Rice flower development determines grain yield. Here we reveal the genetic interactions between the rice meristem maintenance gene FON4 and six floral homeotic genes in flower development.
Keywords: Floral homeotic genes, floral meristem, flower development, FON4, genetic interaction, rice
Abstract
The floral meristem (FM) is self-maintaining at the early stages of flower development, but it is terminated when a fixed number of floral organs are produced. The FLORAL ORGAN NUMBER4 (FON4; also known as FON2) gene, an ortholog of Arabidopsis CLAVATA3 (CLV3), is required for regulating FM size and determinacy in rice. However, its interactions with floral homeotic genes remain unknown. Here, we report the genetic interactions between FON4 and floral homeotic genes OsMADS15 (an A-class gene), OsMADS16 (also called SUPERWOMAN1, SPW1, a B-class gene), OsMADS3 and OsMADS58 (C-class genes), OsMADS13 (a D-class gene), and OsMADS1 (an E-class gene) during flower development. We observed an additive phenotype in the fon4 double mutant with the OsMADS15 mutant allele dep (degenerative palea). The effect on the organ number of whorl 2 was enhanced in fon4 spw1. Double mutant combinations of fon4 with osmads3, osmads58, osmads13, and osmads1 displayed enhanced defects in FM determinacy and identity, respectively, indicating that FON4 and these genes synergistically control FM activity. In addition, the expression patterns of all the genes besides OsMADS13 had no obvious change in the fon4 mutant. This work reveals how the meristem maintenance gene FON4 genetically interacts with C, D, and E floral homeotic genes in specifying FM activity in monocot rice.
Introduction
Plants possess the ability to produce organs throughout their life due to the continuous activity of meristems. Maintenance of meristem activity is dependent on the balance between differentiation and self-renewal of stem cells located in the central zone (Steeves and Sussex, 1989). In the eudicot Arabidopsis thaliana, the feedback loop consisting of the homeodomain transcription factor WUSCHEL (WUS) and the CLAVATA (CLV) ligand–receptor plays a prominent role in the stem cell maintenance of the shoot apical meristem (SAM) and floral meristem (FM) (Fletcher et al., 1999; Brand et al., 2000; Schoof et al., 2000; Carles and Fletcher, 2003; Aichinger et al., 2012; Perales and Reddy, 2012). WUS is expressed in the organizing center (OC) of meristems and migrates into overlying cells of the central zone (CZ), where it specifies stem cell fate and activates a small secreted peptide CLV3 (Mayer et al., 1998; Schoof et al., 2000; Yadav et al., 2011; Daum et al., 2014). CLV3 activates receptor kinase signaling and, in turn, restricts WUS activity (Fletcher et al., 1999; Brand et al., 2000; Schoof et al., 2000). To date, there are at least four receptors known to be required for perceiving CLV3 peptide, including the leucine-rich repeat (LRR) receptor-like kinase CLV1 (Clark et al., 1997; Ogawa et al., 2008), the LRR receptor-like protein CLV2 (Jeong et al., 1999), the pseudokinase CORYNE (CRN)/SUPPRESSOR OF LLP1 2(SOL2) (Müller et al., 2008; Miwa et al., 2008; Bleckmann et al., 2010; Nimchuk et al., 2011), and RECEPTOR-LIKE PROTEIN KINASE 2 (RPK2)/TOADSTOOL 2 (TOAD2) (Kinoshita et al., 2010). Through the feedback regulatory loop between WUS and CLV, the stem cell population within meristems is maintained at a relatively constant number.
Unlike the indeterminate SAM, the FM ceases to have stem cell activity after the formation of a certain number of floral organs (Steeves and Sussex, 1989), whose identities have been proposed to be specified by A, B, C, D, and E floral homeotic genes. This is described by the ‘ABCDE’ model which is based on the research in model eudicot plants (Coen and Meyerowitz, 1991; Angenent et al., 1995; Colombo et al., 1995; Pelaz et al., 2000; Theißen, 2001; Theissen and Saedler, 2001; Ditta et al., 2004; Krizek and Fletcher, 2005). In A. thaliana, the ABCDE model includes floral organ identity genes which are also responsible for the specification or termination of the FM, such as the A-class genes APETALA1 (AP1) and APETALA2 (AP2), the C-class gene AGAMOUS (AG), and the four E-class genes SEPALLATA1 (SEP1), SEP2, SEP3, and SEP4 (Irish and Sussex, 1990; Yanofsky et al., 1990; Mandel et al., 1992; Jofuku et al., 1994; Pelaz et al., 2000; Lenhard et al., 2001; Lohmann et al., 2001; Ditta et al., 2004). Among them, the C-class gene AG plays a major role in the termination of the FM, beside its function in specifying stamen and carpel identities (Yanofsky et al., 1990; Lenhard et al., 2001; Lohmann et al., 2001). Furthermore, at the early floral developmental stage, WUS, together with the meristem identity gene LEAFY (LFY), activates AG expression in the inner two whorls (Lenhard et al., 2001; Lohmann et al., 2001). At the later stage, AG represses WUS activity through two independent mechanisms: the activation of KNUCKLES (KNU), a WUS repressor, and via the recruitment of Polycomb Group (PcG) proteins to WUS (Ming and Ma, 2009; Sun et al., 2009, 2014; Liu et al., 2011; Zhang, 2014). Although both AG and the CLV pathways negatively regulate WUS expression, CLV genes and AG appear to repress WUS independently, as the phenotype of clv1 ag double mutants was substantially additive and the WUS expression domain was larger in clv1 ag double mutants than in ag single mutants (Clark et al., 1993; Lohmann et al., 2001).
In grasses, the basic structural unit of the inflorescence is called the spikelet, and each consists of glumes and one or several florets (Zhang et al., 2013; Zhang and Yuan, 2014; Yuan and Zhang, 2015). Normally, each floret contains two bract-like organs (lemma and palea), lodicules, stamens, and a pistil (Kellogg, 2001; Rudall and Bateman, 2004; Yuan et al., 2009). The lodicules are considered to be the counterparts to the eudicot petals, whereas the origin of the palea and the lemma still remains controversial. Inflorescence and flower development in the grass species are markedly distinct from those in eudicots. Nevertheless, genetic studies on two model plants, maize (Zea mays L.) and rice (Oryza sativa L.), have demonstrated that the CLV signaling pathway of meristem maintenance and the ABCDE model of floral organ specification are partially conserved between grasses and eudicots (Ferrario et al., 2004; Bommert et al., 2005b; Thompson and Hake, 2009; Ciaffi et al., 2011; Pautler et al., 2013; Tanaka et al., 2013; Zhang et al., 2013; Zhang and Yuan, 2014; Wang et al., 2015; Dreni and Zhang, 2016).
In maize, mutations in THICK TASSEL DWARF1 (TD1) and FASCIATED EAR2 (FEA2) genes, which encode orthologs of CLV1 and CLV2, respectively, affect the size of the inflorescence meristem and FM (Taguchi-Shiobara et al., 2001; Bommert et al., 2005a). In addition, TD1 and FEA2 function in different pathway since the td1 fea2 double mutant shows an additive or synergistic phenotype (Bommert et al., 2005a). This may imply a different mechanism in maize, because CLV1 and CLV2 were once thought to act in a common pathway in Arabidopsis (Kayes and Clark, 1998). However, subsequent evidence has revealed that CLV2 functions separately from CLV1 by forming heteromers with CRN/SOL2 (Müller et al., 2008; Miwa et al., 2008; Bleckmann et al., 2010; Nimchuk et al., 2011). Apart from these two CLV-like genes, maize COMPACT PLANT2 (CT2) encoding the α-subunit (Gα) of a heterotrimeric GTP-binding protein, was also shown to be directly involved in the CLV pathway. Biochemical and genetic analyses indicate that CT2/Gα has an interaction with FEA2 in controlling meristem development (Bommert et al., 2013).
In rice, FLORAL ORGAN NUMBER1 (FON1) and FON4 (also known as FON2) are closely related to CLV1 and CLV3, respectively. Both fon1 and fon4 mutants have enlarged FMs, and an increased floral organ number, especially stamens and pistils (Suzaki et al., 2004,2006; Chu et al., 2006). Moreover, it has been shown that FON4 and FON1 function in a common pathway in specifying FM maintenance, mimicking that of CLV3 and CLV1 (Chu et al., 2006; Suzaki et al., 2006; Chu and Zhang, 2007).
Taken together, these studies suggest that the CLV pathway is conserved in the regulation of meristem size between eudicot and grass species. However, there are some differences between CLV genes and corresponding grass orthologs in expression patterns and mutant phenotypes. CLV1 expression is mainly detected in the L3 layers of meristems (Clark et al., 1997; Fletcher et al., 1999), whereas TD1 and FON1 are uniformly expressed throughout the meristems, as well as floral organ primordia (Suzaki et al., 2004; Bommert et al., 2005a). Unlike the clv1 mutant, which displays enlargement of the inflorescence meristem and FM, the rice fon1 mutant has no evident defect in inflorescence meristem size, although it produces an enlarged FM.
In grass species, AP1-like genes are required for the phase transition from vegetative to reproductive growth, such as VRN1 of Triticum monococcum or WAP1 of T. aestivum (Murai et al., 2003; Yan et al., 2003), which differs from Arabidopsis AP1 with a role in establishing FM identity and specifying the identities of the outer two whorls, the sepal and petal (Mandel et al., 1992). The rice genome has four AP1-like MADS-box genes, OsMADS14 (RAP1B), OsMADS15 (RAP1A), OsMADS18, and OsMADS20 (Litt and Irish, 2003; Kater et al., 2006). On the basis of their expression pattern and phenotypic analyses on available mutants or transgenic plants, it was proposed that rice AP1-like genes have a function in FM identity specification (Jeon et al., 2000b; Kyozuka et al., 2000; Masiero et al., 2002; Pelucchi et al., 2002; Lee et al., 2003,2004; Fornara et al., 2004; Komiya et al., 2008; Wang et al., 2010; Taoka et al., 2011; Lu et al., 2012). In contrast, B-class MADS-box genes have conserved functions in both eudicots and grasses. Rice OsMADS16 (also called SUPERWOMAN1, SPW1) and maize Silky1, two orthologs of the Arabidopsis B-function gene APETALA3 (AP3), are both essential for determining lodicule and stamen identity (Ambrose et al., 2000; Nagasawa et al., 2003; Whipple et al., 2007). Genetic analyses also reveal that OsMADS16 and Silky1 are involved in the control of the FM determinacy together with C-class genes (Ambrose et al., 2000; Yun et al., 2013). A recent work suggested that, in maize, the PI/GLO-like B-class genes may also have a similar function (Bartlett et al., 2015). C-class genes have been partially subfunctionalized by means of gene duplication during grass evolution (Kramer et al., 2004; Zahn et al., 2006; Dreni et al., 2013; Dreni and Kater, 2014). In rice, two C-class genes, OsMADS3 and OsMADS58, redundantly regulate the identity of reproductive organs and FM determinacy (Yamaguchi et al., 2006; Dreni et al., 2011; Hu et al., 2011). Likewise, maize has three AG orthologs: ZAG1 (ZEA AGAMOUS1), ZMM2 (ZEA MAYS MADS2), and ZMM23 (Schmidt et al., 1993; Mena et al., 1996; Münster et al., 2002). In the zag1 mutant, FM partially lost determinacy, but the identity of reproductive organs was almost normal, suggesting that other class C genes may be required for stamen and carpel specification, such as ZMM2 and ZMM23, whose functions remain unknown to date (Schmidt et al., 1993; Mena et al., 1996; Münster et al., 2002). In addition, rice OsMADS13, one ortholog of Arabidopsis SEEDSTICK (STK) and petunia FLORAL BINDING PROTEIN7 (FBP7) and FBP11 D-class genes, controls ovule identity and FM determinacy (Angenent et al., 1995; Colombo et al., 1995; Pinyopich et al., 2003; Dreni et al., 2007, 2011; Li et al., 2011b). However, the Arabidopsis stk single mutant does not display the conversion of ovule identity because of functional redundancy with AG, SHATTERPROOF1 (SHP1), and SHP2 (Favaro et al., 2003; Pinyopich et al., 2003). A number of SEP subfamily (E-class) genes with diverse function have been identified from grasses. There are five members [OsMADS1/LEAFY HULL STERILE1 (LHS1)/NAKED SEED RICE (NSR), OsMADS5, OsMADS7, OsMADS8, and OsMADS34] in rice and eight in maize (Malcomber and Kellogg, 2005; Zahn et al., 2005). Rice OsMADS1 was shown to determine lemma and palea identity and to promote FM specification by co-ordinating transcriptional control and hormone signaling pathways (Jeon et al., 2000a; Prasad et al., 2001, 2005; Agrawal et al., 2005; Chen et al., 2006; Gao et al., 2010; Li et al., 2010; Khanday et al., 2013; Hu et al., 2015).
