Abstract
Context
Experimentally controlled studies of estrogenic regulation of lipid measures and inflammatory cytokines in men are rare.
Objective
To delineate the effect of estradiol (E2) on lipids and inflammatory markers.
Design
This was a placebo-controlled, single-masked, prospectively randomized study comprising experimentally degarelix-downregulated healthy men [n = 74; age 65 years (range, 57 to 77)] assigned to four treatment groups: (1) IM saline and oral placebo; (2) IM testosterone and oral placebo; (3) IM testosterone and oral anastrozole (aromatase inhibitor); and (4) IM testosterone, oral anastrozole, and transdermal E2 for 22 (±1) days.
Results
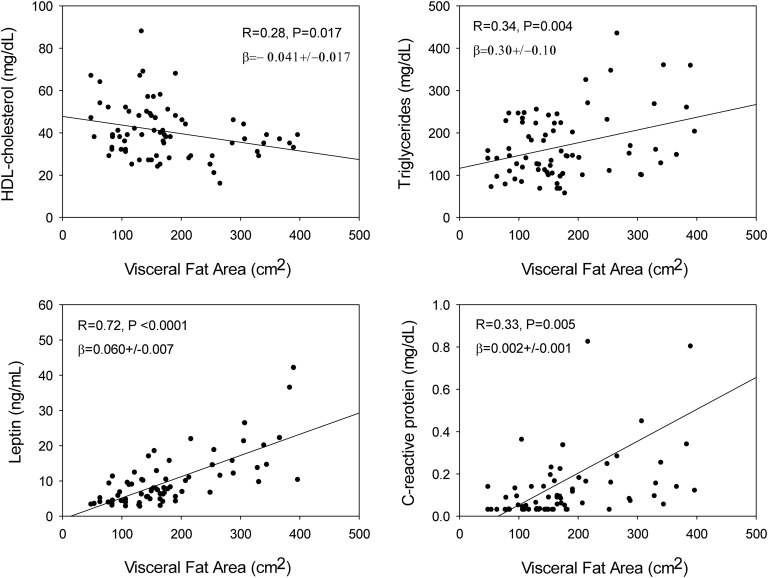
Mean mass spectrometry–quantified serum E2 concentrations ranged from 1.2 to 82 pg/mL in the four treatment groups. E2 extremes did not alter total cholesterol, triglyceride, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein cholesterol (HDL-C) , non–HDL-C, apolipoprotein B, lipoprotein (a), IL-6, or high-sensitivity C-reactive protein (hsCRP) concentrations. Higher E2 concentrations elevated both sex hormone-binding globulin and prolactin as positive controls. LDL cholesterol, adiponectin, and leptin were higher in hypogonadal subjects without testosterone or E2 addback (P = 0.018, 0.039, and 0.023, respectively). Abdominal visceral fat area by CT (independent variable) correlated negatively with HDL-C (P = 0.017), and positively with triglycerides (P = 0.004), hsCRP (P = 0.005), and leptin (P < 0.0001).
Conclusion
In this placebo-controlled prospectively randomized study, wide variations in circulating E2 did not influence lipid measures and inflammatory markers when testosterone concentrations were controlled experimentally. However, medically induced central hypogonadism in older men was accompanied by increased LDL cholesterol and metabolic cytokines, adiponectin and leptin. Abdominal visceral fat correlated strongly and positively with triglycerides, hsCRP, and leptin, but negatively with HDL.
Keywords: age, estradiol, human, inflammation, lipids, testosterone
Marked E2 variations in testosterone-clamped healthy older men do not affect key lipid measures and inflammatory markers.
Sex steroids are used extensively in estrogen treatment of menopausal symptoms, androgen supplementation in men with low serum testosterone (T) levels, and androgen or estrogen administration to transsexual patients [1–4]. Clinical concerns are potential cardiovascular complications (myocardial infarction and cerebrovascular accidents), venous thromboses, and hormone-related cancers (breast, uterine, and prostatic) [5–7]. Investigation of the lipid spectrum is one tool for assessing the safety of hormone treatment. Many studies have been published on side effects of estrogen replacement in menopausal women, but less attention has been devoted to potential drawbacks of estrogen repletion or deprivation in men. To assess direct effects of sex steroids on lipid profiles, short-term studies are required, before any substantial change in body composition occurs [7–9]. Nonetheless, many clinical sex-hormone treatment studies are focused on long-term results [10–12]. In women, the choice of a particular estrogen, its dose and route of administration, and concomitant use of progestin with more or less intrinsic androgenic effects can confound the interpretation of metabolic results [13].
The degree to which estrogen regulates lipid or metabolic outcomes in men is unclear [7, 14–16]. Prior investigations have been handicapped by pathological and/or pharmacologic settings and interventions, such as (diabetic) hypogonadism or surgical castration, high-dose estrogen or human chorionic gonadotropin administration or mixed antiestrogen/partial estrogen agonist treatment [5, 9, 15–19]. In most models, there are profound concurrent changes in T availability, thus confounding interpretation of any estrogen effect per se. Under such circumstances, low estrogen milieus tend to result in lower high-density lipoprotein (HDL) concentrations, and conversely for high-estrogen milieus. Moreover, as suggested by others, longer term interventions also alter body composition, typically lowering HDL whenever abdominal visceral fat increases as under prolonged T deprivation [8, 20].
