal transit. Our study demonstrates an aspect of host physiology under control of a bacterial metabolite that can be exploited as a therapeutic modality.
eTOC Blurb
Bhattarai et. al. uncovered the mechanism by which a bacteria-derived small molecule can alter host gastrointestinal function. Tryptamine produced by bacterial decarboxylation of dietary tryptophan accelerates gastrointestinal transit by activating the epithelial G-protein coupled receptor (GPCR), serotonin receptor-4 (5-HT4R) and increasing anion-dependent fluid secretion in the proximal colon.
Introduction
The gut is home to trillions of microbes, the composition of which correlates with many disease states. While recent studies have started to unravel the effect of this vast microbial community and its complex metabolic repertoire on intestinal physiology, its true potential remains underexplored. Microbes are well adapted to sensing their environment. These signals are often small, freely diffusible molecules that not only help microbes establish community behavior (Visick and Fuqua, 2005, Davies et al., 1998, Swift et al., 2001) but also define their interactions with the host (Cohen et al., 2017, Jones, 2016, Palmer et al., 2005, Stacy et al., 2016, Rhee et al., 2009, Clarke et al., 2014, Kelly et al., 2015, Holmes et al., 2012).
Microbial conversion of dietary substrates into small bioactive molecules represents a potential regulatory mechanism by which gut microbes can alter intestinal physiology (Kabouridis et al., 2015, Reigstad et al., 2015, Anitha et al., 2016, Dey et al., 2015). Among dietary substrates, amino acids are of particular importance since they are not only an important source of energy for gut microbiota, but also contribute to the regulation of host physiology and protein homeostasis (Neis et al., 2015). Tryptophan, an essential amino acid, is particularly important because it embeds an indole nucleus, which is an important precursor and a core building block of myriads of bioactive compounds in humans such as kynurenine, 5-hydroxytryptamine (5-HT; serotonin), melatonin and other indole derivatives (Richard et al., 2009, Bender, 1983). While the majority of tryptophan (90–95%) is metabolized by the host to synthesize these compounds (Gevi et al., 2016), microbial metabolism of tryptophan resulting in indole derivatives such as indole acetic acid and indole propionic acid have recently been a focus of several studies investigating their contributions to host physiology (Rothhammer et al., 2016, Marsland, 2016, Dodd et al., 2017, Venkatesh et al., 2014, Zelante et al., 2013, Zhang and Davies, 2016, Chimerel et al., 2014).
Tryptamine, another tryptophan-derived indole containing monoamine, is abundant in human and rodent feces (Anderson, 1975, Brooks et al., 1984). Tryptamine concentrations increase nearly 200 fold in feces following colonization of germ free (GF) mice with human gut microbiota suggesting that bacterial metabolism of tryptophan generates luminal tryptamine (Marcobal et al., 2013). While this pathway is not as common among human commensal bacteria, Ruminococcus gnavus and Clostridium sporogenes were recently found to express tryptophan decarboxylase, the enzyme responsible for decarboxylation of tryptophan to tryptamine (Williams et al., 2014), and the genes encoding homologs of this enzyme were found in at least 10% of the representative human gut microbiota (Williams et al., 2014).
Although the physiologic effect of tryptamine in the gastrointestinal (GI) tract remains unknown, tryptamine is a ligand for 5-HT receptors (5-HTRs) (Van Oekelen et al., 2002) suggesting a potential contribution of tryptamine in 5-HTR mediated GI functions. Of the seven families of 5-HTRs, the receptor with highest exposure to luminal contents of the GI tract is the G-protein coupled receptor (GPCR) 5-HT4R, which is expressed in the epithelium of the entire colon (Hoffman et al., 2012). GPCRs are ubiquitous across the GI tract representing the largest class of membrane receptors. As a result, it is not surprising that GPCRs are a common target of pharmaceutical agents; 5-HT4R has been targeted in diseases associated with slow GI transit such as constipation predominant irritable bowel syndrome (IBS-C), given its contribution to the control of intestinal secretion (Cole and Rabasseda, 2004). Given the widespread distribution of GPCRs along the GI tract, it is not surprising that like the host, gut microbiota also produce GPCR ligands that mediate host-microbiome interactions by acting on the same host receptors as the endogenous ligands (Cohen et al., 2017). In this study we investigated the effect of exogenous and bacterially produced tryptamine on intestinal secretion as well as studied the interaction of tryptamine with the GPCR 5-HT4R expressed in the colonic epithelium.
Results
1. Tryptamine increases colonic secretion in the mouse proximal colon irrespective of sex and colonization status
The physiological effect of tryptamine is unknown, however, given its structural similarity to 5-HT, a neurotransmitter that affects colonic fluid and anion secretion (Bhattarai et al., 2017b, Yeo et al., 1989, McGowan et al., 1983, Borman and Burleigh, 1997), and its affinity for 5-HTRs (Van Oekelen et al., 2002), we sought to determine if tryptamine affects colonic secretion. Ussing chamber experiments were used to determine the effect of exogenous tryptamine on ionic transport across mouse colonic mucosa-submucosa preparations as a surrogate for colonic secretion. We tested the effects of exogenous tryptamine application on both the mucosal (apical) and the submucosal (basolateral) surface as tryptamine was previously shown to cross physiologic membranes (Paley et al., 2013) and the cellular location of 5-HT4R remains unknown. A change in short circuit current (ΔIsc) in response to exogenous tryptamine application is reflective of ionic transport across the epithelium. We found that 3 mM tryptamine applied both mucosally (apical) and submucosally (basolateral) increased Isc in proximal colon mucosa-submucosa preparations in GF mice. The increase in Isc was seen in both male and female mice (Figure 1A–D). This suggests that tryptamine interacts directly with the host colon, independent of gut microbiota, to alter ionic flux.
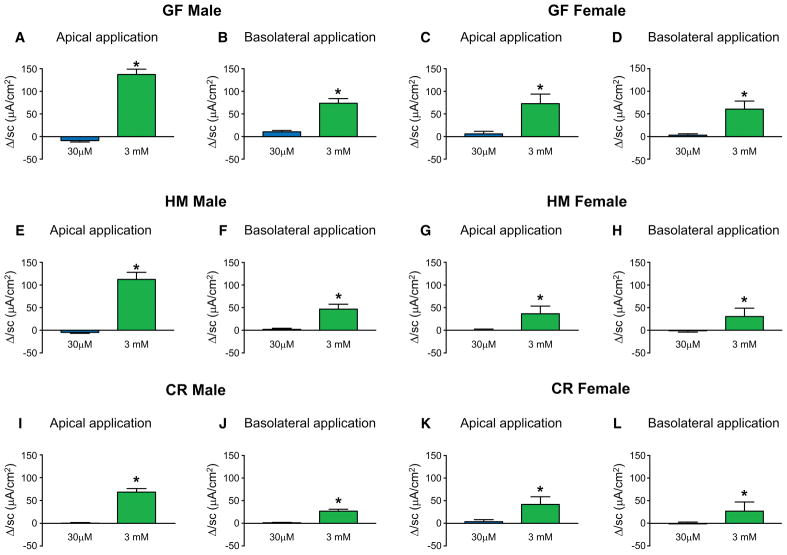
Figure 1.
Tryptamine increases Isc in vitro in mice irrespective of sex and microbial colonization. ΔIsc in response to apically and basolaterally applied concentrations (30μM and 3mM) of tryptamine respectively in GF male (A, B) and GF female mice (C, D). ΔIsc in response to apically and basolaterally applied concentrations (30μM and 3mM) of tryptamine respectively in HM male (E, F) and HM female mice (G, H). ΔIsc in response to apically and basolaterally applied concentrations (30μM and 3mM) of tryptamine respectively in CR male (I, J) and CR female mice (K, L). n=4–6, paired t-test, *P < 0.05.
Bacteria are an integral component of the gut environment and gut microbiota can alter expression of receptors and function in the GI epithelium as well as GI physiology (Bhattarai et al., 2017b, Yano et al., 2015, Reigstad et al., 2015). Therefore to determine if the effects of tryptamine on host tissues described above are also seen in the gut which has been previously primed by the presence of microbiota (Yano et al., 2015), the Ussing chamber studies were repeated in humanized (HM) and conventionally raised (CR) mice. HM mice were used because they are translationally relevant given that GF mice are colonized with fecal bacteria from a healthy human donor. As was observed in GF mice, HM and CR mice also had increased Isc in proximal colon mucosa-submucosa preparations, when stimulated with 3mM tryptamine on either the apical or basolateral surface, an effect that was not sex specific (Figure 1E–L). This suggests that the host secretory response to tryptamine is an endogenous function that is not pre-primed, stimulated or nullified by the presence of microbes. The maximum ΔIsc (Imax) in response to apical tryptamine application was significantly higher in GF and HM males compared to CR males and there was no significant difference in female mice (Supplemental Figure 1A, C). The maximum ΔIsc (Imax) in response to basolateral tryptamine application was significantly higher in GF compared to HM and CR males and there was no significant difference in female mice (Supplemental Figure 1B, D). This suggests that the magnitude of response may depend on the sex and colonization status.
Changes in Isc cannot differentiate between ionic absorption and secretion. To assess the directionality of ionic flow, we tested the effect of tryptamine in the presence of inhibitors of absorption and secretion. Amiloride, an inhibitor of epithelial sodium channels and Na+/H+ exchangers, which are primarily responsible for ion absorption, did not affect tryptamine-evoked ΔIsc following application of cumulative concentrations of tryptamine in either GF or HM mice (Supplemental Figure 2A, C). Next we used 5-nitro-2-(3-phenylpropylamino) benzoic acid (NPPB; anion blocker), an inhibitor of calcium-sensitive chloride channel and bicarbonate exchanger responsible for anion secretion. This compound significantly decreased tryptamine-evoked ΔIsc following application of cumulative concentrations of tryptamine in both GF and HM mice (Supplemental Figure 2B, D), suggesting that anion (Cl− and HCO3−) secretion forms the ionic basis of tryptamine-evoked ΔIsc.
