Abstract
Essentials.
Post thrombotic syndrome (PTS) is a complication of deep vein thrombosis (DVT) with limited treatment options.
Uniformity of the diagnostic strategy and the use of risk prediction models might improve treatment outcomes.
Extending knowledge on the pathophysiology and improving therapeutic options is paramount for progress.
PTS management should evolve to a multimodal approach with treatment tailored to individual patients' needs.
Post thrombotic syndrome (PTS) is a common chronic complication of deep vein thrombosis of the leg (DVT). Treatment options are limited therefore emphasis is placed on its prevention. Several risk factors have been recognized, but were so far not used for risk stratification or translation into prediction models. Early interventions did not yet result in more successful preventive treatment strategies; for the acute phase of DVT there is equipoise on the value of elastic compression, as well as on catheter directed thrombolysis. There are no drugs specifically targeted at PTS prevention. The use of anticoagulant medication such as direct oral anticoagulants (DOACs) might decrease PTS incidence, but this needs to be corroborated. Both research into more effective treatment options as well as future PTS management may benefit from a uniform diagnostic strategy and the use of prediction rules to better allocate treatment and thereby increase treatment efficacy.
Keywords: deep vein thrombosis, management, post thrombotic syndrome
1. INTRODUCTION
The clinical presentation of post thrombotic syndrome (PTS) is characterized by edema and skin changes such as venous ectasia, varicose veins, redness, eczema, hyperpigmentation, and in severe cases fibrosis of the subcutaneous adipose tissue. This condition, known as lipodermatosclerosis results in impaired skin perfusion and poses patients at an increased risk of venous ulceration. Venous ulceration is the ultimate and most severe presentation of PTS. In addition to this array of skin problems, patients may experience leg symptoms such as heaviness, pain, itching, cramps, and paresthesia, with a symptom pattern that is worse with activity (standing, walking) and better with rest (elevation of the leg). Some patients experience “bursting” pain upon exercise, known as venous claudication. This is a result of venous outflow restriction, which is most often situated in the iliofemoral tract.1
Post thrombotic syndrome is an independent determinant of health‐related quality of life (HRQoL)2 with a differential decrease in quality of life associated with disease severity.3, 4 Also variations in patients' or disease characteristics may impact HRQoL differently. The “disease” specific VEINES‐QoL is influenced by a variety of patient factors such as comorbidity (Charlson score), gender, age, and obesity.3, 5 Both PTS and obesity (BMI > 30/m2) were found to be independently associated with impaired HRQoL with a five to seven times larger impact for PTS.5
Post thrombotic syndrome is a chronic condition that is diagnosed based on a clinical score. Although not all symptoms and signs are irreversible once PTS is diagnosed this diagnosis is deemed permanent. There is no gold standard for the diagnosis of PTS and there might not ever be one, as PTS is a syndrome and thus a combination of patient reported symptoms and physician assessed physical signs. The choice for a clinical score incorporating these features may therefore be the best option. This is even more so when it is considered that a large proportion of the disease burden is formed by the impact on HRQoL. Just objectifying lesions by imaging techniques or measuring ambulant venous pressure will not encompass the impact of the condition but may be used as confirmation and tool to assist in allocation of different treatment modalities.
Post thrombotic syndrome not a rare condition, occurring in about 20%‐50% of patients. Plurality of diagnostic scoring systems may be a likely contributor to the lack of precision in the reported prevalence and incidence of the condition. There are at least six scores that have been used in the recent past, and some of them are still being used today: the more or less PTS‐specific scores by Villalta, Brandjes, and Ginsberg, as well as the scores that were intended for the classification of venous disease: the VCSS, CEAP, and Widmer score.6, 7, 8, 9, 10, 11, 12 None of the so‐called “PTS‐specific” scores have been formally validated. It is remarkable that the incidence of PTS appears not to have changed over the years, in spite of many improvements in the management of acute DVT, including better anticoagulation, early mobilization, and adequate compression therapy when needed.
2. BARRIERS TO PROGRESS AND FUTURE PERSPECTIVES
2.1. Lack of gold standard for the diagnosis
At the International Society on Thrombosis and Haemostasis (ISTH) subcommittee meeting in 2008 consensus was reached to establish the diagnosis of PTS on a single Villalta score ≥5, at least 6 months after the acute event of DVT (ISTH consensus scoring method).13
The Villalta scale has many advantages, as it has good measurement properties and it is easy to apply.13 The Villalta score combined with a venous disease–specific quality‐of‐life questionnaire to standardize the subjective criteria was even suggested as the “gold” standard for the diagnosis of PTS.14 However, the Villalta scale also has limitations: venous claudication is not incorporated, and venous ulceration cannot be graded for severity.6, 15, 16
Post thrombotic syndrome is a chronic condition and therefore a diagnosis based on just one observation might not be ideal. Villalta scores tend to be unstable.17, 18 This might lead to overestimation of the diagnosis, especially in patients with mild PTS. With little or no irreversible skin changes, total scores become more dependent on complaints. Making a diagnosis on just one observation is therefore likely to result in a less precise estimation than making a diagnosis based on repeated scores. This is illustrated by Figure 1 where the incidences of PTS are given for one and the same population of patients based on either on one single Villalta score ≥5 (ISTH definition) or on two consecutive scores ≥5 (original definition).
Figure 1.
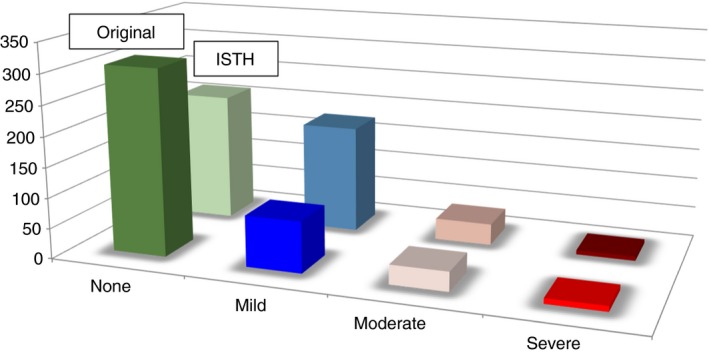
Difference in patient numbers for the severity categories of the Villalta score in relation to scoring method used for the diagnosis, original Villalta scoring method by Prandoni (2 scores of ≥5 with at least 3 months apart) vs the ISTH consensus scoring method based on data from the IDEAL study19. The ISTH scoring method “overestimates” mild post thrombotic syndrome (PTS), while there is no difference for moderate and severe PTS
With a diagnosis based on just one assessment, patients will more often be diagnosed as having PTS; most of these patients will be classified as having mild PTS.19 For the incidence of moderate and severe PTS it makes no difference, which definition of the Villalta score is used as the observed symptoms and signs are less likely to be reversible.
For future clinical trials on PTS treatment it might be better to select patients with moderate to severe PTS based on the Villalta score and hence study the effect of a treatment in a less heterogeneous population to focus on those patients that are most likely to benefit.
2.2. Lack of prediction models
Up till now there are no prediction models to identify patients at low or high risk for PTS. The fact that patients at risk cannot be identified at an early point in time hampers timely and adequately directed therapy. One of the challenges for the construction of a prediction model for PTS is lack of an objective diagnosis.
2.2.1. ISTH Berlin 2017 reports
Two models were presented at the 2017 ISTH meeting in Berlin.
One prediction model by Amin et al.20 was presented as a two‐step model. The diagnosis of PTS was based on the ISTH consensus score. The baseline model (step one) was based on easily available variables at the time of presentation.