Although estrogen administration is a straightforward procedure for the assessment of estrogen effects on the lipid profile in women, this is not the case for men. A major confounding action of administered estrogen in men is downregulation of the gonadotropic axis, resulting in decreased T production. Methods for increasing T in older men are more complicated. Treatment with aromatase blockers causes T levels to increase and estradiol (E2) levels to decrease, whereas exogenously administering T elevates not only T but also E2 concentrations [21]. Thus, for valid mechanistic studies of estrogen action per se, both T and E2 levels must be controlled experimentally. This goal was accomplished here rapidly and precisely by: (1) downregulation of the gonadotropic axis using a potent and selective GnRH antagonist; (2) concomitant addback of placebo or T; (3) treatment with placebo or an aromatase inhibitor to decrease endogenously generated E2 from administered T; and (4) administration of E2 along with the aromatase inhibitor and T in a clamp model [22]. This fourfold strategy allowed us to investigate the effects of E2 under experimentally controlled T levels. The purpose of this explorative study was to evaluate potential short-term effects of sex steroids (especially E2) under controlled (T-clamped) conditions on lipids and selective inflammation markers in 74 healthy older men.
1. Material and Methods
The study was designed to address whether E2 levels at fixed T availability affect lipid concentrations and inflammatory markers measured in fasting blood obtained after 3 weeks of the endocrine clamp.
A. Subjects
Seventy-four healthy, ambulatory, community-dwelling older men [mean age, 65 years (range, 57 to 77)] participated in the overnight clinical unit–based study. The mean body mass index (BMI) was 26.9 kg/m2 (range, 20 to 36). Volunteers were recruited by newspaper advertisements, local posters, the Clinical Trials Center Web page, and community (general and minority) bulletin boards. In this single-blind, prospectively randomized, placebo-controlled study, all qualifying volunteers underwent gonadotropin downregulation using the GnRH antagonist, degarelix (Ferring Pharmaceuticals, NY). The primary randomization was to IM T vs IM saline, and transdermal E2vs no E2, and oral placebo vs anastrozole, an aromatase inhibitor (AstraZeneca Pharmaceuticals, Wilmington, DE). Thereby, the sex-hormone clamp comprised: (1) degarelix 80 mg (given as two subcutaneous injections of 40 mg) once (called day 1) (Ferring Pharmaceuticals, Parsippany, NY); (2) T enanthate or T cypionate (Cardinal Health, Hudson, WI) 100 mg or placebo IM given on day 1 and repeated on days 8 and 15, range ± 1 day; (3) oral placebo or anastrozole (AstraZeneca Pharmaceuticals, Wilmington, DE) 2.0 mg once daily for 23 days; and (4) no patch or an E2 patch calibrated to deliver 0.05 mg/d E2 transdermally (Novartis, Morris Plains, NJ) beginning on day 1 and changed every 3 days through day 22. Statistical comparison was among the four resulting groups: (1) degarelix/T/placebo/no patch; (2), degarelix/T/anastrozole/no patch; (3) degarelix/T/anastrozole/E2 patch; and (4) degarelix/placebo/placebo/no patch.
Individuals arrived in the clinical research unit at or before 6:00 pm to permit placement of bilateral forearm IV catheters for overnight fasting and blood sampling. A blood sample was obtained at 8:00 am for sex-hormone, lipids, inflammatory markers, and peptide measurements. The study was embedded in a protocol in which the influence of sex steroids on nocturnal GH secretion and the effect of GHRH on GH response were assessed (unpublished). Ambulation was allowed to the lavatory. The volunteer was allowed to sleep. To reduce nutritional confounds, subjects were given a prescribed meal to ingest at 6:00 pm on the evening before. Men received a standardized 10 kcal/kg meal (vegetarian or nonvegetarian) with a macronutrient composition of 20% protein, 50% carbohydrate, and 30% fat. Participants then remained fasting for 12 hours overnight (except for allowable intake of noncaloric and noncaffeinated liquids).
The protocol was approved by Mayo Institutional Review Board. Witnessed voluntary written informed consent was obtained before study enrollment. A complete medical history, physical examination, and screening tests of hematological, renal, hepatic, metabolic, and endocrine function were normal. Subjects underwent a single-slice CT of the abdomen, level L3-L4, as an exploratory test of the impact of relative visceral adiposity on lipid responses.
B. Exclusion Criteria
Exclusion criteria were acute or chronic systemic diseases, HIV positivity by medical history, anemia, endocrine disorders (except hypothyroid subjects who were biochemically euthyroid on replacement), psychiatric illness, alcohol or drug abuse, deep venous or arterial thromboses, cancer of any type (except localized basal or squamous cell cancer of the skin treated surgically without recurrence), recent use (within 6 weeks) of anabolic steroids or glucocorticoids, history of stroke, myocardial infarction or angina, allergy to sex steroids used in the study; substantial recent weight change (loss/gain of ≥6 lb over 6 weeks), transmeridian travel (exceeding 3 time zones within the preceding 3 weeks), current or recent night shift work, systemic drugs, abnormal renal, hepatic or hematologic function, concomitant sex-hormone replacement, and unwillingness to provide written informed consent.
