Abstract
Essentials.
Clinical trials demonstrated the gain of extended anticoagulation among patients with VTE.
In a real‐world setting, we evaluated outcomes of extended rivaroxaban use for unprovoked VTE.
Extended rivaroxaban treatment lowered the risk of recurrent VTE among unprovoked VTE patients.
Extended rivaroxaban treatment was not associated with increased risk of major bleeding.
Background
Randomized trial data demonstrate the gain of extended duration anticoagulation in patients with venous thromboembolic events (VTE); however, real‐world data are limited.
Objectives
Assess the risk of recurrent VTE and major bleeding in a real‐world setting of patients who experienced unprovoked VTE and received extended treatment with rivaroxaban.
Methods
US claims databases (February 2011–April 2015) were used in this retrospective study. The study population included adult patients initiated on rivaroxaban within 7 days after their first unprovoked VTE (ie, deep vein thrombosis, pulmonary embolism) and received ≥3 months continuous rivaroxaban treatment (index date: end of 3‐month treatment). Patients who were treated beyond 3 months formed the continued cohort and the remainder formed the discontinued cohort (ie, discontinued at 3 months). Adjusted Kaplan‐Meier rates for recurrent VTE and major bleeding events were compared between cohorts with confounders being controlled through a propensity score weighting approach.
Results
Patients in the continued cohort (N = 3763) had significantly lower rates of recurrent VTE than those who discontinued (N = 1051): 0.57% vs 1.19% (P = .042), 1.07% vs 2.10% (P = .017), and 1.45% vs 2.60% (P = .023) at 3, 6, and 12 months, respectively. No significant differences in the rate of major bleeding were observed between cohorts. A sensitivity analysis among unprovoked VTE patients receiving rivaroxaban for ≥6 months showed similar results.
Conclusions
Continued rivaroxaban treatment beyond an initial 3‐ or 6‐month treatment period significantly lowered the risk of recurrent VTE without a significant increase of major bleeding, compared to treatment discontinued at 3 or 6 months.
Keywords: anticoagulants, recurrence, rivaroxaban, treatment, venous thromboembolism
1. INTRODUCTION
Each year in the United States (US), venous thromboembolism (VTE) affects an estimated 900 000 people and is responsible for approximately 60 000‐100 000 deaths.1 The annual cost of VTE events in the US has been conservatively estimated at $7‐10 billion (in 2014 dollars) and is expected to increase with upward trends in per patient costs (primarily driven by increased hospitalization costs) and in total patient number, which is expected to double to 1.82 million by 2050.2, 3, 4 The two major subtypes of VTE are deep vein thrombosis (DVT) and pulmonary embolism (PE). Patients who have had their first VTE episode have increased risk of recurrence,5, 6, 7, 8, 9 with the risk being highest in the first few months after the initial episode and remaining high through the first year.10 About a quarter of patients will have a recurrent episode within 5 years of their initial VTE episode and 30% are expected to have a recurrent episode within 10 years.10
Although anticoagulant therapy is recognized as the standard of care for treatment and prophylaxis against VTE, there is still uncertainty over the optimal duration of treatment as long‐term use of anticoagulants is associated with reduced VTE recurrence risk but also increased bleeding risk.11, 12 Current guidelines from the American College of Chest Physicians (ACCP) recommend anticoagulant treatment for at least 3 months and suggest extended anticoagulant therapy beyond 3 months for patients with a first unprovoked VTE who have low or moderate bleeding risk.11, 12
Rivaroxaban, an oral direct factor Xa inhibitor anticoagulant indicated for acute and extended treatment of VTE,13 has been demonstrated as a viable alternative therapy to traditional vitamin K antagonist (VKA) therapy with advantages of minimal drug and food interactions and no requirement for routine coagulation monitoring.13, 14, 15 Findings from a pair of phase III clinical trials have shown that extended treatment with rivaroxaban for an additional 6–12 months is safe and effective to decrease VTE recurrence risk in patients with VTE who have previously completed 6 or 12 months of anticoagulant therapy.14, 16 The double‐blind placebo‐controlled EINSTEIN‐Extension trial (EINSTEIN‐EXT) reported a VTE recurrence rate of 1.3% in patients receiving extended treatment with once‐daily 20 mg rivaroxaban vs 7.1% in patients receiving placebo, representing a statistically significant 82% relative risk reduction (P < .001).17 A marginal increase in the rate of major bleeding that did not reach statistical significance was observed in the rivaroxaban cohort (0.7% vs 0% for placebo; P = .11). More recently, data from the double‐blind EINSTEIN‐CHOICE trial reported VTE recurrence rates of 1.5% and 1.2% in patients receiving up to 12 months of extended treatment with once‐daily 20 mg or 10 mg rivaroxaban respectively vs 4.4% for patients receiving once‐daily 100 mg aspirin, representing statistically significant 66% and 74% relative risk reductions (P < .001 for both).16 Observed rates of major bleeding were comparable at 0.5% and 0.4% in the 20 mg and 10 mg rivaroxaban cohorts vs 0.3% in the aspirin cohort. Although the results from the EINSTEIN‐EXT and EINSTEIN‐CHOICE trials suggest extended anticoagulant therapy could yield additional benefit in patients who have experienced VTE, the external validity of the trial results may be limited due to their limited study population size, restrictive entry criteria, close treatment monitoring, etc. Data that corroborate such findings in real‐world clinical practice settings are limited.
Recently, we have conducted a claims analysis covering US patients with VTE (including both provoked and unprovoked VTEs) in a real‐world clinical practice setting and found an association between extended treatment with rivaroxaban therapy and a lower risk of VTE recurrence.18 However, since patients with unprovoked VTE are known to have a higher risk of recurrent VTE compared to those with provoked VTE (ie, VTE caused by transient risk factors such as surgery),5, 6, 8 the extent to which the finding in the overall VTE population could be applicable to the subset of patients with unprovoked VTE remains unclear.