To reveal whether FON4 interacts with floral homeotic genes in specifying rice flower development, we constructed and analyzed the double mutants of FON4 with OsMADS15, OsMADS16, OsMADS3, OsMADS58, OsMADS13, and OsMADS1, respectively. Therefore, we concluded that FON4 and C, D, and E floral homeotic genes play a synergistic role in specifying FM activity and flower development. This work provides insight into the mechanism controlling FM activity in rice.
Materials and methods
Plant materials
In this study, we used the rice (Oryza sativa) mutants fon4-2, fon4-1, dep (degenerative palea), spw1-1, osmads3-4, osmads58, osmads13-3, and osmads1-z. The fon4-2, fon4-1, osmads3-4, osmads58, osmads13-3, and osmads1-z mutants were previously reported (Chu et al., 2006; Gao et al., 2010; Dreni et al., 2011; Hu et al., 2011; Li et al., 2011b). dep was kindly provided by Professor Zhukuan Cheng (Chinese Academy of Sciences), and spw1-1 was provided by Professor Hajime Sakai and Professor Yasuo Nagato (University of Tokyo). Double mutants were isolated by genotyping and phenotype observation. Primers for genotyping are listed in Supplementary Table S1 at JXB online. All the mutants and wild-type rice (9522 cultivar) were grown in the paddy field or greenhouse of Shanghai Jiao Tong University, China.
Histological analysis and microscopy observation
Fresh spikelets were photographed with a Leica S8 APO stereo microscope. For histological analysis, samples were prepared and observed following the method reported by Hu et al. (2015). Scanning electron microscopy (SEM) observations were performed as described previously (Li et al., 2006). Images were processed through Adobe Photoshop CS6 software.
In situ hybridization
The inflorescences of wild-type rice plants were fixed overnight at 4 °C in FAA (5% acetic acid, 50% ethanol, and 3.7% formaldehyde in water), dehydrated in an ethanol series, and embedded in Paraplast Plus (Sigma). The hybridization signals were detected according to the previous description (Chu et al., 2006). The probes for OsMADS15, OsMADS16, OsMADS3, OsMADS58, OsMADS13, OsMADS1, and OSH1 were prepared as previously reported (Li et al., 2010, 2011a, b).
qRT–PCR
Total RNAs were extracted with TRIZOL reagent (Sigma-Aldrich), and ~1 μg of RNA was reverse transcribed using the PrimeScript RT reagent kit with genomic DNA eraser (DRR047A; Takara). The 10-fold diluted cDNA samples were used as templates for the quantitative reverse transcription–PCR (qRT–PCR) experiment. The qRT–PCRs were performed on a Bio-Rad CFX96 Real-Time System using the iQ SYBR Green Supermix (Bio-Rad). The amplifying program was as follows: 95 °C for 30 s, 40 cycles of 95 °C for 5 s and 60 °C for 30 s each. Three biological replicates were conducted with three technical replicates each, and the relative expression levels of the genes were quantified using a relative quantitation method (Δ cycle threshold). Data were normalized by the reference gene ACTIN (LOC_Os03g50885.1). Primers for qRT–PCR analyses are listed in Supplementary Table S1.
Results
FON4 and OsMADS15 function in different pathways
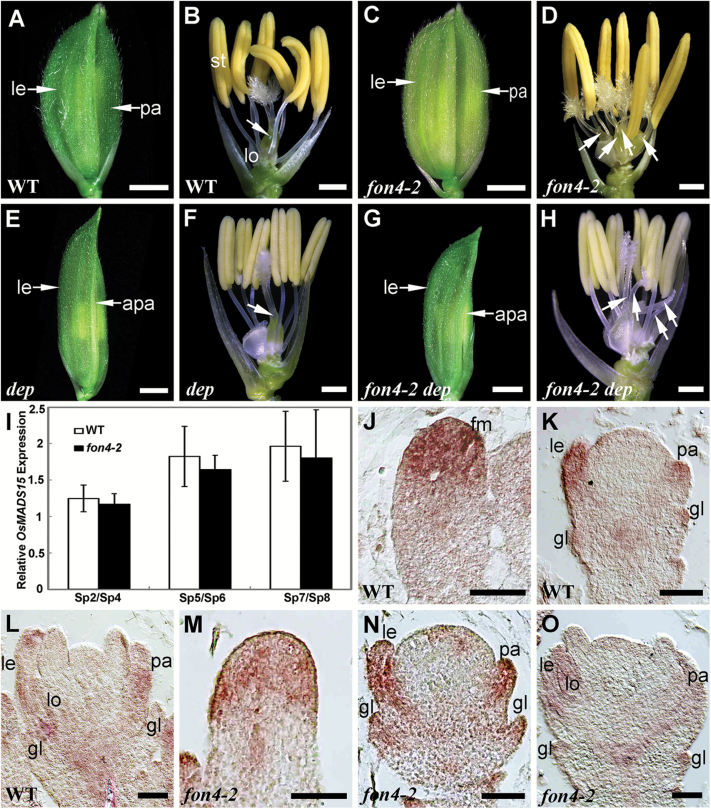
As a result of FM determinacy, a wild-type rice floret consists of a fixed number of floral organs, including two leaf-like organs, the lemma and palea, two lodicules, six stamens, and one pistil from the outer to inner whorls (Fig. 1A, B; Table 1) (Yuan et al., 2009; Zhang et al., 2013). Mutations in FON4 cause an increase in the number of floral organs, especially stamens and pistils (Chu et al., 2006; Suzaki et al., 2006). The fon4-2 allele, which contains a G-to-A base alteration at the 3' end of the first intron of FON4, generated 6–8 stamens and 2–4 pistils, whereas the number of paleas, lemmas, and lodicules showed almost no change (Fig. 1C, D; Table 1) (Chu et al., 2006). OsMADS15, an ortholog of Arabidopsis AP1, is required for flowering time regulation and palea identity specification during rice flower development. The OsMADS15 mutant, dep, displays shrunken paleas, and weak defects in lemmas and glumes (Fig. 1E, F; Table 1) (Wang et al., 2010). To examine whether FON4 has a genetic interaction with OsMADS15, a fon4-2 dep double mutant was created by genetic crosses. The fon4-2 dep double mutant exhibited additive phenotypes of the two single mutants, as both the abnormal paleas and the increased number of floral organs were observed (Fig. 1G, H; Table 1). Furthermore, we compared the expression pattern of OsMADS15 in the wild type and fon4-2 during flower development. The qRT–PCR result showed that OsMADS15 expression was not obviously changed in the fon4-2 mutant from stage Sp2 to stage Sp8 (Fig. 1I) (the stages defined by Ikeda et al., 2004). Consistently, the expression pattern of OsMADS15 in fon4-2 was similar to that in the wild type via in situ hybridization analysis. In the flowers of both the wild type and fon4-2, OsMADS15 was initially expressed in the apical region of the FM at the early stage of flower development (Fig. 1J, M). When the lemma and palea primordia were formed, OsMADS15 expression was detected in the glumes, paleas, and lemmas (Fig. 1K, N). After the stamen primordia initiated, OsMADS15 was expressed in glumes, lemmas, paleas, and lodicules (Fig. 1L, O; Supplementary Fig. S1) (Kyozuka et al., 2000). Thus, we proposed that FON4 and OsMADS15 function in parallel pathways in specifying flower development.
Fig. 1.
Phenotype of fon4-2 dep. (A, B) Wild-type (WT) flower with one lemma, one palea, two lodicules, six stamens, and one pistil. (C, D) fon4-2 flower with one lemma, one palea, two lodicules, seven stamens, and four pistils. (E, F) dep flower with one lemma, one abnormal palea, two lodicules, six stamens, and one pistil. (G, H) fon4-2 dep flower with one lemma, one abnormal palea, two lodicules, six stamens, and four pistils. The lemma and palea were removed in (B, D, F, H), and arrows indicate pistils. (I) qRT–PCR analysis of OsMADS15 expression in wild-type and fon4-2 flowers at stage Sp2/Sp4, Sp5/Sp6, and Sp7/Sp8. (J–O) OsMADS15 expression pattern in wild-type (J–L) and fon4-2 (M–O) flowers at different stages of flower development. apa, abnormal palea; fm, floral meristem; gl, glume; le, lemma; lo, lodicule; pa, palea; st, stamen. Scale bars=2 mm (A, C, E, G), 1 mm (B, D, F, H), and 50 μm (J–O).
Table 1.