The predictors in the baseline model are: age, BMI, gender, previous DVT iliofemoral DVT (IFDVT), varicose veins–provoked DVT, and smoking. The secondary model (step two) for decision making in the sub‐acute phase 6 months after the onset of DVT contains one additional variable: residual thrombosis. The rationale for the derivation of a secondary model was, that for some forms of treatment it is conceivable that they are most effective in the acute and sub‐acute phase and might therefore be stopped in selected patients at low risk after this initial period. Rabinovich et al.21 presented a model, which based the outcome PTS on the Ginsberg score.9 The final model included four independent predictors: index DVT iliac vein, BMI, and moderate or severe Villalta score at diagnosis. Based on the performance of the models it may be anticipated that PTS can be predicted based on baseline characteristics only. At the time of presentation neither model was validated in an external cohort.
2.3. Lack of unequivocally effective interventions
In the absence of unequivocally effective interventions for the prevention and treatment of PTS, the incentive for the identification of patients at risk has been limited. Moreover, without a clearly defined population at risk the identification of potentially successful treatment modalities is challenging. These two issues are mutually demotivating and may have played a role in the lack of research progress.
2.4. One standard treatment for all patients
Up until now the mainstay of treatment for the prevention of PTS has been elastic compression therapy. So far, eight studies have assessed the value of elastic compression therapy for the prevention of PTS with compression starting in the acute or sub‐acute phase after DVT.8, 22, 23, 24, 25, 26, 27, 28 Three studies have found a significant preventive effect of compression8, 22, 23 and two did not.24, 25 Other studies were focused on either shortened duration of therapy28, 29 or late onset of therapy9 or on comparative effectiveness of different types of compression stockings.30
Over the years, several meta‐analyses have been published on this topic.31, 32, 33 Different choices were made concerning the studies that were included, such as the diagnostic strategy used for PTS, the emphasis placed on the assessment of compliance to therapy, and the methodological quality of the studies. These differences have hampered the synthesis of evidence; heterogeneity is high in all published meta‐analyses.
A more recent online meta‐analysis with continuous updates34 focused on compression initiated in the acute and sub‐acute phase, also including the recently published 1 year vs 2 years of elastic compression stockings for prevention of post‐thrombotic syndrome (OCTAVIA study): randomized controlled trial which found that continuing elastic compression stocking (ECS) treatment after initial treatment for 1 year in patients without PTS was significantly better than cessation of treatment, with a number needed to treat (NNT) of 14.29. At this stage the overall conclusion is that the quality of evidence is low, but that the overall direction of the effect is more towards a favourable effect of ECS as an intervention with a potential for PTS prevention (RR 0.62, 95% CI 0.38‐1.01; P = .05).35
In addition, most investigated interventions for established PTS were directed at the reduction of edema and enhancement of calf muscle pump action including complex lymphedema therapy,36 veno‐wave therapy,37 or exercise therapy,38, 39 all with or without additional elastic compression therapy. All interventions resulted in an improvement in the severity of PTS,36, 37, 38, 39 and all but one resulted also in an improved health‐related quality of life (HRQoL).36 Complex lymphedema therapy on top of elastic compression therapy was only superior in patients with severe PTS36 while other studies such as the veno‐wave study were solely directed at patients with severe PTS.37 In addition to these mechanical studies, a limited number of pharmacological studies in which veno active drugs (VADs) were investigated for their properties of edema reduction and improvement of PTS symptomatology have been performed; the quality of evidence is, however, deemed to be low.40
2.5. Individualized duration of ECS treatment
In a previously reported management study, patients without reflux and with two consecutive low Villalta scores from 6 months on were allowed to stop compression. This strategy resulted in shortened treatment duration for about 50% of patients. Wearing ECS shorter than 1 year resulted in incidences for mild to moderate PTS of 21.1% and for severe PTS of 2.56%, suggesting that individualized shortening of therapy based on Villalta scores might be possible without loss of efficacy.18 Based on these findings, a randomized non‐inferiority trial was designed. This trial investigated whether the main therapeutic effect of individualized ECS therapy following initial 6 months of ECS therapy would not be unacceptably different from that of standard duration of therapy, and whether the accepted loss of efficacy of the new therapy would be balanced by a reduction in costs and or an increase in HRQoL.41 The upper margin of the loss of efficacy that was deemed to be acceptable was set at 7.5% (the noninferiority margin).42
2.5.1. ISTH Berlin 2017 reports
The IDEAL DVT study (individualized duration of elastic compression therapy vs longterm duration), which randomized 865 patients to either shortened duration of therapy (based on a clinical assessment) or standard duration therapy of 24 months, showed noninferiority for individualized shortened duration of ECS. The incidences of PTS were 28.9% and 27.8%, respectively. The absolute difference was 1.1% (95% CI upper limit of 7.2%), thereby not exceeding the upper limit of the predefined noninferiority margin of 7.5%.19 The IDEAL study essentially confirmed the findings of the previous management study,18 concluding that individualized shortened duration of compression for 6 months is similar to standard duration of compression for 24 months. The IDEAL trial selected patients based on clinical signs and symptoms captured in the Villalta score. Overall, the outcomes show that with this selection of patients no harm is done. The fact that compression can be stopped in more than 50% of patients after 6 months is likely to be highly cost effective.
2.6. The onset of PTS
The pathophysiology of PTS is thought to be a combination of valvular reflux and venous outflow restriction, the combination of which causes ambulatory venous hypertension, and eventually results in edema and skin changes associated with PTS.43 This principle is illustrated in Figure 2.
Figure 2.
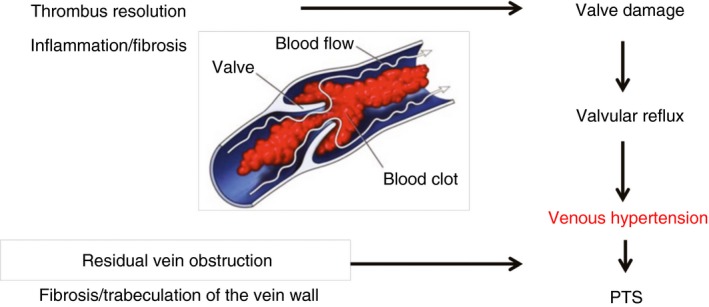
Schematic representation for the proposed pathophysiology of post thrombotic syndrome (PTS)
There is a substantial overlap in complaints between PTS and chronic venous disease (CVD). The incidence of CVD in the population ranges from 13.7% to 19.7%44, 45, 46, 47, 48 the prevalence of disease is age dependent and therefore increases gradually over time, in patients <30 years this is <10% for both sexes, but the incidence increases with age to 77% in patients aged ≥70 years.49 BMI and family history of CVD are important predictors for CVD.50 Contrary to the gradual buildup of venous hypertension in the pathophysiology of CVD, the pathophysiology of PTS is more suggestive of an immediate onset of venous hypertension with a subsequent relatively rapid appearance of end organ manifestations such as skin changes and edema. The obstruction of the vein by the thrombus will result in a sudden increase in venous pressure; this venous pressure will remain elevated until the vascular lumen is opened up by (sufficient) thrombus resolution. Hence, most cases of post thrombotic syndrome are diagnosed within the first year of the acute event of DVT. The incidence is much lower in the second year and even lower thereafter.51
It is therefore important to assess post thrombotic complaints in the context of DVT within a year of the acute DVT, and to do this with validated and disease specific tools in order to not confuse CVD with PTS. Importantly, although the clinical presentations may appear to be similar, treatment options may differ as venous hypertension in the context of DVT might be alleviated by stenting of venous segments with outflow restriction.