The current study is an extension of our previous claims study and aims to assess the risk of recurrent VTE and major bleeding in a real‐world setting among US patients who had their first unprovoked VTE and continued (also noted as extended treatment) or discontinued rivaroxaban therapy after a minimum duration of treatment. For the main analyses, the minimum duration of treatment was set at 3 months per ACCP guideline recommendations,12 and a sensitivity analysis was adopting an alternative minimum duration of treatment of 6 months as used in the EINSTEIN‐EXT and EINSTEIN‐CHOICE trials.14, 16
2. MATERIALS AND METHODS
2.1. Data source
This study used the US‐based Truven Health Analytics MarketScan claims databases from February 2011 to April 2015, which comprise the Commercial Claims and Encounters database and the Medicare Supplemental and Coordination of Benefits database. The former includes claims for employees and their dependents covered by employer‐sponsored private health insurance plans from approximately 100 employers and a number of health plans, while the latter focuses on patients aged 65 years old and above with Medicare coverage plus employer‐paid commercial plans. Institutional review board approval was not required for this study due to the preexisting and de‐identified nature of the data.
2.2. Study design
A retrospective longitudinal study design was used in this study. The study population included adult patients (≥18 years old) newly initiated on rivaroxaban within 7 days of a first unprovoked VTE (ie, index VTE) identified from hospitalization discharge or outpatient/emergency room visit with a primary or secondary VTE diagnosis. The definition of unprovoked VTE used in the current study was previously defined as a VTE occurring in the absence of malignancy, neurosurgery, orthopedic surgery, trauma, acute spinal cord injury, fracture, estrogen therapy, pregnancy/postpartum state, or oral contraceptive use during the 6‐month period prior to the VTE date.19
Patients in the study had to have received at least 3 months of continuous rivaroxaban treatment (ie, without a gap of more than 30 days between two adjacent dispensings for rivaroxaban) after their first unprovoked VTEs. The end of the initial 3‐month rivaroxaban treatment was defined as the index date. Patients whose continuous treatment ended within the 30‐day window (inclusively) after the index date formed the discontinued cohort, while patients whose continuous treatment ended after this 30‐day window formed the continued cohort. The baseline period was defined as the 12‐month continuous health plan enrollment period prior to the index date and the observation period was defined as the period from the index date until whichever event of the following occurred first: end of 12 months of follow‐up, end of continuous health plan enrollment, end of data availability, end of continuous treatment (applied for the continued cohort only), or re‐initiation of oral anticoagulants (applied for the discontinued cohort only). Variables measured during the baseline period include demographics, year of index date, type of index VTE (DVT, PE, or both), VTE identified in the hospital or outpatient/ER, time from first VTE to first rivaroxaban dispensing, Quan‐Charlson comorbidity index (CCI), RIETE bleeding score,20 baseline healthcare resource utilization and costs, risk factors for VTE (including risk factors from the ACCP Evidence‐Based Clinical Practice Guidelines list)21 and bleeding.15
Patients who had used anticoagulant agents during baseline, had a diagnosis of atrial fibrillation or major bleeding event during baseline, or had recurrent VTEs between the index VTE and the index date were excluded. Sensitivity analyses were conducted among patients who received at least 6 months of continuous rivaroxaban treatment.
2.3. Outcome measures
The study endpoints include recurrent VTE (DVT or PE) requiring hospitalization and major bleeding event requiring hospitalization. Recurrent VTEs were defined as hospitalizations with a primary diagnosis of VTE (International Classification of Disease, Ninth Revision, Clinical Modification [ICD‐9‐CM]: DVT: 451.1, 451.2, 453.4, 453.8, 453.9, or PE: 415.1). This restrictive definition helps assure identification of true recurrent VTE (improved specificity), however, recurrent VTE treated as an outpatient is missed (less sensitivity). Major bleeding events were defined as hospitalizations for major bleeding events identified based on Cunningham et al.'s validated algorithm.22 Time to first recurrent VTE and time to first major bleeding event from the index date were measured and patients who did not have the event of interest were censored at the end of the observation period.
2.4. Statistical analyses
Patient demographics and baseline clinical characteristics of both cohorts were reported and compared using descriptive statistics. Categorical variables were summarized with frequencies and proportions, continuous variables were summarized with means, standard deviations, and medians, and differences in baseline variables between cohorts were assessed using standardized differences (≥10% indicating imbalance between cohorts).23
To assess the relationship between continued treatment and outcomes (ie, recurrent VTEs and major bleeding events), Kaplan‐Meier curves adjusted with inverse probability of treatment weights (IPTWs) were compared between cohorts. The IPTWs were used to control for baseline confounding and potential selection bias,24 and were estimated in two steps. First, a multivariate logistic regression model was conducted to estimate the probability (ie, the propensity score [PS]) of receiving continued treatment (vs. discontinued treatment) conditional on baseline covariates. IPTWs were then calculated as 1/PS and 1/(1‐PS) for the continued and discontinued cohorts, respectively, and normalized within each cohort (ie, dividing each weight by the mean of the weights per cohort). The baseline covariates included in the logistic regression model were age, gender, region, insurance type, year of index date, episode type of first VTE (inpatient vs. outpatient settings), type of first VTE (DVT vs PE), time from first VTE to first rivaroxaban dispensing, baseline risk factors for bleeding and VTE, Quan‐Charlson comorbidity index, RIETE score, and healthcare resource utilization and costs. All the analyses in the study were performed with SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Baseline patient characteristics
Among the 4814 unprovoked VTE patients who had received initial treatment with rivaroxaban for at least 3 months, 3763 (78.2%) and 1051 (21.8%) patients formed the continued and discontinued cohorts, respectively (Figure 1). Patient baseline characteristics between cohorts are compared in Tables 1 and 2. The mean observation period was 160 days and 178 days in the continued cohort and the discontinued cohort, respectively. Continued cohort patients were older, had a lower proportion of female patients, more likely to have an initial PE, have more hospitalizations within the baseline period, had slightly higher RIETE bleeding scores, and had higher Quan‐CCI scores compared with discontinued cohort patients. After weighting, the two cohorts had well‐balanced baseline characteristics (Tables 1 and 2).