The number of floral organs in the wild type and mutants
| Genotype | No. of spikelets examined | Lemma/ palea | Glume- like organs | Normal lodicules | Normal stamens | Lodicule- like organs | Fused gynoecia | Stigmas |
|---|---|---|---|---|---|---|---|---|
| Wild type | 20 | 2 | 0 | 2 | 6 | 0 | 1 | 2 |
| fon4-2 | 103 | 2 | 0 | 2.16 ± 0.39 | 6.46 ± 0.75 | 0 | 2.96 ± 1.03 | 7.04 ± 2.25 |
| dep | 20 | 2 | 0 | 2 | 6 | 0 | 1 | 2 |
| fon4-2 dep | 97 | 2 | 0 | 2.32 ± 0.59 | 6.10 ± 0.55 | 0 | 2.44 ± 0.82 | 5.41 ± 1.70 |
| spw1-1 | 56 | 2 | 2.21 ± 0.46 | 0 | 0 | 0 | 7.71 ± 1.11 | 16.52 ± 2.17 |
| fon4-2 spw1-1 | 57 | 2 | 3.49 ± 0.91 | 0 | 0 | 0 | 7.77 ± 1.52 | 20.40 ± 3.88 |
| osmads3-4 | 100 | 2 | 0 | 2.30 ± 0.63 | 4.94 ± 1.39 | 1.30 ± 1.57 | 1.06 ± 0.33 | 2.13 ± 0.77 |
| fon4-2 osmads3-4 | 73 | 2 | 0 | 3.66 ± 1.52 | 0.12 ± 0.44 | 6.15 ± 0.83 | 7.89 ± 2.11 | 18.73 ± 5.17 |
| fon4-1 | 100 | 2.18 ± 0.38 | 0 | 2.51 ± 0.62 | 6.44 ± 0.59 | 0 | 2.86 ± 0.78 | 6.08 ± 1.94 |
| osmads58 | 109 | 2 | 0 | 2 | 6 | 0 | 1 | 2.25 ± 0.45 |
| fon4-1 osmads58 | 102 | 2.16 ± 0.37 | 0 | 2.52 ± 0.66 | 6.48 ± 1.14 | 0 | 4.53 ± 1.19 | 8.11 ± 1.97 |
| osmads13-3 | 150 | 2 | 0 | 2 | 6 | 0 | 1 | 2.19 ± 0.42 |
| fon4-1 osmads13-3 | 100 | 2.09 ± 0.29 | 0 | 2.30 ± 0.55 | 6.35 ± 0.67 | 0 | 7.98 ± 1.97 | 19.84 ± 4.93 |
The average number is shown as the mean ±SD.
FON4 acts in parallel with OsMADS16 in specifying flower development
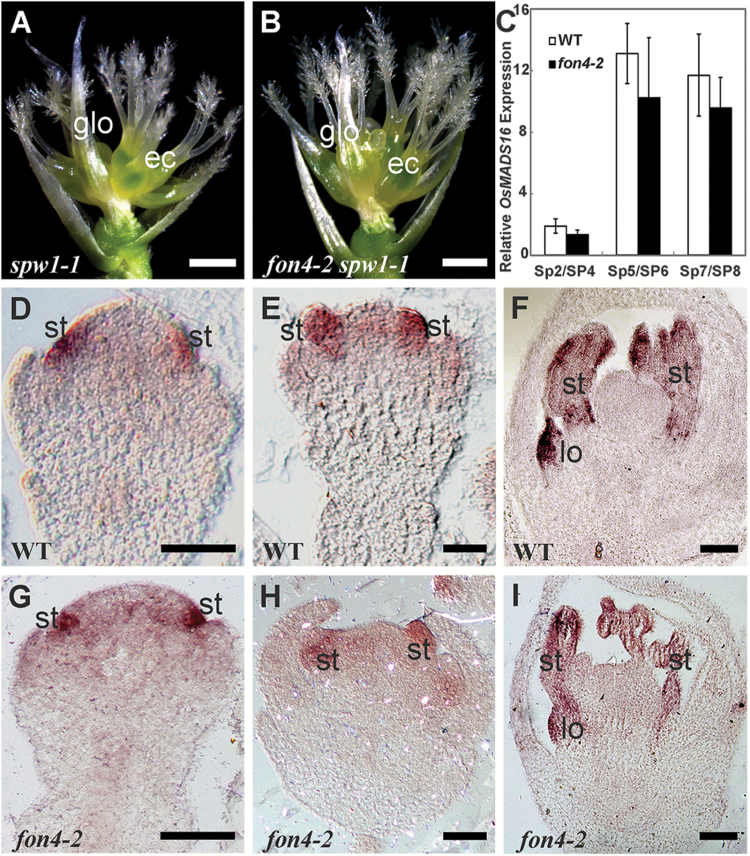
The rice B-class gene OsMADS16 was shown to specify the identity of lodicules and stamens (Nagasawa et al., 2003; Li et al., 2011a; Yun et al., 2013). spw1-1 flowers display the conversion of lodicules and stamens into glume-like organs and carpel-like organs, respectively (Fig. 2A; Table 1) (Li et al., 2011a; Yun et al., 2013). Our recent discoveries revealed that OsMADS16 is also involved in the control of the FM determinacy together with C-class genes OsMADS3 and OsMADS58 or the E-class gene OsMADS6 (Li et al., 2011a; Yun et al., 2013). We observed that the number of glume-like organs in whorl 2 of the fon4-2 spw1-1 double mutant showed a distinct increase compared with spw1-1 (Fig. 2B; Table 1). Since the fon4-2 mutant only exhibited a slight change in the number of lodicules, there may be a synergistic interaction between FON4 and OsMADS16 in whorl 2. On the other hand, the number of fused gynoecia and stigmas in the inner whorls had no apparent change in fon4-2 spw1-1 compared with spw1-1, suggesting that FON4 may have a less obvious interaction with OsMADS16 in specifying the inner flower organ development. To test whether OsMADS16 and FON4 have a transcriptional regulatory relationship, we performed qRT–PCR and in situ hybridization (Fig. 2C–I). In the wild-type flower, OsMADS16 expression was first detected in the incipient lodicule and stamen primordia, and then in the developing lodicule and stamen primordia (Fig. 2D–F; Supplementary Fig. S1) (Nagasawa et al., 2003; Yun et al., 2013). Analogously, we observed that OsMADS16 expression in fon4-2 was restricted to the two floral organs (Fig. 2G–I). Collectively, we concluded that FON4 and OsMADS16 synergistically regulate the organ number of the whorl 2, and they might function independently during flower development.
Fig. 2.
Phenotype of fon4-2 spw1-1. (A) spw1-1 flower with glume-like organs and ectopic carpels. (B) fon4-2 spw1-1 flower. The palea and lemma were removed in (A) and (B). (C) qRT–PCR analysis of OsMADS16 expression in wild-type and fon4-2 flowers. (D–I) OsMADS16 expression pattern in wild-type (D–F) and fon4-2 (G–I) flowers. ec, ectopic carpel; glo, glume-like organ; lo, lodicule; st, stamen. Scale bars=1 mm (A, B) and 50 μm (D–I).
Interactions of FON4 with OsMADS3 and OsMADS58
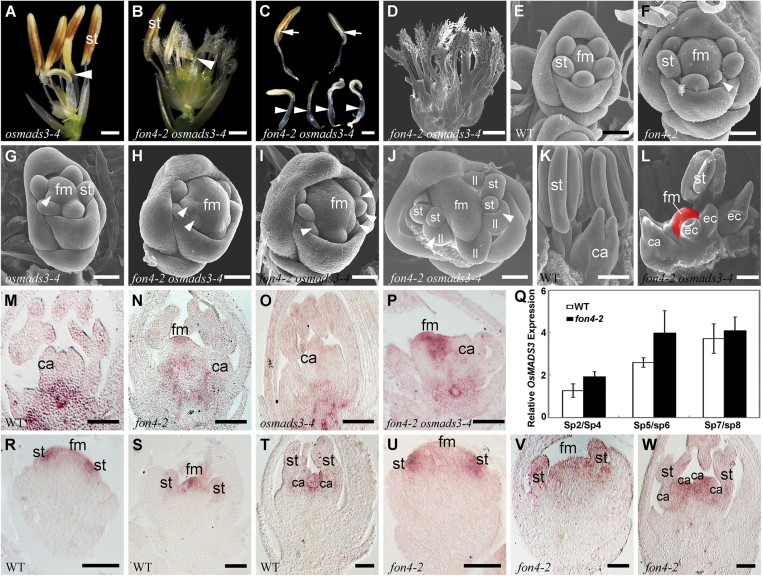
The rice genome contains two duplicated C-class genes, OsMADS3 and OsMADS58, which redundantly regulate reproductive organ identity and FM determinacy (Yamaguchi et al., 2006; Dreni et al., 2011). In the osmads3-4 mutant, an intermediate allele of OsMADS3, the stamens in whorl 3 were partially converted into lodicule-like organs, whereas the carpel in whorl 4 developed almost normally (Fig. 3A; Table 1) (Hu et al., 2011). fon4-2 osmads3-4 showed additive effects on the number of floral organs in whorl 3, but the number of lodicule-like organs that transformed from stamens was significantly increased in the double mutant compared with that of the osmads3-4 mutant (Fig. 3B, C; Table 1), suggesting that fon4-2 enhances the stamen identity defect seen in osmads3-4. Moreover, the fon4-2 osmads3-4 double mutant displayed dramatically enhanced defects of FM determinacy, where more carpel-like structures were produced than either of the single mutants (Fig. 3D; Table 1).
Fig. 3.
Phenotype of fon4-2 osmads3-4. (A) osmads3-4 flower. (B) fon4-2 osmads3-4 flower. The lemma and palea were removed in (A) and (B), and arrowheads indicate lodicule-like organs. (C) A close-up of normal stamens (arrows) and lodicule-like organs (arrowheads) in (B). (D) SEM observation of numerous carpels in fon4-2 osmads3-4. (E–J) SEM images of wild-type (E), fon4-2 (F), osmads3-4 (G), and fon4-2 osmads3-4 (H–J) flowers at the stage when stamen primordia are formed in whorl 3. Arrowheads in (F–J) indicate organ primordia developed from the additional whorl. A partial palea was removed in (J). (K) SEM image of a wild-type flower at the stage when the pistil with two stigmas is produced. (L) SEM image of a fon4-2 osmads3-4 flower at the stage that corresponds to that of (K). The floral meristem (red region) remains in fon4-2 osmads3-4 even after several carpels have been formed. (M–P) OSH1 expression in wild-type (M), fon4-2 (N), osmads3-4 (O), and fon4-2 osmads3-4 (P) flowers after the formation of carpels. OSH1 expression completely disappears when the carpels are formed in the wild-type and osmads3-4 flowers, whereas it continues to be expressed in the FM around the carpel primordia in fon4-2 and fon4-2 osmads3-4 flowers. Moreover, the OSH1 expression region is larger in fon4-2 osmads3-4 than in the fon4-2 single mutant. (Q) qRT–PCR analysis of OsMADS3 expression in wild-type and fon4-2 flowers. (R–W) OsMADS3 expression pattern in wild-type (R–T) and fon4-2 (U–W) flowers. ca, carpel; ec, ectopic carpel; fm, floral meristem; ll, lodicule-like organ; st, stamen. Scale bars=1 mm (A–C), 500 μm (D), 50 μm (E–J, R–W), and 100 μm (K–P).