2.6.1. ISTH Berlin 2017 reports
To this end, an abstract was presented on risk factors for PTS in a cohort of patients with a first DVT from the MEGA study.52 PTS complaints were assessed with an alternative clinical classification score based on the Villalta score. The 1‐ and 7‐year cumulative incidence of PTS was determined. Sex, height and weight were identified as independent risk factors for PTS. The risk of PTS was found to be substantial up to 7 years after the first DVT and to be highest in women and overweight individuals. It can, however, not be excluded that these patients were actually suffering from CVD instead of PTS.
3. THROMBUS RESOLUTION
Venous flow is an important factor in the process of thrombus resolution. In a mouse model, it was elegantly shown that reduced flow is associated with impaired thrombus resolution and sustained inflammation of the vein wall compared to the normal flow condition.53 The inflammatory response increases the capillary permeability leading to edema and a cascade of inflammatory events, which eventually results in post thrombotic skin changes.54
3.1. Mechanisms of ECS treatment
External compression reduces the vein diameter and improves the venous flow velocity; as a consequence, there is a reduction of edema and a better efficiency of the calf muscle pump. This reduction of vein diameter and improvement of calf muscle efficiency is already significant at pressures as low as 20‐30 mm Hg.55, 56 Fluid speed increases when the vessel lumen narrows, thereby restoring the ejection volume (ejected volume divided by the total volume) into the normal ranges.56 This is in concordance with the observed efficacy of elastic compression therapy even for lower compression classes.29
The optimal onset of compression therapy for the prevention of PTS is poorly defined and scarcely investigated. Compression in the acute phase might be important for thrombus resolution, the prevention of venous hypertension, and consequently the reduction of the inflammatory response and secondary skin changes. As compression is also expected to reduce edema and pain, it might also positively impact HRQoL. When only studies that assessed compression applied within 24 hours of the DVT are considered, thus far only three small studies, with patient numbers varying from 53 to 73 patients, evaluated the effectiveness of early compression therapy.22, 26, 27 All studies showed improved clinical scores for pain and edema; one study showed improved recanalization and patency,27 and one study could substantiate a reduction in PTS.22 A major limitation of these studies was the limited sample size. For all other studies that have assessed the efficacy of compression, treatment was started in the sub‐acute phase when the initial edema had already receded.8, 23, 24 In a sub‐study to the compression stockings to prevent post‐thrombotic syndrome: a randomized placebo‐controlled (SOX) trial no differences were found for pain reduction and edema at respectively 14, 30, and 60 days after DVT between the active treatment with compression stockings (30‐40 mm Hg) and the sham stockings (5 mm Hg), not even in highly compliant patients.24
3.1.1. ISTH Berlin 2017 reports
The IDEAL DVT study also performed a pre‐planned sub‐study on the value of initial compression therapy. Time to compression was within 24 hours of the diagnosis. There were three prespecified protocols for compression in the acute phase.19 Initial compression was associated with a 15% relative reduction of (predominantly irreversible) skin signs. Compression therapy was anticipated to positively influence HRQoL.57 However, only compression with hosiery had a significant and clinically relevant positive effect on all HRQOL measures.58 An explanation for this lack of positive effect on complaints and HRQOL of compression therapy combined may be that bandaging has a negative impact on quality of life, as it involves a substantial loss of autonomy, which is an important trade‐off for treatment efficacy.59
3.2. Anticoagulant treatment
Over the years, several risk factors for PTS have been established.60 Many associations were found for PTS and hypercoagulable conditions. Biomarker research so far however could not confirm that hypercoagulability is an important driver for PTS. Neither inherited nor acquired thrombophilia were associated with PTS.61, 62 Only D‐dimer was more often associated with PTS with overall OR of 2.04 (1.02‐4.08) in several prospective cohorts.61, 63, 64 D‐dimer is non‐specific, and high D‐dimer levels can be a result of high fibrin‐turnover in the context of coagulation, but may also be associated with processes of inflammation.65 Inadequate inhibition of coagulation or hypercoagulability may lead to alterations in clot structure and thereby also affect clot lysis. In connection to this, it was observed that patients with PTS make denser clots with thinner fibers that are harder to lyse. In addition, those types of clots were also associated with higher probability of recurrent DVT.66
Several studies have shown a negative effect of sub‐therapeutic INR on the incidence of PTS.67, 68, 69 Rapid and adequate anticoagulation may therefore be an important therapeutic tool in the prevention of this complication. Low molecular weight heparin (LMWH) was found to be associated with significantly better outcomes compared to warfarin for both thrombus resolution and PTS.70 This might be explained by the fact that LMWHs provide not only a stable anticoagulant effect, but also have additional pleiotropic properties that are endothelial protective, anti‐inflammatory and anti‐angiogenic.71 Sulodexide, a heparin‐like substance that is active parentally (displaying anti‐thrombotic properties) as well as orally (with limited effect on the coagulation system) shows similar anti‐inflammatory and endothelial protective effects and might be an interesting target for long‐term preventive therapy following initial anticoagulant treatment for thrombosis.72 It is not known whether direct oral anticoagulants (DOACs) have pleiotropic effects that could be beneficial to the vessel wall. DOACs are simply assumed to provide more effective anticoagulation, hence less PTS due to the more stable thrombin inhibition. Only one post hoc analysis in a limited selection of patients (336) from a large randomized clinical trial that compared rivaroxaban to warfarin with LMWH (Einstein study) was performed thus far. The PTS incidence at 60 months follow‐up was 29% in the rivaroxaban group and 40% in the enoxaparin/VKA group. After adjusting for age, gender, body mass index, previous VTE, ipsilateral recurrent DVT, extent of DVT, idiopathic DVT, duration of anticoagulant treatment, compliance to assigned study medication, elastic compression stocking use, and active malignancy, the outcome did not reach statistical significance, the HR of PTS development for rivaroxaban was 0.76 (95% CI: 0.51‐1.13).73
3.2.1. ISTH Berlin 2017 reports
In a cross‐sectional study Utne and collaborators assessed whether treatment with rivaroxaban might have lowered the rate of PTS compared to warfarin treatment.74 The 2‐year rate of PTS for patients treated with rivaroxaban and patients treated with enoxaparin/warfarin was compared. PTS was assessed at one point in time at a mean of 24 ± 6 months after DVT using a patient reported Villalta score.74 PTS was scored according to the ISTH consensus method (one score ≥5 established the diagnosis). HRQOL was assessed with generic (EQ5D) and disease‐specific (Veines Qol/Sym) questionnaires. Of 390 patients studied, 161 patients (52%) had been treated with rivaroxaban and 148 (48%) with enoxaparin/warfarin. The prevalence of PTS was 45% for rivaroxaban users vs 59% for warfarin users, p = .01. After adjustment for confounders in a multivariate analysis the OR was 0.5 95% CI 0.3‐0.9; p = .02.
Considering the findings of the two studies analysed so far, the protective effect of rivaroxaban treatment seems credible and may be based on a more stable inhibition of thrombin generation and thereby limiting activation of thrombin activatable fibrinolysis inhibitor (TAFI) and thus leaving the cofactor function of fibrin intact rendering the fibrinolysis far more efficient.75, 76 The suggested potential benefit of reaching an absolute reduction in the incidence of PTS of 11%‐14%, is similar to the reduction in PTS obtained with catheter directed thrombolysis (CDT).77 However, while there is promise, based on the available evidence we cannot yet conclude that rivaroxaban is superior in PTS prevention, and certainly cannot make any statement regarding any of the other DOACs, for which we still don't have any evidence.