2. Tryptamine induced colonic secretion is mediated by 5-HT4Rs irrespective of sex and microbial colonization
Tryptamine, a monoamine structurally similar to serotonin (5-HT), is a physiological ligand for several 5-HTRs (Van Oekelen et al., 2002). Among the 5-HTRs, 5-HT3R and 5-HT4R in the colonic epithelium and submucosal neurons are the receptors primarily responsible for regulating intestinal secretion (Bhattarai et al., 2017b, Mawe and Hoffman, 2013). To determine if tryptamine-evoked ionic secretion was mediated by these receptors, we examined the effects of 5-HT3R and 5-HT4R specific antagonists on tryptamine-evoked ΔIsc following application of cumulative concentrations of tryptamine on both the apical and the basolateral surface. Tryptamine evoked ΔIsc was almost completely blocked by the 5-HT4R antagonist irrespective of the sex and colonization status (Figure 2A–L). No difference in 5-HT4R mRNA expressions among the sexes and colonization states were observed (Supplemental Figure 3) suggesting that sex and colonization based differences in tryptamine response is not due to altered 5-HT4R expression. Tryptamine-evoked ΔIsc was not inhibited by ondansetron, a 5-HT3R antagonist (Supplemental Figure 4A–H). This highlights a specific function of 5-HT4R in tryptamine-mediated intestinal secretion.
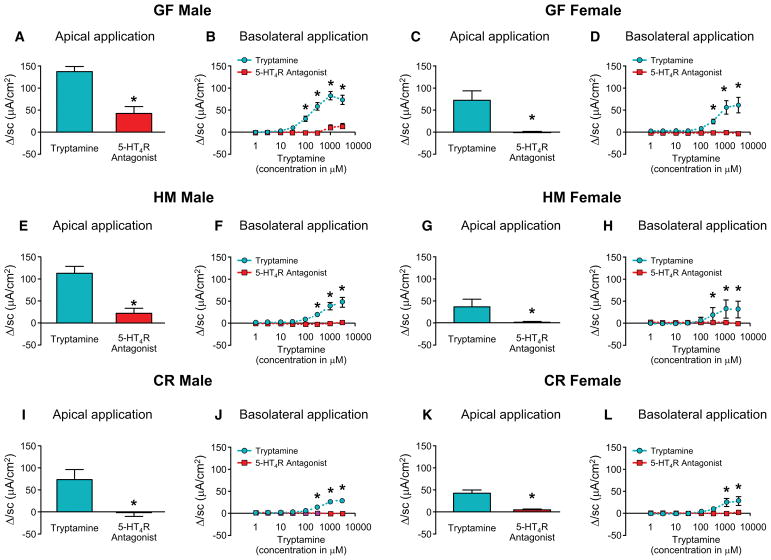
Figure 2.
Tryptamine evoked ΔIsc is blocked by 5-HT4R antagonist irrespective of sex and microbial colonization. ΔIsc in response to tryptamine applied apically (3mM, paired t-test) and cumulative concentrations of tryptamine applied basolaterally (two-way ANOVA) respectively either alone or in the presence of 5-HT4R antagonist, GR-113808 (30nM) in GF male (A, B) and GF female mice (C, D). ΔIsc in response to tryptamine applied apically (3mM, paired t-test) and cumulative concentrations of tryptamine applied basolaterally (two-way ANOVA) respectively either alone or in the presence of 5-HT4R antagonist, GR-113808 (30nM) in HM male (E, F) and HM female mice (G, H). ΔIsc in response to tryptamine applied apically (3mM, paired t-test) and cumulative concentrations of tryptamine applied basolaterally (two-way ANOVA) respectively either alone or in the presence of 5-HT4R antagonist, GR-113808 (30nM) in CR male (I, J) and CR female mice (K, L). n=5–6, *P < 0.05.
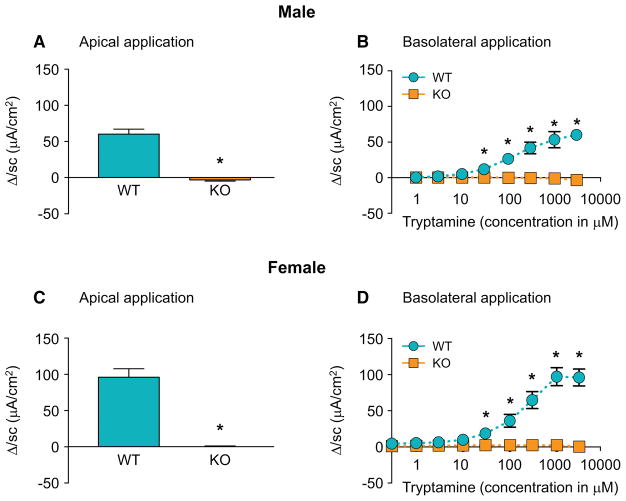
Though GR-113808 is a specific 5-HT4R antagonist in the dose used in this study, in order to boost confidence in this finding we complemented these assays using similarly designed experiments in genetically modified 5-HT4R−/− (KO) mice. While tryptamine significantly increased ΔIsc in wild type (WT) mice following application of cumulative concentrations of tryptamine on the apical and basolateral surface, it did not elicit a ΔIsc response in 5-HT4R−/− male and female mice (Figure 3A–D), further supporting the contribution of 5-HT4R in mediating the effect of tryptamine on secretion. Since these effects were seen in both male and female mice, suggesting a similar mechanism of action of tryptamine in both sexes, we performed the majority of the subsequent mechanistic experiments in male mice.
Figure 3.
Tryptamine evoked increase in Isc is absent in 5-HT4R−/− mice. ΔIsc in response to tryptamine applied apically (3mM, paired t-test) and cumulative concentrations of tryptamine applied basolaterally (two-way ANOVA) respectively in WT and in 5-HT4R−/− male (A, B) and female mice (C, D). n=5, *P < 0.05.
In addition to 5-HTRs, tryptamine is also a physiologic ligand for the aryl hydrocarbon receptor (AHR) (Jin et al., 2014, Heath-Pagliuso et al., 1998), widely expressed in the immune system and implicated in immune regulation and epithelial barrier function (Gargaro et al., 2016, Prager et al., 2016, Han et al., 2016, Esser and Rannug, 2015). We evaluated the effect of pharmacological inhibition of AHR using an AHR antagonist, CH-223191 in Ussing chamber experiments as described above. CH-223191 did not inhibit tryptamine-evoked ΔIsc in either GF or HM mice (Supplemental Figure 4I, J) suggesting that AHRs are not involved in mediating the effect of tryptamine on colonic secretion.
3. Tryptamine acts on epithelial 5-HT4Rs to modulate colonic secretion
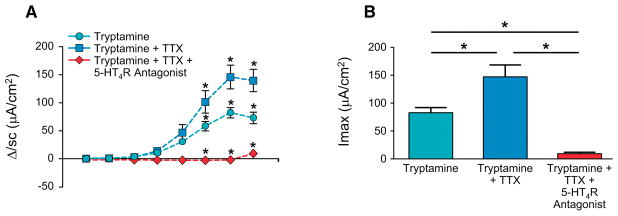
5-HT4Rs are expressed in the colonic epithelium as well as the submucosal neurons (Hoffman et al., 2012b, Sakurai-Yamashita et al., 1999). To delineate the contribution of neuronal 5-HT4R and non-neuronal epithelial 5-HT4R in mediating tryptamine-evoked intestinal secretion, we tested the effect of application of cumulative concentrations of tryptamine in the presence of tetrodotoxin (TTX; 500nM), a submucosal neuronal blocker. Application of TTX alone did not have an effect on Isc, but it significantly increased tryptamine-evoked ΔIsc, whereas co-application of TTX with 5-HT4R antagonist GR-113808 completely blocked the tryptamine-evoked Isc response suggesting that the effect of tryptamine can be mediated by epithelial 5-HT4R alone (Figure 4A, B).
Figure 4.
Tryptamine-evoked increase in Isc can be mediated by epithelial 5-HT4R. ΔIsc in response to application cumulative concentration of tryptamine basolaterally (A, two-way ANOVA) and maximum ΔIsc (Imax) following application of tryptamine basolaterally (B, one-way ANOVA) either alone, or in presence of TTX (500nM) or in presence of TTX (500nM) and GR-113808 (30nM) in GF mice. n=4–5, *P < 0.05
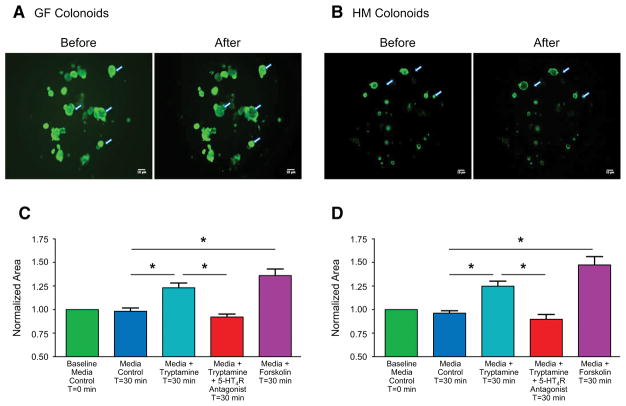
Ussing chamber experiments enable the study of anion secretion across the epithelium, which in turn is known to be accompanied by fluid secretion. Ussing chamber experiments however cannot directly assess fluid secretion. In order to determine if tryptamine is a secretagogue with effects on intestinal fluid secretion, we performed the swelling assay in GF and HM mice colonoids (organoids prepared from colonic epithelial stem cells), which has been previously validated for studying intestinal fluid secretion (Dekkers et al., 2013, In et al., 2016). The colonoids were labelled with Calcein-AM, a cell-permeant green fluorescent dye that is retained within living cells after metabolic conversion, enabling assessment of change in surface area of colonoids following addition of tryptamine. To first determine the validity of our assay, we used the known secretagogue forskolin, which raises intracellular cyclic AMP (cAMP) concentration (Domingue et al., 2014, Ao et al., 2013) and increases fluid secretion via the cystic fibrosis transmembrane regulator (CFTR) (Dekkers et al., 2013) resulting in swelling of colonoids. Application of forskolin in colonoid preparations expanded the colonoids (swelling) after 30 minutes, determined by the relative increase in the surface area of fluorescent colonoids in each well validating the use of the swelling assay to assess fluid secretion in the colonoid preparations. Incubation of colonoids with 1mM tryptamine for thirty minutes significantly increased the surface area in both GF and HM colonoids (Figure 5A, B). The tryptamine dependent increase in colonoid surface area was not observed in vehicle treated colonoids or colonoids pre-treated with the 5-HT4R antagonist GR-113808 in both GF and HM colonoids (Figure 5C, D). Hence the swelling assay with tryptamine in colonoids agrees with the ionic flux measurements obtained from Ussing chamber experiments and together they support the contribution of epithelial 5-HT4R in mediating the effect of tryptamine on intestinal secretion.