Furthermore, our SEM analysis revealed that at the stage when the stamen primordia were formed in whorl 3 of the wild-type flower (Fig. 3E), fon4-2 showed an increased size of the FM, and extra stamens in the same whorl or in an additional whorl compared with the wild type (Fig. 3F), and osmads3-4 generated extra organ primordia with an irregular shape inside whorl 3, reflecting a homeotic transformation of stamens (Fig. 3G). fon4-2 osmads3-4 exhibited a more severe homeotic conversion of stamens, indicated by fewer normal stamen primordia in whorl 3 and more abnormal primordia detected in an additional whorl compared with the osmads3-4 single mutant. The double mutant also had a dramatically enlarged FM compared with that of either single mutant (Fig. 3H–J; Supplementary Table S2). After the formation of carpel primordium in whorl 4, the FM activity was terminated in the wild type (Fig. 3K), but the FM of fon4-2 osmads3-4 appeared to be still active, even after a number of carpels had formed (Fig. 3L). Furthermore, in situ analysis showed that OSH1, a molecular marker of undifferentiated cells in rice meristems (Sato et al., 1996), continued to be expressed in the central region after several ectopic carpels formed in fon4-2 osmads3-4 flowers, whereas OSH1 expression disappeared after the formation of carpels in the wild type (Fig. 3M, P). In addition, the region of OSH1 expression was larger in the double mutant than in either of the single mutants (Fig. 3N–P). These results suggest that FON4 and OsMADS3 synergistically regulate reproductive organ identity and FM determinacy, and loss of function of both genes causes an enlarged FM. To dissect further the relationship between FON4 and OsMADS3, we performed qRT–PCR assays and observed that there was no obvious expression change of OsMADS3 in fon4-2 from stage Sp2 to stage Sp8 (Fig. 3Q). Consistently, in situ hybridization analysis showed that OsMADS3 expression in fon4-2 mirrored that in the wild type (Fig. 3R–W; Supplementary Fig. S1) (Dreni et al., 2011).
In rice, another C-class gene, OsMADS58, has a partially divergent function from OsMADS3 (Yamaguchi et al., 2006; Dreni et al., 2011). An insertional mutant of OsMADS58 carrying a dSpm element in the second intron displayed a normal phenotype, except that few flowers generated bifurcated stigmas (Fig. 4A; Table 1) (Dreni et al., 2011). We combined osmads58 and fon4-1, another allele of FON4, which has a deletion of ~200 kb (Chu et al., 2006). fon4-1 osmads58 formed three outer floral whorls similar to those of fon4-1, whereas the number of pistils in the double mutant was slightly increased compared with that of fon4-1 (Fig. 4B, C; Table 1), suggesting that osmads58 enhances the defect of fon4 in FM activity, similar to that of osmads3. In the wild type, OsMADS58 expression is first detectable when OsMADS3 transcripts start to accumulate, but it is uniformly distributed in the FM. After the stamen primordia initiated, OsMADS58 was persistently expressed in the developing inner two whorls, displaying an expression profile similar to that of OsMADS3 (Dreni et al., 2011). Although the FM of fon4 mutants appeared larger than that of the wild type, leading to more carpel-like organs at the late stage (Chu et al., 2006), our expression analysis showed that the OsMADS58 expression level had no significant change in fon4-2 (Fig. 4D–J; Supplementary Fig. S1). Based on these results, we concluded that FON4 synergistically interacts with C-class genes OsMADS3 and OsMADS58 in the regulation of reproductive organ identity and FM determinacy.
Fig. 4.
Phenotype of fon4-1 osmads58. (A–C) The flowers of osmads58 (A), fon4-1 (B), and fon4-1 osmads58 (C) with the removal of the lemma and half the palea. Arrows indicate pistils. (D) qRT–PCR analysis of OsMADS58 expression in wild-type and fon4-2 flowers. (E–J) OsMADS58 expression pattern in wild-type (E–G) and fon4-2 (H–J) flowers. ca, carpel; fm, floral meristem; st, stamen. Scale bars=1 mm (A–C) and 50 μm (E–J).
FON4 and OsMADS13 synergistically specify FM determinacy
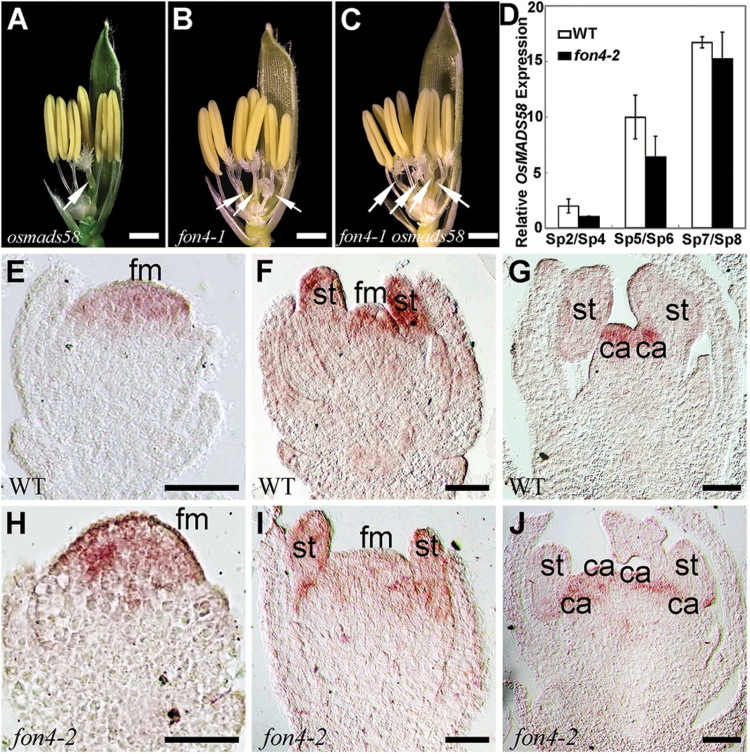
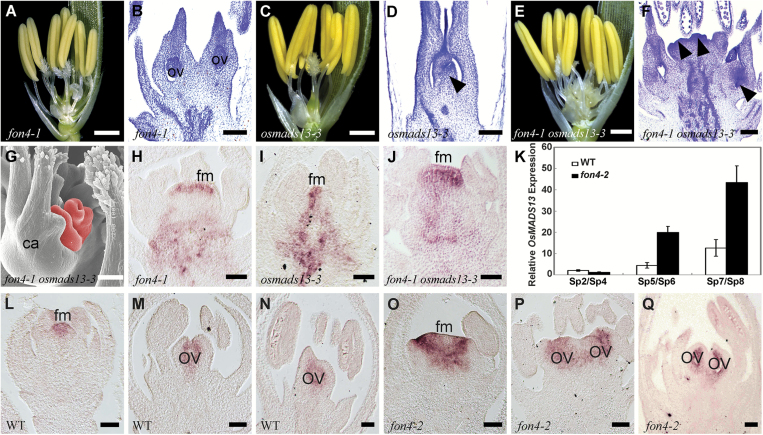
The rice D-class gene OsMADS13 regulates ovule identity and specifies FM termination redundantly with OsMADS3 and OsMADS58 (Dreni et al., 2007, 2011; Li et al., 2011b). In osmads13-3, the ovules were homeotically transformed into carpelloid structures, and 3–4 stigmas formed in some flowers (Fig. 5C, D; Table 1) (Li et al., 2011b). fon4-1 osmads13-3 displayed changes in the number of floral organs of the outer three whorls, which may be attributable to the mutation in fon4-1, but the number of carpelloid structures in the double mutant was greatly increased in comparison with fon4-1 (Fig. 5A, B, E–G; Table 1). The expression domain of OSH1 in fon4-1 osmads13-3 appeared wider than that in either single mutant at the stage when the FM terminated in the wild type (Fig. 5H–J). Accordingly, we propose that FON4 and OsMADS13 synergistically specify FM determinacy. Notably, the qRT–PCR result showed that the OsMADS13 expression level was higher in the fon4-2 mutant than in the wild type (Fig. 5K). In addition, the expression region of OsMADS13 became much larger in fon4-2 (Fig. 5L–Q; Supplementary Fig. S1) (Lopez-Dee et al., 1999; Dreni et al., 2007). One possible explanation is that FON4 may repress OsMADS13 expression, and the increased expression of OsMADS13 could represent a molecular response which compensates the loss of fon4-2, to alleviate the expansion of the meristem. Therefore, we concluded that OsMADS13 and FON4 may regulate each other at the transcriptional level.
Fig. 5.
Phenotype of fon4-1 osmads13-3. (A, C, E) The flowers of fon4-1 (A), osmads13-3 (C), and fon4-1 osmads13-3 (E) with the removal of the lemma and half the palea. (B, D, F) Longitudinal section of fon4-1 (B), osmads13-3 (D), and fon4-1 osmads13-3 (F) stained with 0.1% Toluidine blue showing carpel and ovule development. The arrowheads in (D) and (F) indicate the carpelloid structures converted from the ovules. (G) SEM image of carpelloid structures (red region) in fon4-1 osmads13-3. (H–J) OSH1 expression in fon4-1 (H), osmads13-3 (I), and fon4-1 osmads13-3 (J). (K) qRT–PCR analysis of OsMADS13 expression in wild-type and fon4-2 flowers. (L–Q) OsMADS13 expression pattern in wild-type (L–N) and fon4-2 (O–Q) flowers. ca, carpel; fm, floral meristem; ov, ovule. Scale bars=1 mm (A, C, E), 100 μm (B, D, F, G), and 50 μm (H–J, L–Q).
FON4 and OsMADS1 synergistically maintain FM identity
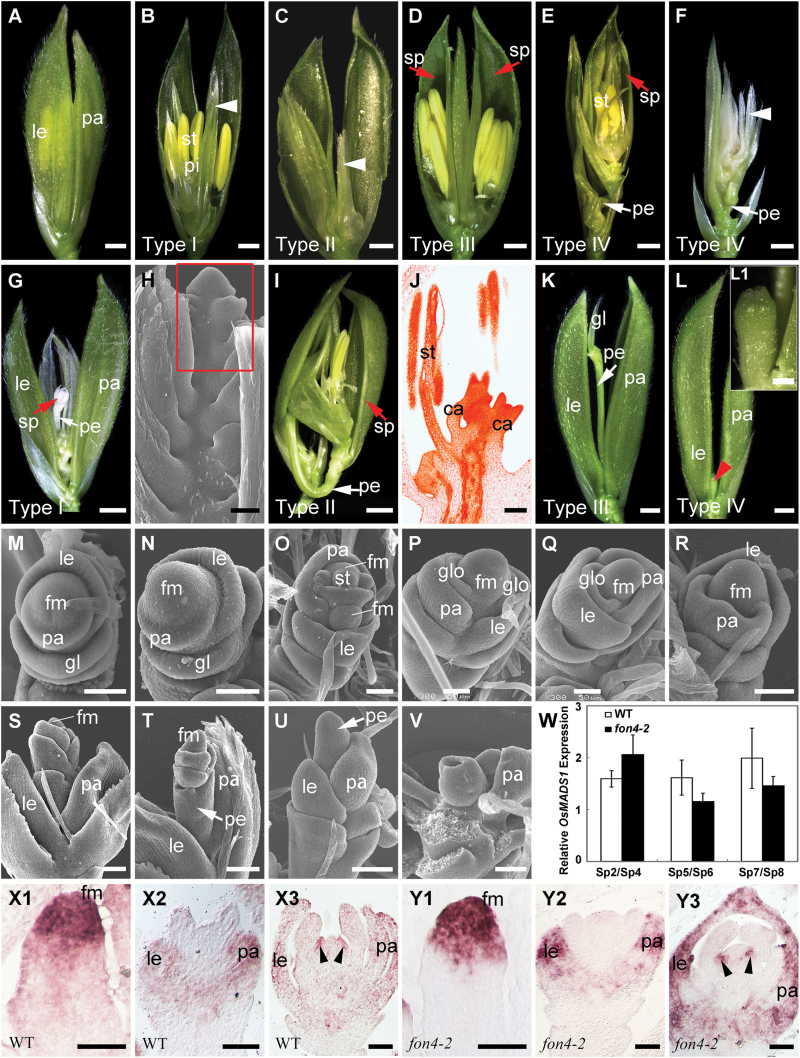
OsMADS1, an E-class gene, has been shown to be involved in promoting FM identity and specifying floral organ identity (Jeon et al., 2000a; Prasad et al., 2001, 2005; Agrawal et al., 2005; Chen et al., 2006; Gao et al., 2010; Li et al., 2010; Khanday et al., 2013; Hu et al., 2015). As we described previously (Gao et al., 2010; Hu et al., 2015), the osmads1-z allele produced a leafy lemma and palea, showing open flowers with four types of flower patterning for the inner whorls (Fig. 6A–F): type I (69%, n=320) with a change in the number of stamens and pistils (Fig. 6B); type II (16%, n=320) with glume-like organs in place of inner floral organs (Fig. 6C); type III (9%, n=320) with twin flowers in each spikelet (Fig. 6D); and type IV (6%, n=320) with a new spikelet along a pedicel comprised of stamens and carpels or only glume-like organs in the inner whorls (Fig. 6E, F). Despite the fact that fon4-2 osmads1-z produced abnormal lemma and palea resembling those of osmads1-z, the double mutant lacked the inner floral organs; instead, they generated a new spikelet along with the long pedicel or an undefined organ in the inner whorls (Fig. 6G–L). fon4-2 osmads1-z flowers were mainly classified into four types: type I showing an indeterminate spikelet composed of repetitious glume-like organs (44%, n=352) (Fig. 6G, H); type II displaying a determinate spikelet containing stamens and carpels in the inner whorls (22%, n=352) (Fig. 6I, J); type III bearing glumes or no organs along with the pedicel (13%, n=352) (Fig. 6K); and type IV having an undefined organ in the center (22%, n=352) (Fig. 6L, L1). Overall, fon4-2 enhanced the defect of osmads1-z in FM identity, and thus we inferred that FON4 and OsMADS1 synergistically maintain FM identity. Detailed observation showed that fon4-2 osmads1-z flowers failed to generate stamen primordia after the lemma and palea were formed in osmads1-z flowers. Instead, the double mutant either continued to produce glume-like organs at the flank of the FM or remained an indeterminate meristem (Fig. 6M–R), causing the formation of different types of spikelets inside the lemma and palea (Fig. 6S–V). Further expression analysis showed that no obvious expression change of OsMADS1 was observed in fon4-2 at various stages during flower development (Fig. 6W–Y3; Supplementary Fig. S1).