Figure 1.
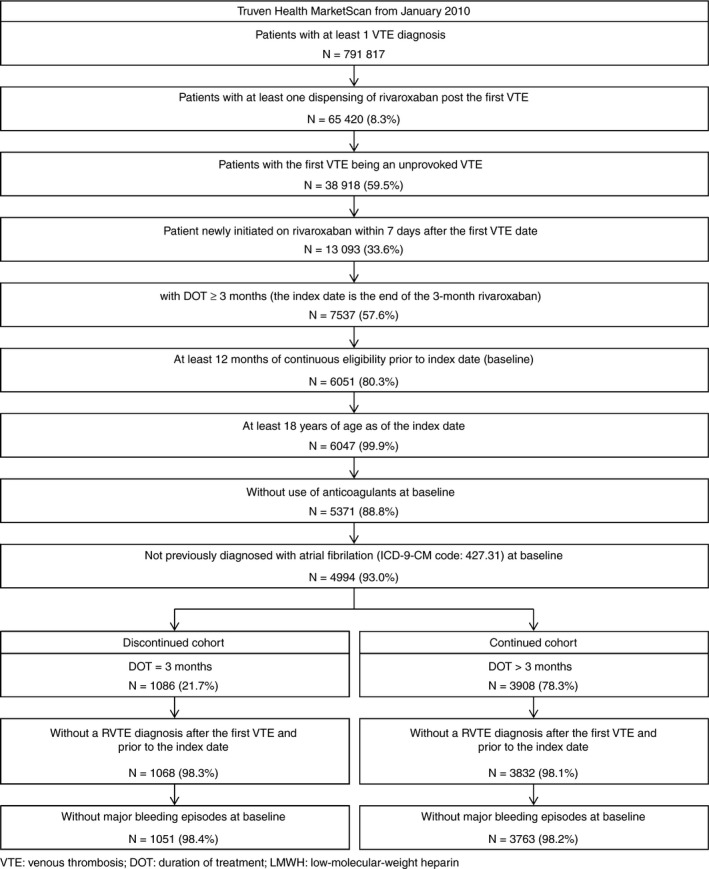
Patient disposition–3‐month therapy
Table 1.
Demographic and clinical characteristics assessed during the 12‐month baseline period among rivaroxaban users who continued vs. discontinued therapy after 3 months
| Characteristics | Unweighted cohorts | IPTW‐weighted cohorts1 | ||||
|---|---|---|---|---|---|---|
| Discontinued cohort | Continued cohort | Standardized difference | Discontinued cohort | Continued cohort | Standardized difference | |
| (N = 1051) | (N = 3763) | (N = 1051) | (N = 3763) | |||
| Observation period, days, mean ±SD [median]2 | 178 ± 193 [101] | 160 ± 133 [112] | – | 203 ± 195 [149] | 154 ± 131 [109] | – |
| Total duration of treatment (including 3 months of rivaroxaban therapy), days, mean ± SD [median]3 | 105 ± 9 [106] | 252 ± 134 [203] | – | 106 ± 9 [107] | 247 ± 132 [200] | – |
| Propensity score variables | ||||||
| Demographics | ||||||
| Age, years, mean ± SD [median] | 55.8 ± 14.5 [56] | 57.2 ± 13.7 [57] | 10.0% | 57.0 ± 14.5 [57] | 56.8 ± 13.8 [57] | 1.0% |
| Gender, female, n (%) | 445 (42.3%) | 1432 (38.1%) | 8.7% | 413 (39.3%) | 1469 (39.1%) | 0.5% |
| Insurance type, n (%) | ||||||
| Consumer directed health plan | 619 (58.9%) | 2184 (58.0%) | 1.7% | 614 (58.4%) | 2186 (58.1%) | 0.6% |
| Comprehensive | 76 (7.2%) | 291 (7.7%) | 1.9% | 83 (7.9%) | 289 (7.7%) | 0.9% |
| Exclusive provider organization | 118 (11.2%) | 505 (13.4%) | 6.7% | 136 (12.9%) | 485 (12.9%) | 0.1% |
| High‐deductible health plan | 68 (6.5%) | 228 (6.1%) | 1.7% | 65 (6.1%) | 235 (6.2%) | 0.4% |
| Health maintenance organization | 77 (7.3%) | 316 (8.4%) | 4.0% | 86 (8.1%) | 309 (8.2%) | 0.3% |
| Point‐of‐service | 3 (0.3%) | 19 (0.5%) | 3.5% | 5 (0.4%) | 17 (0.5%) | 0.4% |
| Point‐of‐service capitated | 3 (0.3%) | 16 (0.4%) | 2.3% | 3 (0.3%) | 15 (0.4%) | 2.3% |
| Preferred provider organization | 72 (6.9%) | 163 (4.3%) | 11.0% | 49 (4.7%) | 183 (4.9%) | 0.9% |
| Not specified | 15 (1.4%) | 41 (1.1%) | 3.0% | 12 (1.1%) | 43 (1.2%) | 0.4% |
| Year of index date, n (%) | ||||||
| 2012 | 6 (0.6%) | 15 (0.4%) | 2.5% | 4 (0.4%) | 17 (0.4%) | 0.3% |
| 2013 | 233 (22.2%) | 1059 (28.1%) | 13.8% | 285 (27.1%) | 1009 (26.8%) | 0.7% |
| 2014 | 474 (45.1%) | 2185 (58.1%) | 25.9% | 572 (54.5%) | 2075 (55.1%) | 1.4% |
| 2015 | 338 (32.2%) | 504 (13.4%) | 44.7% | 189 (18.0%) | 662 (17.6%) | 1.0% |
| First VTE diagnosis identified in hospital, n (%) | 399 (38.0%) | 1807 (48.0%) | 20.3% | 474 (45.1%) | 1721 (45.7%) | 1.3% |
| Type of first VTE diagnosis, n (%) | ||||||
| Deep vein thrombosis | 672 (63.9%) | 1904 (50.6%) | 27.0% | 564 (53.7%) | 2015 (53.6%) | 0.3% |