C. Assays
Total cholesterol (TC), triglycerides (TG), and HDL cholesterol (HDL-C) were measured using the Roche Cobas c311 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN) with interassay coefficients of variation (CV) of 2.2%, 0.8%, and 0.6% at 249, 178, and 51 mg/dL, respectively. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald equation. Apolipoprotein B (ApoB) was measured immunoturbimetrically on the Hitachi Chemistry Analyzer using the DiaSorin ApoB SPQ II Reagent Set (DiaSorin Inc., Stillwater, MN) with an interassay CV of 4% at 94 mg/dL. Lipoprotein (a) [Lp(a)] was measured by a turbidimetric immunoassay on the Roche Cobas c311 Chemistry Analyzer. The interassay CV was 4.3% at 21 mg/dL. Interleukin-6 was measured by a high-sensitivity two-site enzyme-linked immunoassay from R&D Systems (Minneapolis, MN). The interassay CV was 3.6% at 3.88 pg/mL. Leptin and adiponectin were measured by specific immunoassays (Linco Research, Inc., St. Louis, MO). The interassay CVs were 11% and 4.7% at 20.4 ng/mL and 29.9 ng/mL, respectively. High-sensitivity C-reactive protein (hsCRP) was measured by a high-sensitivity immunoturbimetric assay on the Roche Cobas c311 Chemistry Analyzer. The interassay CV was 1.7% at 0.17 mg/dL. E2 and total T were measured using liquid chromatography-tandem mass spectrometry (Agilent Technologies, Inc., Santa Clara, CA). Intraassay CVs were: E2, 10.8% at 0.29 pg/mL and 5.1% at 32 pg/mL; and T, 8.9% at 0.69 ng/dL, 4.0% at 45 ng/dL, and 3.5% at 841 ng/dL. Sex hormone–binding globulin (SHBG), IGF-I, and IGFBP3 were quantified by solid-phase chemiluminescent assay on the Siemens Immulite 2000 Automated Immunoassay System (Siemens Health Care Diagnostics, Deerfield, IL). Intraassay CV for SHBG was 4.0% at 5.4 nmol/L and 5.9% at 74 nmol/L; for IGF-I, 4.9% at 37 µg/L and 5% at 225 µg/L; and for IGFBP3, 4% and 3.9% at 1.0 and 4.3 mg/L. IGFBP1 was determined by a two-site immunoradiometric assay (Diagnostic Systems Laboratories, Webster, TX). Interassay CVs were 10.2% at 0.49 µg/L and 6.7% at 4.5 µg/L. The interassay CV was 1.7% at 0.17 mg/dL. Insulin was measured by a two-site immunoenzymatic sandwich assay on the Roche e411 (Roche Diagnostics). Intraassay CVs were 3.3%, 2.8%, and 2.5% at 18, 61, and 172 mU/L. Prolactin, FSH, and LH were measured by two-site chemiluminescent sandwich immunoassays on a DXL 800 automated immunoassay system (Beckman Instruments, Chaska, MN). Intraassay CVs for prolactin were 3.7%, 2.1%, and 4.8% at 6.1, 16.4, and 34.5 μg/L; for FSH, 3.6%, 3.2%, and 4.7% at 6.5, 16.7, and 58.0 IU/L; and for LH, 9.3%, 6.0%, and 6.0% at 1.4, 15.6, and 48.8 IU/L, respectively.
D. Statistics
Data were analyzed by one-way ANOVA for the four randomly assigned treatment groups. In case of nonnormal distribution, the Kruskal-Wallis nonparametric ANOVA test was used. This was followed by the Dwass-Steel-Critchlow test for pairwise comparisons. Linear regression analysis was applied to identify concentration-dependent effects of T and/or E2, and effects of abdominal visceral fat. The statistical power of this study was 80% based upon a change of 20% of mean lipid concentrations with a standard deviation of 20% when studying 12 to 15 subjects in each group.
Calculations were performed with Systat 13 (Systat Software, Inc., San Jose, CA). P < 0.05 was construed as significant for the overall study.
2. Results
Demographic and anthropomorphic data of the volunteers are presented in Table 1. Statistically, the four subject groups were balanced before any treatment protocol started. Interventions were well tolerated and there were no dropouts. Serum concentrations of IGF-I and its binding proteins IGFBP3 and IGFBP1 were similar after treatment in the four groups. Total T and (calculated) free T levels were low in subjects with downregulated gonadotropins without T addback, whereas T addback in the other three groups yielded statistically comparable T concentrations (Table 2). E2 concentrations were lowest in subjects treated with anastrozole, which inhibits the conversion of T in E2, followed by the control group with no T addback. Serum SHBG concentrations in E2-depleted men (group C) were lower than in men with E2 addback (group D); namely, 30.0 ± 2.6 and 38.0 ± 2.7 nmol/L, respectively (P = 0.03). Serum prolactin was higher in subjects treated with E2 compared with men treated with placebo (8.0 ± 0.7 µg/L vs 6.2 ± 0.3 µg/L, P = 0.02). Furthermore, serum gonadotropin concentrations were higher in the two groups with low E2 concentrations (post hoc contrasts for LH and FSH, P < 0.0001), consistent with known negative feedback by E2 on gonadotropin secretion. However, likely because of differences in sensitivity to degarelix between individuals, LH and FSH were not completely suppressed in all subjects. This is obviously important for subjects in group A, whereas the other groups received testosterone addback. Nevertheless, subjects of group A were all hypogonadal with 80% decrease of free T levels. Addback of E2 or T or the combination had no influence on serum lipids, including TC, HDL-C, LDL-C, non-HDL-C, TG, ApoB, and LP(a). Adiponectin and leptin concentrations were higher in men treated with degarelix only (group A) compared with the other three groups, who received addback of one or both sex steroids (Table 3). Absolute values of the inflammatory markers, hsCRP and IL-n-6, were higher in subjects treated with degarelix only (group A). However, differences with men who received E2 and/or T addback were not important.
Table 1.