3.3. Plausible targets for future preventive therapy
3.3.1. Cell adhesion molecules
Endothelial cells and platelets are activated upon endothelial cell injury, promoting the expression of Cellular adhesion molecules (CAM). These molecules are implicated in perivascular inflammation as CAM expression facilitates leukocyte rolling and binding onto the endothelium with subsequent transmigration into the perivascular space.78 Therefore also CAM may be a target for preventive therapy. Two small studies so far showed that increased levels of CAM are associated with an increased incidence of PTS, this has been observed for levels of CAM at baseline,79 but also for levels of CAM assessed at a later point in time.80 Kellermaier et al. studied 48 patients following acute DVT and showed that increased baseline soluble platelet derived CAM (sPECAM‐1) plasma levels were associated with an increase in PTS development. Moreover, patients with higher levels of sPECAM‐1 also had significantly delayed thrombus resolution.79 Another relatively small case‐control study including 53 patients (26 patients with PTS and 27 patients without PTS) and 26 age and sex matched controls, observed that levels of vascular endothelium derived CAM (VCAM‐1), assessed 63 months after the thrombosis were increased in patients with PTS.80 In addition, Diaz and colleagues showed that inhibition of P‐selectin promoted thrombus resolution and prevented vein wall fibrosis better than enoxaparin or von Willebrand factor (VWF) inhibition in baboons. P‐selectin inhibition does not inflict anticoagulant effects and therefore may have less impact on hemostasis, in contrast to enoxaparin or VWF inhibition. P‐selectin inhibition therefore might be a safe and effective adjunctive treatment option for the prevention of PTS.81
3.3.2. Veno active drugs
Veno active drugs (VAD) have been on the market for decades and have shown to be effective for the reduction of PTS‐like complaints in patients with primary CVD. VADs such as rutosides have also been found to express CAM inhibiting properties.82 Meta‐analyses of randomized clinical trials in patients with CVD showed consistent effectiveness for VAD concerning edema and PTS‐like complaints.83, 84 With the use of VAD a clinically and statistically significant mean reduction of 30% was achieved for edema83 and a reduction of 15% for complaints of pain, swelling, and paresthesia.84 Venous hypertension is the central feature in the pathophysiology of both PTS and CVD and therefore the potential effectiveness of VAD in patients with PTS is credible. Only two RCTs and one registry studying rutosides have been undertaken in patients with PTS. All these studies showed significant reductions in complaints and edema, while only one study assessed PTS severity.85, 86, 87 Based on current available evidence the effectiveness and safety of VAD for the treatment of PTS is still uncertain and needs to be further investigated.88
3.3.3. Statins
Statins have not only been suggested as possible candidate preventive drugs for VTE but also for prevention of PTS. Statins are lipid‐lowering agents with anti‐thrombotic and anti‐inflammatory properties.89, 90 Treatment with daily atorvastatin or rosuvastatin significantly reduced thrombus burden (by 25%) without affecting lipid levels, blood coagulation parameters, or blood cell counts in a study of murine stasis and non‐stasis chemical‐induced venous thrombosis. In addition, statins reduced DVT‐induced vein wall scarring by 50% durably up to day 21 in stasis induced VT.91 Blood from statin‐treated mice showed significant reductions in platelet aggregation and clot stability. Statins additionally reduced thrombus plasminogen activator inhibitor‐1 (PAI‐1), tissue factor, neutrophils, myeloperoxidase, neutrophil extracellular traps (NETs), and macrophages. These effects were most notable in the earlier time points after DVT formation.91 That adjunctive therapy with statins can also reduce the incidence of PTS in humans was shown in an open label, RCT that included 230 patients with DVT. The effect of rosuvastatin given on top of LMWH on levels of CRP, D‐dimer and PTS at 3 months of follow‐up was compared to LMWH alone. No significant differences were observed in D‐dimer levels, but patients treated with statins displayed significantly lower levels of CRP (4.2 [4.3] vs 22.4 [97.5], p = .018) after 3 months of follow‐up. There was a significant decrease in PTS incidence (Villalta score > 5) in the rosuvastatin group (38.3% vs. 48.5%, p = .019), with scores between groups of 3.5 (6.0) and (7.8 [5.6], p = .035. There were no differences in EuroQol score between groups.92
3.4. Invasive clot removal strategies and stenting
The “open vein” hypothesis relates to the belief that fast removal of the thrombus prevents venous reflux, venous outflow restriction and thereby PTS. Impaired fibrinolysis may result in inadequate thrombus resolution with trabeculation, which together with a scarred and stiff vein wall will result in venous hypertension and consequent onset of PTS. The longer the thrombus is adjacent to the vessel wall, the higher the risk of vein wall damage. Fast removal of the thrombus may therefore prevent PTS.93
3.4.1. Thrombolysis
Since the early 1990s, thrombolysis has been used as an adjunctive treatment to swiftly remove the thrombus in the acute phase of the DVT. Four RCTs have been published so far, with only two reporting on long‐term effects on PTS incidence using the Villalta scale.94, 95, 96, 97 Thrombolysis is not without risk; there is an enhanced risk of bleeding induced by the administration of a thrombolytic drug in addition to the anticoagulant treatment. Solid data supporting the net clinical benefit of thrombolysis is scarce.
The Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial (183 patients) used pharmacomechanical CDT with percutaneous endovenous intervention (PEVI) a combination of thrombectomy, balloon venoplasty, stenting, and/or local low‐dose thrombolytic therapy. PTS was assessed with a non‐standardized scoring system and the intervention was heterogeneous. PTS at a mean follow up of 30 ± 5 months was lower for the PEVI +anticoagulation group (6.8%) than for the anticoagulant only group (29.6%), p < .001. Also for recurrent thrombosis PEVI+ anticoagulation (4.5%) was superior to anticoagulation alone (16%) p = .02. The lack of long‐term PTS data, the use of an alternative PTS score and the nature of the PEVI intervention hamper comparison with outcomes of other studies.98
The Catheter‐Directed Venous Thrombolysis in Acute Iliofemoral Vein Thrombosis (CaVenT) trial a medium‐sized RCT (209 patients) showed a significant benefit for additional catheter directed thrombolysis (CDT) on top of anticoagulant treatment: 41.1% patients allocated to additional CDT presented with PTS compared with 55.6% in the control group (p = .047), for an absolute risk reduction of 14.4% (95% CI 0.2‐27.9) with a NNT of 7 (95% CI 4‐5.02). CDT resulted in three major and five clinically relevant bleeds. The 5‐year follow up data showed a further improvement in scores for the CDT group compared to anticoagulant treatment alone in a limited sample left for assessment. Quality‐of‐life scores with either assessment scale did not differ between the treatment groups.
The North American Thrombus Removal with Adjunctive Catheter‐Directed Thrombolysis (ATTRACT) trial was recently completed and its results have been published.99 Data showed that 46.7% of the patients that received pharmacomechanical CDT developed PTS, compared to 48.2% of those who received anticoagulation alone (p = .56). There was also no significant difference in quality of life scores. There were no fatal or intracranial bleeds in either arm of the trial. More major bleeds were observed in the interventional arm (1.7%) than in the in the control arm (0.3%; p = .049). There were also significantly more instances of any bleeding: 4.5% in the interventional arm vs 1.7% in the control arm (p = .049). As factors explaining the rather poor outcome of this trial the selection of patients (CDT not restricted to iliofemoral DVT) the relatively low percentage of stenting and the incomplete follow‐up (with overrepresentation in the control group) were mentioned.