| Pulmonary embolism | 235 (22.4%) | 1073 (28.5%) | 14.1% | 295 (28.1%) | 1024 (27.2%) | 1.9% |
| Both diagnoses on the same day | 144 (13.7%) | 786 (20.9%) | 19.0% | 192 (18.3%) | 724 (19.2%) | 2.5% |
| Time from first VTE to first rivaroxaban dispensing | ||||||
| Mean ± SD [median] | 0.7 ± 1.2 [0] | 0.7 ± 1.2 [0] | 3.4% | 0.7 ± 1.2 [0] | 0.7 ± 1.2 [0] | 1.2% |
| Same day, n (%) | 629 (59.8%) | 2328 (61.9%) | 4.1% | 638 (60.7%) | 2311 (61.4%) | 1.5% |
| 1 day, n (%) | 270 (25.7%) | 928 (24.7%) | 2.4% | 262 (25.0%) | 936 (24.9%) | 0.2% |
| 2 days, n (%) | 74 (7.0%) | 218 (5.8%) | 5.1% | 69 (6.6%) | 229 (6.1%) | 2.0% |
| 3 days, n (%) | 25 (2.4%) | 131 (3.5%) | 6.5% | 33 (3.2%) | 122 (3.2%) | 0.4% |
| 4–7 days, n (%) | 53 (5.0%) | 158 (4.2%) | 4.0% | 48 (4.6%) | 165 (4.4%) | 1.0% |
| Comorbidity index scores | ||||||
| Quan‐Charlson comorbidity index, mean ± SD [median] | 1.0 ± 1.6 [0] | 1.1 ± 1.6 [1] | 6.0% | 1.2 ± 1.7 [1] | 1.1 ± 1.6 [1] | 5.2% |
| N (%) | ||||||
| 0 | 540 (51.4%) | 1796 (47.7%) | 7.3% | 496 (47.2%) | 1824 (48.5%) | 2.6% |
| 1 | 273 (26.0%) | 980 (26.0%) | 0.2% | 273 (25.9%) | 979 (26.0%) | 0.2% |
| 2 | 102 (9.7%) | 434 (11.5%) | 5.9% | 125 (11.9%) | 419 (11.1%) | 2.5% |
| ≥3 | 136 (12.9%) | 553 (14.7%) | 5.1% | 157 (15.0%) | 541 (14.4%) | 1.7% |
| RIETE score, mean ±SD [median] | 1.1 ± 1.2 [1] | 1.2 ± 1.2 [1] | 7.7% | 1.2 ± 1.2 [1] | 1.2 ± 1.2 [1] | 4.7% |
| N (%) | ||||||
| 0 | 440 (41.9%) | 1287 (34.2%) | 15.8% | 371 (35.3%) | 1349 (35.8%) | 1.1% |
| 1–4 | 580 (55.2%) | 2377 (63.2%) | 16.2% | 652 (62.1%) | 2314 (61.5%) | 1.2% |
| >4 | 31 (2.9%) | 99 (2.6%) | 1.9% | 27 (2.6%) | 100 (2.7%) | 0.3% |
| Baseline healthcare utilization, mean ± SD [median] | ||||||
| Hospitalizations | 0.57 ± 0.71 [0] | 0.64 ± 0.67 [1] | 10.8% | 0.62 ± 0.69 [1] | 0.62 ± 0.68 [1] | 0.7% |
| ER visits | 0.93 ± 1.27 [1] | 0.95 ± 1.39 [1] | 1.3% | 1.00 ± 1.38 [1] | 0.95 ± 1.36 [1] | 3.3% |
| Outpatient visits | 16.73 ± 13.99 [13] | 15.79 ± 13.08 [12] | 6.9% | 16.20 ± 13.28 [13] | 16.00 ± 13.28 [12] | 1.5% |
| Baseline healthcare cost, $US 2015, mean ± SD | ||||||
| Total healthcare cost | $24 383 ± 30 185 | $25 620 ± 29 023 | 4.2% | $26 353 ± 30 321 | $25 414 ± 29 001 | 3.2% |
| Hospitalizations | $10 740 ± 21 931 | $11 883 ± 20 784 | 5.3% | $12 126 ± 22 478 | $11 696 ± 20 958 | 2.0% |
| ER visits | $1956 ± 4254 | $2027 ± 4341 | 1.7% | $2123 ± 4718 | $2018 ± 4270 | 2.3% |
| Outpatient visits | $7159 ± 12 425 | $6804 ± 11 590 | 3.0% | $7235 ± 12 738 | $6923 ± 11 672 | 2.6% |
| Pharmacy | $3698 ± 5620 | $4326 ± 8356 | 8.8% | $4174 ± 6562 | $4185 ± 7852 | 0.1% |
VTE: venous thromboembolism; SD: standard deviation; ER: emergency room; RIETE: Registro Informatizado de Enfermedad TromboEmbólica.