Figure 5.
Tryptamine increases fluid secretion in colonoids. Representative example of Calcein-AM –labeled colonoids before and after application of tryptamine (1mM) for thirty minutes in GF (A) and HM (B) colonoids. Arrows represent colonoids that show prominent change in area. Change in surface area is measured relative to vehicle at t = 0 (Baseline; 100%). Change in surface area normalized to baseline (t=0) after application of vehicle (medium alone), media with tryptamine (1mM), media with tryptamine (1mM) co-applied with GR-113808 (100 nM), and media with forskolin (10 μM) in colonoids from GF male (C) and HM male (D) mice for thirty minutes from three independent wells. n=5, one-way ANOVA, *P < 0.05
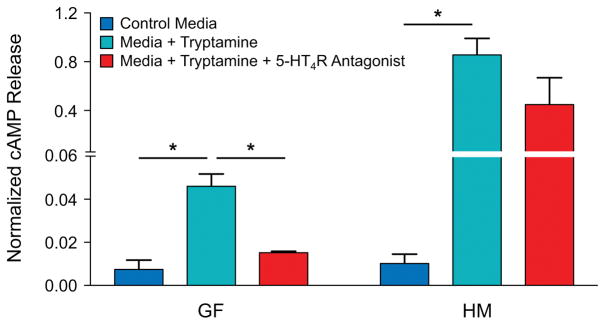
5-HT4R is a GPCR; hence its activation increases cAMP. Therefore, to determine if tryptamine-mediated activation of 5-HT4R increases cAMP, we measured the change in total cAMP in GF and HM mice colonoids incubated with 1mM tryptamine for an hour with or without pre-treatment with GR-113808. There was a significant increase in absolute concentration of cAMP (Supplemental Figure 5) as well as forskolin normalized (to control for cell density) concentration of cAMP (Figure 6) in both GF and HM colonoids incubated with tryptamine alone. The tryptamine dependent increase in cAMP was not observed in GF or HM colonoids pre-treated with the 5-HT4R antagonist GR-113808.
Figure 6.
Tryptamine increases cAMP in colonoids irrespective of microbial colonization. Total cAMP (normalized to forskolin response) in colonoids from GF and HM male mice after incubation for an hour with either control media alone, media with tryptamine (1mM) and media with tryptamine (1mM) thirty minutes after pre-treatment with GR-113808 (100 nM). n=3, *P < 0.05, one-way ANOVA. See also Figure S5.
4. In vivo tryptamine production by a gut microbe accelerates whole gut transit
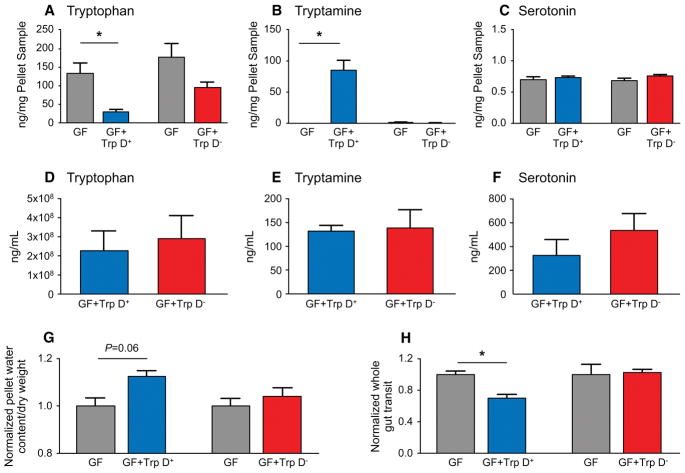
In humans, luminal tryptamine can be produced from decarboxylation of tryptophan via enzymatic activity of gut microbes that express tryptophan decarboxylase (Trp D) (Williams et al., 2014). We have previously described tryptophan decarboxylase activity in two human commensals, C. sporogenes and R. gnavus (Williams et al., 2014). While the decarboxylation of tryptophan to tryptamine is rare among bacteria in general (Facchini et al., 2000), we reported that at least 10% of a representative human population harbor a homolog of this enzyme in the gut. Tryptamine production by gut bacteria may represent a selective way for gut microbiota to alter host function (Williams et al., 2014) though several microbial metabolites can have overlapping effects on host physiology (Hurst et al., 2014, Soret et al., 2010). Nevertheless the specific effect of tryptamine on intestinal secretion described above makes it an attractive therapeutic candidate for diseases associated with slow GI transit such as IBS-C (Bhattarai et al., 2017a) and functional constipation similar to the currently used pharmacological secretagogues in these diseases. Interestingly, some of the most effective therapeutics in IBS-C have been 5-HT4R agonists yet the success of such agonists in clinical applications has been limited given the systemic adverse effects resulting from activation of cardiac 5-HT4Rs (Drolet et al., 1998). We wanted to assess the therapeutic potential of bacterially produced tryptamine as a way of altering host physiology without systemic effects. To test this, the gene encoding tryptophan decarboxylase in R. gnavus was transferred to Bacteroides thetaiotaomicron (B. thetaiotaomicron Trp D+), a common, genetically tractable commensal, which effectively colonizes the gut, and can potentially be used as a biotherapeutic. To facilitate robust expression in an exogenous host, the R. gnavus copy of tryptophan decarboxylase was codon optimized for expression in B. thetaiotaomicron, placed under the control of a constitutively active phage derived promoter (Whitaker et al., 2017) and integrated into the host chromosome of wild type B. thetaiotaomicron (B. thetaiotaomicron Trp D+). The ability of the engineered B. thetaiotaomicron Trp D+ strain to produce tryptamine was assessed in vitro by inoculating B. thetaiotaomicron Trp D+ in 0.25% tryptophan supplemented tryptone-yeast extract-glucose (TYG) medium. At 24 hours post inoculation, significantly higher tryptamine concentrations were detected in culture supernatants of B. thetaiotaomicron Trp D+ compared to a control B. thetaiotaomicron with empty vector insertion (Trp D−) as well as medium only controls (Supplemental Figure 6). Next, to assess if engineered B. thetaiotaomicron Trp D+ produces tryptamine in vivo, we colonized GF mice with either B. thetaiotaomicron Trp D+ or vector only control B. thetaiotaomicron Trp D− and supplemented the drinking water with 0.25% tryptophan (See Supplemental Figure 7A for experimental design and timeline). GF mice colonized with B. thetaiotaomicron Trp D+ displayed significantly decreased tryptophan and increased tryptamine concentrations in the feces following colonization for five days although there was no change in tryptophan or tryptamine concentrations in the feces of GF mice colonized with the empty vector control B. thetaiotaomicron Trp D− strains (Figure 7A, B). This confirmed engineered B. thetaiotaomicron can produce tryptamine in vivo. There was no significant difference in serum tryptophan or tryptamine concentrations in GF mice colonized either with B. thetaiotaomicron Trp D+ or and B. thetaiotaomicron Trp D− control strains, suggesting that bacterially produced tryptamine does not significantly affect circulating tryptamine concentrations (Figure 7D, E). Tryptamine, like 5-HT, is catabolized by monoamine oxidase (Jones, 1982), which is abundantly expressed in the colonic epithelium (Mustala et al., 1969) and likely prevents a significant increase in circulating levels of tryptamine. The colonization of GF mice with either B. thetaiotaomicron Trp D+ or control B. thetaiotaomicron Trp D− did not alter 5-HT concentrations in feces or serum suggesting that the physiologic effects of engineered B. thetaiotaomicron Trp D+ are not mediated by an increase in serotonin release (Figure 7C, F). To determine if bacterially produced tryptamine has a physiologic effect in vivo, we assessed the differences in pellet water content and whole gut transit time, common surrogate measures to assess differences in intestinal secretion in vivo (Cil et al., 2016) and to assess efficacy of pharmaceutical secretagogues in human subjects (Thomas and Allmond, 2013). We found that GF mice colonized with B. thetaiotaomicron Trp D+ strain had higher normalized and absolute pellet water content (Figure 7G, Supplementary Figure 7B) and significantly faster normalized and absolute whole gut transit time (Figure 7H, Supplemental Figure 7C) compared to GF mice colonized with the control strain. Together, the data suggest that engineered B. thetaiotaomicron can produce tryptamine in vivo, which remains localized to the gut and accelerates whole gut transit by increasing colonic secretion.