Therefore, considering acute thrombolysis for the prevention of PTS, there is a situation of equipoise. Whether or not acute thrombolysis reduces the occurrence of (more severe) PTS and whether the enhanced risk of bleeding will be sufficiently balanced by the decreased incidence of (more severe) PTS still has to be demonstrated. The Dutch Catheter directed thrombolysis vs anticoagulation alone (CAVA) study is still ongoing, and the results are expected end of 2018.
3.4.2. Stent placement in chronic PTS
In chronic PTS with a substantial outflow problem, angioplasty and stent placement may maintain long‐term venous patency and relieve symptoms. The evidence up until now is derived from mainly retrospective studies that are conducted in single centers and have limited sample sizes. More recently guidelines have been issued to improve and better standardize treatment.100 Progress has been made with the development of dedicated venous stents, which are longer and wider and more flexible than arterial stents.101 Improvement in HRQoL and decrease in Villalta scores were so far only shown in small, uncontrolled studies.102
At this moment in time it is still unclear what the implications for anticoagulant therapy in cases of venous stenting should be. No conclusive data are available for type or duration of anticoagulant treatment. A systematic review including 14 stenting studies showed that in 12 studies anticoagulant treatment (warfarin INR 2‐3) was administered for all patients for at least 6 months, in two studies anticoagulation treatment was only administered in those patients that were already on anticoagulant treatment preceding the stenting procedure with antiplatelet therapy for the remaining patients. The addition of antiplatelet therapy to warfarin treatment in four studies did not improve the outcomes for patency and re‐occlusion, nor did it increase the risk of bleeding.101 The most important risk factors associated with the outcome re‐occlusion were found to be the location and age of the thrombus and the stenting procedure itself. The optimal type, timing, and duration of peri‐procedural antithrombotic treatment for venous stenting need to be established.
4. CONCLUSION
Despite advances in DVT care in recent decades, the incidence of PTS has not decreased. Current interventions such as catheter directed thrombolysis have not proven to be unequivocally effective. ECS the longtime single and most effective form of PTS prevention is now disputed for its preventive properties, but is still valued for its role in the reduction of PTS symptomatology. So far there are no drugs specifically targeted at the prevention or treatment of PTS, and at the same time the available drugs for PTS‐like symptoms in CVD such as VAD have not been sufficiently tested in patients with PTS.
Ideally, PTS management should evolve from a “one treatment fits all” strategy with ECS as the only treatment modality to a more complex approach with treatment tailored to individual patients' needs (Figure 3). In that scenario, PTS risk prediction is based on prediction models and is followed by a multicomponent approach. Better allocation of therapy based on a uniform diagnostic strategy and risk stratification supported by risk prediction models might also improve the outcome of existing therapies. In addition, more research should be undertaken to translate pathophysiologic mechanisms of thrombus resolution and PTS development, into identification of PTS specific targets for pharmacotherapeutic intervention.
Figure 3.
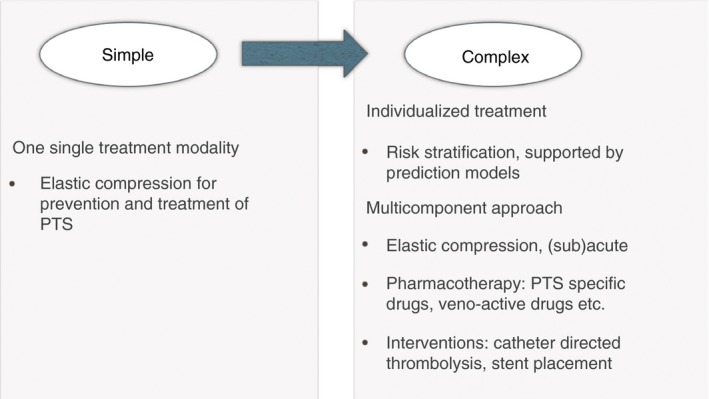
Proposed management strategy for post thrombotic syndrome (PTS) prevention, with multiple adjunctive preventive treatment modalities in addition to anticoagulant therapy
RELATIONSHIP DISCLOSURE
The author has no disclosures relevant to this paper.
ten Cate‐Hoek AJ. Prevention and treatment of the post‐thrombotic syndrome. Res Pract Thromb Haemost. 2018;2:209–219. 10.1002/rth2.12085
REFERENCES
- 1. Killewich LA, Martin R, Cramer M, Beach KW, Strandness DE Jr. Pathophysiology of venous claudication. J Vasc Surg. 1984;1:507–11. [PubMed] [Google Scholar]
- 2. Lubberts B, Paulino Pereira NR, Kabrhel C, Kuter DJ, DiGiovanni CW. What is the effect of venous thromboembolism and related complications on patient reported health‐related quality of life? A meta‐analysis. Thromb Haemost. 2016;116:417–31. [DOI] [PubMed] [Google Scholar]
- 3. Kahn SR, Hirsch A, Shrier I. Effect of postthrombotic syndrome on health‐related quality of life after deep venous thrombosis. Arch Intern Med. 2002;162:1144–8. [DOI] [PubMed] [Google Scholar]
- 4. Roberts LN, Patel RK, Donaldson N, Bonner L, Arya R. Post‐thrombotic syndrome is an independent determinant of health‐related quality of life following both first proximal and distal deep vein thrombosis. Haematologica. 2014;99:e41–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Utne KK, Tavoly M, Wik HS, et al. Health‐related quality of life after deep vein thrombosis. Springerplus. 2016;5:1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kolbach DN, Neumann HAM, Prins MH. Definition of the post‐thrombotic syndrome, differences between existing classifications. Eur J Vasc Endovasc Surg. 2005;30:404–14. [DOI] [PubMed] [Google Scholar]
- 7. Villalta S, Bagatella P, Piccioli A, Lensing A, Prins M, Prandoni P. Assessment of validity and reproducibility of a clinical scale for the post‐thrombotic syndrome. Haemostasis. 1994;24:158a. [Google Scholar]
- 8. Brandjes DP, Büller HR, Heijboer H, et al. Randomised trial of effect of compression stockings in patients with symptomatic proximal‐vein thrombosis. Lancet. 1997;349:759–62. [DOI] [PubMed] [Google Scholar]
- 9. Ginsberg JS, Hirsh J, Julian J, et al. Prevention and treatment of postphlebitic syndrome: results of a 3‐part study. Arch Intern Med. 2001;161:2105–9. [DOI] [PubMed] [Google Scholar]
- 10. Rutherford RB, Padberg FTJR, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–12. [DOI] [PubMed] [Google Scholar]
- 11. Eklöf B, Rutherford RB, Bergan JJ, et al. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–52. [DOI] [PubMed] [Google Scholar]
- 12. Widmer LK, Stathelin HB, Nissen C, Sd A. Venen‐Arterien, Krankheiten, koronaire Herzkrankhei bei berufstatigen. Bern: Verlag Hans Huber; 1981: pp. 66–82. [Google Scholar]
- 13. Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of post‐thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7:879–83. [DOI] [PubMed] [Google Scholar]
- 14. Soosainathan A, Moore HM, Gohel MS, Davies AH. Scoring systems for the post‐thrombotic syndrome. J Vasc Surg. 2013;57:254–61. [DOI] [PubMed] [Google Scholar]