Notes:
1. The propensity score of receiving continued treatment (vs. discontinued treatment) was estimated using a multivariate logistic regression model conditional on baseline covariates including age, gender, region, insurance type, year of index date, episode type of first VTE, type of first VTE, time from first VTE to first rivaroxaban dispensing, baseline risk factors for bleeding and VTE, Quan‐Charlson comorbidity index, RIETE score, and healthcare resource utilization and costs.
2. From the index date to the earliest date between initiation of a new anticoagulant therapy, end of data availability (April 2015), or end of insurance coverage as well as the end of the rivaroxaban therapy for the continued cohort.
3. Duration of treatment was defined as the duration of continuous use of rivaroxaban from the first dispensing until a 30‐day interruption or end of follow‐up.
Table 2.
Baseline risk factorsa among rivaroxaban users who continued vs. discontinued therapy after 3 months
| Characteristics | Unweighted cohorts | IPTW‐weighted cohortsb | ||||
|---|---|---|---|---|---|---|
| Discontinued cohort | Continued cohort | Standardized difference | Discontinued cohort | Continued cohort | Standardized difference | |
| (N = 1051) | (N = 3763) | (N = 1051) | (N = 3763) | |||
| VTE and bleeding risk factors, n (%) | ||||||
| Hypertension | 501 (47.7%) | 1929 (51.3%) | 7.2% | 541 (51.5%) | 1903 (50.6%) | 1.8% |
| Diabetes | 160 (15.2%) | 724 (19.2%) | 10.6% | 200 (19.0%) | 692 (18.4%) | 1.6% |
| VTE risk factors, n (%) | ||||||
| Hyperlipidemia | 424 (40.3%) | 1627 (43.2%) | 5.9% | 442 (42.0%) | 1603 (42.6%) | 1.1% |
| Trauma | 275 (26.2%) | 850 (22.6%) | 8.3% | 240 (22.8%) | 880 (23.4%) | 1.3% |
| Other serious infections | 198 (18.8%) | 647 (17.2%) | 4.3% | 185 (17.6%) | 658 (17.5%) | 0.2% |
| Obesity | 181 (17.2%) | 697 (18.5%) | 3.4% | 193 (18.4%) | 688 (18.3%) | 0.3% |
| Abdominal surgery | 131 (12.5%) | 406 (10.8%) | 5.2% | 130 (12.4%) | 421 (11.2%) | 3.6% |
| Arrhythmia | 123 (11.7%) | 455 (12.1%) | 1.2% | 130 (12.3%) | 452 (12.0%) | 1.0% |
| Pneumonia | 113 (10.8%) | 438 (11.6%) | 2.8% | 118 (11.2%) | 430 (11.4%) | 0.7% |
| Major surgery | 70 (6.7%) | 271 (7.2%) | 2.1% | 84 (8.0%) | 269 (7.1%) | 3.4% |
| Varicose veins | 69 (6.6%) | 195 (5.2%) | 5.9% | 57 (5.4%) | 204 (5.4%) | 0.1% |
| Bleeding risk factors, n (%) | ||||||
| NSAID use | 300 (28.5%) | 970 (25.8%) | 6.2% | 281 (26.7%) | 991 (26.3%) | 0.9% |
| Excessive fall risk (Parkinson's disease, etc.) | 185 (17.6%) | 589 (15.7%) | 5.2% | 173 (16.4%) | 605 (16.1%) | 1.0% |
| Renal disease | 171 (16.3%) | 638 (17.0%) | 1.8% | 177 (16.9%) | 634 (16.8%) | 0.1% |
| Anemia | 157 (14.9%) | 524 (13.9%) | 2.9% | 153 (14.6%) | 534 (14.2%) | 1.1% |
| Hepatic disease | 64 (6.1%) | 241 (6.4%) | 1.3% | 70 (6.7%) | 239 (6.3%) | 1.3% |
| Chronic kidney disease | 59 (5.6%) | 246 (6.5%) | 3.9% | 69 (6.6%) | 237 (6.3%) | 1.1% |
VTE: venous thromboembolism; NSAID: nonsteroidal anti‐inflammatory drugs; COPD: chronic obstructive pulmonary disease; SERM: selective estrogen receptor modulator.
Notes:
Table shows only factors observed in at least 5% of the sample. Additional propensity score variables not reported in this table include the following bleeding and VTE risk factors: cancer, cerebrovascular accident (stroke), congestive heart failure, COPD, thrombophilia, myocardial infarction, rheumatoid arthritis, left ventricular dysfunction, inflammatory bowel disease, surgical resection of abdominal or pelvic cancer, pregnancy, hip, pelvis, or leg fracture, immobility, treatment with aromatase inhibitors, contraceptive pill (use of oral), spinal cord injury, treatment with erythropoiesis stimulating agents, total hip replacement, total knee replacement, treatment with serms, coagulation defect, thrombocytopenia (low platelet count), central venous catheter, ethanol abuse, peptic ulcer, and bleeding diathesis.
The propensity score of receiving continued treatment (vs. discontinued treatment) was estimated using a multivariate logistic regression model conditional on baseline covariates including age, gender, region, insurance type, year of index date, episode type of first VTE, type of first VTE, time from first VTE to first rivaroxaban dispensing, baseline risk factors for bleeding and VTE, Quan‐Charlson comorbidity index, RIETE score, and healthcare resource utilization and costs.