Baseline Demographic and Endocrine Data in 74 Healthy Older Men
| Variable | D/T(−) | D/T(+) | D/A/E2(−)/T(+) | D/A/E2(+)/T(+) | ANOVA P |
|---|---|---|---|---|---|
| Number of subjects | 16 | 18 | 20 | 20 | |
| Age (y) | 64 ± 0.8 | 66 ± 1.0 | 65 ± 1.0 | 65 ± 1.2 | 0.69 |
| BMI (kg/m2) | 28.1 ± 1.1 | 27.7 ± 0.8 | 25.4 ± 0.5 | 26.9 ± 0.9 | 0.12 |
| Visceral fat (cm2) | 193 ± 25 | 194 ± 22 | 144 ± 13 | 176 ± 23 | 0.29 |
| HOMA-IR | 1.28 ± 0.19 | 1.28 ± 0.20 | 1.00 ± 0.15 | 1.30 ± 0.26 | 0.67 |
| LH (U/L) | 5.0 ± 0.9 | 4.0 ± 0.4 | 5.5 ± 0.8 | 4.0 ± 0.4 | 0.23 |
| FSH (U/L) | 9.1 ± 1.0 | 6.6 ± 0.7 | 9.9 ± 1.3 | 6.8 ± 0.6 | 0.12 |
| T (ng/dL) | 430 ± 30 | 430 ± 21 | 480 ± 22 | 500 ± 28 | 0.11 |
| E2 (pg/mL) | 22.6 ± 2.5 | 21.4 ± 1.6 | 27.2 ± 1.3 | 24.7 ± 1.3 | 0.08 |
| SHBG (nmol/L) | 45 ± 3.3 | 43 ± 3.2 | 42 ± 2.7 | 50 ± 3.3 | 0.35 |
| TSH (mU/L) | 2.33 ± 0.2 | 2.76 ± 0.3 | 2.83 ± 0.3 | 2.90 ± 0.3 | 0.44 |
| PRL (µg/L) | 9.2 ± 0.9 | 8.4 ± 0.6 | 8.7 ± 0.7 | 8.3 ± 0.6 | 0.81 |
Data are shown as the mean ± SEM. P values were estimated by one-way ANOVA across the four study groups.
Abbreviations: A, anastrozole; D, degarelix; E2(–), no 17β estradiol; E2(+), 17β estradiol; T(−), no testosterone addback; T(+), testosterone addback.
Table 2.
IGF-I, IGF-Binding Proteins, and Sex Hormones During Hormone Administration in 74 Older Men
| Group A | Group B | Group C | Group D | ANOVA | |
|---|---|---|---|---|---|
| IGF-I (µg/L) | 106 ± 8.4 | 111 ± 7.4 | 106 ± 6.6 | 114 ± 6.1 | 0.80 |
| IGFBP1 (µg/L) | 1.51 ± 0.19 | 1.31 ± 0.12 | 1.40 ± 0.15 | 1.14 ± 0.07 | 0.25 |
| IGFBP3 (mg/L) | 2.96 ± 0.19 | 2.84 ± 0.12 | 3.06 ± 0.14 | 2.89 ± 0.12 | 0.68 |
| Estradiol (pg/mL) | 9.4 ± 1.9a | 31.2 ± 3.5b | 1.21 ± 0.24c | 82 ± 18d | <0.0001 |
| Free testosterone (ng/dL) | 3.1 ± 0.7a | 19.5 ± 1.9b | 19.9 ± 2.0b | 20.4 ± 1.8b | <0.0001 |
| Testosterone (ng/dL) | 164 ± 35a | 760 ± 61b | 748 ± 71b | 845 ± 66b | <0.0001 |
| SHBG (nmol/L) | 37 ± 3.3 | 33 ± 2.9 | 30 ± 2.4 | 38 ± 2.7 | 0.22 |
| Prolactin (µg /L) | 6.5 ± 0.6a | 10.4 ± 0.8b | 6.2 ± 0.3a | 8.0 ± 0.7a,b | <0.0001 |
| FSH (U/L) | 4.0 ± 0.9a | 0.29 ± 0.04b | 5.02 ± 1.18a | 0.33 ± 0.06b | <0.0001 |
| LH (U/L) | 2.4 ± 0.5a | 0.21 ± 0.1b | 1.50 ± 0.34a | 0.24 ± 0.09b | <0.0001 |
Data are shown as mean ± SEM. Differing superscripts denote significant post hoc contrasts by multiple-comparison testing among the four treatment groups. Boldface values denote P < 0.01 level of significance.
Table 3.
Lipid Profiles and Inflammatory Markers During Hormone Administration in 74 Older Healthy Men
| Parameter | Group A | Group B | Group C | Group D | ANOVA |
|---|---|---|---|---|---|
| TC (mg/dL) | 175 ± 7.1 | 166 ± 6.3 | 158 ± 7.1 | 166 ± 5.0 | 0.34 |
| HDL-C (mg/dL) | 42 ± 3.5 | 37 ± 2.8 | 42 ± 3.3 | 42 ± 2.6 | 0.62 |
| LDL-C (mg/dL) | 100 ± 6.3 | 91 ± 4.3 | 84 ± 5.8 | 90 ± 4.5 | 0.018 a |
| Non-HDL-C (mg/dL) | 133 ± 6.7 | 128 ± 6.2 | 116 ± 7.2 | 124 ± 4.9 | 0.31 |
| Triglycerides (mg/dL) | 161 ± 16.7 | 187 ± 20.9 | 163 ± 16.9 | 168 ± 19.0 | 0.77 |
| Lp(a) (mg/dL) | 27.0 ± 6.3 | 23.3 ± 5.3 | 21.9 ± 4.4 | 16.8 ± 4.1 | 0.55 |
| ApoB (mg/dL) | 0.88 ± 0.044 | 0.84 ± 0.043 | 0.78 ± 0.049 | 0.82 ± 0.029 | 0.34 |
| Adiponectin (mg/dL) | 9840 ± 1190 | 6120 ± 616 | 7690 ± 650 | 8250 ± 980 | 0.039 b |
| HsCRP (mg/dL) | 0.36 ± 020 | 0.17 ± 0.05 | 0.076 ± 0.012 | 0.11 ± 004 | 0.16 |
| Leptin (ng/mL) | 14.6 ± 2.1 | 9.6 ± 1.9 | 6.8 ± 0.7 | 10.2 ± 1.9 | 0.023 c |
| Interleukin 6 (pg/mL) | 5.2 ± 2.1 | 3.8 ± 0.6 | 3.4 ± 0.4 | 3.9 ± 0.7 | 0.67 |
Data are shown as mean ± SEM. Boldface values denote ANOVA P < 0.05.
Post hoc contrasts:
Group A vs group C: P = 0.029.
Group A vs groups B, C, D jointly: P = 0.008.