- 15. ten Cate‐Hoek AJ, Ten Cate H, Henskens Y, van der Meijden PE, Spronk HM, Wittens C. Maastricht Consensus Conference on Thrombosis (MCCT): a roadmap for future research, February 11‐13, 2015, Maastricht, The Netherlands. Thromb Res. 2015;136(suppl 1):S1–2. [DOI] [PubMed] [Google Scholar]
- 16. Comerota AJ, Sandset PM, Konstantinides S, et al. Theme 4: invasive management of (recurrent) VTE and PTS. Thromb Res. 2015;136(suppl 1):S19–25. [DOI] [PubMed] [Google Scholar]
- 17. Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. [DOI] [PubMed] [Google Scholar]
- 18. Ten Cate‐Hoek AJ, Ten Cate H, Tordoir J, Hamulyák K, Prins MH. Individually tailored duration of elastic compression therapy in relation to incidence of the postthrombotic syndrome. J Vasc Surg. 2010;52:132–8. [DOI] [PubMed] [Google Scholar]
- 19. ten Cate‐Hoek AJ, Amin EE, Bouman AC, et al. Individualised versus standard duration of elastic compression therapy for prevention of post‐thrombotic syndrome (IDEAL DVT): a multicenter, randomised, single‐blind, allocation‐concealed, non‐inferiority trial. Lancet Haematol. 2018;5:e25–33. [DOI] [PubMed] [Google Scholar]
- 20. Amin EE, van Kuijk SMJ, Joore MA, ten Cate H, ten Cate‐Hoek AJ. Development and validation of a practical two‐step prediction model for post‐thrombotic syndrome. Res Pract Thromb Haemost 2017;1(suppl 1):195. [DOI] [PubMed] [Google Scholar]
- 21. Rabinovich A, Ducruet T, Kahn SR, SOX Trial investigators . Development of a clinical prediction model for the postthrombotic syndrome in a prospective cohort of patients with proximal deep vein thrombosis. J Thromb Haemost. 2018;16:262–70. [DOI] [PubMed] [Google Scholar]
- 22. Partsch H, Kaulich M, Mayer W. Immediate mobilisation in acute vein thrombosis reduces post‐thrombotic syndrome. Int Angiol. 2004;23:206–12. [PubMed] [Google Scholar]
- 23. Prandoni P, Lensing AW, Prins MH, et al. Below‐knee elastic compression stockings to prevent the post‐thrombotic syndrome: a randomized, controlled trial. Ann Intern Med. 2004;141:249–56. [DOI] [PubMed] [Google Scholar]
- 24. Kahn SR, Shapiro S, Wells PS, et al.; SOX trial investigators . Compression stockings to prevent post‐thrombotic syndrome: a randomised placebo‐controlled trial. Lancet. 2014;383:880–8. [DOI] [PubMed] [Google Scholar]
- 25. Jayaraj A, Meissner M. Impact of graduated compression stockings on the prevention of post‐thrombotic syndrome – results of a randomized controlled trial. Phlebology. 2015;30:541–8. [DOI] [PubMed] [Google Scholar]
- 26. Roumen‐Klappe EM, den Heijer M, van Rossum J, et al. Multilayer compression bandaging in the acute phase of deep‐vein thrombosis has no effect on the development of the post‐thrombotic syndrome. J Thromb Thrombolysis. 2009;27:400–5. [DOI] [PubMed] [Google Scholar]
- 27. Arpaia G, Cimminiello C, Mastrogiacomo O, de Gaudenzi E. Efficacy of elastic compression stockings used early or after resolution of the edema on recanalization after deep venous thrombosis: the COM.PRE Trial. Blood Coagul Fibrinolysis. 2007;18:131–7. [DOI] [PubMed] [Google Scholar]
- 28. Aschwanden M, Jeanneret C, Koller MT, et al. Effect of prolonged treatment with compression stockings to prevent post‐thrombotic sequelae: a randomized controlled trial. J Vasc Surg. 2008;47:1015–21. [DOI] [PubMed] [Google Scholar]
- 29. Mol GC, van de Ree MA, Klok FA, et al. One versus two years of elastic compression stockings for prevention of post‐thrombotic syndrome (OCTAVIA study): randomised controlled trial. BMJ. 2016;353:i2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prandoni P, Noventa F, Quintavalla R, et al. Thigh‐length versus below‐knee compression elastic stockings for prevention of the postthrombotic syndrome in patients with proximal‐venous thrombosis: a randomized trial. Blood. 2012;119:1561–5. [DOI] [PubMed] [Google Scholar]
- 31. Subbiah R, Aggarwal V, Zhao H, Kolluri R, Chatterjee S, Bashir R. Effect of compression stockings on post thrombotic syndrome in patients with deep vein thrombosis: a meta‐analysis of randomised controlled trials. Lancet Haematol. 2016;3:e293–300. [DOI] [PubMed] [Google Scholar]
- 32. Burgstaller JM, Steurer J, Held U, Amann‐Vesti B. Efficacy of compression stockings in preventing post‐thrombotic syndrome in patients with deep venous thrombosis: a systematic review and metaanalysis. Vasa. 2016;45:141–7. [DOI] [PubMed] [Google Scholar]
- 33. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. https://github.com/openMetaAnalysis/Compression-stockings-to-prevent-post-phlebitic-syndrome/blob/master/README.md
- 35. Appelen D, van Loo E, Prins MH, Neumann MH, Kolbach DN. Compression therapy for prevention of post‐thrombotic syndrome. Cochrane Database Syst Rev. 2017;9:CD004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmes CE, Bambace NM, Lewis P, Callas PW, Cushman M. Efficacy of a short course of complex lymphedema therapy or graduated compression stocking therapy in the treatment of post‐thrombotic syndrome. Vasc Med. 2014;19:42–8. [DOI] [PubMed] [Google Scholar]
- 37. O'Donnell MJ, McRae S, Kahn SR, et al. Evaluation of a venous‐return assist device to treat severe post‐thrombotic syndrome (VENOPTS). A randomized controlled trial. Thromb Haemost. 2008;99:623–9. [DOI] [PubMed] [Google Scholar]
- 38. Kahn SR, Shrier I, Shapiro S, et al. Six‐month exercise training program to treat post‐thrombotic syndrome: a randomized con‐ trolled two‐centre trial. CMAJ. 2011;183:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Padberg FT Jr, Johnston MV, Sisto SA. Structured exercise improves calf muscle pump function in chronic venous insufficiency: a randomized trial. J Vasc Surg. 2004;39:79–87. [DOI] [PubMed] [Google Scholar]
- 40. Morling JR, Yeoh SE, Kolbach DN. Rutosides for treatment of post‐thrombotic syndrome. Cochrane Database Syst Rev. 2015;9:CD005625. [DOI] [PubMed] [Google Scholar]
- 41. Ten Cate‐Hoek AJ, Bouman AC, Joore MA, Prins M, Ten Cate H; IDEAL DVT trial investigators . The IDEAL DVT study, individualised duration elastic compression therapy against long‐term duration of therapy for the prevention of post‐thrombotic syndrome: protocol of a randomised controlled trial. BMJ Open. 2014;4:e005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bouman AC, ten Cate‐Hoek AJ, Ramaekers BL, Joore MA. Sample size estimation for non‐inferiority trials: frequentist approach versus decision theory approach. PLoS ONE. 2015;10:e0130531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Phillips LJ 2nd, Sarkar R. Molecular characterization of post‐thrombotic syndrome. J Vasc Surg. 2007;45(suppl A):A116–22. [DOI] [PubMed] [Google Scholar]