Figure 7.
Colonization of GF mice with engineered B. thetaiotaomicron Trp D+ increases luminal conversion of tryptophan to tryptamine, without increasing circulating tryptamine and accelerates whole gut transit. Change in fecal (A–C) and serum (D–F) tryptophan, tryptamine and 5-HT concentrations following colonization of GF mice with either engineered B. thetaiotaomicron Trp D+ or B. thetaiotaomicron Trp D− empty vector control. Change in normalized pellet water content (G) and whole gut transit (H) in GF male mice colonized with B. thetaiotaomicron Trp D+ or Trp D−. Mean ± SEM, n=5, un-paired t-test and one-way ANOVA, *P < 0.05. See also Figure S7.
Discussion
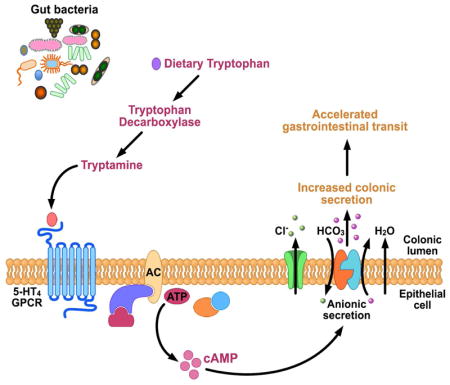
Our study highlights the ability of bacterially produced bioactive molecules to modulate a specific host function via interaction with a host GPCR. Here, we show that tryptamine, a microbial metabolite, increases colonic secretion as evidenced by tryptamine-evoked increase in Isc in Ussing chamber experiments as well as swelling of colonoids following application of tryptamine. The effect of tryptamine on colonic secretion is blocked by a 5-HT4R antagonist and is absent in 5-HT4R−/− mice. Although 5-HT4Rs are expressed both in colonic mucosa as well as neuronal plexus, the effects of tryptamine on secretion can be mediated by epithelial 5-HT4R alone. The activation of epithelial 5-HT4R by tryptamine is further supported by an increase in cAMP, the downstream mediator of GPCR stimulation (Frizzell and Hanrahan 2012), in colonoids. Engineered B. thetaiotaomicron optimized to express tryptophan decarboxylase produces tryptamine in vivo and accelerates GI transit. Together these data show that the bacterial metabolite tryptamine increases anion dependent fluid secretion via its effect on epithelial 5-HT4R in proximal colon and can alter host GI transit. The identification of a bacterial metabolite activating a host GPCR to alter host function represents an important advance in the field, and provides a distinct example of a small molecule mediated host-microbe interaction.
5-HT4Rs mediate important physiological functions in the GI tract including GI motility, visceral pain, immune regulation and epithelial secretion (Grider et al., 1998, Jin et al., 1999, Hoffman et al., 2012, Kaji et al., 2015). Epithelial secretion in the gut is important for a variety of host functions including regulation of the immune response to enteric pathogens, maintenance of luminal fluid concentrations, and GI transit (Kagnoff and Eckmann, 1997, Frizzell and Hanrahan, 2012). Pharmaceutical agents that target 5-HT4R have been effective in treating diseases associated with delayed GI transit such as IBS-C (Cole and Rabasseda, 2004) but were abandoned following reports of off target adverse cardiovascular events (Drolet et al., 1998, Tack et al., 2012). This highlights the shortcomings of systemically administered 5-HT4R agonists. Increasing the production of endogenous ligands such as 5-HT can also modulate secretion, although these ligands frequently activate multiple receptor subtypes, which complicate yielding the desired physiological change with pharmaceutical approaches. The ability of engineered B. thetaiotaomicron to increase luminal tryptamine without affecting systemic concentrations highlights a way to overcome those barriers while maintaining a local therapeutic effect as shown in our study. Further, engineered B. thetaiotaomicron did not affect other tryptophan-derived host or microbial metabolites suggesting that increasing luminal tryptamine concentrations by bacteria will likely not lead to off target effects. Hence B. thetaiotaomicron, a common genetically tractable Gram negative commensal that is well adapted to the human gut, engineered to produce tryptamine, is attractive as a therapeutic for diseases with slow GI transit such as IBS-C. Though probiotic engineered bacteria producing a specific effector molecule acting on a host receptor present an attractive therapeutic approach, challenges associated with functional long term establishment of probiotic strains in complex human communities and regulating the expression of desired metabolites need to be overcome before functional supplementation will be an effective strategy.
The absence of reliable 5-HT4R antibodies makes it difficult to determine if 5-HT4R localizes primarily to the apical or basolateral surface of colonic epithelial cells. However we observed secretory effects of tryptamine with both apical and basolateral application suggesting that tryptamine can stimulate secretion irrespective of the polarity of its application. Further, tryptamine readily crosses physiological membranes (Wurtman et al., 1980, Richard et al., 2009), suggesting that the subcellular location of 5-HT4R within the colonic epithelial cells is not a limiting factor for tryptamine mediated secretory effects. Besides the epithelium, 5-HT4R is also expressed in submucosal neurons (Mawe and Hoffman, 2013, Hoffman et al., 2012), yet we found that the effect of tryptamine on intestinal secretion can be mediated by epithelial 5-HT4R alone, as neuronal blockade does not inhibit tryptamine-evoked secretion. Indeed, the effect of tryptamine on ionic flux was significantly greater following submucosal neuronal blockade with TTX. This observation is consistent with previous study by Kaji et al., in which submucosal neurons, particularly the nitric oxide (NO) neurons, were found to inhibit 5-HT evoked Isc (Kaji et al., 2015). Removal of submucosa and/or application of NOS blocker increased 5-HT evoked Isc reversing the inhibitory effect of submucosal NO neurons. The inhibitory action of submucosal neurons may also explain the differences in response to tryptamine in GF and HM mice when comparing results from Ussing chamber experiments with cAMP measurements in colonoids. The Ussing chamber experiments are performed with intact submucosa while cAMP release is measured in colonoids derived from the epithelium alone.
Tryptamine increases 5-HT release in myenteric neurons; hence it is reasonable to hypothesize that 5-HT is mediating the effects of tryptamine. Nevertheless, our data do not support this hypothesis. We have previously shown the effect of 5-HT on anion secretion is significantly inhibited by both 5-HT3R and 5-HT4R antagonists (Bhattarai et al., 2017b), however the effect of tryptamine on secretion is not inhibited by 5-HT3R antagonists suggesting that it is unlikely the effect of tryptamine is mediated via 5-HT. Further engineered B. thetaiotaomicron increased luminal tryptamine concentrations in gnotobiotic mice but did not increase luminal or serum 5-HT concentrations.
The effect of tryptamine on intestinal secretion was mediated via 5-HT4R irrespective of the sex or colonization status suggesting a conserved mechanism of action. Nonetheless, there were relative differences in the magnitude of response between sexes. The factors underlying these differences remain unclear as there were no significant differences in 5-HT4R expression among the sexes and three colonization states; GF, HM and CR mice. Studies designed to determine the basis for sex and colonization based differences in response and the impact of these differences in response on host physiology in vivo are active areas of pursuit.
As with any animal study, there are certain limitations in our model, and hence need to be further validated prior to clinical application. While GF mice are ideal for mechanistic studies to determine the physiological effects of individual bacteria or bacterial metabolites, we cannot directly extrapolate findings to humans. GF mice exhibit altered physiology including an immature immune system, which represents an adaptation to the absence of microbes. Similarly humanized mice are ideal as a translational model but at the same time, the lack of prior adaptation of human microbiota to mouse GI tract and the shorter duration of colonization is a potential limitation. In addition, isolator-to-isolator variability in gnotobiotic experiments necessitates using mice as their own control. Though every model has its limitations, our use of multiple models in parallel increases the confidence in our findings. There are several studies that now highlight the advantages of gnotobiotic studies as preclinical models to determine mechanism and test efficacy of microbiota-based therapies.
In conclusion, our data suggests that tryptamine acts on epithelial GPCR 5-HT4R to increase cAMP and colonic anion and fluid secretion. Engineered B. thetaiotaomicron carrying the tryptophan decarboxylase gene produces tryptamine in vivo, which localizes in the gut and can accelerate whole gut transit time. This study demonstrates an aspect of host physiology under control of a bacterial metabolite that can be exploited as a gut localized therapeutic modality in GI disorders associated with constipation.
STAR Methods
Contact for Reagent and Resource Sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Purna C. Kashyap (kashyap.purna@mayo.edu).