Fig. 6.
Phenotype of fon4-2 osmads1-z. (A) osmads1-z flower with leaf-like lemma and palea. (B–D) osmads1-z flowers of type I (B), type II (C), and type III (D). A half of a leafy lemma and palea were removed, and arrowheads in (B) and (C) indicate glume-like organs. (E, F) Type IV flower of osmads1-z with a new spikelet in the center. The spikelet in (E) has stamens and carpels in the inner whorls, whereas the spikelet in (F) only contains repetitious glume-like organs (arrowheads). A half of a leafy lemma and palea of primary and secondary flowers were removed in (E), and the whole leafy lemma and palea were removed in (F). (G) Type I flower of fon4-2 osmads1-z generating a new indeterminate composed of only glume-like organs. (H) SEM image of that in (G). The red square indicates the indeterminate FM. (I) Type II flower of fon4-2 osmads1-z generating a new determinate spikelet with stamens and carpels in the inner whorls. (J) Longitudinal section of the spikelet in (I). (K) Type III flower of fon4-2 osmads1-z containing only a glume on the pedicel. (L) Type IV flower of fon4-2 osmads1-z with an undefined structure (red arrowhead) inside the flower. (L1) A close-up view of the undefined structure in (L). (M, N) SEM images of osmads1-z (M) and fon4-2 osmads1-z (N) at the stage when the glume, lemma, and palea primordia are formed. (O) SEM image of the osmads1-z flower at the stage when stamen primordia initiate. (P–V) SEM images of fon4-2 osmads1-z flowers. (W) qRT–PCR analysis of OsMADS1 expression in wild-type and fon4-2 flowers. (X1–Y3) OsMADS1 expression pattern in wild-type (X1–X3) and fon4-2 (Y1–Y3) flowers. Arrowheads in (X3) and (Y3) indicate carpels. sp, spikelet; fm, floral meristem; gl, glume; le, lemma; pa, palea; pe, pedicel; glo, glume-like organ; st, stamen. Scale bars=1 mm (A–G, I, K, L), 500 μm (L1), 100 μm (H, J, S–V), and 50 μm (M–R, X1–Y3).
Discussion
The FM is determinate, and its activity is maintained until all the floral organs are formed (Steeves and Sussex, 1989). On the other hand, the rice FM still persists after the carpel develops and, instead, it is completely consumed when the ovule forms (Dreni et al., 2007). In rice, FON1 and FON4 are the orthologs of CLV1 and CLV3, respectively (Suzaki et al., 2004, 2006; Chu et al., 2006; Chu and Zhang, 2007), but which factor(s) is(are) functionally similar to WUS is still unknown. It has been indicated that rice C-class genes OsMADS3 and OsMADS58, and the D-class gene OsMADS13 redundantly regulate FM determinacy (Dreni et al., 2011; Li et al., 2011b). In this work, we have investigated the genetic relationship between FON4 and floral homeotic genes. FON4 showed an additive interaction with OsMADS15 and a synergistic interaction with OsMADS16 in the control of the organ number of whorl 2. On the other hand, the phenotypic analysis of double mutant combinations of fon4 with osmads3, osmads58, osmads13, and osmads1 individually indicates that FON4 and these genes act in parallel pathways that converge on a common process of FM activity control. However, it is unknown where and how these pathways converge.
Conserved and diversified genetic control of floral organ number and identity
The FM in angiosperms develops into floral organs whose numbers, positions, and identities lead to the diversification of flowers. A lot of genes that regulate floral organ number have been identified in Arabidopsis. For example, mutations in CLV genes, WIGGUM (WIG), and ULTRAPETALA1 (ULT1) cause an increase in floral organ number (Leyser and Furner, 1992; Clark et al., 1993, 1995, 1997; Kayes and Clark, 1998; Running et al., 1998; Fletcher et al., 1999; Jeong et al., 1999; Ziegelhoffer et al., 2000; Fletcher, 2001; Carles et al., 2005), whereas mutations in TOUSLED (TSL), REVOLUTA (REV), FASCIATA (FAS), and WUS result in fewer floral organs (Leyser and Furner, 1992; Roe et al., 1993, 1997; Talbert et al., 1995; Mayer et al., 1998; Kaya et al., 2001). In these mutants, the changes in organ number are related to FM size during the time of organ primordia initiation. After organ primordia initiation at the correct position, floral homeotic genes sequentially specify organ identities. Therefore, the mechanisms of floral organ number regulation and determination of floral organ identity seem to be two separate processes. Clark and co-workers reported that double mutant combinations of clv1 with ap2, ap3, pistillata (pi), and ag were all additive in phenotype, indicating that CLV1 regulates the FM structure independently of these homeotic gene functions. The double mutant with ap1 displayed the enhanced defects in FM identity. Furthermore, expression patterns of AG and AP1 were altered in the clv1 mutant.
In this study, we analyze the phenotypes of the double mutant combinations of the CLV3 ortholog FON4 and rice floral homeotic genes. Some differences are discovered in comparison with the corresponding double mutants of clv1. First of all, the fon4 dep double mutant shows an additive phenotype (Fig. 1A–H), whereas clv1 ap1 exhibits an enhancement in FM identity defects. Occasionally, a new inflorescence meristem is generated in the center of clv1 ap1 flowers that was not observed in the single mutants. This difference might result from the distinct effects of ap1 and dep mutations on FM activity. Unlike the ap1 mutation which affects sepal and petal development, and causes a partial transformation of a floral meristem into an inflorescence meristem (Irish and Sussex, 1990; Mandel et al., 1992), the dep mutant only shows a stable degenerative palea phenotype (Wang et al., 2010). In the rice genome, there are four orthologs of Arabidopsis AP1, namely OsMADS14, OsMADS15, OsMADS18, and OsMADS20 (Litt and Irish, 2003; Kater et al., 2006). We speculate that these AP1-like genes work redundantly, and thus neither dep nor fon4 dep shows abnormality in FM identity. Secondly, the double mutants of FON4 with AG-like genes OsMADS3 or OsMADS58 display the enhanced defect in FM size and determinacy, and the fon4 mutation enhances the stamen identity defect of the osmads3-4 allele (Figs 3A–P, 4 A–C), which has not been observed in the clv1 ag double mutant (Clark et al., 1993). However, since the reported double mutant contained a strong ag allele, consisting of only sepals and petals, it is unknown whether the clv mutants can also enhance the ag phenotype of floral organ identity transformation. Additionally, the expression patterns of OsMADS15, OsMADS3, and OsMADS58 in the fon4 mutant mirror those in the wild type (Figs 1I–O, 3Q–W, 4D–J). In contrast, AP1 and AG are altered in clv1 flowers in Arabidopsis (Clark et al., 1993). In the wild type, AG was uniformly expressed throughout the FM at the early stage (Drews et al., 1991). However, the clv1 mutants lack AG expression in the very center of the FM (Clark et al., 1993). The absence of detectable AG expression in the center of the flower possibly resulted in continued proliferation of these central cells.
FON4 regulates FM activity in parallel with C, D, and E genes
FON4 is responsible for the regulation of FM size. The fon4 mutation causes an increase in the number of all floral organs owing to the enlarged FM (Chu et al., 2006; Suzaki et al., 2006). In addition, FON4 also controls FM determinacy at the later stage. In the wild type, the FM activity terminates after the carpel develops in whorl 4. Conversely, the FM in the fon4 mutant still remains and the meristem marker gene OSH1 continues to be expressed even after a number of carpels have been formed (Fig. 3N) (Chu et al., 2006; Suzaki et al., 2006). Among the floral homeotic genes selected in this study, OsMADS15 plays a minor role in FM determinacy. The fon4-2 dep double mutant displays an additive phenotype, indicating that FON4 acts independently of OsMADS15 (Fig. 1A–H). OsMADS16 has been reported to have a function in FM determinacy (Li et al., 2011a; Yun et al., 2013). fon4-2 spw1-1 displays an enhanced effect in the organ number of whorl 2, although this change in the internal whorls is not as clear (Fig. 2A, B; Table 1), since it is difficult to measure carpel number directly in the mutant where multiple carpels could form one fused gynoecium, and not all of them will produce a stigma. OsMADS3, OsMADS58, and OsMADS13 are instead important for FM determinacy, and FM determinacy is enhanced in all the double mutant combinations of fon4 with these genes (Figs 3A–P, 4A–C, 5A–J). The altered phyllotaxy (from whorled to distichous) seen occasionally in osmads1-z (Fig. 6F) is significantly enhanced in fon4-2 osmads1-z (Fig. 6G, H), suggesting that FON4 acts synergistically with OsMADS1 to promote the transition from spikelet meristem to floral meristem, and to maintain FM identity. Intriguingly, these data suggest that there could be feedback between FM size and FM identity, and that it is difficult to maintain FM identity as FM size abnormally increases. It is possible that fon4-2 enhances the loss of stamen identity in osmads3-4 (Fig. 3A–C) through a similar mechanism. Furthermore, the expression patterns of OsMADS3, OsMADS58, and OsMADS1 have no obvious change in the fon4 mutants (Figs 3Q–W, 4D–J, 6W–Y3). Therefore, it seems that FON4 genetically controls FM activity together with OsMADS3, OsMADS58, and OsMADS1, but without regulating each other’s transcription. The OsMADS13 expression level is up-regulated in the fon4 mutant, though its expression remains restricted to the FM and ovule primordia (Fig. 5K–Q), suggesting that FON4 may indirectly repress OsMADS13 expression. A feedback loop could exist so that loss of FON4 is compensated by an increased expression of OsMADS13, which contributes to reducing meristem expansion.