Group A vs groups B, and C and D jointly: P = 0.02, group A vs group B; P = 0.005.
Based upon linear regression analysis, TC, HDL-C, LDL-C, non-HDL-C, TG, ApoB, and Lp(a) were not related to serum E2 or T concentrations (Table 4). Restricting analysis to subjects treated with anastrozole (groups C and D), serum E2 and lipid concentrations also were not correlated (Table 4). On the other hand, CT-estimated abdominal visceral fat area correlated positively with TG (R = 0.34, P = 0.004), nonsignificantly with non-HDL (R = 0.20, P = 0.07) and negatively with HDL-C (R = −0.28, P = 0.017). Additionally, visceral fat area correlated positively with leptin (R = 0.72, P < 0.0001) and hsCRP (R = 0.33, P = 0.005) (Fig. 1). Serum SHBG concentrations were linearly related to Lp(a) (R = −0.30, P = 0.011) and leptin (R = 0.37, P = 0.001), but nonsignificantly to adiponectin (R = 0.22, P = 0.06).
Table 4.
Linear Regressions Among Serum Sex-Hormone Concentrations and Lipids, Lipoproteins, and Inflammatory Proteins, and Analogously for Visceral Fat in 74 Older Men
| T | Free T | E2 | E2 in Anastrozole-Treated Subjects | Visceral Fat Area | |
|---|---|---|---|---|---|
| Total cholesterol | −0.065/0.58 | −0.10/0.39 | +0.024/0.84 | +0.052/0.75 | +0.071/0.56 |
| Triglycerides | +0.02/0.87 | −0.00/0.99 | +0.09/0.94 | +0.023/0.89 | +0.34/0.004 |
| HDL-C | +0.008/0.95 | −0.004/0.98 | +0.052/0.67 | +0.031/0.85 | -0.28/0.017 |
| LDL-C | −0.088/0.46 | −0.015/0.31 | −0.006/0.96 | +0.028/0.87 | +0.008/0.95 |
| Non-HDL-C | −0.062/0.60 | −0.10/0.39 | +0.001/0.99 | +0.038/0.82 | +0.20/0.07 |
| ApoB | +0.07/0.55 | −0.109/0.36 | +0.046/0.70 | +0.023/0.89 | +0.12/0.32 |
| Lp(a) | −0.030/0.80 | +0.066/0.58 | −0.047/0.69 | −0.045/0.78 | +0.002/0.99 |
| hsCRP | −0.14/0.23 | −0.17/0.16 | +0.061/0.06 | −0.051/0.76 | +0.33/0.005 |
| Leptin | −0.078/0.51 | −0.22/0.06d | +0.036/0.76 | +0.010/0.52 | +0.72/<0.0001 |
| Adiponectin | −0.007/0.95 | −0.063/0.60 | −0.004/0.97 | +0.020/0.87 | −0.22/0.06 |
| IL-6 | −0.15/0.22 | −0.17/0.15 | −0.06/0.62 | −0.078/0.64 | +0.17/0.16 |
Data are the linear correlation coefficient (β) and P value. Boldface values denote that individual data are plotted in Figure 1.
Figure 1.
Linear regressions between abdominal visceral fat area and each of HDL-C, TG, leptin, or hsCRP in 74 healthy older men. P values are for the indicated slope (β coefficient) and R (correlation) values.
3. Discussion
This prospectively randomized, placebo-controlled, single-masked study investigated separate and combined effects of E2 and T addback in older healthy men under a sex-steroid clamp enforced after gonadal-axis downregulation with a potent, selective, and long-acting GnRH antagonist, degarelix. T was added back in some groups, and its conversion to E2 was inhibited by the aromatase blocker, anastrozole, in two other groups. Placebo or E2 was then added back under anastrozole block. Sex-steroid concentrations were quantified by mass spectrometry, yielding an absolute range of mean E2 concentrations of 1.2 to 82 pg/mL across the four study groups.
The current study circumvents many earlier issues by experimentally fixing systemic T concentrations for 22 ± 1 days, whereas adjusting serum E2 concentrations over a nearly 80-fold range verified by mass spectrometry to ensure accurate quantification of very low E2 levels. We are unaware of any prior investigations constraining E2 in men over a comparable range while fixing T concentrations. The short study interval of 3 weeks obviates major shifts in body composition otherwise observed over more prolonged intervals, although minor changes may occur short term in severe acute hypogonadism [23]. In the current study, only group A had reduced testosterone levels, whereas the other three groups had normal levels under T addback, limiting concerns about a possible (minor) change in body composition. Under the present conditions, marked E2 variations did not detectably alter LDL-C, HDL-C, TG, TC, non-HDL-C, Lp(a), ApoB, hsCRP or IL-6 concentrations. In contrast, extensive available literature establishes that nonaromatizable anabolic steroids and androgenic progestins consistently suppress HDL, often markedly [24], depending upon chemical structure, dose, route, and duration of exposure [14, 25].
Estradiol administration for 22 ± 1 day in the current study resulted in well-known stimulatory effects on serum prolactin and SHBG concentrations. This verifies adequacy of the E2 clamp to modulate known endocrine targets of E2. Furthermore, in the presence of low E2 and/or low T levels, the competitively antagonistic effect of degarelix on gonadotropin secretion was partly disinhibited. Thus, expected hypothalamopituitary and hepatic effects of sex steroids were present in these sex steroid–clamped healthy subjects.
In the overall cohort of 74 men, no effects of either E2 or T on the lipid profile could be demonstrated, including on TC, HDL-C, LDL-C, non-HDL-C, TG, ApoB, and Lp(a). The power of these observations was emphasized by the absence of important (linear) relationships between serum T and E2 concentrations on one hand and lipid concentrations on the other. In women, the beneficial effects of endogenous estrogens including E2 on lipids and cardiovascular risk are well accepted, inasmuch as premenopausal women are protected from cardiovascular mortality compared with age-matched men. After menopause, a less favorable lipid profile emerges, along with higher vascular risk. The menopausally defined low estrogen, androgen, and progesterone milieu is associated with increased TC, LDL-C, and TG, but decreased HDL-C and Lp(a) [26]. Administration of estrogens to postmenopausal women can improve the lipid profile, depending on the choice of estrogen; the dose, duration, and route of administration; and accompanying progestin [27].