Experimental Model and Subject Details
Mouse Husbandry and Ethics
All animal studies were approved by Mayo Clinic Institutional Animal Care and Use Committee (Protocol# A49415) and conducted in compliance with regulatory guidelines. Age matched male and female mice between 8–12 weeks were used for experiments. All mice used in the study were maintained in 12 hr light-dark cycle. The following strains of mice were used for the study; Swiss Webster (outbred strain), 129/Sv WT and 5-HT4R knock out (KO, 5-HT4R −/−) on 129/Sv background. Mice used in the study were monitored daily for signs of any obvious physical stress and behavioral changes, and euthanized per protocol if found in distress. GF Swiss Webster mice were obtained from Mayo Clinic gnotobiotic facility; HM mice were generated from GF Swiss Webster mice; age matched conventionally raised (CR) Swiss Webster mice were obtained from Taconic Farms (Taconic farms, Hudson, NY), and 129/Sv WT and 5-HT4R KO mice were obtained from our collaborator (Compan et al., 2004). Germ free (GF), gnotobiotic (GF colonized with B. thetaiotaomicron) and humanized mice, were maintained in flexible film vinyl isolators (Class Biologically Clean, Madison, WI) in the Mayo Clinic Germ Free Facility where they were housed in open top cages with autoclaved Sani-Chips bedding and given autoclaved diet (Purina Lab diet, 5K67, Collins feed and seed center, Rochester, MN), and autoclaved nanopure water. Mice feed and bedding were changed every week and earlier if needed. GF status was confirmed prior to start of experiments with two consecutive cultures of fecal pellet, feed and bedding on brain heart infusion (BHI), Sabouraud dextrose, and nutrient media under both anaerobic and aerobic condition as well as PCR of 16s rRNA gene in fecal DNA using universal primer and turicibacter primer (Bhattarai and Kashyap, 2016). If needed, gram stain was also performed to further confirm GF status. Gnotobiotic mice (GF colonized with B. thetaiotaomicron) were singly housed and given 0.25% tryptophan in drinking water for duration of in-vivo experiment. HM mice were generated by delivering stool from a healthy human donor by oral gavage to GF mice at 4–5 weeks of age. Stool suspensions were generated by mixing equal volumes of stool and sterile pre-reduced PBS in a screw cap vial inside the anaerobic chamber (Coy Laboratory Products, Grass Lake, MI). The vial was then sealed before removing from the anaerobic chamber to be transferred to GF isolators. Mice were gavaged with 300 μL of the prepared stool suspension and allowed 4–5 weeks for the microbiota to adapt to the mouse gut. All mice were co-housed except as indicated above. Specific pathogen free CR founder 129/Sv WT and 5-HT4R KO mice (Compan et al., 2004) were bred, co-housed and maintained in a micro-isolator caging system using conventional caging and husbandry practices. All WT and 5-HT4R KO CR mice were given irradiated diet (Purina Lab Diet, 5053, Collins feed and seed center, Rochester, MN) for the duration of the experiment.
Generation of Bacterial Strains and Growth Conditions
A Bacteroides thetaiotaomicron variant capable of producing tryptamine was constructed as follows. The Ruminococcus gnavis encoded tryptamine decarboxylase (Trp D+) gene was codon-optimized for efficient expression in a B. thetaiotaomicron background, synthesized by DNA2.0, and cloned into the conjugation and integration vector pNBU2 under the phage promoter described previously (Whitaker et al., 2017). The resulting plasmid was transferred to B. thetaiotaomicron via conjugation using E. coli S17 λ-pir and inserted into the host chromosome via the activity of the co-encoded tyrosine integrase IntN2 on the plasmid backbone as described previously (Whitaker et al., 2017). Successful integration of the plasmid into the B. thetaiotaomicron chromosome was confirmed by PCR and in vitro production of tryptamine when cultured in minimal media containing tryptophan. A vector only control strain of B. thetaiotaomicron was constructed by conjugation and insertion of an empty pNBU2 plasmid backbone into the host chromosome.
For preparation of bacterial gavage sample, engineered B. thetaiotaomicron frozen stocks were first grown on pre-reduced tryptone-yeast extract-glucose (TYG) agar. Single colonies were selected from agar plates and grown in TYG liquid medium containing erythromycin (25 μg/mL) and gentamycin (200 μg/mL) inside an anaerobic growth chamber, kept at 37°C, and supplied with 15% CO2, 5% H2 and 80% N2 (Coy Laboratory Products, Grass Lake, MI) for 24 hours. The liquid culture was aliquoted into sterile 2mL cryovials and sealed before removing from the anaerobic chamber to prevent oxygen exposure. The sealed aliquots were then transferred into two separate sterile experimental isolators containing GF mice. The investigators were blinded to assignment of engineered B. thetaiotaomicron Trp D+/− strains. 300 μL of B. thetaiotaomicron Trp D+/− bacterial culture were delivered to each mouse by oral gavage and any remaining culture was stored at −80 °C for metabolite analysis. At the end of experiment, blood samples were collected using cardiac puncture for serum metabolite measurements.
Method Details
In vitro Measurements
Assessment of Epithelial Transport Using Ussing Chamber
To measure tryptamine induced epithelial ionic transport in vitro proximal colon tissues from GF, HM, CR, WT and 5-HT4R −/− male and female mice were collected at 8–12 weeks of age. At necropsy, a proximal colon segment 2–3 cm below the cecum was removed, opened along the mesenteric border, and washed with ice-cold Krebs solution (composition in mM/l: 11.5 D-glucose, 120.3 NaCl, 15.5 NaHCO3, 5.9 KCl, 1.2 NaH2PO4, 2.5 CaCl2·2H2O, and 1.2 MgCl2; pH 7.3–7.4). The tissue was then pinned flat on the Sylgard® (Dow Corning, Auburn, MI) with the serosal side up and the underlying muscle layer was peeled away under a stereomicroscope. The resulting mucosa-sub-mucosa epithelial sheet was mounted in an Ussing chamber cassette (Physiologic Instruments, San Diego, USA) with an aperture of 0.31 cm2. The basolateral side of the chamber was bathed with 4mL of glucose containing Krebs solution (composition in mM: 11.5 D-glucose, 120.3 NaCl, 15.5 NaHCO3, 5.9 KCl, 1.2 NaH2PO4, 2.5 CaCl2·2H2O, and 1.2 MgCl2; pH 7.3–7.4) while the apical side was bathed with 4 mL of Krebs Mannitol solution (composition in mM: 11.5 D-mannitol, 120.3 NaCl, 15.5 NaHCO3, 5.9 KCl, 1.2 NaH2PO4, 2.5 CaCl2·2H2O, and 1.2 MgCl2; pH 7.3–7.4). The chamber was bubbled with a 97% O2 and 3% CO2 gas mixture. Changes in short circuit current (ΔIsc) were continuously recorded using Acquire and Analyze® software (Physiologic instruments, San Diego, USA). ΔIsc values were measured before and after application of drugs to the apical and the basolateral side and normalized to the tissue area. Tissue viability was confirmed by adding 100 μM acetylcholine on the submucosal side prior to the start of experiments. We assessed the response using concentration (1 μM-3 mM) response measurements allowing assessment of a range on concentrations. The experiments were performed in tissues from 4–6 GF, HM, CR, WT and 5-HT4R KO male and female mice which serve as biological replicates. The use of tryptamine at 1–3 mM was supported by previously published study which showed that in vitro concentration of tryptamine in bacterial culture reaches ~1.7mM (Williams et al., 2014). The dose of 5-HT3R and 5-HT4R antagonist, ondansetron (100 nM) and GR-113808 (30 nM) used in the study was based on previously published studies by (Tuladhar et al., 2002, Tuladhar et al., 1996, Tonini et al., 1994, Weissenburger et al., 2009, Sagrada et al., 1994), which suggest that at these concentrations ondansetron and GR-113808 act as a specific receptor antagonists for 5-HT3R and 5-HT4R respectively.
Quantitative Real-Time PCR
To detect if sex and colonization status affects 5-HT4R mRNA expression, we performed qRT-PCR on proximal colon mucosa-submucosa preparation from male and female GF, HM and CR mice. RNA was extracted, isolated and transcribed using previously established protocol (Bhattarai et al., 2017b). Briefly, proximal colon mucosa-submucosa preparation (as described above) was used to extract RNA. RNA was isolated using a RNeasy Mini Kit (Qiagen, Valencia, CA) and was reverse transcribed to cDNA using SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA) which was then used for PCR analysis. The qRT-PCR assays were performed in 25 μl reactions, which contained 1 X LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland) and 300-nM gene-specific primers. cDNA synthesis reactions were set up along with additional samples lacking reverse transcriptase which served as a control for genomic DNA contamination. Samples were analyzed in triplicate using a LightCycler 480 Instrument II (Roche). Total mRNA expression was calculated after normalization to L32 mRNA (housekeeping) using the comparative cycle threshold (ΔΔCT) analysis method. Relative mRNA expression (fold difference) was calculated on the basis of the 5-HT4R mRNA expression in GF control after normalization. 5-HT4R primer sequences were synthesized at the Integrated DNA Technologies facility at Mayo Clinic (Rochester, MN).
Colonoid Experiments
To prepare three dimensional epithelial colonoids an entire colon segment caudal to the cecum was removed from 3–5, 8–12 weeks old GF and HM mice and immediately placed in cold sterile Ca2+ and Mg2+ free PBS (Corning, 21-040-CV). After collection, colon contents were removed and the tissue was rinsed using a 10mL syringe filled with sterile PBS. The resulting clean colon tissue was minced into ~1mm2 pieces and washed again with cold Ca2+ and Mg2+ free PBS (Corning; 21-040-CV) until the supernatant was clear of any luminal debris. The minced tissue pieces were then incubated for fifteen minutes in gentle cell dissociation medium (Stem cell technologies, 7174) and then rinsed vigorously using a 10mL Pasteur pipette pre-wet with freshly prepared filter-sterilized 0.1% PBS-BSA solution to prevent tissues from sticking to the pipette. The tissue suspension was filtered through a 70μm nylon mesh (Fisher Scientific, 22363548), and filtrate containing the crypt stem cells were centrifuged in a 15mL conical at 290 g at 4°C for 5 minutes. The supernatant liquid was removed and the centrifuged cell pellets were suspended in 6–7mL of DMEM-F/12 medium (Life technologies, 11330-032) and centrifuged again at 290g at 4°C for 5 minutes. The cell pellets were then gently lifted using a 1mL pipette, and re-suspended and mixed with 150μL of IntestiCult™ organoid growth medium (IOGM) (Stem cell technologies, 06000) and 150μL of reduced Matrigel® (1:1 ratio, Corning, 356231). 50μL domes of cell-Matrigel® mixture were plated in a 24well plate, supplemented with 1mL (1:20 of Matrigel® dome/IOGM mix) of warm IOGM medium and maintained at 37°C with 5% CO2 for seven days. After seven days, the colonoids from both GF and HM mice were split into two separate plates after passaging. The first sets of plates were used for swelling assays and the second sets were used for cAMP measurements.