- 44. Andreozzi GM, Signorelli S, Di Pino L, et al. Varicose symptoms without varicose veins: the hypotonic phlebopathy, epidemiology and pathophysiology. Minerva Cardioangiol. 2000;48:277–85. [PubMed] [Google Scholar]
- 45. Andreozzi GM. Prevalence of patients with chronic venous disease‐related symptoms but without visible signs (described as C0s in the CEAP classification): the Italian experience. Phlebolymphology. 2006;13:28–35. [Google Scholar]
- 46. Langer RD, Ho E, Denenberg JO, et al. Relationships between symptoms and venous disease: the San Diego population study. Arch Intern Med. 2005;165:1420–4. [DOI] [PubMed] [Google Scholar]
- 47. Guex JJ, Rabe E, Escotto SI, et al. The “C0s”' patient: worldwide results from the Vein Consult Program. Phlebolymphology. 2012;19:182–92. [Google Scholar]
- 48. Vuylsteke ME, Colman R, Thomis S, et al. The influence of age and gender on venous symptomatology. An epidemiological survey in Belgium and Luxembourg. Phlebology. 2016;31:325–33. [DOI] [PubMed] [Google Scholar]
- 49. Serra R, Grande R, Butrico L, et al. Epidemiology, diagnosis and treatment of chronic venous disease: a systematic review. Chirurgia. 2016;29:34–45. [Google Scholar]
- 50. Chiesa R, Marone EM, Limoni C, et al. Chronic venous disorders: correlation between visible signs, symptoms, and presence of functional disease. J Vasc Surg. 2007;46:322–30. [DOI] [PubMed] [Google Scholar]
- 51. Prandoni P, Lensing AW, Cogo A, et al. The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. [DOI] [PubMed] [Google Scholar]
- 52. Ende‐Verhaar YM, Tick LW, Klok FA, Huisman MV, Rosendaal FR, Cannegieter SC. Short and long‐term incidence of and risk factors for post thrombotic syndrome after a first deep vein thrombosis. Res Pract Thromb Haemost. 2017;1(suppl 1):193. [Google Scholar]
- 53. Cooley BC, Chen CY, Hess R, Schmeling G. Incomplete resolution of deep vein thrombosis under reduced flow conditions. Thromb Res. 2013;131:55–8. [DOI] [PubMed] [Google Scholar]
- 54. Perrin M, Ramelet AA. Pharmacological treatment of primary chronic venous disease: rationale, results and unanswered questions. Eur J Vasc Endovasc Surg. 2011;41:117–25. [DOI] [PubMed] [Google Scholar]
- 55. Flour M, Clark M, Partsch H, et al. Dogmas and controversies in compression therapy: report of an International Compression Club (ICC) meeting, Brussels, May 2011. Int Wound J. 2013;10:516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mosti G, Partsch H. High compression pressure over the calf is more effective than graduated compression in enhancing venous pump function. Eur J Vasc Endovasc Surg. 2012;44:332–6. [DOI] [PubMed] [Google Scholar]
- 57. Amin EE, Joore MA, ten Cate H, ten Cate‐Hoek AJ; the IDEAL DVT Investigators . No compression, multilayer compression bandaging or compression hosiery in the acute phase of deep vein thrombosis in relation to the Villalta score, health‐related quality of life, and costs at 3 months after the diagnosis. Res Pract Thromb Haemost. 2017; 1(suppl 1):196. [Google Scholar]
- 58. Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ‐5D and SF‐6D. Qual Life Res. 2005;14:1523–32. [DOI] [PubMed] [Google Scholar]
- 59. Bouman AC, Ten Cate‐Hoek AJ, Dirksen CD, Joore MA. Eliciting patients' preferences for elastic compression stocking therapy after deep vein thrombosis: potential for improving compliance. J Thromb Haemost. 2016;14:510–7. [DOI] [PubMed] [Google Scholar]
- 60. Tick LW, Doggen CJ, Rosendaal FR, et al. Predictors of the post‐thrombotic syndrome with non‐invasive venous examinations in patients 6 weeks after a first episode of deep vein thrombosis. J Thromb Haemost. 2010;8:2685–92. [DOI] [PubMed] [Google Scholar]
- 61. Bouman AC, Atalay S, ten Cate H, ten Wolde M, ten Cate‐Hoek AJ. Biomarkers for post‐thrombotic syndrome. J Vasc Surg Venous Lymphat Disord. 2014;2:79–88. [DOI] [PubMed] [Google Scholar]
- 62. Rabinovich A, Cohen JM, Kahn SR. Predictive value of markers of inflammation in the postthrombotic syndrome: a systematic review: inflammatory biomarkers and PTS. Thromb Res. 2015;136:289–97. [DOI] [PubMed] [Google Scholar]
- 63. Stain M, Schönauer V, Minar E, et al. The post‐thrombotic syndrome: risk factors and impact on the course of thrombotic disease. J Thromb Haemost. 2005;3:2671–6. [DOI] [PubMed] [Google Scholar]
- 64. Latella J, Desmarais S, Miron MJ, et al. Relation between D‐dimer level, venous valvular reflux and the development of post‐thrombotic syndrome after deep vein thrombosis. J Thromb Haemost. 2010;8:2169–75. [DOI] [PubMed] [Google Scholar]
- 65. Lippi G, Bonfanti L, Saccenti C, Cervellin G. Causes of elevated D‐dimer in patients admitted to a large urban emergency department. Eur J Intern Med. 2014;25:45–8. [DOI] [PubMed] [Google Scholar]
- 66. Bouman AC, McPherson H, Cheung YW, et al. Clot structure and fibrinolytic potential in patients with post thrombotic syndrome. Thromb Res. 2016;137:85–91. [DOI] [PubMed] [Google Scholar]
- 67. Chitsike RS, Rodger MA, Kovacs MJ, et al. Risk of post‐thrombotic syndrome after subtherapeutic warfarin anticoagulation for a first unprovoked deep vein thrombosis: results from the REVERSE study. J Thromb Haemost. 2012;10:2039–44. [DOI] [PubMed] [Google Scholar]
- 68. van Dongen CJ, Prandoni P, Frulla M, Marchiori A, Prins MH, Hutten BA. Relation between quality of anticoagulant treatment and the development of the postthrombotic syndrome. J Thromb Haemost. 2005;3:939–42. [DOI] [PubMed] [Google Scholar]
- 69. Ziegler S, Schillinger M, Maca TH, Minar E. Post‐thrombotic syndrome after primary event of deep venous thrombosis 10 to 20 years ago. Thromb Res. 2001;101:23–33. [DOI] [PubMed] [Google Scholar]
- 70. Hull RD, Pineo GF, Brant R, et al.; LITE Trial Investigators . Home therapy of venous thrombosis with long‐term LMWH versus usual care: patient satisfaction and post‐thrombotic syndrome. Am J Med. 2009;122:762–69.e3. [DOI] [PubMed] [Google Scholar]
- 71. Mousa SA. The low molecular weight heparin, tinzaparin, in thrombosis and beyond. Cardiovasc Drug Rev. 2002;20:199–216. [DOI] [PubMed] [Google Scholar]
- 72. Luzzi R, Belcaro G, Dugall M, et al. The efficacy of sulodexide in the prevention of postthrombotic syndrome. Clin Appl Thromb Hemost. 2014;20:594–9. [DOI] [PubMed] [Google Scholar]
- 73. Cheung YW, Middeldorp S, Prins MH, et al.; Einstein PTS Investigators Group . Post‐thrombotic syndrome in patients treated with rivaroxaban or enoxaparin/vitamin K antagonists for acute deep‐vein thrombosis. A post‐hoc analysis. Thromb Haemost. 2016;116:733–8. [DOI] [PubMed] [Google Scholar]