In uncontrolled epidemiological studies, long-term effects of sex-steroid administration can introduce confounding factors (e.g., changes in lifestyle and/or body composition), which secondarily alter lipoproteins and inflammatory markers. These drawbacks were avoided in a recent placebo-controlled prospective short-term study in postmenopausal women [13]. The paradigm demonstrated that E2 and natural progesterone modify lipids and inflammatory markers after 3 weeks of sex-steroid treatment. The current study exploits an analogous sex-steroid clamp strategy adapted to men, wherein systemic E2 and T concentrations are experimentally controlled. The absence of estrogenic effects on the lipid profile in this paradigm in men raises the possibility that lipoproteins are less sensitive to estrogen in men than women, although mass spectrometry–quantified E2 concentrations were numerically comparable in men (81 pg/mL) and women (99 pg/mL) in the two studies [13]. As positive controls, there were clear effects of administered E2 on prolactin, SHBG, and gonadotropin concentrations in both women and men. Thus, the present clinical model of E2 administration is sufficient to demonstrate expected E2 regulation of well-known physiological endpoints.
Investigations of the effects of estrogens on lipid concentrations in men are limited by cohort selection and clinical context (e.g., male-to-female transsexuals and patients with prostatic carcinoma). In a recent study by Auer et al., 24 previously untreated male-to-female transgender patients were studied at baseline and after 12 months of oral estrogen exposure. In response to estrogen, fat mass and the waist:hip ratio decreased, along with TG, TC, and HDL-C [11]. Another study of E2 administration in men reported increased HDL-C and TG concentrations after 6 months [4]. Potential interpretational problems in these studies are concomitant changes in body composition, simultaneous exposure to antiandrogens and/or antiprogestins, and profound reduction in serum T concentrations. The combined factors do not allow facile interpretation of direct or exclusive E2 effects on lipid measures and inflammatory markers in such individuals.
In men with prostatic carcinoma, orchiectomy with monthly injections of polyphosphate E2 or daily oral administration of ethinyl E2 decreased TC, LDL-C, and ApoB while increasing HDL-C [28]. Similar findings occurred during very high-dose transdermal E2 treatment (0.6 mg/d), which is sixfold the menopausal dose [2]. In these settings, serum E2 concentrations were at least fourfold greater than those in our study, whereas total and free T concentrations were in the hypogonadal or castrate ranges [2]. Some drawbacks of these studies were circumvented by a study using an early-generation GnRH antagonist along with parenteral T addback and a relatively nonspecific aromatase inhibitor, testolactone [29]. In the young men in whom E2 levels decreased, HDL-C decreased by 8%, and apolipoprotein A by 6%. Concentrations of TC, LDL-C, and TG did not change. Unfortunately, mass spectrometry was not used to quantify T or E2, E2 was not added back, and no data on ApoB, Lp(a), or cytokine concentrations were reported. Collectively, these heterogeneous studies in very different populations suggest that, in the face of demonstrably normal adult male T levels, the effects of nonfeminizing concentrations of E2 on LDL-C, HDL-C, and TG are limited.
Adiponectin and leptin concentrations were higher in hypogonadal men, defined here by low serum T, free T, and E2 concentrations after 3 weeks. In chronically hypogonadal men, T repletion normalized initially elevated leptin levels [30]. Analogously, in healthy men, treatment with a GnRH-receptor antagonist reduced T levels in 7 days, and increased adiponectin levels. Concomitant addition of T prevented the rise of adiponectin [31]. Likewise, long-term (6 to 12 months) of T treatment in female-to-male transsexual patients decreased adiponectin [32] as well as leptin [11, 33]. Accordingly, T and/or its metabolites can diminish leptin and adiponectin concentrations. Because the same cytokines did not decrease in the current study after anastrozole’s blockade of T-to-E2 conversion, we infer that T’s restoration of cytokine levels does not depend upon physiological amounts of E2 derived from T’s aromatization. Indeed, in another study, transdermal E2 administration in men with prostate cancer for 8 weeks did not alter leptin levels [2]. On the other hand, short-term aromatase inhibition in young and elderly men resulting in increased T and decreased E2 levels was accompanied by increased leptin, but unchanged adiponectin, concentrations [34]. Although available data do not agree on all points, overall observations on the regulation of serum leptin and adiponectin in sex-hormone controlled men are compatible with androgen and not estrogen effects on these adipokines.
Although in vitro studies have shown a direct negative effect of testosterone and dihydrotestosterone on leptin secretion by rodent adipocytes [35], a direct effect on adiponectin secretion in human adipocytes is not present [36]. Additional investigations have shown that humoral serum components of high molecular weight are involved in the secretion of various molecular forms of adiponectin [37].
The inflammatory markers, IL-6 and hsCRP, assessed in this study were not different among the four groups. In addition, their levels were not related to serum concentrations of E2, T, and free T, suggesting that they are not modulated short-term by T or E2 availabilities in men. Comparable conclusions were reached in other studies based upon T administration in older men, transdermal E2 administration in patients with prostatic carcinoma [2], and anastrozole administration in elderly men with low T [38].
Limitations of the current analysis include the relatively small cohort (n = 74 men), shorter duration of observation (3 weeks), and absence of more exhaustive lipid fractionation by mass spectrometry, PAGE or other laboratory techniques, as well as the restricted age range evaluated. Thus, our outcomes do not necessarily apply to populational data, or reflect expected outcomes after lipid subfractionation, or in young adult cohorts. Likewise, our study does not address cardiovascular outcomes per se, which have been reviewed by others recently [8, 39].