Swelling Assay
Previously prepared colonoids from five GF and HM mice were passaged and 2μl Matrigel® domes were used for experiment. Matrigel® containing colonoids were plated in triplicates on 96 well plates and placed in an incubator maintained at 37°C with 5% CO2 for three days. On the day of the assay, colonoids were incubated with (10μM) Calcein-AM (Invitrogen, C3100MP) for 60 minutes and then either control IOGM media, media containing tryptamine (1mM), media containing tryptamine (1mM), media containing tryptamine and 5-HT4R antagonist (GR-113808 100 nM; Tocris Bioscience, CAS no. 144625-51-4), or media containing Forskolin (10 μM; Sigma-Aldrich, CAS no. 66575-29-9) was added. Organoids were continuously monitored by confocal live cell microscopy (Leica Microsystems, DMi8, Buffalo Grove IL). Images were captured every 10 minutes for 30 minutes using 4X objective and Leica Application Suite X (Leica, Buffalo Grove IL). Image J (Version 1.50i, National Institutes of Health, USA) software was used to manually set the measurement scale at 4X and measure the change in colonoids surface area post treatment by using a freehand selection tool. Colonoid area of 4–5 spherical colonoids from each well were measured using this method and were normalized to baseline control media treatment at t=0 minutes for final calculation.
cAMP Measurements
Colonoids from three GF and HM mice each (biological replicates) were passaged consecutively to generate adequate number of colonoids necessary for the cAMP measurement. Colonoids were plated in triplicate (technical replicates) at similar density on a 20μl Matrigel® domes for cAMP measurements and maintained at 37°C with 5% CO2 for 5–7 days. On the day of the assay the colonoids were incubated in either control IOGM media, media containing tryptamine (1mM), media containing tryptamine (1mM), media containing tryptamine and 5-HT4R antagonist (GR-113808 100 nM; Tocris Bioscience, CAS no. 144625-51-4), or media containing Forskolin (10 μM; Sigma-Aldrich, CAS no. 66575-29-9) and placed in an incubator maintained at 37°C with 5% CO2 for an hour. Free cAMP production (ng/μL) in the samples was assessed using an ELISA cAMP Direct Immunoassay Detection Kit (Abcam, ab138880) according to the manufacturer’s instructions. ELISA plates were analyzed using a Synergy MXplate reader (BiotTek, USA). Only ELISA plates that had R2 >0.90 in their standard curve were included in the analysis. Tryptamine induced cAMP release was normalized to forskolin dependent cAMP release as an additional control for cell density.
In vivo Measurements
Pellet Water Content
Pre-weighed Eppendorf tubes were used to collect 2 – 3 freshly collected fecal pellets from mice. The Eppendorf tubes were tightly closed and removed from the isolators to measure the wet weight. The Eppendorf tubes containing the pellets were placed in an oven maintained at 60°C overnight to measure dry weight. The protocol was repeated 3 times every 48 hours before and after colonization with B. thetaiotaomicron Trp D+/−. Pellet water content to dry pellet mass ratio was measured by subtracting dry weight from wet weight and normalizing it to the dry pellet mass. Pellet water content to dry pellet mass ratio was presented either as absolute or normalized values.
Carmine Red Assay
Mice were acclimatized to the transit protocol by delivering a sham water gavage using a 20 gauge round-tip feeding needle within the gnotobiotic isolator and placing them on top of a perforated metal rack installed in the cage bottom for 4–5 hours. This acclimatization was repeated twice in the week preceding all transit measurements. The day after the second acclimatization, mice were given a 300μL aliquot of the prepared carmine red solution by oral gavage to measure total GI transit time and this was done both before and after gavage with B. thetaiotaomicron Trp D+/−. A solution of carmine red was prepared by mixing 6% carmine red (weight/volume Sigma-Aldrich, 1390-65-4) in 0.5% methylcellulose (wt/volume, Sigma-Aldrich, 9004-67-5). After gavage, the mice were returned to their cages with installed metal rack and white sheet of paper in the cage bottom for ease of tracking pellet color. Fecal pellets were monitored after one hour at 15 min intervals for the presence of carmine red in the fecal pellet. Total time taken for the appearance of the first red pellet was recorded as the whole gut transit time. The assay was repeated after 48 hours.
Metabolite Measurements
Medium Supernatant Sample Preparation for LC-MS Analysis
Supernatants were thawed on ice and 75 μL was aliquoted into a 1.5 mL microfuge tube. 0.3 mL of ice-cold methanol (80% v/v, labeled with IS mix) was added and sample vortexed for 20 minutes at RT. Proteins were precipitated out of solution overnight at −80 °C. Samples were centrifuged at 16,000 G, 20 minutes at 4°C. Methanol (0.01 mL) extract was removed and a QC pool of extract(s), are prepared before storage. Extracts are stored at −80C until liquid chromatography-mass spectroscopic (LC-MS) analysis.
Fecal Samples Preparation for LC-MS Analysis
Five days after bacterial gavage, fresh fecal samples from mice were collected in a cryovials and stored at −80°C until they were ready to be shipped to the Proteomics and Metabolomics Facility at the University of Colorado. There pellets were extracted (with IS Methanol solvent; 0.025 mL per mg of pellet), sonicated and vortexed for 10 minutes at 4°C. Samples were then incubated overnight to allow protein precipitation. The next day, samples were analyzed using LC-MS analysis.
Serum Samples Preparation for LC-MS Analysis
Serum samples were thawed on ice and 100μL was removed for bi-phasic extraction with met-tert-butyl ether (MTBE; labeled with IS mix). Briefly, 1 mL of MTBE was added and sample was vortexed for 20 minutes at 4°C at the end of which 0.125 mL of LC-MS water was added to induce the bi-phase. Samples were centrifuged 16,000 G for 10 minutes at 4°C. 100 μL of the bottom polar layer was then removed and a 10 μL from each sample was pooled for the QC sample. Extracts were stored at −80°C for L C-MS analysis.
LC-MS/MS Analysis
LC-MS/MS was performed on a Waters Acquity UPLC coupled to a Waters Xevo TQ-S triple quadrupole mass spectrometer. Chromatographic separations were carried out on a Waters UPLC T3 C18 stationary phase (1 × 100 mm, 1.7 μM) column. Mobile phases were 100% methanol (B) and water with 0.1% formic acid (A). The analytical gradient was as follows: time = 0 min, 0.1% B; time = 1.0 min, 0.1% B; time = 12 min, 55% B; time = 15 min, 97% B; time 15.5 min, 0.1% B; time 20 min, 0.1% B. Flow rate was 120 μl/min and injection volume was 2 μL. Samples were held at 4°C in the autosampler, and the column was operated at 45°C. The MS was operated in selected reaction monitoring (SRM) mode, where a parent ion is selected by the first quadrupole, fragmented in the collision cell, then a fragment ion selected for by the third quadrupole. Product ions, collision energies, and cone voltages were optimized for each analyte by direct injection of individual synthetic standards. Inter-channel delay was set to 3 ms. The MS was operated in both negative and positive ionization modes with the capillary voltage set to 3.2 kV. Source temperature was 150°C and desolvation temperature 500°C. Desolvation gas flow was 1000 L/hr, cone gas flow was 150 L/hr, and collision gas flow was 0.2 mL/min. Nebulizer pressure (nitrogen) was set to 7 Bar. Argon was used as the collision gas. A calibration curve was generated using authentic standards for each compounds and their corresponding stable isotope labeled internal standards in 100% methanol solution.