- 74. Utne KK, Ghanima W, Foyn S, Kahn S, Sandset PM, Wik HS. Development and validation of a tool for patient reporting of symptoms and signs of the post‐thrombotic syndrome. Thromb Haemost. 2016;115:361–7. [DOI] [PubMed] [Google Scholar]
- 75. Ursoy T, Tekinalp G, Yigit S, Kirazli S, Korkmaz A, Gurgey A. Thrombin activatable fibrinolysis inhibitor activity (TAFIa) levels in neonates with meconium‐stained amniotic fluid. J Matern Fetal Neonatal Med. 2008;21:123–8. [DOI] [PubMed] [Google Scholar]
- 76. Bajzar L, Nesheim M, Morser J, Tracy PB. Both cellular and soluble forms of thrombomodulin inhibit fibrinolysis by potentiating the activation of thrombin‐activable fibrinolysis inhibitor. J Biol Chem. 1998;273:2792–8. [DOI] [PubMed] [Google Scholar]
- 77. Enden T, Haig Y, Kløw NE, et al.; CaVenT Study Group . Long‐term outcome after additional catheter‐directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–8. [DOI] [PubMed] [Google Scholar]
- 78. Myers DD, Wakefield TW. Inflammation‐dependent thrombosis. Front Biosci. 2005;10:2750–7. [DOI] [PubMed] [Google Scholar]
- 79. Kellermair J, Redwan B, Alias S, et al. Platelet endothelial cell adhesion molecule 1 deficiency misguides venous thrombus resolution. Blood. 2013;122:3376–84. [DOI] [PubMed] [Google Scholar]
- 80. Bouman AC, Cheung YW, Spronk HM, et al. Biomarkers for post thrombotic syndrome: a case‐control study. Thromb Res. 2014;134:369–75. [DOI] [PubMed] [Google Scholar]
- 81. Diaz JA, Wrobleski SK, Alvarado CM, et al. P‐selectin inhibition therapeutically promotes thrombus resolution and prevents vein wall fibrosis better than enoxaparin and an inhibitor to von Willebrand factor. Arterioscler Thromb Vasc Biol. 2015;35:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Son EW, Lee KR, Rhee DK, et al. Effect of rutin on adhesion molecules expression and no production induced by ‐irradiation in human endothelial cells. J Appl Pharmacol. 2001;9:156–61. [Google Scholar]
- 83. Martinez MJ, Bonfill X, Moreno RM, Vargas E, Capellà D. Phlebotonics for venous insufficiency. Cochrane Database Syst Rev 2005;3:CD003229. [DOI] [PubMed] [Google Scholar]
- 84. Coleridge‐Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta‐analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg 2005;30:198–208. [DOI] [PubMed] [Google Scholar]
- 85. Prandoni P. Elastic stockings, hydroxyethylrutosides or both for the treatment of post‐thrombotic syndrome. Thromb Haemost. 2005;93:183–5. [PubMed] [Google Scholar]
- 86. de Jongste AB, Jonker JJ, Huisman MV, ten Cate JW, Azar AJ. A double blind three center clinical trial on the short‐term efficacy of 0‐(beta‐hydroxyethyl)‐rutosides in patients with post thrombotic syndrome. Thromb Haemost. 1989;62:826–9. [PubMed] [Google Scholar]
- 87. Ippolito E, Belcaro G, Dugall M, et al. Venoruton: post thrombotic syndrome. Clinical improvement in venous insuffi‐ ciency (signs and symptoms) with Venoruton. A five‐year, open‐registry, efficacy study. Panminerva Med. 2011;53(3 suppl 1):13–9. [PubMed] [Google Scholar]
- 88. Kahn SR, Comerota AJ, Cushman M, et al.; American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing . The postthrombotic syndrome: evidence‐based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636–61. [DOI] [PubMed] [Google Scholar]
- 89. Rodriguez AL, Wojcik BM, Wrobleski SK, Myers DD Jr, Wakefield TW, Diaz JA. Statins, inflammation and deep vein thrombosis: a systematic review. J Thromb Thrombolysis. 2012;33:371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Squizzato A, Galli M, Romualdi E, et al. Statins, fibrates, and venous thromboembolism: a meta‐analysis. Eur Heart J. 2010;31:1248–56. [DOI] [PubMed] [Google Scholar]
- 91. Rahimi K, Bhala N, Kamphuisen P, et al. Effect of statins on venous thromboembolic events: a meta‐analysis of published and unpublished evidence from randomized controlled trials. PLoS Med. 2012;9:e1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kessinger CW, Kim JW, Henke PK, et al. Statins improve the resolution of established murine venous thrombosis: reductions in thrombus burden and vein wall scarring. PLoS ONE. 2015;10:e0116621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. San Norberto EM, Gastambide MV, Taylor JH, García‐Saiz I, Vaquero C. Effects of rosuvastatin as an adjuvant treatment for deep vein thrombosis. Vasa. 2016;45:133–40. [DOI] [PubMed] [Google Scholar]
- 94. ten Cate‐Hoek AJ, Henke PK, Wakefield TW. The post thrombotic syndrome: ignore it and it will come back to bite you. Blood Rev. 2016;30:131–7. [DOI] [PubMed] [Google Scholar]
- 95. Elsharawy M, Elzayat E. Early results of thrombolysis vs anticoagulation in iliofemoral venous thrombosis. A randomised clinical trial. Eur J Vasc Endovasc Surg. 2002;24:209–14. [DOI] [PubMed] [Google Scholar]
- 96. Sharifi M, Bay C, Mehdipour M, Sharifi J; TORPEDO Investigators . Thrombus Obliteration by Rapid Percutaneous Endovenous Intervention in Deep Venous Occlusion (TORPEDO) trial: midterm results. J Endovasc Ther. 2012;19:273–80. [DOI] [PubMed] [Google Scholar]
- 97. Haig Y, Enden T, Grøtta O, et al. Post‐thrombotic syndrome after catheter‐directed thrombolysis for deep vein thrombosis (CaVenT): 5‐year follow‐up results of an open‐label, randomised controlled trial. Lancet Haematol. 2016;3:e64–71. [DOI] [PubMed] [Google Scholar]
- 98. Vedantham S, Sista AK, Klein SJ, et al.; Society of Interventional Radiology and Cardiovascular and Interventional Radiological Society of Europe Standards of Practice Committees . Quality improvement guidelines for the treatment of lower‐extremity deep vein thrombosis with use of endovascular thrombus removal. J Vasc Interv Radiol. 2014;25:1317–25. [DOI] [PubMed] [Google Scholar]
- 99. de Wolf MA, de Graaf R, Kurstjens RL, Penninx S, Jalaie H, Wittens CH. Short‐term clinical experience with a dedicated venous nitinol stent: initial results with the sinus‐venous stent. Eur J Vasc Endovasc Surg. 2015;50:518–26. [DOI] [PubMed] [Google Scholar]
- 100. Falcoz MT, Falvo N, Aho‐Glélé S, et al.; Burgundy Research; Study Group on Treatment of Venous Diseases . Endovascular stent placement for chronic post‐thrombotic symptomatic ilio‐femoral venous obstructive lesions: a single‐center study of safety, efficacy and quality‐of‐life improvement. Quant Imaging Med Surg. 2016;6:342–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eijgenraam P, ten Cate H, ten Cate‐Hoek AJ. Venous stenting after deep venous thrombosis and antithrombotic therapy: a systematic review. Rev Vasc Med. 2014;2:88–97. [Google Scholar]
- 102. Vedantham S, Goldhaber SZ, Julian JA, et al. Pharmacomechanical catheter‐directed thrombolysis for deep‐vein thrombosis. N Engl J Med. 2017;377:2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