3.2. Recurrent VTE
In the weighted population, 47 patients with recurrent VTEs were identified at 12 months of follow‐up, with a corresponding Kaplan‐Meier rate of 1.72% at 12 months. Among these patients with recurrent VTEs, 21 had a diagnosis of PE and 26 had a diagnosis of DVT. Patients in the continued cohort had significantly lower rates of recurrent VTE than those in the discontinued cohort at all different points of time over follow‐up: 3 months (0.57% vs 1.19%, P = .042), 6 months (1.07% vs 2.10%, P = .017), and 12 months (1.45% vs 2.60%, P = .023) (Figure 2). The difference of KM rates between cohorts increased slightly over time. The analysis by types of recurrent VTE showed that patients in the continued cohort had a significantly lower rate of recurrent PE at 12 months compared to those in the discontinued cohort (0.52% vs. 1.52%, P = .005), while the rate of the recurrent DVT did not differ significantly between the two cohorts (rates at 12 months, continued and discontinued: 0.93% and 1.09%, P = .630).
Figure 2.
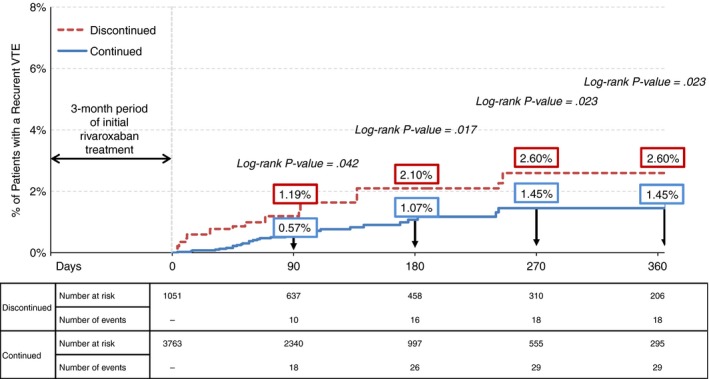
IPTW adjusted Kaplan‐Meier rates of recurrent VTE among rivaroxaban users who continued vs. discontinued therapy after 3 months
3.3. Major bleeding events
In the weighted population, 30 patients with 37 major bleeding events were identified at 12 months of follow‐up. Of these events, 21 were gastrointestinal, four were genitourinary, one was cerebral, and 11 were other bleeding. No statistically significant differences in the rates of major bleeding were observed between cohorts. The rates of major bleeding in the continued and the discontinued cohort were 0.51% and 0.72% at 3 months (P = .367), 0.79% and 0.72% at 6 months (P = .756), and 1.06% and 1.13% at 12 months (P = .780), respectively (Figure 3).
Figure 3.
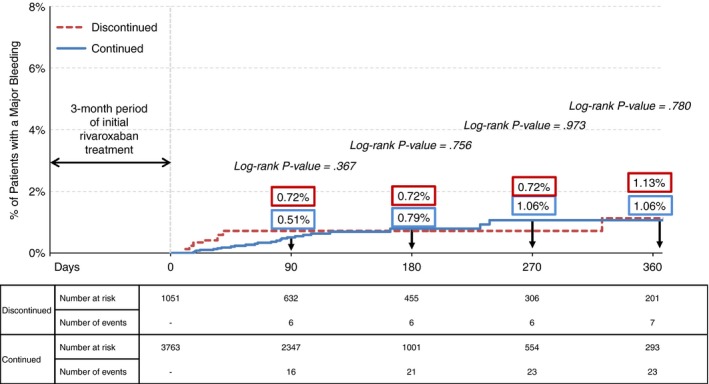
IPTW adjusted Kaplan‐Meier rates of major bleeding events among rivaroxaban users who continued vs. discontinued therapy after 3 months
3.4. Sensitivity analysis
A sensitivity analysis was conducted among the 2557 patients who received rivaroxaban treatment for at least 6 months. Comparisons of patient baseline characteristics between the discontinued (N = 793) and continued cohort (N = 1764) are presented in Table S1. This analysis also showed that the continued treatment was associated with a reduced risk of recurrent VTE with no significant difference in major bleeding (Figures S1 and S2).
4. DISCUSSION
Currently, there is no clear consensus on the optimal time that unprovoked VTE patients should remain on anticoagulants as long‐term use of anticoagulants will reduce the risk of recurrence but may increase the risk of bleeding. The current study assessed the impact of the 3‐ or 6‐month extended treatment with rivaroxaban on the risk of recurrent VTE and major bleeding among patients with unprovoked VTEs in the real‐world settings using US‐based claims databases. Compared with those who stopped rivaroxaban at 3 months, patients who received rivaroxaban beyond 3 months (ie, the continued cohort) had a 44% lower risk of recurrent VTE at 12 months (1.45% vs 2.60%, P = .023). The number needed to treat for 12 months to avoid one recurrent VTE was 87. Similarly, the analysis of continuous treatment with rivaroxaban for at least 6 months (vs stopped at 6 months) showed that patients in the continued cohort had a 65% lower risk of recurrent VTE at 12 months (from 4.62% vs 1.64% for discontinued vs continued cohorts, P = .002). Both 3‐ and 6‐month analyses showed no statistically significant differences in the risk of major bleeding event between the continued and discontinued cohorts.
The stratified analysis by type of recurrent VTE (PE or DVT) in the current study demonstrated that patients in the continued cohort had a significantly lower risk of recurrent PE compared to those in the discontinued cohort. Although the rate of recurrent DVT was also lower in the continued cohort, this difference was not statistically significant. This may be explained by insufficient statistical power to detect an effect due to the short duration of follow‐up (up to 12 months) and by the potential underestimation of the number of recurrent VTEs due to using a hospitalization‐based approach to identify recurrent VTEs (ie, only VTEs diagnosed in hospitalizations were defined as recurrent VTEs). Since patients with DVT are more likely to be treated in outpatient settings than patients with PE, underestimation of recurrent events using a hospitalization‐based approach may have a more profound impact on the rate estimation of recurrent DVT than recurrent PE.