Quantification and Statistical Analysis
Statistical Analysis
Statistical analyses were conducted with Prism 7.0a software (GraphPad). Image J (Version 1.50i, National Institutes of Health, USA) was used to quantify change in colonoids surface area. For pairwise and two independent group comparison Student’s t test was used, while for multiple group comparison ANOVA was performed. To compare multiple concentration response curves between groups Two-way ANOVA with Bonferroni post hoc test was performed. Data are presented as mean ± SEM and P < 0.05 was considered statistically significant. The n number refers to the number of mice for each experiment. The number of mice for each experiment was based on our previous studies (Bhattarai et al., 2017b) to achieve power of 80% with α of 0.05 as well as constraints within gnotobiotic isolators. Any additional technical replicates are described within the method details as well as the results.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial Strains | ||
| Bacteroides thetaiotaomicron VPI-5482 | ATCC | ATCC 29148 |
| Bacteroides thetaiotaomicron VPI-5482 pNBU2 | This work | N/A |
| Bacteroides thetaiotaomicron VPI-5482 pNBU2::trpD | This work | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Ca2+ Mg free PBS | Corning | 21-040-CV |
| Gentle cell dissociation media | Stem cell technologies | Cat# 7174 |
| Bovine Serum Albumin | Sigma Life Science | Cas# 9048-46-8 |
| DMEM-F/12 media | Life technologies | Cat# 11330-032 |
| IntestiCultTM organoid growth media | Stem cell technologies | Cat# 6000 |
| Matrigel | Corning | Cat# 356231 |
| Calcein green | Invitrogen | Cat# C3100MP |
| Tryptamine hydrochloride | Aldrich | Cas# 343-92-2 |
| GR-113808 | Tocris Bioscience | Cas# 144625-51-4 |
| Ondansetron | Sigma-Aldrich | Cas# 99614-01-04 |
| Forskolin | Sigma-Aldrich | Cas# 66575-29-9 |
| Erythromycin | Sigma Life Science | Cas# 114-07-8 |
| Gentamycin | Sigma Life Science | Cas# 1405-41-0 |
| Methanol | Sigma-Aldrich | Cas# 67-56-1 |
| Methylcellulose | Sigma-Aldrich | Cas#9004-67-5 |
| Carmine red | Sigma-Aldrich | Cas# 1390-65-4 |
| 5-Nitro-2-(3-phenylpropylamino) benzoic acid | Sigma-Aldrich | Cas# 107254-86-4 |
| Amiloride hdrochloride | Sigma-Aldrich | Cas# 2016-88-8 |
| CH-223191 | Tocris | Cat# 3858 |
| Tetrodotoxin | EMD Biosciences Inc. | Cat# 554412 |
| Critical Commercial Assays | ||
| cAMP Direct Immunoassay Detection Kit | Abcam | Cat# ab138880 |
| Experimental Models: Organisms/Strains | ||
| GF and HM mice | Taconic Farms | Swiss Webster |
| 5-HT4R−/− and WT mice | Compan et al.,2004 | 129/Sv |
| Recombinant DNA | ||
| pNBU2 | Whitaker et al.2017 | N/A |
| Primers sequence | ||
| 5-HT4R Forward: 5′-AGTTCCAACGAGGGTTTCAGG-3′ Reverse: 5′-CAGCAGGTTGCCCAAGATG-3′ |
Bhattarai et al. 2017 | N/A |
| Turicibacter Forward: 5′-GCGCGCAGGTGGTTAATTAAGTCT-3′ Reverse: 5′-TCAGTGTCAGTTGCAGACCAGGAA-3′ |
This work | N/A |
| Universal Forward: 5′-AGAGTTTGATCCTGGCTCAG-3′ Reverse: 5′-GACGGGCGGTGWGTRCA-3′ |
Bhattarai and Kashyap, 2016 | N/A |
| Software and Algorithms | ||
| ImageJ | NIH | Version 1.50i |
| GraphPad PRISM | GraphPad Software | Version 7.0a |
| Acquire and Analyze | Physiologic instruments | Version 2.0 |
| Leica Application Suite X | Leica | N/A |
| Other | ||
| Synergy MXplate reader | BiotTek | Gen5 1.09 |
| Confocal live cell microscopy | Leica Microsystems | DMi8 |
| Ussing Chamber | Physiologic Instruments | Cat# P2300 |
| Voltage-clamp amplifier | Physiologic Instruments | Version VCC MC8 |
| LC-MS/MS | Waters | N/A |
Supplementary Material
Highlights.
Tryptamine increases anion and fluid secretion in the proximal colon
Tryptamine induced effect is mediated by the GPCR serotonin receptor-4 (5-HT4R)
Tryptamine activates epithelial 5-HT4R to increase cAMP level and drive fluid secretion
In vivo tryptamine production by an engineered microbe accelerates whole gut transit
Acknowledgments
We would like to thank Bradley Schmidt, Nella Gabrielová, Cheryl Bernard and William Moor for technical assistance, Dr. Lisa M. Wolfe and Dr. Corey Broeckling from the metabolomics facility at Colorado State University for assistance with metabolomics analysis, Dr. John Grider for valuable scientific discussions, Dr. Valérie Compan for providing 5-HT4R−/− mice, Julie Nielsen and Donna T DeSmet for help with figures and Lyndsay Busby for administrative assistance. Figures for graphical abstract and animation for Figure360 were made using Biomedical PowerPoint Toolkit Suite (www.motifolio.com). Funding: This work was made possible by funding from NIH DK100638, DK111850, DK114007 (PCK), NIDDK K23 103911 (MG), Integrated Physiology Core of the Hopkins Conte Digestive Disease Basic and Translational Research Core Center (P30 DK-089502) as well as the Global Probiotic Council (PCK), the Center for Individualized Medicine, Mayo Clinic, Rochester, MN (PCK) and VA merit review funds (JDK).
Footnotes
Author Contributions
YB, MAF, JLS, GF and PCK, designed the experiments and the overall data analysis; YB, EJB and PCK wrote the manuscript with input from co-authors; YB, LT, YA, EJB contributed to the mouse experiments; BBW, WRW, EJB, MAF, JLS, PCK and YB contributed to bacterial culture and manipulation and in vivo study design, YB, NZ contributed to colonoid experiments, YB, MAG, DRL, KK, JDK, GF, PCK contributed to design and conduct of in vitro and Ussing chamber experiments.
Declaration of Interests
The authors have no financial interests to declare. We have a patent (US20170042860A1) related to this work- “Methods and materials for using Ruminococcus gnavus or Clostridium sporogenes to treat gastrointestinal disorders.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson GM. Quantitation of tryptophan metabolites in rat feces by thin-layer chromatography. J Chromatogr. 1975;105(2):323–8. doi: 10.1016/s0021-9673(01)82261-5. [DOI] [PubMed] [Google Scholar]
- Anitha M, Reichardt F, Tabatabavakili S, Nezami BG, Chassaing B, Mwangi S, Vijay-Kumar M, Gewirtz A, Srinivasan S. Intestinal dysbiosis contributes to the delayed gastrointestinal transit in high-fat diet fed mice. Cell Mol Gastroenterol Hepatol. 2016;2(3):328–339. doi: 10.1016/j.jcmgh.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl(−) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Physiol Cell Physiol. 2013;305(4):C447–56. doi: 10.1152/ajpcell.00416.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender DA. Biochemistry of tryptophan in health and disease. Mol Aspects Med. 1983;6(2):101–97. doi: 10.1016/0098-2997(83)90005-5. [DOI] [PubMed] [Google Scholar]
- Bhattarai Y, Kashyap PC. Germ-Free Mice Model for Studying Host-Microbial Interactions. Methods Mol Biol. 2016;1438:123–35. doi: 10.1007/978-1-4939-3661-8_8. [DOI] [PubMed] [Google Scholar]
- Bhattarai Y, Muniz Pedrogo DA, Kashyap PC. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. 2017a;312(1):G52–G62. doi: 10.1152/ajpgi.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai Y, Schmidt BA, Linden DR, Larson ED, Grover M, Beyder A, Farrugia G, Kashyap PC. Human Derived Gut Microbiota Modulates Colonic Secretion in Mice by Regulating 5-HT3 Receptor Expression via Acetate Production. Am J Physiol Gastrointest Liver Physiol. 2017b;313(1):G80–G87. doi: 10.1152/ajpgi.00448.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman RA, Burleigh DE. Heterogeneity of 5-HT receptors mediating secretion in the human intestine. Ann N Y Acad Sci. 1997;812:224–5. doi: 10.1111/j.1749-6632.1997.tb48183.x. [DOI] [PubMed] [Google Scholar]
- Brooks JB, Nunez-Montiel OL, Basta MT, Hierholzer JC. Studies of stools from pseudomembranous colitis, rotaviral, and other diarrheal syndromes by frequency-pulsed electron capture gas-liquid chromatography. J Clin Microbiol. 1984;20(3):549–60. doi: 10.1128/jcm.20.3.549-560.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9(4):1202–8. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cil O, Phuan PW, Lee S, Tan J, Haggie PM, Levin MH, Sun L, Thiagarajah JR, Ma T, Verkman AS. CFTR activator increases intestinal fluid secretion and normalizes stool output in a mouse model of constipation. Cell Mol Gastroenterol Hepatol. 2016;2(3):317–327. doi: 10.1016/j.jcmgh.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28(8):1221–38. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LJ, Esterhazy D, Kim SH, Lemetre C, Aguilar RR, Gordon EA, Pickard AJ, Cross JR, Emiliano AB, Han SM, Chu J, Vila-Farres X, Kaplitt J, Rogoz A, Calle PY, Hunter C, Bitok JK, Brady SF. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549(7670):48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P, Rabasseda X. Tegaserod: a serotonin 5-HT4 receptor agonist for treatment of constipation-predominant irritable bowel syndrome. Drugs Today (Barc) 2004;40(12):1013–30. doi: 10.1358/dot.2004.40.12.872576. [DOI] [PubMed] [Google Scholar]
- Compan V, Zhou M, Grailhe R, Gazzara RA, Martin R, Gingrich J, Dumuis A, Brunner D, Bockaert J, Hen R. Attenuated response to stress and novelty and hypersensitivity to seizures in 5-HT4 receptor knock-out mice. J Neurosci. 2004;24(2):412–9. doi: 10.1523/JNEUROSCI.2806-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280(5361):295–8. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19(7):939–45. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- Dey N, Wagner VE, Blanton LV, Cheng J, Fontana L, Haque R, Ahmed T, Gordon JI. Regulators of Gut Motility Revealed by a Gnotobiotic Model of Diet-Microbiome Interactions Related to Travel. Cell. 2015;163(1):95–107. doi: 10.1016/j.cell.2015.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551(7682):648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingue JC, Ao M, Sarathy J, George A, Alrefai WA, Nelson DJ, Rao MC. HEK-293 cells expressing the cystic fibrosis transmembrane conductance regulator (CFTR): a model for studying regulation of Cl− transport. Physiol Rep. 2014;2(9) doi: 10.14814/phy2.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet B, Khalifa M, Daleau P, Hamelin BA, Turgeon J. Block of the rapid component of the delayed rectifier potassium current by the prokinetic agent cisapride underlies drug-related lengthening of the QT interval. Circulation. 1998;97(2):204–10. doi: 10.1161/01.cir.97.2.204. [DOI] [PubMed] [Google Scholar]