FON4 enhances the function of C, D, and E genes at different stages
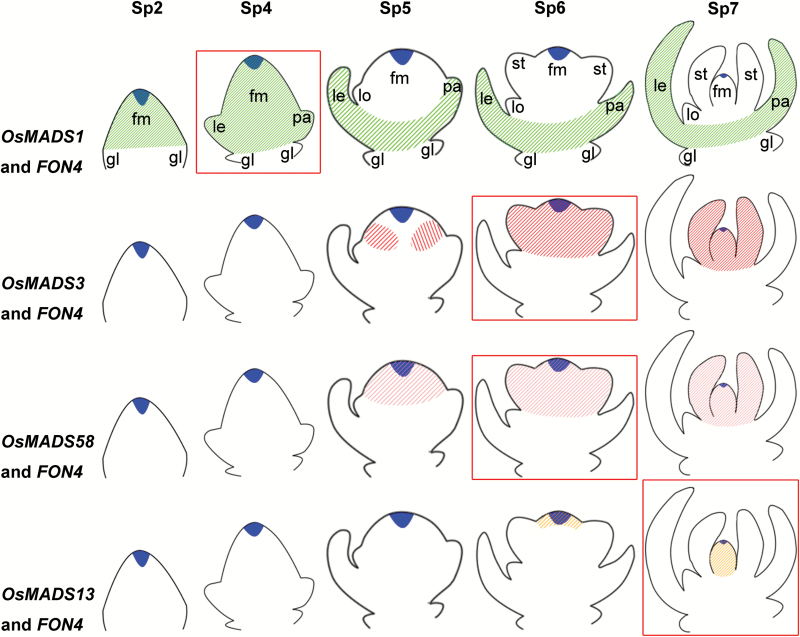
In rice flower development, FON4 is persistently expressed in the central region of the FM apex until the carpel is formed (Fig. 7) (Chu et al., 2006; Suzaki et al., 2006). We compared the expression regions of FON4 and OsMADS1, OsMADS3, OsMADS58, and OsMADS13 in the wild type at the stage when the double mutants started to display the obviously enhanced defects (Fig. 7). OsMADS1 expression is first detectable in the incipient FM, and later it is restricted in the developing lemma/palea and carpel (Prasad et al., 2001, 2005). fon4-2 osmads1-z displays apparent abnormality after the formation of the lemma and palea primordia (stage Sp4; Fig. 6M–R). At this stage, OsMADS1 is expressed in the lemma, palea, and FM in the wild type, encompassing the expression region of FON4. OsMADS3 is initially expressed in the founder cells recruited to stamen primordia, then its expression is retained in the two inner whorls (Dreni et al., 2011). When the stamen primordia emerge in whorl 3 (stage Sp6; Fig. 3E–J), fon4-2 osmads3-4 shows an enhanced phenotype. The expression of OsMADS3 at this stage is observed in stamen primordia and in the FM, including the FON4 expression region. The fon4-1 osmads58 double mutant has enhanced defects in FM determinacy similar to fon4 osmads3. OsMADS13 is expressed in the FM before the differentiation of carpel and ovule primordia. Subsequently, it continues to be expressed in the ovule primordium and in the inner layer of the carpel wall (Lopez-Dee et al., 1999; Dreni et al., 2007, 2011). In the fon4-1 osmads13-3 double mutant, the OSH1 expression region is much larger than that of either single mutant at the stage when the stamen differentiates into anther and filament, whereas carpel and ovule primordia have not emerged (stage Sp7; Fig. 5H–J). At this stage, the expression region of OsMADS13 includes that of FON4. Taken together, these data suggest that FON4 enhances the function of OsMADS1, OsMADS3, OsMADS58, and OsMADS13 at different stages, and that the expression region of FON4 partially overlaps with these genes in the FM.
Fig. 7.
Comparison of the expression pattern of FON4 and floral homeotic genes from stage Sp2 to Sp7. Blue indicates the expression region of FON4, and the green, red, pink, and orange oblique lines indicate the expression region of OsMADS1, OsMADS3, OsMADS58, and OsMADS13, respectively. At the stages (red square) when apparent abnormalities are observed in the double mutants, the expression region of FON4 partially overlaps with these genes in the floral meristem. fm, floral meristem; gl, glume; le, lemma; lo, lodicule; pa, palea; st, stamen.
In Arabidopsis, AG and CLV pathways appear to mediate WUS repression in partially independent ways, since the phenotype of the clv1 ag double mutant was substantially additive and the WUS expression domain was larger in ag clv1 double mutants than in ag single mutants. In our study, fon4 synergistically interacts with osmads3, osmads58, osmads13, and osmads1 in FM activity; therefore, we speculate that FON4 and these genes regulate a common target gene, similarly to WUS regulation, through parallel pathways at the stage when the significantly enhanced defects are observed. However, such a factor has not been identified in rice. Phylogenetic analysis revealed that rice contained a single WUS ortholog OsWUS (also called MONOCULM3, MOC3 and TILLERS ABSENT1, TAB1) (Nardmann and Werr, 2006; Zhang et al., 2010; Lu et al., 2015; Tanaka et al., 2015). Nardmann and Werr (2006) reported that the expression pattern of OsWUS was markedly different from that of WUS in Arabidopsis because it was not expressed in the OC of shoot meristems. Later studies showed that OsWUS transcript was detected in the pre-meristem zone during axillary meristem development (Tanaka et al., 2015). In contrast to the Arabidopsis wus mutant, which produced numerous adventitious shoots, mutations in OsWUS resulted in no tiller formation (Lu et al., 2015; Tanaka et al., 2015), demonstrating that WUS may have a diverged function between rice and Arabidopsis. Moreover, OsWUS is expressed in the apical region of branch and spikelet meristems (Tanaka et al., 2015). The moc3-1 allele seemed to be female sterile, whereas the tab1-1 allele showed reduced spikelet number and abnormal spikelet structure (Lu et al., 2015; Tanaka et al., 2015). Another WOX gene, OsWOX4, is required for the maintenance of the vegetative meristem and is negatively regulated by FON2-LIKE CLE PROTEIN1 (FCP1) (Ohmori et al., 2013). Although OsWOX4 expression is detected in all reproductive meristems, its role in inflorescence and spikelet development is still unknown. Therefore, the functional analysis of CLV–WUS-like pathways in rice will provide clues about their conservation and diversification in meristem maintenance between grasses and eudicots.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Sense probes were used as negative controls for in situ hybridization experiments.
Table S1. Primers used in this study.
Table S2. Floral meristem sizes in the wild type and mutants.
Supplementary Material
Acknowledgements
We thank Professor Zhukuan Cheng for providing dep; Professor Hajime Sakai and Professor Yasuo Nagato for spw1-1; Changsong Yin and Jie Wang for the assistance in situ hybridization; Li Yang for genotyping; and Deborah L. Devis and Rupert Fray for revising this paper. This work was supported by funds from the National Natural Science Foundation of China (31230051); National Key Research and Development Program of China (2016YFD0100902); China Postdoctoral Science Foundation (2014M560328); China Innovative Research Team, Ministry of Education; and the Programme of Introducing Talents of Discipline to Universities (111 Project, B14016).
References
- Agrawal GK, Abe K, Yamazaki M, Miyao A, Hirochika H. 2005. Conservation of the E-function for floral organ identity in rice revealed by the analysis of tissue culture-induced loss-of-function mutants of the OsMADS1 gene. Plant Molecular Biology 59, 125–135. [DOI] [PubMed] [Google Scholar]
- Aichinger E, Kornet N, Friedrich T, Laux T. 2012. Plant stem cell niches. Annual Review of Plant Biology 63, 615–636. [DOI] [PubMed] [Google Scholar]
- Ambrose BA, Lerner DR, Ciceri P, Padilla CM, Yanofsky MF, Schmidt RJ. 2000. Molecular and genetic analyses of the Silky1 gene reveal conservation in floral organ specification between eudicots and monocots. Molecular Cell 5, 569–579. [DOI] [PubMed] [Google Scholar]
- Angenent GC, Franken J, Busscher M, van Dijken A, van Went JL, Dons HJ, van Tunen AJ. 1995. A novel class of MADS box genes is involved in ovule development in petunia. The Plant Cell 7, 1569–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett ME, Williams SK, Taylor Z, DeBlasio S, Goldshmidt A, Hall DH, Schmidt RJ, Jackson DP, Whipple CJ. 2015. The maize PI/GLO ortholog Zmm16/sterile tassel silky ear1 interacts with the zygomorphy and sex determination pathways in flower development. The Plant Cell 27, 3081–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckmann A, Weidtkamp-Peters S, Seidel CA, Simon R. 2010. Stem cell signaling in Arabidopsis requires CRN to localize CLV2 to the plasma membrane. Plant Physiology 152, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommert P, Je BI, Goldshmidt A, Jackson D. 2013. The maize Gα gene COMPACT PLANT2 functions in CLAVATA signalling to control shoot meristem size. Nature 502, 555–558. [DOI] [PubMed] [Google Scholar]
- Bommert P, Nardmann J, Vollbrecht E, Running M, Jackson D, Hake S, Werr W. 2005a thick tassel dwarf1 encodes a putative maize ortholog of the Arabidopsis CLAVATA1 leucine-rich repeat receptor-like kinase. Development 132, 1235–1245. [DOI] [PubMed] [Google Scholar]
- Bommert P, Satoh-Nagasawa N, Jackson D, Hirano HY. 2005b Genetics and evolution of inflorescence and flower development in grasses. Plant and Cell Physiology 46, 69–78. [DOI] [PubMed] [Google Scholar]
- Brand U, Fletcher JC, Hobe M, Meyerowitz EM, Simon R. 2000. Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289, 617–619. [DOI] [PubMed] [Google Scholar]
- Carles CC, Choffnes-Inada D, Reville K, Lertpiriyapong K, Fletcher JC. 2005. ULTRAPETALA1 encodes a SAND domain putative transcriptional regulator that controls shoot and floral meristem activity in Arabidopsis. Development 132, 897–911. [DOI] [PubMed] [Google Scholar]
- Carles CC, Fletcher JC. 2003. Shoot apical meristem maintenance: the art of a dynamic balance. Trends in Plant Science 8, 394–401. [DOI] [PubMed] [Google Scholar]
- Chen ZX, Wu JG, Ding WN, Chen HM, Wu P, Shi CH. 2006. Morphogenesis and molecular basis on naked seed rice, a novel homeotic mutation of OsMADS1 regulating transcript level of AP3 homologue in rice. Planta 223, 882–890. [DOI] [PubMed] [Google Scholar]
- Chu H, Qian Q, Liang W, et al. 2006. The floral organ number4 gene encoding a putative ortholog of Arabidopsis CLAVATA3 regulates apical meristem size in rice. Plant Physiology 142, 1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H, Zhang D. 2007. The shoot apical meristem size regulated by FON4 in rice. Plant Signaling and Behavior 2, 115–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaffi M, Paolacci AR, Tanzarella OA, Porceddu E. 2011. Molecular aspects of flower development in grasses. Sexual Plant Reproduction 24, 247–282. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1993. CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119, 397–418. [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM. 1995. CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121, 2057–2067. [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. 1997. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89, 575–585. [DOI] [PubMed] [Google Scholar]
- Coen ES, Meyerowitz EM. 1991. The war of the whorls: genetic interactions controlling flower development. Nature 353, 31–37. [DOI] [PubMed] [Google Scholar]
- Colombo L, Franken J, Koetje E, van Went J, Dons HJ, Angenent GC, van Tunen AJ. 1995. The petunia MADS box gene FBP11 determines ovule identity. The Plant Cell 7, 1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum G, Medzihradszky A, Suzaki T, Lohmann JU. 2014. A mechanistic framework for noncell autonomous stem cell induction in Arabidopsis. Proceedings of the National Academy of Sciences, USA 111, 14619–14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF. 2004. The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Current Biology 14, 1935–1940. [DOI] [PubMed] [Google Scholar]