In summary, this clinical investigation delineates the absence of influence of a very wide range of near-physiological concentrations of E2 under fixed T concentrations on lipid measures and inflammatory markers in older healthy men over 22 days. Acutely induced central hypogonadism in men is associated with increased metabolic cytokines, adiponectin and leptin, which are normalized during T addback whether T’s conversion to E2 is blocked. The last finding indicates that aromatase activity is not required to transduce T’s suppression of these adipocytokines.
Acknowledgments
We thank Jill Smith for support of manuscript preparation, the Mayo Immunochemical Laboratory for assay assistance, and the Mayo research nursing staff for implementing the protocol.
Financial Support: This work was supported in part by National Institutes of Health Grants R01 AG019695, R01 DK073148, and R01 AG031763 to J.D.V., and P30 DK050456 (Metabolic Studies Core of the Minnesota Obesity Center). The project described was supported by National Center for Advancing Translational Sciences Grant UL1 TR000135 and National Institute of Standards and Technology Grant 60NANB10D005Z. Contents are solely the responsibility of the authors and do not necessarily represent the official views of any federal institution.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- ApoB
Apolipoprotein B
- BMI
body mass index
- CV
coefficient of variation
- E2
estradiol
- HDL-C
high-density lipoprotein cholesterol
- hsCRP
high-sensitivity C-reactive protein
- LDL-C
low-density lipoprotein cholesterol
- Lp(a)
lipoprotein (a)
- SHBG
sex hormone–binding globulin
- T
testosterone
- TC
total cholesterol
- TG
triglyceride
References and Notes
- 1. Gartlehner G, Patel SV, Feltner C, Weber RP, Long R, Mullican K, Boland E, Lux L, Viswanathan M. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;318(22):2234–2249. [DOI] [PubMed] [Google Scholar]
- 2. Purnell JQ, Bland LB, Garzotto M, Lemmon D, Wersinger EM, Ryan CW, Brunzell JD, Beer TM. Effects of transdermal estrogen on levels of lipids, lipase activity, and inflammatory markers in men with prostate cancer. J Lipid Res. 2006;47(2):349–355. [DOI] [PubMed] [Google Scholar]
- 3. Yassin A, Almehmadi Y, Saad F, Doros G, Gooren L. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clin Endocrinol (Oxf). 2016;84(1):107–114. [DOI] [PubMed] [Google Scholar]
- 4. Deutsch MB, Bhakri V, Kubicek K. Effects of cross-sex hormone treatment on transgender women and men. Obstet Gynecol. 2015;125(3):605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhindsa S, Ghanim H, Batra M, Kuhadiya ND, Abuaysheh S, Sandhu S, Green K, Makdissi A, Hejna J, Chaudhuri A, Punyanitya M, Dandona P. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care. 2016;39(1):82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jones TH, Kelly DM. Randomized controlled trials - mechanistic studies of testosterone and the cardiovascular system. Asian J Androl. 2018;20(2):120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander GC, Iyer G, Lucas E, Lin D, Singh S. Cardiovascular risks of exogenous testosterone use among men: a systematic review and meta-analysis. Am J Med. 2017;130(3):293–305. [DOI] [PubMed] [Google Scholar]
- 8. Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf). 2005;63(3):280–293. [DOI] [PubMed] [Google Scholar]
- 9. Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2006;154(6):899–906. [DOI] [PubMed] [Google Scholar]
- 10. Asscheman H, Gooren LJ, Megens JA, Nauta J, Kloosterboer HJ, Eikelboom F. Serum testosterone level is the major determinant of the male-female differences in serum levels of high-density lipoprotein (HDL) cholesterol and HDL2 cholesterol. Metabolism. 1994;43(8):935–939. [DOI] [PubMed] [Google Scholar]
- 11. Auer MK, Ebert T, Pietzner M, Defreyne J, Fuss J, Stalla GK, T’Sjoen G. Effects of sex hormone treatment on the metabolic syndrome in transgender individuals: focus on metabolic cytokines. J Clin Endocrinol Metab. 2018;103(2):790–802. [DOI] [PubMed] [Google Scholar]
- 12. Dias JP, Shardell MD, Carlson OD, Melvin D, Caturegli G, Ferrucci L, Chia CW, Egan JM, Basaria S. Testosterone vs. aromatase inhibitor in older men with low testosterone: effects on cardiometabolic parameters. Andrology. 2017;5(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roelfsema F, Yang RJ, Veldhuis JD. Differential effects of estradiol and progresterone on cardiovascular risk factors in postmenopausal women. J Endocr Soc. 2018;2(7):794–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haffner SM, Valdez RA. Endogenous sex hormones: impact on lipids, lipoproteins, and insulin. Am J Med. 1995; 98(1, 1A)40S–47S. [DOI] [PubMed] [Google Scholar]
- 15. Nóvoa FJ, Boronat M, Carrillo A, Tapia M, Díaz-Cremades J, Chirino R. Effects of tamoxifen on lipid profile and coagulation parameters in male patients with pubertal gynecomastia. Horm Res. 2002;57(5-6):187–191. [DOI] [PubMed] [Google Scholar]
- 16. Hiraga T, Shimokawa K, Murase T, Yokoyama M. Reduction of serum lipoprotein (a) by estrogen in men with prostatic cancer. Endocr J. 1993;40(5):507–513. [DOI] [PubMed] [Google Scholar]
- 17. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. [DOI] [PubMed] [Google Scholar]
- 18. Angelin B, Olivecrona H, Reihnér E, Rudling M, Ståhlberg D, Eriksson M, Ewerth S, Henriksson P, Einarsson K. Hepatic cholesterol metabolism in estrogen-treated men. Gastroenterology. 1992;103(5):1657–1663. [DOI] [PubMed] [Google Scholar]
- 19. Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf). 2010;73(5):602–612. [DOI] [PubMed] [Google Scholar]
- 20. Cornoldi A, Caminiti G, Marazzi G, Vitale C, Patrizi R, Volterrani M, Miceli M, Fini M, Spera G, Rosano G. Effects of chronic testosterone administration on myocardial ischemia, lipid metabolism and insulin resistance in elderly male diabetic patients with coronary artery disease. Int J Cardiol. 2010;142(1):50–55. [DOI] [PubMed] [Google Scholar]
- 21. Veldhuis JD, Iranmanesh A. Short-term aromatase-enzyme blockade unmasks impaired feedback adaptations in luteinizing hormone and testosterone secretion in older men. J Clin Endocrinol Metab. 2005;90(1):211–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu PY, Veldhuis JD. Hypothalamo-pituitary unit, testis, and male accessory organs. In: Strauss II, JF, Barbieri RL, Gargiulo A, eds. Yen & Jaffe's Reproductive Endocrinology. 8th ed. Philadelphia, PA: Elsevier; 2018:285–300.