- Esser C, Rannug A. The aryl hydrocarbon receptor in barrier organ physiology, immunology, and toxicology. Pharmacol Rev. 2015;67(2):259–79. doi: 10.1124/pr.114.009001. [DOI] [PubMed] [Google Scholar]
- Facchini PJ, Huber-Allanach KL, Tari LW. Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry. 2000;54(2):121–38. doi: 10.1016/s0031-9422(00)00050-9. [DOI] [PubMed] [Google Scholar]
- Frizzell RA, Hanrahan JW. Physiology of epithelial chloride and fluid secretion. Cold Spring Harb Perspect Med. 2012;2(6):a009563. doi: 10.1101/cshperspect.a009563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargaro M, Pirro M, Romani R, Zelante T, Fallarino F. Aryl Hydrocarbon Receptor-Dependent Pathways in Immune Regulation. Am J Transplant. 2016;16(8):2270–6. doi: 10.1111/ajt.13716. [DOI] [PubMed] [Google Scholar]
- Gevi F, Zolla L, Gabriele S, Persico AM. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol Autism. 2016;7:47. doi: 10.1186/s13229-016-0109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grider JR, Foxx-Orenstein AE, Jin JG. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea pig intestine. Gastroenterology. 1998;115(2):370–80. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- Han B, Sheng B, Zhang Z, Pu A, Yin J, Wang Q, Yang K, Sun L, Yu M, Qiu Y, Xiao W, Yang H. Aryl Hydrocarbon Receptor Activation in Intestinal Obstruction Ameliorates Intestinal Barrier Dysfunction Via Suppression of MLCK-MLC Phosphorylation Pathway. Shock. 2016;46(3):319–28. doi: 10.1097/SHK.0000000000000594. [DOI] [PubMed] [Google Scholar]
- Heath-Pagliuso S, Rogers WJ, Tullis K, Seidel SD, Cenijn PH, Brouwer A, Denison MS. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry. 1998;37(33):11508–15. doi: 10.1021/bi980087p. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Tyler K, MacEachern SJ, Balemba OB, Johnson AC, Brooks EM, Zhao H, Swain GM, Moses PL, Galligan JJ, Sharkey KA, Greenwood-Van Meerveld B, Mawe GM. Activation of colonic mucosal 5-HT(4) receptors accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142(4):844–854 e4. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E, Li JV, Marchesi JR, Nicholson JK. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 2012;16(5):559–64. doi: 10.1016/j.cmet.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26(11):1586–96. doi: 10.1111/nmo.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In JG, Foulke-Abel J, Estes MK, Zachos NC, Kovbasnjuk O, Donowitz M. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol. 2016;13(11):633–642. doi: 10.1038/nrgastro.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin JG, Foxx-Orenstein AE, Grider JR. Propulsion in guinea pig colon induced by 5-hydroxytryptamine (HT) via 5-HT4 and 5-HT3 receptors. J Pharmacol Exp Ther. 1999;288(1):93–7. [PubMed] [Google Scholar]
- Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol. 2014;85(5):777–88. doi: 10.1124/mol.113.091165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM. The Influence of the Gut Microbiota on Host Physiology: In Pursuit of Mechanisms. Yale J Biol Med. 2016;89(3):285–297. [PMC free article] [PubMed] [Google Scholar]
- Jones RS. Tryptamine: a neuromodulator or neurotransmitter in mammalian brain? Prog Neurobiol. 1982;19(1–2):117–39. doi: 10.1016/0301-0082(82)90023-5. [DOI] [PubMed] [Google Scholar]
- Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85(2):289–95. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100(1):6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji I, Akiba Y, Said H, Narimatsu K, Kaunitz JD. Luminal 5-HT stimulates colonic bicarbonate secretion in rats. Br J Pharmacol. 2015;172(19):4655–70. doi: 10.1111/bph.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013;7(10):1933–43. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland BJ. Regulating inflammation with microbial metabolites. Nat Med. 2016;22(6):581–3. doi: 10.1038/nm.4117. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10(8):473–86. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan K, Kane A, Asarkof N, Wicks J, Guerina V, Kellum J, Baron S, Gintzler AR, Donowitz M. Entamoeba histolytica causes intestinal secretion: role of serotonin. Science. 1983;221(4612):762–4. doi: 10.1126/science.6308760. [DOI] [PubMed] [Google Scholar]
- Mustala O, Solatunturi E, Tarpila S. Monoamine oxidase activity in the human small intestine. Acta Med Scand. 1969;185(3):145–6. doi: 10.1111/j.0954-6820.1969.tb07312.x. [DOI] [PubMed] [Google Scholar]
- Neis EP, Dejong CH, Rensen SS. The role of microbial amino acid metabolism in host metabolism. Nutrients. 2015;7(4):2930–46. doi: 10.3390/nu7042930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paley EL, Perry G, Sokolova O. Tryptamine induces axonopathy and mitochondriopathy mimicking neurodegenerative diseases via tryptophanyl-tRNA deficiency. Curr Alzheimer Res. 2013;10(9):987–1004. doi: 10.2174/15672050113106660164. [DOI] [PubMed] [Google Scholar]
- Palmer KL, Mashburn LM, Singh PK, Whiteley M. Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol. 2005;187(15):5267–77. doi: 10.1128/JB.187.15.5267-5277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager M, Büttner J, Grunert P, Ellinghaus D, Büning C. A Promoter Variant Within the Aryl Hydrocarbon Receptor Gene Is Associated with an Epithelial Barrier Defect in Smokers with Crohn’s Disease. Inflamm Bowel Dis. 2016;22(10):2356–68. doi: 10.1097/MIB.0000000000000910. [DOI] [PubMed] [Google Scholar]
- Reigstad CS, Salmonson CE, Rainey JF, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–14. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DM, Dawes MA, Mathias CW, Acheson A, Hill-Kapturczak N, Dougherty DM. L-Tryptophan: Basic Metabolic Functions, Behavioral Research and Therapeutic Indications. Int J Tryptophan Res. 2009;2:45–60. doi: 10.4137/ijtr.s2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kébir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagrada A, Schiavi GB, Cereda E, Ladinsky H. Antagonistic properties of McNeil-A-343 at 5-HT4 and 5-HT3 receptors. Br J Pharmacol. 1994;113(3):711–6. doi: 10.1111/j.1476-5381.1994.tb17051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai-Yamashita Y, Yamashita K, Kanematsu T, Taniyama K. Localization of the 5-HT(4) receptor in the human and the guinea pig colon. Eur J Pharmacol. 1999;383(3):281–5. doi: 10.1016/s0014-2999(99)00642-1. [DOI] [PubMed] [Google Scholar]
- Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138(5):1772–82. doi: 10.1053/j.gastro.2010.01.053. [DOI] [PubMed] [Google Scholar]
- Stacy A, Fleming D, Lamont RJ, Rumbaugh KP, Whiteley M. A Commensal Bacterium Promotes Virulence of an Opportunistic Pathogen via Cross-Respiration. MBio. 2016;7(3) doi: 10.1128/mBio.00782-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift S, Downie JA, Whitehead NA, Barnard AM, Salmond GP, Williams P. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv Microb Physiol. 2001;45:199–270. doi: 10.1016/s0065-2911(01)45005-3. [DOI] [PubMed] [Google Scholar]
- Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Müller-Lissner S, Quigley EM, Schuurkes J, De Maeyer JH, Stanghellini V. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther. 2012;35(7):745–67. doi: 10.1111/j.1365-2036.2012.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RH, Allmond K. Linaclotide (Linzess) for Irritable Bowel syndrome With Constipation and For Chronic Idiopathic Constipation. P T. 2013;38(3):154–60. [PMC free article] [PubMed] [Google Scholar]
- Tonini M, Messori E, Franceschetti GP, Rizzi CA, Castoldi AF, Coccini T, Candura SM. Characterization of the 5-HT receptor potentiating neuromuscular cholinergic transmission in strips of human isolated detrusor muscle. Br J Pharmacol. 1994;113(1):1–2. doi: 10.1111/j.1476-5381.1994.tb16163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar BR, Costall B, Naylor RJ. Pharmacological characterization of the 5-hydroxytryptamine receptor mediating relaxation in the rat isolated ileum. Br J Pharmacol. 1996;119(2):303–10. doi: 10.1111/j.1476-5381.1996.tb15986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar BR, Costall B, Naylor RJ. Modulation of 5-HT4 receptor function in the rat isolated ileum by fluoxetine: the involvement of endogenous 5-hydroxytryptamine. Br J Pharmacol. 2002;136(1):150–6. doi: 10.1038/sj.bjp.0704694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oekelen D, Megens A, Meert T, Luyten WH, Leysen JE. Role of 5-HT(2) receptors in the tryptamine-induced 5-HT syndrome in rats. Behav Pharmacol. 2002;13(4):313–8. doi: 10.1097/00008877-200207000-00008. [DOI] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41(2):296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visick KL, Fuqua C. Decoding microbial chatter: cell-cell communication in bacteria. J Bacteriol. 2005;187(16):5507–19. doi: 10.1128/JB.187.16.5507-5519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenburger J, Funck-Brentano C, Jaillon P, Charbit B. Droperidol and ondansetron in vitro electrophysiological drug interaction study. Fundam Clin Pharmacol. 2009;23(6):719–26. doi: 10.1111/j.1472-8206.2009.00735.x. [DOI] [PubMed] [Google Scholar]
- Whitaker WR, Shepherd ES, Sonnenburg JL. Tunable Expression Tools Enable Single-Cell Strain Distinction in the Gut Microbiome. Cell. 2017;169(3):538–546.e12. doi: 10.1016/j.cell.2017.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, Van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell host & microbe. 2014;16(4):495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtman RJ, Hefti F, Melamed E. Precursor control of neurotransmitter synthesis. Pharmacol Rev. 1980;32(4):315–35. [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–76. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo CJ, Couse NF, Zinner MJ. Serotonin and substance P stimulate intestinal secretion in the isolated perfused ileum. Surgery. 1989;105(1):86–92. [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–85. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang LS, Davies SS. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8(1):46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.