- Dreni L, Jacchia S, Fornara F, Fornari M, Ouwerkerk PB, An G, Colombo L, Kater MM. 2007. The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. The Plant Journal 52, 690–699. [DOI] [PubMed] [Google Scholar]
- Dreni L, Kater MM. 2014. MADS reloaded: evolution of the AGAMOUS subfamily genes. New Phytologist 201, 717–732. [DOI] [PubMed] [Google Scholar]
- Dreni L, Osnato M, Kater MM. 2013. The ins and outs of the rice AGAMOUS subfamily. Molecular Plant 6, 650–664. [DOI] [PubMed] [Google Scholar]
- Dreni L, Pilatone A, Yun D, Erreni S, Pajoro A, Caporali E, Zhang D, Kater MM. 2011. Functional analysis of all AGAMOUS subfamily members in rice reveals their roles in reproductive organ identity determination and meristem determinacy. The Plant Cell 23, 2850–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreni L, Zhang D. 2016. Flower development: the evolutionary history and functions of the AGL6 subfamily MADS-box genes. Journal of Experimental Botany 67, 1625–1638. [DOI] [PubMed] [Google Scholar]
- Drews GN, Bowman JL, Meyerowitz EM. 1991. Negative regulation of the Arabidopsis homeotic gene AGAMOUS by the APETALA2 product. Cell 65, 991–1002. [DOI] [PubMed] [Google Scholar]
- Favaro R, Pinyopich A, Battaglia R, Kooiker M, Borghi L, Ditta G, Yanofsky MF, Kater MM, Colombo L. 2003. MADS-box protein complexes control carpel and ovule development in Arabidopsis. The Plant Cell 15, 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario S, Immink RG, Angenent GC. 2004. Conservation and diversity in flower land. Current Opinion in Plant Biology 7, 84–91. [DOI] [PubMed] [Google Scholar]
- Fletcher JC. 2001. The ULTRAPETALA gene controls shoot and floral meristem size in Arabidopsis. Development 128, 1323–1333. [DOI] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. 1999. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283, 1911–1914. [DOI] [PubMed] [Google Scholar]
- Fornara F, Parenicová L, Falasca G, et al. 2004. Functional characterization of OsMADS18, a member of the AP1/SQUA subfamily of MADS box genes. Plant Physiology 135, 2207–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Liang W, Yin C, Ji S, Wang H, Su X, Guo C, Kong H, Xue H, Zhang D. 2010. The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development. Plant Physiology 153, 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Liang W, Yin C, Cui X, Zong J, Wang X, Hu J, Zhang D. 2011. Rice MADS3 regulates ROS homeostasis during late anther development. The Plant Cell 23, 515–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liang W, Yin C, et al. 2015. Interactions of OsMADS1 with floral homeotic genes in rice flower development. Molecular Plant 8, 1366–1384. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Sunohara H, Nagato Y. 2004. Developmental course of inflorescence and spikelet in rice. Breeding Science 54, 147–156. [Google Scholar]
- Irish VF, Sussex IM. 1990. Function of the apetala-1 gene during Arabidopsis floral development. The Plant Cell 2, 741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Jang S, Lee S, et al. 2000a leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. The Plant Cell 12, 871–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon JS, Lee S, Jung KH, Yang WS, Yi GH, Oh BG, An G. 2000b Production of transgenic rice plants showing reduced heading date and plant height by ectopic expression of rice MADS-box genes. Molecular Breeding 6, 581–592. [Google Scholar]
- Jeong S, Trotochaud AE, Clark SE. 1999. The Arabidopsis CLAVATA2 gene encodes a receptor-like protein required for the stability of the CLAVATA1 receptor-like kinase. The Plant Cell 11, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BG, Van Montagu M, Okamuro JK. 1994. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. The Plant Cell 6, 1211–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater MM, Dreni L, Colombo L. 2006. Functional conservation of MADS-box factors controlling floral organ identity in rice and Arabidopsis. Journal of Experimental Botany 57, 3433–3444. [DOI] [PubMed] [Google Scholar]
- Kaya H, Shibahara KI, Taoka KI, Iwabuchi M, Stillman B, Araki T. 2001. FASCIATA genes for chromatin assembly factor-1 in arabidopsis maintain the cellular organization of apical meristems. Cell 104, 131–142. [DOI] [PubMed] [Google Scholar]
- Kayes JM, Clark SE. 1998. CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125, 3843–3851. [DOI] [PubMed] [Google Scholar]
- Kellogg EA. 2001. Evolutionary history of the grasses. Plant Physiology 125, 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanday I, Yadav SR, Vijayraghavan U. 2013. Rice LHS1/OsMADS1 controls floret meristem specification by coordinated regulation of transcription factors and hormone signaling pathways. Plant Physiology 161, 1970–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Betsuyaku S, Osakabe Y, et al. 2010. RPK2 is an essential receptor-like kinase that transmits the CLV3 signal in Arabidopsis. Development 137, 3911–3920. [DOI] [PubMed] [Google Scholar]
- Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K. 2008. Hd3a and RFT1 are essential for flowering in rice. Development 135, 767–774. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Jaramillo MA, Di Stilio VS. 2004. Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS subfamily of MADS box genes in angiosperms. Genetics 166, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC. 2005. Molecular mechanisms of flower development: an armchair guide. Nature Reviews. Genetics 6, 688–698. [DOI] [PubMed] [Google Scholar]
- Kyozuka J, Kobayashi T, Morita M, Shimamoto K. 2000. Spatially and temporally regulated expression of rice MADS box genes with similarity to Arabidopsis class A, B and C genes. Plant and Cell Physiology 41, 710–718. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim J, Han JJ, Han MJ, An G. 2004. Functional analyses of the flowering time gene OsMADS50, the putative SUPPRESSOR OF OVEREXPRESSION OF CO 1/AGAMOUS-LIKE 20 (SOC1/AGL20) ortholog in rice. The Plant Journal 38, 754–764. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim J, Son JS, et al. 2003. Systematic reverse genetic screening of T-DNA tagged genes in rice for functional genomic analyses: MADS-box genes as a test case. Plant and Cell Physiology 44, 1403–1411. [DOI] [PubMed] [Google Scholar]
- Lenhard M, Bohnert A, Jürgens G, Laux T. 2001. Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105, 805–814. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Furner I. 1992. Characterisation of three shoot apical meristem mutants of Arabidopsis thaliana.Development 116, 397–403. [Google Scholar]
- Li H, Liang W, Hu Y, Zhu L, Yin C, Xu J, Dreni L, Kater MM, Zhang D. 2011a Rice MADS6 interacts with the floral homeotic genes SUPERWOMAN1, MADS3, MADS58, MADS13, and DROOPING LEAF in specifying floral organ identities and meristem fate. The Plant Cell 23, 2536–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liang W, Jia R, Yin C, Zong J, Kong H, Zhang D. 2010. The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice. Cell Research 20, 299–313. [DOI] [PubMed] [Google Scholar]
- Li H, Liang W, Yin C, Zhu L, Zhang D. 2011b Genetic interaction of OsMADS3, DROOPING LEAF, and OsMADS13 in specifying rice floral organ identities and meristem determinacy. Plant Physiology 156, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhang DS, Liu HS, et al. 2006. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. The Plant Cell 18, 2999–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litt A, Irish VF. 2003. Duplication and diversification in the APETALA1/FRUITFULL floral homeotic gene lineage: implications for the evolution of floral development. Genetics 165, 821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Kim YJ, Müller R, Yumul RE, Liu C, Pan Y, Cao X, Goodrich J, Chen X. 2011. AGAMOUS terminates floral stem cell maintenance in Arabidopsis by directly repressing WUSCHEL through recruitment of Polycomb Group proteins. The Plant Cell 23, 3654–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D. 2001. A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105, 793–803. [DOI] [PubMed] [Google Scholar]
- Lopez-Dee ZP, Wittich P, Enrico Pè M, Rigola D, Del Buono I, Gorla MS, Kater MM, Colombo L. 1999. OsMADS13, a novel rice MADS-box gene expressed during ovule development. Developmental Genetics 25, 237–244. [DOI] [PubMed] [Google Scholar]
- Lu SJ, Wei H, Wang Y, Wang HM, Yang RF, Zhang XB, Tu JM. 2012. Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.). Plant Molecular Biology Reporter 30, 1461–1469. [Google Scholar]
- Lu Z, Shao G, Xiong J, et al. 2015. MONOCULM 3, an ortholog of WUSCHEL in rice, is required for tiller bud formation. Journal of Genetics and Genomics 42, 71–78. [DOI] [PubMed] [Google Scholar]
- Müller R, Bleckmann A, Simon R. 2008. The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. The Plant Cell 20, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münster T, Deleu W, Wingen L, et al. 2002. Maize MADS-box genes galore. Maydica 47, 287–301. [Google Scholar]
- Malcomber ST, Kellogg EA. 2005. SEPALLATA gene diversification: brave new whorls. Trends in Plant Science 10, 427–435. [DOI] [PubMed] [Google Scholar]
- Mandel MA, Gustafson-Brown C, Savidge B, Yanofsky MF. 1992. Molecular characterization of the Arabidopsis floral homeotic gene APETALA1. Nature 360, 273–277. [DOI] [PubMed] [Google Scholar]
- Masiero S, Imbriano C, Ravasio F, Favaro R, Pelucchi N, Gorla MS, Mantovani R, Colombo L, Kater MM. 2002. Ternary complex formation between MADS-box transcription factors and the histone fold protein NF-YB. Journal of Biological Chemistry 277, 26429–26435. [DOI] [PubMed] [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jürgens G, Laux T. 1998. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95, 805–815. [DOI] [PubMed] [Google Scholar]
- Mena M, Ambrose BA, Meeley RB, Briggs SP, Yanofsky MF, Schmidt RJ. 1996. Diversification of C-function activity in maize flower development. Science 274, 1537–1540. [DOI] [PubMed] [Google Scholar]
- Ming F, Ma H. 2009. A terminator of floral stem cells. Genes and Development 23, 1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Betsuyaku S, Iwamoto K, Kinoshita A, Fukuda H, Sawa S. 2008. The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis. Plant and Cell Physiology 49, 1752–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai K, Miyamae M, Kato H, Takumi S, Ogihara Y. 2003. WAP1, a wheat APETALA1 homolog, plays a central role in the phase transition from vegetative to reproductive growth. Plant and Cell Physiology 44, 1255–1265. [DOI] [PubMed] [Google Scholar]