- 23. Chao J, Rubinow KB, Kratz M, Amory JK, Matsumoto AM, Page ST. Short-term estrogen withdrawal increases adiposity in healthy men. J Clin Endocrinol Metab. 2016;101(10):3724–3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bagatell CJ, Bremner WJ. Androgen and progestagen effects on plasma lipids. Prog Cardiovasc Dis. 1995;38(3):255–271. [DOI] [PubMed] [Google Scholar]
- 25. Jockenhövel F, Bullmann C, Schubert M, Vogel E, Reinhardt W, Reinwein D, Müller-Wieland D, Krone W. Influence of various modes of androgen substitution on serum lipids and lipoproteins in hypogonadal men. Metabolism. 1999;48(5):590–596. [DOI] [PubMed] [Google Scholar]
- 26. Cifkova R, Krajcoviechova A.. Dyslipidemia and cardiovascular disease in women. Curr Cardiol Rep. 2015;17(7):1–10. [DOI] [PubMed] [Google Scholar]
- 27. Jensen J. Effects of sex steroids on serum lipids and lipoproteins. Baillieres Clin Obstet Gynaecol. 1991;5(4):867–887. [DOI] [PubMed] [Google Scholar]
- 28. Wallentin L, Varenhorst E. Plasma lipoproteins during anti-androgen treatment by estrogens or orchidectomy in men with prostatic carcinoma. Horm Metab Res. 1981;13(5):293–297. [DOI] [PubMed] [Google Scholar]
- 29. Bagatell CJ, Knopp RH, Rivier JE, Bremner WJ. Physiological levels of estradiol stimulate plasma high density lipoprotein2 cholesterol levels in normal men. J Clin Endocrinol Metab. 1994;78(4):855–861. [DOI] [PubMed] [Google Scholar]
- 30. Jockenhövel F, Blum WF, Vogel E, Englaro P, Müller-Wieland D, Reinwein D, Rascher W, Krone W. Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J Clin Endocrinol Metab. 1997;82(8):2510–2513. [DOI] [PubMed] [Google Scholar]
- 31. Page ST, Herbst KL, Amory JK, Coviello AD, Anawalt BD, Matsumoto AM, Bremner WJ. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26(1):85–92. [PubMed] [Google Scholar]
- 32. Berra M, Armillotta F, D’Emidio L, Costantino A, Martorana G, Pelusi G, Meriggiola MC. Testosterone decreases adiponectin levels in female to male transsexuals. Asian J Androl. 2006;8(6):725–729. [DOI] [PubMed] [Google Scholar]
- 33. Elbers JM, Asscheman H, Seidell JC, Frölich M, Meinders AE, Gooren LJ. Reversal of the sex difference in serum leptin levels upon cross-sex hormone administration in transsexuals. J Clin Endocrinol Metab. 1997;82(10):3267–3270. [DOI] [PubMed] [Google Scholar]
- 34. Lapauw B, T’Sjoen G, Mahmoud A, Kaufman JM, Ruige JB. Short-term aromatase inhibition: effects on glucose metabolism and serum leptin levels in young and elderly men. Eur J Endocrinol. 2009;160(3):397–402. [DOI] [PubMed] [Google Scholar]
- 35. Wabitsch M, Blum WF, Muche R, Braun M, Hube F, Rascher W, Heinze E, Teller W, Hauner H. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J Clin Invest. 1997;100(4):808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakashima E, Benet LZ. General treatment of mean residence time, clearance, and volume parameters in linear mammillary models with elimination from any compartment. J Pharmacokinet Biopharm. 1988;16(5):475–492. [DOI] [PubMed] [Google Scholar]
- 37. Körner A, Wabitsch M, Seidel B, Fischer-Posovszky P, Berthold A, Stumvoll M, Blüher M, Kratzsch J, Kiess W. Adiponectin expression in humans is dependent on differentiation of adipocytes and down-regulated by humoral serum components of high molecular weight. Biochem Biophys Res Commun. 2005;337(2):540–550. [DOI] [PubMed] [Google Scholar]
- 38. Dougherty RH, Rohrer JL, Hayden D, Rubin SD, Leder BZ. Effect of aromatase inhibition on lipids and inflammatory markers of cardiovascular disease in elderly men with low testosterone levels. Clin Endocrinol (Oxf). 2005;62(2):228–235. [DOI] [PubMed] [Google Scholar]
- 39. Sharma R, Oni OA, Gupta K, Chen G, Sharma M, Dawn B, Sharma R, Parashara D, Savin VJ, Ambrose JA, Barua RS. Normalization of testosterone level is associated with reduced incidence of myocardial infarction and mortality in men. Eur Heart J. 2015;36(40):2706–2715. [DOI] [PubMed] [Google Scholar]