The findings in the current study indicating a lower risk of recurrent VTE among patients with unprovoked VTE who received the extended treatment with rivaroxaban are consistent with that reported in our previous study conducted among patients with VTE. Specifically, in the 3‐month analysis, the relative risk reduction for recurrent VTE at 12 months was 35% (from 3.01% to 1.97% for discontinued vs continued cohorts, P = .017) among patients with VTE (ie, provoked and unprovoked VTE), compared to 44% observed in our study among patients with unprovoked VTE. Likewise, the relative risk reduction in the 6‐month sensitivity analysis was also similar, with 54% (from 3.70% to 1.72% for discontinued vs continued cohorts, P = .024) observed among the overall VTE population and 65% observed in the current study.18 In addition, as with the current study, our previous study did not find a statistically significant difference in the risk for major bleeding between cohorts.18
Our findings on the association between extended treatment beyond 3 months and a low risk of recurrent VTE without an increasing risk of major bleeding support the ACCP suggestion of using extended anticoagulant therapy over 3 months among patients who have had their first unprovoked VTE and who have low or moderate bleeding risk.11 Moreover, the finding in our sensitivity analysis on the association between the extended treatment beyond 6 months and a low risk of recurrent VTE are consistent with the EINSTEIN‐EXT and EINSTEIN‐CHOICE trials. The relative risk reduction of recurrent VTE event at 12 months was 65% lower for those who continued treatment vs discontinued in our study. The corresponding risk reduction was 82% in EINSTEIN‐EXT (rivaroxaban vs. placebo), and about 70% in EINSTEIN‐CHOICE (rivaroxaban vs. aspirin).14, 16, 25
In both 3‐month and 6‐month analyses, rates of major bleeding events were low and did not differ significantly between the two cohorts (ie, continued and discontinued). Given the short duration of follow‐up (up to 12 months) in our study, insufficient statistical power may explain the inability to detect a significant association of extended treatment with risk of major bleeding. Another possibility is that a potential “reverse causation” may distort the estimated association as patients in the discontinued cohort might have been taken off therapy due to early symptoms of bleeding before the index date and subsequently had a major bleeding event. Therefore, our study may overestimate the rate of major bleeding events in the discontinued cohort in the early period of follow‐up and thus diminish the difference in the risk of major bleeding between cohorts. However, with limited information on clinical factors in a claims database, we were not able to test this “reverse causation” hypothesis. Regarding major bleeding events, the non‐significant rate difference estimated from our 6‐month analysis was smaller than the rate difference reported in the EINSTEIN‐EXT trial but similar to that in the EINSTEIN‐CHOICE trial, although the level of events was higher (our study: 1.51% vs. 1.39% for the continued and discontinued cohorts, respectively; EINSTEIN‐EXT: 0.7% vs 0% in rivaroxaban and placebo cohorts, respectively; EINSTEIN‐CHOICE: 0.5% and 0.4% vs 0.3% for the 10 mg, 20 mg rivaroxaban, and aspirin cohorts, respectively).14, 16 This may be due to a lower risk of bleeding in the population of clinical trials given their restrictive eligibility criteria.
Our study results are consistent with the findings in other studies assessing the benefits and risks associated with long‐term use of other anticoagulants among patients with unprovoked VTE. According to Kearon et al., extended therapy with warfarin decreased the risk of recurrent VTE by 95% compared to placebo (P < .001), with only a small non‐significant increase in the risk of major bleeding (3.8% vs none for placebo; P = .09).26 Their trial evaluated warfarin therapy for 24 months versus placebo in patients who had completed 3 months of anticoagulant therapy due to an initial VTE. At least two additional trials have reported similar findings. One study on PE27 and a second study on DVT28 have reported decreased risk of recurrent VTE among patients who received 9‐month extended treatment with VKAs (warfarin or acenocoumarol) following 3 months of anticoagulant therapy. Both studies also reported that the benefit from extended anticoagulant therapy was not maintained following treatment discontinuation.27, 28 Summarizing the clinical trial (eg, AMPLIFY‐EXT [apixaban vs placebo] and RE‐SONATE [dabigatran vs placebo]) evidence base for recurrent VTE risk and duration of anticoagulant therapy, Smilowitz et al. suggested anticoagulant therapy for at least 3–6 months after an initial VTE episode and then recommended tailoring the duration of subsequent therapy to the individual.29 Two reviews covering multiple phase III studies evaluating newer oral anticoagulants have also reported that the clinical data show the drugs are effective for long‐term prevention of recurrent VTE.30, 31 Finally, a systematic review has reported that long‐term anticoagulant therapy with a VKA is associated with reduced risk of recurrent VTE.32
This study has a number of limitations. First, claims databases are subject to billing inaccuracies or incomplete coding. Second, the actual consumption of rivaroxaban and the duration of treatment may be overestimated since no information is available to assess whether the medication was taken as prescribed. Third, not capturing the recurrent VTE diagnosed in outpatient settings may have underestimated the rate of recurrent VTE and major bleeding, particularly for recurrent DVT, which are likely to be diagnosed and treated in outpatient settings. However, since the extent of event underestimation may be the same across cohorts, such detection bias may be unlikely to affect our estimates on the rate differences. Fourth, death is not available in the claims data used in this study and we were not able to assess its potential impact on our findings. Finally, as with all observational studies, our study results may be subject to unmeasured confounders, such as the potential difference in patients’ severity of illness.
Despite the described limitations, the current study provides real‐world evidence on the benefits and risks of extended anticoagulation therapy among patients with unprovoked VTE. The current findings show that patients with unprovoked VTE who received continuous rivaroxaban treatment for a period longer than 3 months had significantly lower risk of recurrent VTE requiring hospitalization and no increased risk of major bleeding. Similar observations were made when comparing patients who continued rivaroxaban after the first 6 months of treatment with those who stopped at 6 months, corroborating results in randomized clinical trials.