- Nagasawa N, Miyoshi M, Sano Y, Satoh H, Hirano H, Sakai H, Nagato Y. 2003. SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development 130, 705–718. [DOI] [PubMed] [Google Scholar]
- Nardmann J, Werr W. 2006. The shoot stem cell niche in angiosperms: expression patterns of WUS orthologues in rice and maize imply major modifications in the course of mono- and dicot evolution. Molecular Biology and Evolution 23, 2492–2504. [DOI] [PubMed] [Google Scholar]
- Nimchuk ZL, Tarr PT, Meyerowitz EM. 2011. An evolutionarily conserved pseudokinase mediates stem cell production in plants. The Plant Cell 23, 851–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M, Shinohara H, Sakagami Y, Matsubayashi Y. 2008. Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science 319, 294. [DOI] [PubMed] [Google Scholar]
- Ohmori Y, Tanaka W, Kojima M, Sakakibara H, Hirano HY. 2013. WUSCHEL-RELATED HOMEOBOX4 is involved in meristem maintenance and is negatively regulated by the CLE gene FCP1 in rice. The Plant Cell 25, 229–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautler M, Tanaka W, Hirano HY, Jackson D. 2013. Grass meristems I: shoot apical meristem maintenance, axillary meristem determinacy and the floral transition. Plant and Cell Physiology 54, 302–312. [DOI] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF. 2000. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405, 200–203. [DOI] [PubMed] [Google Scholar]
- Pelucchi N, Fornara F, Favalli C, Masiero S, Lago C, Pè EM, Colombo L, Kater MM. 2002. Comparative analysis of rice MADS-box genes expressed during flower development. Sexual Plant Reproduction 15, 113–122. [Google Scholar]
- Perales M, Reddy GV. 2012. Stem cell maintenance in shoot apical meristems. Current Opinion in Plant Biology 15, 10–16. [DOI] [PubMed] [Google Scholar]
- Pinyopich A, Ditta GS, Savidge B, Liljegren SJ, Baumann E, Wisman E, Yanofsky MF. 2003. Assessing the redundancy of MADS-box genes during carpel and ovule development. Nature 424, 85–88. [DOI] [PubMed] [Google Scholar]
- Prasad K, Parameswaran S, Vijayraghavan U. 2005. OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. The Plant Journal 43, 915–928. [DOI] [PubMed] [Google Scholar]
- Prasad K, Sriram P, Kumar CS, Kushalappa K, Vijayraghavan U. 2001. Ectopic expression of rice OsMADS1 reveals a role in specifying the lemma and palea, grass floral organs analogous to sepals. Development Genes and Evolution 211, 281–290. [DOI] [PubMed] [Google Scholar]
- Roe JL, Nemhauser JL, Zambryski PC. 1997. TOUSLED participates in apical tissue formation during gynoecium development in Arabidopsis. The Plant Cell 9, 335–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe JL, Rivin CJ, Sessions RA, Feldmann KA, Zambryski PC. 1993. The Tousled gene in A. thaliana encodes a protein kinase homolog that is required for leaf and flower development. Cell 75, 939–950. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Bateman RM. 2004. Evolution of zygomorphy in monocot flowers: iterative patterns and developmental constraints. New Phytologist 162, 25–44. [Google Scholar]
- Running MP, Fletcher JC, Meyerowitz EM. 1998. The WIGGUM gene is required for proper regulation of floral meristem size in Arabidopsis. Development 125, 2545–2553. [DOI] [PubMed] [Google Scholar]
- Sato Y, Hong S-K, Tagiri A, Kitano H, Yamamoto N, Nagato Y, Matsuoka M. 1996. A rice homeobox gene, OSH1, is expressed before organ differentiation in a specific region during early embryogenesis. Proceedings of the National Academy of Sciences, USA 93, 8117–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Veit B, Mandel MA, Mena M, Hake S, Yanofsky MF. 1993. Identification and molecular characterization of ZAG1, the maize homolog of the Arabidopsis floral homeotic gene AGAMOUS. The Plant Cell 5, 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jürgens G, Laux T. 2000. The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635–644. [DOI] [PubMed] [Google Scholar]
- Steeves TA, Sussex IM. 1989. Patterns in plant development. Cambridge: Cambridge University Press. [Google Scholar]
- Sun B, Looi LS, Guo S, He Z, Gan ES, Huang J, Xu Y, Wee WY, Ito T. 2014. Timing mechanism dependent on cell division is invoked by Polycomb eviction in plant stem cells. Science 343, 1248559. [DOI] [PubMed] [Google Scholar]
- Sun B, Xu Y, Ng KH, Ito T. 2009. A timing mechanism for stem cell maintenance and differentiation in the Arabidopsis floral meristem. Genes and Development 23, 1791–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzaki T, Sato M, Ashikari M, Miyoshi M, Nagato Y, Hirano HY. 2004. The gene FLORAL ORGAN NUMBER1 regulates floral meristem size in rice and encodes a leucine-rich repeat receptor kinase orthologous to Arabidopsis CLAVATA1. Development 131, 5649–5657. [DOI] [PubMed] [Google Scholar]
- Suzaki T, Toriba T, Fujimoto M, Tsutsumi N, Kitano H, Hirano HY. 2006. Conservation and diversification of meristem maintenance mechanism in Oryza sativa: function of the FLORAL ORGAN NUMBER2 gene. Plant and Cell Physiology 47, 1591–1602. [DOI] [PubMed] [Google Scholar]
- Taguchi-Shiobara F, Yuan Z, Hake S, Jackson D. 2001. The fasciated ear2 gene encodes a leucine-rich repeat receptor-like protein that regulates shoot meristem proliferation in maize. Genes and Development 15, 2755–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbert PB, Adler HT, Parks DW, Comai L. 1995. The REVOLUTA gene is necessary for apical meristem development and for limiting cell divisions in the leaves and stems of Arabidopsis thaliana. Development 121, 2723–2735. [DOI] [PubMed] [Google Scholar]
- Tanaka W, Ohmori Y, Ushijima T, Matsusaka H, Matsushita T, Kumamaru T, Kawano S, Hirano HY. 2015. Axillary meristem formation in rice requires the WUSCHEL ortholog TILLERS ABSENT1. The Plant Cell 27, 1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka W, Pautler M, Jackson D, Hirano HY. 2013. Grass meristems II: inflorescence architecture, flower development and meristem fate. Plant and Cell Physiology 54, 313–324. [DOI] [PubMed] [Google Scholar]
- Taoka K, Ohki I, Tsuji H, et al. 2011. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476, 332–335. [DOI] [PubMed] [Google Scholar]
- Theißen G. 2001. Development of floral organ identity: stories from the MADS house. Current Opinion in Plant Biology 4, 75–85. [DOI] [PubMed] [Google Scholar]
- Theissen G, Saedler H. 2001. Plant biology. Floral quartets. Nature 409, 469–471. [DOI] [PubMed] [Google Scholar]
- Thompson BE, Hake S. 2009. Translational biology: from Arabidopsis flowers to grass inflorescence architecture. Plant Physiology 149, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang L, Cai Q, et al. 2015. OsMADS32 interacts with PI-like proteins and regulates rice flower development. Journal of Integrative Plant Biology 57, 504–513. [DOI] [PubMed] [Google Scholar]
- Wang K, Tang D, Hong L, Xu W, Huang J, Li M, Gu M, Xue Y, Cheng Z. 2010. DEP and AFO regulate reproductive habit in rice. PLoS Genetics 6, e1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple CJ, Zanis MJ, Kellogg EA, Schmidt RJ. 2007. Conservation of B class gene expression in the second whorl of a basal grass and outgroups links the origin of lodicules and petals. Proceedings of the National Academy of Sciences, USA 104, 1081–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav RK, Perales M, Gruel J, Girke T, Jönsson H, Reddy GV. 2011. WUSCHEL protein movement mediates stem cell homeostasis in the Arabidopsis shoot apex. Genes and Development 25, 2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Lee DY, Miyao A, Hirochika H, An G, Hirano HY. 2006. Functional diversification of the two C-class MADS box genes OSMADS3 and OSMADS58 in Oryza sativa. The Plant Cell 18, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. 2003. Positional cloning of the wheat vernalization gene VRN1. Proceedings of the National Academy of Sciences, USA 100, 6263–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM. 1990. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346, 35–39. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Gao S, Xue DW, et al. 2009. RETARDED PALEA1 controls palea development and floral zygomorphy in rice. Plant Physiology 149, 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Z, Zhang D. 2015. Roles of jasmonate signalling in plant inflorescence and flower development. Current Opinion in Plant Biology 27, 44–51. [DOI] [PubMed] [Google Scholar]
- Yun D, Liang W, Dreni L, Yin C, Zhou Z, Kater MM, Zhang D. 2013. OsMADS16 genetically interacts with OsMADS3 and OsMADS58 in specifying floral patterning in rice. Molecular Plant 6, 743–756. [DOI] [PubMed] [Google Scholar]
- Zahn LM, Kong H, Leebens-Mack JH, Kim S, Soltis PS, Landherr LL, Soltis DE, Depamphilis CW, Ma H. 2005. The evolution of the SEPALLATA subfamily of MADS-box genes: a preangiosperm origin with multiple duplications throughout angiosperm history. Genetics 169, 2209–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn LM, Leebens-Mack JH, Arrington JM, Hu Y, Landherr LL, dePamphilis CW, Becker A, Theissen G, Ma H. 2006. Conservation and divergence in the AGAMOUS subfamily of MADS-box genes: evidence of independent sub- and neofunctionalization events. Evolution and Development 8, 30–45. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yuan Z. 2014. Molecular control of grass inflorescence development. Annual Review of Plant Biology 65, 553–578. [DOI] [PubMed] [Google Scholar]
- Zhang D, Yuan Z, An G, Dreni L, Hu J, Kater MM. 2013. Panicle development. In: Zhang Q, Wing RA, eds. Genetics and genomics of rice. New York: Springer, 279–295. [Google Scholar]
- Zhang X. 2014. Plant science. Delayed gratification—waiting to terminate stem cell identity. Science 343, 498–499. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zong J, Liu J, Yin J, Zhang D. 2010. Genome-wide analysis of WOX gene family in rice, sorghum, maize, Arabidopsis and poplar. Journal of Integrative Plant Biology 52, 1016–1026. [DOI] [PubMed] [Google Scholar]
- Ziegelhoffer EC, Medrano LJ, Meyerowitz EM. 2000. Cloning of the Arabidopsis WIGGUM gene identifies a role for farnesylation in meristem development. Proceedings of the National Academy of Sciences, USA 97, 7633–7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.