AUTHOR CONTRIBUTIONS
J.S. Berger, R. Seheult, F. Laliberté, C. Crivera, D. Lejeune, Y. Xiao, J. Schein, P. Lefebvre, and S. Kaatz participated in the study concept and design and data interpretation. F. Laliberté, D. Lejeune, Y. Xiao performed the data collection and statistical analyses. Writing of the manuscript was shared by F. Laliberté, D. Lejeune, Y. Xiao. Revision of the manuscript was shared by J.S. Berger, R. Seheult, C. Crivera, J. Schein, P. Lefebvre, and S. Kaatz. All approved this version of the manuscript to be published and agreed to be accountable for all aspects of the study.
RELATIONSHIP DISCLOSURES
This research was funded by Janssen Scientific Affairs, LLC, Titusville, NJ, United States. The study sponsor, Janssen LLC, was involved in some stages of the study research and manuscript preparation (study design, collection, analysis of data), but had no influence on the interpretation of the results and the decision to submit the final paper, which were made by each author independently. F. Laliberté, D. Lejeune, P. Lefebvre, and Y. Xiao are employees of Groupe d'analyse, Ltée., a consulting company that has received research grants from Janssen Scientific Affairs. C. Crivera and J. Schein are employees of Janssen Scientific Affairs, LLC.
Supporting information
Berger JS, Seheult R, Laliberté F, et al. Clinical outcomes of prolonged anticoagulation with rivaroxaban after unprovoked venous thromboembolism. Res Pract Thromb Haemost. 2018;2:58–68. 10.1002/rth2.12050
Parts of this study were presented at the CHEST Annual Meeting (October 2016) in Los Angeles, USA and at the ESC Congress (August 2016) in Rome, Italy
REFERENCES
- 1. Centers for disease control and, prevention . Deep vein Thrombosis/pulmonary embolism (DVT/PE) [Internet]. [cited 2016 Jun 16]. Available from: https://www.cdc.gov/ncbddd/dvt/index.html
- 2. Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011;86:217–20. [DOI] [PubMed] [Google Scholar]
- 3. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez M, Hogue SL, Preblick R, Kwong WJ. Review of the cost of venous thromboembolism. Clin Outcomes Res. 2015;7:451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107:I22–30. [DOI] [PubMed] [Google Scholar]
- 6. Zhu T, Martinez I, Emmerich J. Venous thromboembolism: risk factors for recurrence. Arterioscler Thromb Vasc Biol. 2009;29:298–310. [DOI] [PubMed] [Google Scholar]
- 7. Prandoni P, Lensing AW, Cogo A, et al. The long‐term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125:1–7. [DOI] [PubMed] [Google Scholar]
- 8. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. [DOI] [PubMed] [Google Scholar]
- 9. Hansson PO, Sörbo J, Eriksson H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Arch Intern Med. 2000;160:769–74. [DOI] [PubMed] [Google Scholar]
- 10. Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 13. Janssen Pharmaceuticals I . Prescribing Information for XARELTO. 2015.
- 14. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 15. Laliberté F, Cloutier M, Nelson WW, et al. Real‐world comparative effectiveness and safety of rivaroxaban and warfarin in nonvalvular atrial fibrillation patients. Curr Med Res Opin. 2014;30:1317–25. [DOI] [PubMed] [Google Scholar]
- 16. Weitz JI, Lensing AWA, Prins MH, et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–22. [DOI] [PubMed] [Google Scholar]
- 17. The EINSTEIN Investigators . Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. [DOI] [PubMed] [Google Scholar]
- 18. Khorana AA, Berger JS, Wells PS, et al. Risk for venous thromboembolism recurrence among rivaroxaban‐treated patients who continued versus discontinued therapy: analyses among patients with VTE. Clin Ther. 2017;39:1396–408. [DOI] [PubMed] [Google Scholar]
- 19. Laliberté F, Coleman CI, Bookhart B, et al. Weekly risk of venous thromboembolism recurrence in patients receiving oral anticoagulants. Curr Med Res Opin. 2014;30:1513–20. [DOI] [PubMed] [Google Scholar]
- 20. Ruíz‐Giménez N, Suárez C, González R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008;100:26–31. [DOI] [PubMed] [Google Scholar]
- 21. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients. Chest. 2012;141:e195S–226S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA. An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf. 2011;20:560–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin P. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat‐Simul Comput. 2009;38:1228–34. [Google Scholar]
- 24. Hernán MA, Robins JM. Estimating causal effects from epidemiological data. J Epidemiol Community Health. 2006;60:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen AT, Dobromirski M. The use of rivaroxaban for short‐ and long‐term treatment of venous thromboembolism. Thromb Haemost. 2012;107:1035–43. [DOI] [PubMed] [Google Scholar]
- 26. Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med. 1999;340:901–7. [DOI] [PubMed] [Google Scholar]
- 27. Agnelli G, Prandoni P, Becattini C, et al. Extended oral anticoagulant therapy after a First episode of pulmonary embolism. Ann Intern Med. 2003;139:19. [DOI] [PubMed] [Google Scholar]
- 28. Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. N Engl J Med. 2001;345:165–9. [DOI] [PubMed] [Google Scholar]
- 29. Smilowitz NR, Mega JL, Berger JS. Duration of anticoagulation for venous thromboembolic events. Circulation. 2014;130:2343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen AT. Long‐term benefits of preventing venous thromboembolic events. Curr Med Res Opin. 2012;28:877–89. [DOI] [PubMed] [Google Scholar]
- 31. Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kearon C, Akl EA. Duration of anticoagulant therapy for deep vein thrombosis and pulmonary embolism. Blood. 2014;123:1794–801. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


